Abstract
Introduction
Diabetic retinopathy remains one of the most feared complications of diabetes. Despite extensive research in the field, the molecular mechanism responsible for the development of this slow progressing disease remains unclear. In the pathogenesis of diabetic retinopathy, mitochondria are damaged and inflammatory mediators are elevated before the histopathology associated with the disease can be observed. Matrix metalloproteinases (MMPs) regulate a variety of cellular functions including apoptosis and angiogenesis. Diabetic environment stimulates the secretion of several MMPs that are considered to participate in complications, including retinopathy, nephropathy and cardiomyopathy. Patients with diabetic retinopathy and also animal models have shown increased MMP-9 and MMP-2 in their retina and vitreous. Recent research has shown that MMPs have dual role in the development of diabetic retinopathy; in the early stages of the disease (pre-neovascularization), MMP-2 and MMP-9 facilitate the apoptosis of retinal capillary cells, possibly via damaging the mitochondria, and in the later phase, they help in neovascularization.
Areas covered
This article reviews the literature to evaluate the role of MMPs, especially MMP-9, in the development of diabetic retinopathy, and presents existing evidence that the inhibitors targeted toward MMP-9, depending on the duration of diabetes at the times their administration could have potential to prevent the progression of this blinding disease, and protect the vision loss.
Expert opinion
Inhibitors of MMPs could have dual role: in the early stages of the diseases, inhibit capillary cell apoptosis, and if the disease has progressed to the angiogenic stage, inhibit the growth of new vessels.
Keywords: diabetes, diabetic retinopathy, matrix metalloproteinases, retina
1. Diabetic retinopathy
Diabetic retinopathy is one of the most common eye disease faced by diabetic patients. It is a slow progressing complication that results from damage to the blood vessels of the retina. In the initial stages of diabetic retinopathy, the disease may remain asymptomatic, but eventually, if not treated, can result in blindness [1]. The development of retinopathy is directly related to the duration of diabetes, by 10 years of diabetes approximately 50% of the patients, and by 20 – 25 years, nearly 90% of the diabetic patients have some stage of retinopathy [2,3]. Sustained hyperglycemia is considered as the major initiator of the development of diabetic retinopathy, but the mechanism by which hyperglycemia results in the retinal pathology remains unclear. In the pathogenesis of diabetic retinopathy, retinal cells, including capillary cells, Müller cells and ganglion cells undergo accelerated apoptosis [4–7], and the apoptosis of capillary cells precedes the appearance of microvascular histopathology characteristic of diabetic retinopathy suggesting that accelerated apoptosis can account for the pericyte `drop-out' and formation of `ghosts' [1,8,9]. Diabetes Control and Complications Trial and the United Kingdom Prospective Diabetes Study have clearly suggested that the control of circulating blood sugar and blood pressure has potential to delay the progression of retinopathy in diabetic patients [10,11], but it is difficult, or at times not possible, to maintain normal blood glucose levels, and adjacent therapeutic treatments are required. Elucidation of therapeutic targets to prevent this disease based on the molecular mechanism of its development is needed.
The development of diabetic retinopathy is complex involving interplay from several molecular and biochemical mechanisms affecting molecular, cellular and physiological environment of the retinal vasculature. Leading laboratories are actively pursuing research to understand the pathogenesis of diabetic retinopathy. Diabetes increases oxidative stress via a number of different mechanisms including auto-oxidation of glucose, increased superoxide production/decreased scavenging, activation of polyol pathway and protein kinase C and increased formation of advanced glycation end products [12]. Retinal mitochondria become dysfunctional, and overexpression of manganese superoxide dismutase in mice prevents these mitochondrial abnormalities and the development of diabetic retinopathy, suggesting a major role of mitochondria in the development of this disease [13–22]. The exact etiology of this multifactorial disease, however, remains elusive.
2. Matrix metalloproteinases
Matrix metalloproteinases (MMPs), a family of over 25 zinc-dependent proteinases which degrade at least one component of the extracellular matrix (ECM), regulate many normal and pathological processes [23,24]. They play a central role in organ development and subsequent tissue remodeling, and in inflammation and injury. MMPs have similar domain structures which consisting of a `pre' region to target for secretion, a `pro' region to maintain latency and an active catalytic region with a zinc-binding active site. They are synthesized and secreted as inactive pro-enzymes that subsequently become proteolytically cleaved and activated [25,26]. Activity of MMPs is modulated by endogenous tissue inhibitors of metalloproteinases (TIMPs), a four-member family of small proteins that bind to MMPs in a 1:1 stoichiometric ratio. TIMP-1 shows greater preference for MMP-9 than any other MMPs [27] and TIMP-2 for MMP-2. Normally, there is a tight balance between MMP and TIMP, but in pathological conditions this balance is impaired resulting in excess of activated MMP. Although both MMP-2 and MMP-9 are active in the degradation of type IV, MMP-2 is the most ubiquitous member and MMP-9 is the largest and the most complex member of the MMP family [28–31]. This review is mainly focused on the role of MMP-9 in the development of diabetic retinopathy, and has a brief discussion about the role of MMP-2.
MMPs are generally associated with the degradation of most ECM proteins, but recent studies have shown their presence within the cells in nuclear, mitochondrial and cytoplasmic compartments [26]. They have been shown to cleave other non-ECM proteins, including growth factors, cytokines and cell receptors [32]. Modification of the membrane-associated proteins by MMPs is crucial for communication between cells and the extracellular milieu, and determines cell fate and the integrity of tissues. Depending on the extracellular or subcellular localization, the same MMP may affect physiology and pathology in opposing manner. MMP-9 is secreted in the body in a latent form, but on activation it acts on many inflammatory substrates and also on mitochondria [33]. The enzyme is under strict control at various levels: gene transcription, synthesis, secretion, activation, inhibition and glycosylation [34,35].
MMP-2 helps advancing front of the migrating column of endothelial cells to migrate through the basement membrane and ECM. Membrane type-1 matrix metalloproteinase (MT1-MMP) initiates the activation pathway by converting pro-MMP-2 into an activation intermediate that further undergoes autocatalytic conversion to generate the mature MMP-2. In contrast to MMPs, MT1-MMP is a cell membrane-bound proteinase with relatively short transmembrane domain and a cytoplasmic tail [36]. It helps associate these enzymes with discrete regions of the plasma membrane and the intracellular compartment. MT1-MMP also acts as a receptor for TIMP-2, and forms a tri-molecular complex with MMP-2 and TIMP-2 [28]. Although MT1-MMP has been identified as a key player during the angiogenic response, its regulation and participation in angiogenesis depends on the nature of the angiogenic stimulus [37].
3. Regulation of MMPs
Various regulators have been shown to regulate MMPs; a small molecular weight G-protein, H-Ras, regulates MMP-9, possibly via its downstream signaling pathway [38,39]. H-Ras-mediated activation of ERK1/2, followed by activation of nuclear transcription factor-kappa B (NF-kB), is shown to directly regulate the induction of MMP-9, suggesting a close relationship between H-Ras-ERK-NF-kB and MMP-9 activation [40,41].
The transcription of MMP-9 is mostly controlled by 670 base pairs of upstream sequence which includes AP-1, NF-kB, PEA3 and Sp1 binding sites. Human MMP-9 promoter contains cis-acting regulatory elements for binding of the transcription factors, including AP-1 (−533 bp, −79 bp), NF-kB (−600 bp) and Sp1 (−588 bp), which participate in the regulation of the MMP-9 gene and these NF-kB, AP-1, and Sp1 binding sites are considered indispensable [42–44]. Chromatin structure of the MMP-9 promoter is remodeled in coordination with the activation of MMP-9 gene transcription, and the composition of AP-1 and NF-kB on the MMP-9 promoter dynamically changes over the course of MMP-9 induction [45]. Inhibition of SIRT1, a member of the sirtuin family of proteins, has been reported to increase histone-4 acetylation at NF-kB binding sites (−594 to −604) at the MMP-9 promoter increasing the binding of NF-kB to the MMP-9 promoter [46], and these studies have suggested a role of epigenetic modifications in the regulation of MMP-9. In addition, the recent studies have shown that retinal MMP-9 promoter is epigenetically modified in diabetes, further contributing to its expression (Zhong & Kowluru, manuscript in preparation).
MMPs are highly sensitive to oxidative stress, and they are induced by increase in reactive oxygen species (ROS) [47], possibly via direct oxidation of crucial cysteine residues contained within the DNA-binding domain [48]. MMPs are also the prime nitric oxide targets, and peroxynitrite, formed between ROS and nitric oxide can activate pro-MMPs via interacting with cytosolic glutathione [49,50]. Mitochondrial oxidative stress is shown to regulate MMP-9 activation [51], and also, the induction of MMP-9 is considered as a negative regulator of mitochondrial function. MMPs also have potential to impair mitochondrial function via damaging mitochondrial membrane potential by disrupting mitochondrial connexin-43 protein [17,52,53].
4. MMPs and apoptosis
Activation of MMPs is shown to accelerate the apoptosis process; increased MMPs activate apoptosis possibly by disrupting mitochondrial connexin-43 protein and impairing mitochondrial membrane potential [17,20,52]. Inhibition of MMP-2 in myocytes is shown to decrease β-adrenergic receptor-stimulated apoptosis, and JNK-dependent mitochondrial death is considered as the major pathway [54]. Activation of MMP-2 cleaves the nuclear poly(ADP-ribose)polymerase (PARP) and results in apoptosis via mitochondrial pathway releasing apoptosis-inducing factor from the mitochondria [55–57].
5. MMPs and diabetic retinopathy
Diabetic environment stimulates the secretion of several MMPs that are considered to participate in many diabetic complications, including retinopathy, nephropathy and cardiomyopathy [58–65]. Patients with diabetic retinopathy and also animal models have shown increased MMP-9 and MMP-2 in their retina and vitreous [66–68]. Retinal mRNA levels of MMP-2, MMP-9 and MT1-MMP are elevated in diabetes [20,60,61,69], and the pro-forms of MMP-2 and MMP-9 are significantly elevated in the neovascular retinal membranes [62,70]. Although the exact mechanism operating in the retina in diabetes is not clear, MMPs could contribute in the disease process via number of different pathways. MMPs have an important role in maintaining the integrity of the blood–retinal barrier (BRB), and in the development of diabetic retinopathy, BRB damage is an early event [71]. Increased retinal MMPs in diabetes facilitate the increase in vascular permeability via proteolytic degradation of the tight junction protein occludin and disruption of the overall tight junction complex [67,71]. Pro-MMP-2 is efficiently activated in the fibrovascular tissues from patients with proliferative diabetic retinopathy, and interactions with MT1-MMP and TIMP-2 are suggested to play important role in this. Increased expression of MT1-MMP and MMP-2 are observed in retinal pericytes incubated in high glucose and in the retina in diabetes, and increased MMP-2 activity is considered to compromise retinal pericyte survival [72]. Heavily oxidized and glycated low-density lipoprotein (LDL), which is elevated in diabetes, increases MMP-2 in retinal pericytes [73]. MMP-9 is also up-regulated in retinal microvascular cells cultured under high glucose conditions [67,69,71]. Increased MMP-9 is also observed in the human retina showing active neovascularization [62,74]. The exact mechanism by which MMP-9 could contribute to the development of diabetic retinopathy is not clear.
5.1 MMPs and inflammation
MMPs act on pro-inflammatory mediators to regulate varied aspects of inflammation, and can act as switch in acute and chronic inflammation [75]. IL-1β is considered as a critical substrate for MMP-9, and MMP-9 activates the pro-forms of IL-1β increasing the cleaved forms of IL-1β in the dorsal root ganglion [76]. MMP-9 knockout mice, after permanent focal ischemia, have reduced inflammatory mediators, cell apoptosis and ischemic lesions, and these mice also have decreased immune complex-induced arthritis [77]. MMP-9 is also an important effector molecule in inflammatory cells; it can act as a switch in acute and chronic inflammation, and is postulated to be involved in both the initial phase of inflammation and the later phase of tissue remodeling [75,78]. In the development of diabetic retinopathy, subclinical inflammation has been considered to play a role in the vascular lesions associated with diabetic retinopathy [79,80]. The levels of cytokines, including IL-1β and TNF-α, are increased in the vitreous fluid of the patients with proliferative diabetic retinopathy and in the retina from diabetic rats and mice [81,82]. The capillaries become non-perfused and ischemic, and the number of platelet-fibrin thrombi increases; these pro-inflammatory changes and leukostasis constitute as some of the earliest changes observed in the retina of diabetic animals [83]. Intracellular adhesion molecule-1 and CD18 upregulation, leukocytes adherence to the retinal microvasculature, and endothelial cell damage are also reported early in the development of retinopathy in diabetic rats [83]. Intravitreal injection of IL-1β increases TUNEL-stained capillary cells in the retina of normal rats; the apoptosis process is mediated via activation of NF-kB [84]. Diabetes-induced TNF-α up-regulation is shown to contribute to increased apoptosis of retinal capillary cells [85]. How MMP-9 increases inflammatory mediators is not clear, but by degrading a major component of the basement membrane, it has potential to enable invasiveness of immune system cells to the injured tissue (suggesting MMP-9 as a byproduct) or to the tissue that is about to be injured, acting as a direct etiological factor [34]. MMPs also facilitate tissue availability of bound VEGF [86], and VEGF is considered one of the major growth factors in the development of diabetic retinopathy [87]. Activated MMP increases vascular permeability in the retina in diabetes by proteolytic degrading occludin and disrupting the overall tight junction complex, and breakdown of the BRB is considered an early event in the pathogenesis of diabetic retinopathy [67,71]. Thus, there appears a strong relationship between MMP-9 and inflammation in the development of diabetic retinopathy.
5.2 MMPs and mitochondrial dysfunction
In the pathogenesis of diabetic retinopathy, Ras-Raf-MEK-ERK cascade is responsible for the activation of MMP-9 in the retina resulting in the apoptosis of its capillary cells [61]. As stated above, MMPs are regulated by mitochondrial oxidative stress, and, by contrast, the induction of MMPs serves as a negative regulator of mitochondrial function, suggesting a vicious cycle of mitochondria damage and activation of MMPs [17,52]. The recent studies have shown increased mitochondrial localization of MMP-2 and MMP-9 in the pathogenesis of diabetic retinopathy, and have demonstrated a pro-apoptotic role for these MMPs in the pre-angiogenic stages of diabetic retinopathy [17,20,60]. Furthermore, there appears to be a direct role of MMP-9 in the development of diabetic retinopathy; the retinal vasculature of diabetic mice with MMP-9 gene abrogated is protected from accelerated apoptosis, and from the histopathology characteristic of diabetic retinopathy. These MMP-9 gene knockout mice are protected from diabetes-induced mitochondria damage suggesting a direct role of activated MMP-9 in mitochondria damage and in membrane permeability. Damaged mitochondria allow Bax to move into the mitochondria, and apoptotic machinery is activated. The molecular mechanism via which MMP-9 is increased in the retinal mitochondria appears to be via the regulation of chaperons, and Hsp70 and Hsp60 appear to be important in chaperoning MMP-9 to the mitochondria [17,20,60,69].
Similarly, diabetes also activates MMP-2 in the retinal mitochondria damaging their integrity, and the process is mediated via the regulation of Hsp60 and connexin-43; mitochondrial MMP-2 damages retinal mitochondria by modulation of Hsp60 and connexin-43, allowing cytochrome c to leak out and activate the apoptotic machinery. Thus, the damage of mitochondria appears to be one of the pathways via which increased MMPs can contribute to the development of diabetic retinopathy [20,60,69].
5.3 MMPs and neovascularization
During the advanced stages of the development of diabetic retinopathy, subsequent to capillary basement membrane thickening and loss of pericytes and endothelial cells, neovascularization begins and collateral vessels start to appear. These new retinal vessels are fragile, and are prone to bleeding resulting in vitreous hemorrhage, and if not treated, lead to the retinal detachment [1]. MMPs, in particular, MMP-2 and MMP-9, assist in angiogenesis, and multiple mechanisms are implicated. MMPs degrade the capillary basement membrane, which is a requirement for the penetration of endothelial cells in the subendothelial matrix and for the formation of a new lumen. MMPs are active players in the VEGF-mediated cell proliferation and the development of new vasculature [88–90]. MMPs also facilitate tissue availability of bound VEGF [86], and VEGF is considered one of the major growth factors in the development of diabetic retinopathy [87,91]. Alternatively, MMP-9 could also act as an angiogenesis antagonist; it is shown to activate the angiostatin an angiogenesis inhibitor factor [92]. The activity of both, MMP-9 and MMP-2, are increased in epiretinal neovasculature membrane of patients with proliferative diabetic retinopathy [67,74,93]. Further, patients with proliferative diabetic retinopathy present activated pro-MMP-2, and the activation of pro-MMP-2 in the fibrovascular tissues is postulated to be mediated via its interaction with MT1-MMP and TIMP-2 [72].
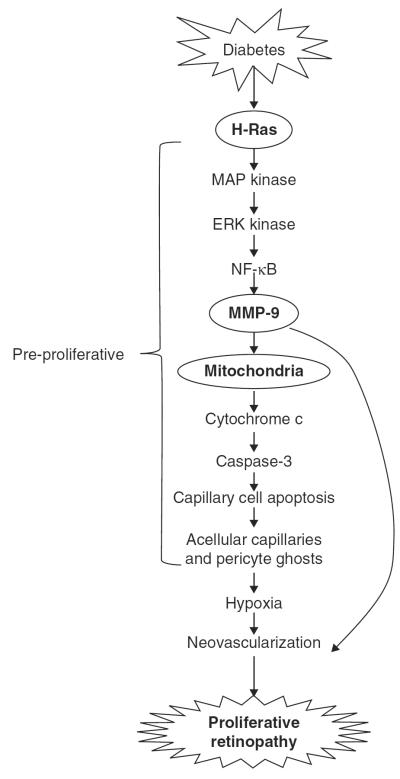
Thus, MMPs appear to have dual role in the development of diabetic retinopathy: in the earlier stages of diabetic retinopathy (pre-neovascularization), they facilitate accelerated apoptosis of retinal capillary cells, making retinal microvasculature low in endothelial cells and pericytes, and in later stages, assisting in the new vessel formation (Figure 1).
Figure 1. In diabetes, activation of H-Ras in retinal capillary cells activates MMP-9 via Raf-1-MAP kinase-ERK kinase-NF-kB cascade.
In the early stages of diabetic retinopathy, activated MMP-9 damages the mitochondria, and cytochrome c is released from the mitochondria. This accelerates capillary cell apoptosis, ultimately resulting in acellular capillaries and pericyte ghosts (the pathology characteristic of early stages of diabetic retinopathy). With time, the capillaries become hypoxic ultimately leading to neovascularization. Furthermore, MMP-9, via degrading the capillary basement membrane and assisting the penetration of endothelial cells in the subendothelial matrix, can also result in neovascularization.
6. MMPs and other ocular diseases
Upregulation of MMPs is also observed in many other ocular diseases, including age-related macular degeneration, proliferative vitreoretinopathy, secondary cataract and conjunctivochalasis [94]. In age-related macular degeneration, several MMPs, including MMP-2 and MMP-9 are elevated in the Bruch's membrane; especially high levels are observed in the areas of choroidal new vessel, and are associated with the choroidal neovascularization [95]. In glaucomatous eyes, alterations in MMPs are considered to contribute to the increased outflow resistance [96], and increased activity of MMP-9 has an important role in the corneal stroma pathology observed in the dysfunctional tear state [97]. Furthermore, high MMP-9 activity is observed in the eyes with cortical cataract, and the activity increases with age in the lens epithelial cells of patients with age-related cataract [98]. Thus, there is a great deal of evidence that MMPs play important roles in the pathogenesis of other ocular diseases.
7. Inhibitors of MMPs
As reviewed above, MMPs are involved in many diseases, including diabetic retinopathy making them viable drug targets in the therapy of these diseases. There are several classes of pharmacological MMP inhibitors with a majority of them based on the binding to the zinc site of the MMP to block its activity. Doxycycline is considered to be the most potent MMP inhibitor with a broad-spectrum range and inhibits MMP-1, -2, -7, -8, -9, -12 and -13 [99]. Other synthetic MMP inhibitor, bisphosphonates, has potent MMP-inhibitory properties, probably through cation-chelation of zinc [100]. Because of a close relationship between MMP-9 and inflammation, non-steroidal anti-inflammatory drugs, such as indomethacin, are shown to reduce PGE2 synthesis and consequently MMP-9 production [101]. In the development of diabetic retinopathy, pharmacological inhibition of MMPs is shown to prevent retinal and choroidal neovascularization [102], and inhibit MMP-9-mediated retinal vascular permeability and inflammation [71,103]. Furthermore, administration of a synthetic MMP inhibitor prevents the induction of proliferative vitreoretinopathy [104]. MMP-2 and MMP-9 inhibition in presence of COX inhibitor is shown to prevent retinal abnormalities [105]. However, there have been no clinical trials to use the inhibitors of MMPs to treat diabetic retinopathy patients. The recent studies using mice genetically modified for MMP-9 have shown that these mice, when made diabetic, are protected from the accelerated loss of retinal capillary cells, and also the development of early signs of diabetic retinopathy [17], and this strengthens the possible use of therapies to target MMPs to ameliorate the development of diabetic retinopathy in diabetic patients.
Since the late 70s, more than 70 inhibitors of MMPs have been pursued as clinical candidates for cancer, arthritis, cardiovascular diseases and many others, but the results have been less than satisfactory. This can be attributed to either poor selectivity of the inhibitors, or target validation. Lessons from previous failures, and recent research showing an association between mitochondrial damage and MMPs, novel non-matrix-related intra- and extracellular targets of MMPs and their regulation by epigenetic modifications, are opening channels to develop new inhibitors targeting MMPs. It is believed that the understanding of the molecular mechanism(s) regulating the induction and repression of MMPs should help provide valuable insights for developing therapeutic agents.
The delivery of a drug to the retina, however, presents additional challenges for the treatment of diabetic retinopathy. The BRB impedes delivery of many compounds which fail (or poorly) to pass this barrier, and targeted delivery (via intravitreal injections) though now in practice for anti-VEGF treatment, comes with some complications, including the possibility of undesired cataracts and retinal infections. The other major issue is the safety of the drug for this lifelong disease. Furthermore, since the duration of diabetes is an important contributor in the development of this slowly progressing disease, the time of initiation of a therapy has major role in the outcome of the therapy, and initiation of a therapy during early stages has better potential for a positive outcome than at later stages of the disease. But lack of screening of patients leaves many high-risk patients without any treatment for longer durations. Recent ongoing research is coming up with novel means to deliver the drug, for example, the use of nanoparticles, should help provide clinicians a more feasible means for drug delivery to the retina.
Furthermore, the authors recognize that the treatment strategies that could enhance the degree of inhibition of MMPs represent one possible direction for clinical research, but it is unlikely that the development of retinopathy could be completely prevented by just one drug. It is believed that inhibitors of MMPs, however, could become an important part of the complex approach to inhibit the pathogenesis of diabetic retinopathy.
8. Expert opinion
One of the most devastating complications of diabetes is the retinopathy. This progressive disease, which remains asymptomatic in the early stages, if not treated, can rob patient's vision. The pathogenesis of diabetic retinopathy is complex, and involves several molecular and biochemical mechanisms affecting cellular metabolism of the retina. But, despite extensive cutting-edge research in the field, the mechanism remains unclear.
MMPs have been shown to play an important role in many chronic diseases including cancer and arthritis. Although higher levels of MMPs were observed in the vitreous of patients with proliferative retinopathy over two decades ago, their role in the development of this blinding disease has remained unclear. In the pathogenesis of diabetic retinopathy, MMPs in addition to being pro-angiogenic during the neovascularization phase, could act as pro-apoptotic in the early stages, and the accelerated apoptosis of retinal capillary cells is considered to predict the development of retinopathy in diabetes.
With the data available implicating the role MMPs in diabetic complications, it is believed that the inhibitors of MMPs could have dual role: in the early stages of the diseases, inhibit capillary cell apoptosis, and if the disease has progressed to the angiogenic stage, inhibit the growth of new vessels. This will help patients save their vision from deteriorating, and prevent them becoming blind.
Article highlights
Retinal MMPs are activated in diabetes.
Epigenetic modifications regulate retinal MMP-9 in diabetes.
In the early stage of diabetic retinopathy, MMPs, especially MMP9 and MMP2, damage mitochondria and accelerate the apoptosis of retinal capillary cells, and in the advanced stage of the disease, these MMPs assist in angiogenesis.
Inhibitors of MMPs may have potential to halt the development of diabetic retinopathy via inhibiting the early events, and also the formation of neovesculature.
This box summarizes key points contained in the article.
Acknowledgments
This was supported in part by grants from the National Institutes of Health, Juvenile Diabetes Research Foundation, the Thomas Foundation and Research to Prevent Blindness.
Footnotes
Declaration of interest The authors have no other competing interests to declare.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Frank RN. Diabetic Retinopathy. N Engl J Med. 2004;350:48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]; • Overall review of diabetic retinopathy.
- 2.Klein R, Klein BEK, Jensen SC, Moss SE. The relation of socioeconomic factors to the incidence of proliferative diabetic retinopathy and loss of vision. Ophthalmology. 1994;101:68–76. doi: 10.1016/s0161-6420(94)31354-6. [DOI] [PubMed] [Google Scholar]
- 3.Chew EY. Epidemiology of diabetic retinopathy. Hosp Med. 2003;64:396–9. doi: 10.12968/hosp.2003.64.7.2275. [DOI] [PubMed] [Google Scholar]
- 4.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–90. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Accelerated apoptosis of retinal capillary cells in diabetic retinopathy.
- 5.Barber AJ, Lieth E, Khin SA, et al. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest. 1998;102:783–91. doi: 10.1172/JCI2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowluru RA, Odenbach S. Effect of long-term administration of alpha lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53:3233–8. doi: 10.2337/diabetes.53.12.3233. [DOI] [PubMed] [Google Scholar]
- 7.Feit-Leichman RA, Kinouchi R, Takeda M, et al. Vascular damage in a mouse model of diabetic retinopathy: relation to neuronal and glial changes. Invest Ophthalmol Vis Sci. 2005;46:4281–7. doi: 10.1167/iovs.04-1361. [DOI] [PubMed] [Google Scholar]
- 8.Frank RN. On the pathogenesis of diabetic retinopathy. A 1990 update. Ophthalmology. 1991;98:586–93. doi: 10.1016/s0161-6420(91)32253-x. [DOI] [PubMed] [Google Scholar]
- 9.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Control and Complications Trial Research Group Early worsening of diabetic retinopathy in the diabetes control and complication trial. Arch Ophthalm. 1998;116:874–86. doi: 10.1001/archopht.116.7.874. [DOI] [PubMed] [Google Scholar]
- 11.UKPDS Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 12.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 13.Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci. 2003;44:5327–34. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]; • Mitochondrial dysfunction in diabetic retinopathy.
- 14.Kowluru RA, Kowluru V, Ho YS, Xiong Y. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic Biol Med. 2006;41:1191–6. doi: 10.1016/j.freeradbiomed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Madsen-Bouterse SA, Mohammad G, Kanwar M, Kowluru RA. Role of mitochondrial DNA damage in the development of diabetic retinopathy, and the metabolic memory phenomenon associated with its progression. Antioxid Redox Signal. 2010;13:797–805. doi: 10.1089/ars.2009.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madsen-Bouterse S, Zhong Q, Mohammad G, et al. Oxidative damage of mitochondrial DNA in diabetes, and its protection by manganese superoxide dismutase. Free Radic Res. 2010;44:313–21. doi: 10.3109/10715760903494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowluru RA, Mohammad G, Dos Santos JM, Zhong Q. Abrogation of MMP9 gene protects against the development of retinopathy in diabetic mice by preventing mitochondrial damage. Diabetes. 2011;60:3023–33. doi: 10.2337/db11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• MMP-9 and mitochondria damage in diabetic retinopathy.
- 18.Santos JM, Mohammad G, Zhong Q, Kowluru RA. Diabetic retinopathy, superoxide damage and antioxidant. Curr Pharm Biotechnol. 2011;12:352–61. doi: 10.2174/138920111794480507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos JM, Tewari S, Goldberg AFX, Kowluru RA. Mitochondria biogenesis and the development of diabetic retinopathy. Free Radic Biol Med. 2011;51:1849–60. doi: 10.1016/j.freeradbiomed.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammad G, Kowluru RA. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:3832–41. doi: 10.1167/iovs.10-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Apoptotic role of MMP-2 in the development of diabetic retinopathy.
- 21.Zhong Q, Kowluru RA. Epigenetic changes in mitochondrial superoxide dismutase in the retina and the development of diabetic retinopathy. Diabetes. 2011;60:1304–13. doi: 10.2337/db10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Epigenetic changes and diabetic retinopathy.
- 22.Zhong Q, Kowluru RA. Diabetic retinopathy and damage to mitochondrial structure and transport machinery. Invest Ophthalmol Vis Sci. 2011;52:8739–46. doi: 10.1167/iovs.11-8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagase H, Woessner JJF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Hill JW, Rosenberg GA. Multiple roles of metalloproteinases in neurological disorders. Prog Mol Biol Transl Sci. 2011;99:241–63. doi: 10.1016/B978-0-12-385504-6.00006-3. [DOI] [PubMed] [Google Scholar]; •• MMPs in diseases.
- 25.Fridman R, Toth M, Chvyrkova I, et al. Cell surface association of matrix metalloproteinase-9 (gelatinase B) Cancer Metastasis Rev. 2003;22:153–66. doi: 10.1023/a:1023091214123. [DOI] [PubMed] [Google Scholar]
- 26.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–90. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Review of MMPs.
- 27.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–83. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 28.Liotta LA. Probing the depths of degradation: matrix metalloproteinase-2 and endometrial menstrual breakdown. J Clin Invest. 1996;97:273–4. doi: 10.1172/JCI118412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]; •• MMPs in diseases.
- 30.Itoh Y, Seiki M. CMT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 31.Dufour A, Sampson NS, Zucker S, Cao J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J Cell Physiol. 2008;217:643–51. doi: 10.1002/jcp.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Overall CM, Dean RA. Degradomics: systems biology of the protease web. Pleiotropic roles of MMPs in cancer. Cancer Metastasis Rev. 2011;25:69–75. doi: 10.1007/s10555-006-7890-0. [DOI] [PubMed] [Google Scholar]
- 33.Ovechkin AV, Tyagi N, Rodriguez WE, et al. Role of matrix metalloproteinase-9 in endothelial apoptosis in chronic heart failure in mice. J Appl Physiol. 2005;99:2398–405. doi: 10.1152/japplphysiol.00442.2005. [DOI] [PubMed] [Google Scholar]; •• MMP-9 and apoptosis.
- 34.Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006;26:299–307. doi: 10.1007/s10875-006-9022-6. [DOI] [PubMed] [Google Scholar]
- 35.Van den Steen PE, Dubois B, Nelissen I, et al. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 36.Sounni NE, Janssen M, Foidart JM, Noel A. Membrane type-1 matrix metalloproteinase and TIMP-2 in tumor angiogenesis. Matrix Biol. 2003;22:55–61. doi: 10.1016/s0945-053x(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 37.Chetty C, Lakka SS, Bhoopathi P, Rao JS. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer. 2010;127:1081–95. doi: 10.1002/ijc.25134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee KW, Kim MS, Kang NJ, et al. H-Ras selectively up-regulates MMP-9 and COX-2 through activation of ERK1/2 and NF-kappaB: an implication for invasive phenotype in rat liver epithelial cells. Int J Cancer. 2006;119:1767–75. doi: 10.1002/ijc.22056. [DOI] [PubMed] [Google Scholar]; • Regulation of MMP-9.
- 39.Yoon SO, Shin S, Lee HL, et al. Isoginkgetin inhibits tumor cell invasion by regulating phosphatidylinositol 3-kinase/Akt-dependent matrix metalloproteinase-9 expression. Mol Cancer Ther. 2006;5:2666–75. doi: 10.1158/1535-7163.MCT-06-0321. [DOI] [PubMed] [Google Scholar]
- 40.Kim SD, Yang SI, Kim HC, et al. Inhibition of GSK-3beta mediates expression of MMP-9 through ERK1/2 activation and translocation of NF-kappaB in rat primary astrocyte. Brain Res. 2007;1186:12–20. doi: 10.1016/j.brainres.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Takada Y, Khuri FR, Aggarwal BB. Protein farnesyltransferase inhibitor (SCH 66336) abolishes NF-kappaB activation induced by various carcinogens and inflammatory stimuli leading to suppression of NF-kappaB-regulated gene expression and up-regulation of apoptosis. J Biol Chem. 2004;279:26287–99. doi: 10.1074/jbc.M400963200. [DOI] [PubMed] [Google Scholar]
- 42.Ma Z, Shah R, Chang M, Benveniste E. Coordination of cell signaling, chromatin remodeling, histone modifications, and regulator recruitment in human matrix metalloproteinase 9 gene transcription. Mol Cell Biol. 2004;24:5496–509. doi: 10.1128/MCB.24.12.5496-5509.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mancini A, Di Battista JA. Transcriptional regulation of matrix metalloprotease gene expression in health and disease. Front Biosci. 2006;11:423–46. doi: 10.2741/1809. [DOI] [PubMed] [Google Scholar]
- 44.Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol. 2007;211:19–26. doi: 10.1002/jcp.20948. [DOI] [PubMed] [Google Scholar]; • Epigenetic modification of MMPs.
- 45.Robert I, Aussems M, Keutgens A, et al. Metalloproteinase-9 gene induction by a truncated oncogenic NF-kappaB2 protein involves the recruitment of MLL1 and MLL2 H3K4 histone methyltransferase complexes. Oncogene. 2009;28:1626–8. doi: 10.1038/onc.2009.6. [DOI] [PubMed] [Google Scholar]
- 46.Liu P, Wilson MJ. miR-520c and miR-373 target mTOR and SIRT1, activate the Ras/Raf/MEK/Erk pathway and NF-?B, with up-regulation of MMP9 in human fibrosarcoma cells. J Cell Physiol. 2010 doi: 10.1002/jcp.22993. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasebe Y, Egawa K, Shibanuma M, Nose K. Induction of matrix metalloproteinase gene expression in an endothelial cell line by direct interaction with malignant cells. Cancer Sci. 2007;98:58–67. doi: 10.1111/j.1349-7006.2006.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768–84. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Pfeilschifter J, Eberhardt W, Huwiler A. Nitric oxide and mechanisms of redox signalling: matrix and matrix-metabolizing enzymes as prime nitric oxide targets. Eur J Pharmacol. 2001;429:279–86. doi: 10.1016/s0014-2999(01)01326-7. [DOI] [PubMed] [Google Scholar]
- 50.Valentin F, Bueb JL, Kieffer P, et al. Oxidative stress activates MMP-2 in cultured human coronary smooth muscle cells. Fundam Clin Pharmacol. 2005;19:661–7. doi: 10.1111/j.1472-8206.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- 51.Moshal KS, Singh M, Sen U, et al. Homocysteine-mediated activation and mitochondrial translocation of calpain regulates MMP-9 in MVEC. Am J Physiol Heart Circ Physiol. 2006;11:H2825–35. doi: 10.1152/ajpheart.00377.2006. [DOI] [PubMed] [Google Scholar]
- 52.Moshal KS, Tipparaju SM, Vacek TP, et al. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol. 2008;295:H890–7. doi: 10.1152/ajpheart.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhoua H-Z, Maa X, Graye MO, et al. Transgenic MMP-2 expression induces latent cardiac mitochondrial dysfunction. Biochem Biophys Res Commun. 2007;358:189–95. doi: 10.1016/j.bbrc.2007.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amin P, Singh M, Singh K. beta-Adrenergic receptor-stimulated cardiac myocyte apoptosis: Role of beta1 integrin. Signal Transduct. 2011;2011:179057. doi: 10.1155/2011/179057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwan JA, Schulze CJ, Wang W, et al. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18:690–2. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 56.Dawson VL, Dawson TM. Deadly conversations: nuclear-mitochondrial cross-talk. J Bioenerg Biomembr. 2004;36:287–94. doi: 10.1023/B:JOBB.0000041755.22613.8d. [DOI] [PubMed] [Google Scholar]
- 57.Rosenberger D, Gargoum R, Tyagi N, et al. Homocysteine enriched diet leads to prolonged QT interval and reduced left ventricular performance in telemetric monitored mice. Nutr Metab Cardiovasc Dis. 2011;21:492–8. doi: 10.1016/j.numecd.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tyagi SC, Rodriguez WPA, Roberts AM, et al. Hyperhomocysteinemic diabetic cardiomyopathy: oxidative stress, remodeling, and endothelial-myocyte uncoupling. J Cardiovasc Pharmacol Ther. 2005;10:1–10. doi: 10.1177/107424840501000101. [DOI] [PubMed] [Google Scholar]
- 59.Thrailkill KM, Clay Bunn R, Fowlkes JL. Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine. 2009;35:1–10. doi: 10.1007/s12020-008-9114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohammad G, Kowluru RA. Matrix metalloproteinase-2 in the development of diabetic retinopathy and mitochondrial dysfunction. Lab Invest. 2010;90:1365–72. doi: 10.1038/labinvest.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• MMP-2 and diabetic retinopathy.
- 61.Mohammad G, Kowluru R. Diabetic retinopathy and signaling mechanism for activation of matrix metalloproteinases-9. J Cell Physiol. 2011 doi: 10.1002/jcp.22822. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Mechanism of activation of MMP-9 in diabetic retinopathy.
- 62.Das A, McGuire PG, Eriqat C, et al. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Invest Ophthalmol Vis Sci. 1999;40:809–13. [PubMed] [Google Scholar]; •• MMPs and diabetic retinopathy.
- 63.Hayden MR, Sowers JR, Tyagi SC. The central role of vascular extracellular matrix and basement membrane remodeling in metabolic syndrome and type 2 diabetes: the matrix preloaded. Cardiovasc Diabetol. 2005;28:9. doi: 10.1186/1475-2840-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; • MMPs and diabetic cardiomyopathy.
- 64.McKittrick IB, Bogaert Y, Nadeau K, et al. Urinary matrix metalloproteinase activities: biomarkers for plaque angiogenesis and nephropathy in diabetes. Am J Physiol Renal Physiol. 2011;301:F326–33. doi: 10.1152/ajprenal.00267.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams JM, Zhang J, North P, et al. Evaluation of metalloprotease inhibitors on hypertension and diabetic nephropathy. Am J Physiol Renal Physiol. 2011;300:F983–8. doi: 10.1152/ajprenal.00262.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin M, Kashiwagi K, Iizuka Y, et al. Matrix metalloproteinases in human diabetic and nondiabetic vitreous. Retina. 2001;21:28–33. doi: 10.1097/00006982-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 67.Giebel SJ, Menicucci G, McGuire PG, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 2005;85:567–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]; •• MMPs and BRB.
- 68.Yang R, Liu H, Williams I, Chaqour B. Matrix metalloproteinase-2 expression and apoptogenic activity in retinal pericytes: implications in diabetic retinopathy. Ann NY Acad Sci. 2007;1103:196–201. doi: 10.1196/annals.1394.000. [DOI] [PubMed] [Google Scholar]; • MMP-2 and diabetic retinopathy.
- 69.Kowluru RA, Kanwar M. Oxidative stress and the development of diabetic retinopathy: contributory role of matrix metalloproteinase-2. Free Radic Biol Med. 2009;46:1677–85. doi: 10.1016/j.freeradbiomed.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffmann S, He S, Ehren M, et al. MMP-2 and MMP-9 secretion is stimulated by angiogenic molecules found in choroidal neovascular membranes. Retina. 2006;26:454–61. doi: 10.1097/01.iae.0000238549.74626.33. [DOI] [PubMed] [Google Scholar]
- 71.Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes. 2007;56:2380–7. doi: 10.2337/db06-1694. [DOI] [PubMed] [Google Scholar]; •• Role of MMPs in BRB degradation in diabetic retinopathy.
- 72.Noda K, Ishida S, Inoue M, et al. Production and activation of matrix metalloproteinase-2 in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2003;44:2163–70. doi: 10.1167/iovs.02-0662. [DOI] [PubMed] [Google Scholar]
- 73.Barth JL, Yu Y, Song W, et al. Oxidised, glycated LDL selectively influences tissue inhibitor of metalloproteinase-3 gene expression and protein production in human retinal capillary pericytes. Diabetologia. 2007;50:2200–8. doi: 10.1007/s00125-007-0768-z. [DOI] [PubMed] [Google Scholar]
- 74.Das A, McLamore A, Song W, McGuire PG. Retinal neovascularization is suppressed with a matrix metalloproteinase inhibitor. Arch Ophthalmol. 1999;117:498–503. doi: 10.1001/archopht.117.4.498. [DOI] [PubMed] [Google Scholar]; •• MMS and neovascularization.
- 75.Hu J, Van den Steen P, Sang Q-X, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–98. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 76.Kawasaki Y, Xu ZZ, Wang X, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–6. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asahi M, Asahi K, Jung JC, et al. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–9. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 78.Chadzinska M, Baginski P, Kolaczkowska E, et al. Expression profiles of matrix metalloproteinase 9 in teleost fish provide evidence for its active role in initiation and resolution of inflammation. Immunology. 2008;125:601–10. doi: 10.1111/j.1365-2567.2008.02874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joussen AM, Poulaki V, Le ML, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–2. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 80.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004;88:1343–7. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan PS, Kanwar M, Kowluru RA. Resistance of retinal inflammatory mediators to suppress after re-institution of good glycemic control: novel mechanism for metabolic memory. J Diabetes Complications. 2010;24:55–63. doi: 10.1016/j.jdiacomp.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Joussen AM, Murata T, Tsujikawa A, et al. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–52. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kowluru RA, Odenbach S. Role of interleukin-1beta in the development of retinopathy in rats: effect of antioxidants. Invest Ophthalmol Vis Sci. 2004;45:4161–6. doi: 10.1167/iovs.04-0633. [DOI] [PubMed] [Google Scholar]
- 85.Behl Y, Krothapalli P, Desta T, et al. Diabetes-enhanced tumor necrosis factor-alpha production promotes apoptosis and the loss of retinal microvascular cells in type 1 and type 2 models of diabetic retinopathy. Am J Pathol. 2008;172:1411–18. doi: 10.2353/ajpath.2008.071070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee CZ, Xue Z, Zhu Y, et al. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke. 2007;38:2563–8. doi: 10.1161/STROKEAHA.106.481515. [DOI] [PubMed] [Google Scholar]
- 87.Aiello LP. Vascular endothelial growth factor and the eye: biochemical mechanisms of action and implications for novel therapies. Ophthalmic Res. 1997;29:354–62. doi: 10.1159/000268033. [DOI] [PubMed] [Google Scholar]
- 88.Busti C, Falcinelli E, Momi S, Gresele P. Matrix metalloproteinases and peripheral arterial disease. Intern Emerg Med. 2009;5:13–25. doi: 10.1007/s11739-009-0283-y. [DOI] [PubMed] [Google Scholar]
- 89.Ishizaki E, Takai S, Ueki M, et al. Correlation between angiotensin-converting enzyme, vascular endothelial growth factor, and matrix metalloproteinase-9 in the vitreous of eyes with diabetic retinopathy. Am J Ophthalmol. 2006;141:129–34. doi: 10.1016/j.ajo.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 90.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–3. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aiello LP, Wong JS. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int. 2000;77:S113–19. doi: 10.1046/j.1523-1755.2000.07718.x. [DOI] [PubMed] [Google Scholar]
- 92.Chung AW, Hsiang YN, Matzke LA, et al. Reduced expression of vascular endothelial growth factor paralleled with the increased angiostatin expression resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in human type 2 diabetic arterial vasculature. Circ Res. 2006;99:140–8. doi: 10.1161/01.RES.0000232352.90786.fa. [DOI] [PubMed] [Google Scholar]
- 93.Das S, Mandal M, Chakraborti T, et al. Isolation of MMP-2 from MMP-2/TIMP-2 complex: characterization of the complex and the free enzyme in pulmonary vascular smooth muscle plasma membrane. Biochim Biophys Acta. 2004;1674:158–74. doi: 10.1016/j.bbagen.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 94.Federici TJ. The non-antibiotic properties of tetracyclines: clinical potential in ophthalmic disease. Pharmacol Res. 2011;64:614–23. doi: 10.1016/j.phrs.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 95.Hussain AA, Lee Y, Zhang JJ, Marshall J. Disturbed matrix metalloproteinase activity of Bruch's membrane in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:4459–66. doi: 10.1167/iovs.10-6678. [DOI] [PubMed] [Google Scholar]
- 96.Ghanem AA, Arafa LF, El-Baz A. Connective tissue growth factor and tissue inhibitor of matrix metalloproteinase-2 in patients with exfoliative glaucoma. Curr Eye Res. 2011;36:540–5. doi: 10.3109/02713683.2011.565541. [DOI] [PubMed] [Google Scholar]
- 97.Sambursky R, O'Brien TP. MMP-9 and the perioperative management of LASIK surgery. Curr Opin Ophthalmol. 2011;22:294–303. doi: 10.1097/ICU.0b013e32834787bb. [DOI] [PubMed] [Google Scholar]
- 98.Alapure BV, Praveen MR, Gajjar D, et al. Matrix metalloproteinase-9 activity in human lens epithelial cells of cortical, posterior subcapsular, and nuclear cataracts. J Cataract Refract Surg. 2008;34:2063–7. doi: 10.1016/j.jcrs.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 99.Wang X, Li KF, Adams E, Van Schepdael A. Matrix metalloproteinase inhibitors: a review on bioanalytical methods, pharmacokinetics and metabolism. Curr Drug Metab. 2011;12:395–410. doi: 10.2174/138920011795202901. [DOI] [PubMed] [Google Scholar]; •• Inhibitors of MMPs.
- 100.Hidalgo M, Eckhardt SC. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:178–93. doi: 10.1093/jnci/93.3.178. [DOI] [PubMed] [Google Scholar]
- 101.Yiqin Y, Meilin X, Jie X, Keping Z. Aspirin inhibits MMP-2 and MMP-9 expression and activity through PPARalpha/gamma and TIMP-1-mediated mechanisms in cultured mouse celiac macrophages. Inflammation. 2009;32:233–41. doi: 10.1007/s10753-009-9125-3. [DOI] [PubMed] [Google Scholar]
- 102.Samtani S, Amaral J, Campos MM, et al. Doxycycline-977 mediated inhibition of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50:5098–106. doi: 10.1167/iovs.08-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reddy AB, Ramana KV, Srivastava S, et al. Aldose reductase regulates high glucose-induced ectodomain shedding of tumor necrosis factor (TNF)-alpha via protein kinase C-delta and TNF-alpha converting enzyme in vascular smooth muscle cells. Endocrinology. 2005;150:63–74. doi: 10.1210/en.2008-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ozerdem U, Mach-Hofacre B, Cheng L, et al. The effect of prinomastat (AG3340), a potent inhibitor of matrix metalloproteinases, on a subacute model of proliferative vitreoretinopathy. Curr Eye Res. 2000;20:447–53. [PubMed] [Google Scholar]
- 105.Bhatt LK, Addepalli V. Attenuation of diabetic retinopathy by enhanced inhibition of MMP-2 and MMP-9 using aspirin and minocycline in streptozotocin-diabetic rats. Am J Transl Res. 2010;2:181–9. [PMC free article] [PubMed] [Google Scholar]