Abstract
The dopamine D5 receptor (D5R) exhibits a wide distribution in prefrontal cortex (PFC) but its role in this region has not yet been elucidated. In the present study we identified a novel physiological function for the D5R as a regulator of BDNF and Akt signalling in PFC. Specifically, acute activation of the D5R by the dopamine agonist SKF 83959 enhanced BDNF expression and signalling through its receptor, TrkB, in rats and in mice gene-deleted for the D1 receptor but not the D5R. These changes were concomitant with increased expression of GAD67, a protein whose downregulation has been implicated in the etiology of schizophrenia. Furthermore, D5R activation increased phosphorylation of Akt at the Ser473 site, consequently decreasing the activity of its substrate GSK-3β. These findings could have wide-reaching implications given evidence showing activation of these pathways in PFC has therapeutic effects in neuropsychiatric disorders such as drug addiction, schizophrenia and depression.
Keywords: prefrontal cortex, dopamine D5 receptor, BDNF, Akt, GSK-3β
Introduction
Dysfunction of D1-like (D1, D5) receptor signalling in PFC has been implicated in disorders of cognitive dysfunction such as schizophrenia (Goldman-Rakic et al., 2004). However, as a result of the inability of D1-like receptor agonists and antagonists to distinguish between the D1 receptor (D1R) and D5R, the relative contribution of these receptors to PFC functioning remains difficult to ascertain. In rodent PFC, the abundance of D5R is higher than D1R (Luedtke et al., 1999), suggesting that this receptor likely has an important, and distinct, functional role in this region. The most common tool that has been utilized to elucidate this role is through the use of mice gene-deleted for the D1R or the D5R. A potential pharmacological tool, however, involves the use of the atypical dopamine agonist SKF 83959. SKF 83959 does not activate the Gs-linked D1R (Jin et al., 2003), but has been linked to phospholipase C (PLC) activation mediated either by the D5R (Sahu et al., 2009), or the dopamine D1-D2 receptor heteromer (Lee et al., 2004). With both the D5R and D1-D2 heteromer, activation of PLC is regulated by the Gq protein and to result in calcium signalling (Rashid et al., 2007; So et al., 2009).
The effects of SKF 83959 on D5R-mediated signalling have not been elucidated, however regulation of brain-derived neurotrophic factor (BDNF) and Akt (protein kinase B)-glycogen synthase kinase (GSK-3) signalling are implicated. Specifically, SKF 83959-induced activation of the D1-D2 receptor heteromer resulted in the phosphorylation of calcium/calmodulin dependent protein kinase IIα (CaMKIIα) (Rashid et al., 2007), and increased BDNF expression in nucleus accumbens (NAc) (Hasbi et al., 2009). This ability of SKF 83959 to induce BDNF expression, via PLC, suggests that this signalling pathway may also be regulated by the D5R in PFC, a suggestion supported by evidence demonstrating that SKF 83959 stimulated inositol phosphate production in rat frontal cortex (Jin et al., 2003), but to a much lesser degree in D5R knock-out mice (Sahu et al., 2009). SKF 83959 has also been shown to regulate GSK-3β activation in cortical neurons and consequently increase the expression of inducible nitric oxide synthase (iNOS) (Yu et al., 2008), an effect potentially mediated via D5R or D1-D2 receptor heteromer-induced Gq activation as the Gq protein has been demonstrated to attenuate GSK-3β activity in vitro (Salmanian et al., 2010).
The regulation of BDNF and Akt-GSK-3 signalling in PFC are of relevance as these pathways are implicated in the etiology of mood disorders, drug addiction and schizophrenia (Beaulieu, 2012; Berglind et al., 2009; Whitfield et al., 2011). Therefore, in the present study we sought to elucidate the effect of D5R activation on the expression of proteins associated with BDNF and Akt signalling in rodent PFC. We showed the D5R regulated the activity of both pathways thereby identifying a novel function for the D5R in PFC.
Methods
Subjects
Adult male Sprague-Dawley rats (n = 8–9/group per experiment) and adult male gene-deleted (D1R−/− or D5R−/−) or wildtype control mice (C57BL/6J) (n = 6–7/group per experiment) of approximately 12 weeks of age were used. Homozygous D1R−/− and D5R−/− mice were produced from a genetic background of 129/SvJ1 and C57BL/6J. The mice were congenic, having been backcrossed for 14 and 12 successive generations (N14, N12) to the parent C57BL/6J strain. Animals were handled for 3 days daily before each experiment. Animal housing and experimental procedures followed the guidelines described in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care).
Drugs
SKF 83959 hydrobromide (Tocris Bioscience) was dissolved in physiological saline with 5% dimethyl sulfoxide. Animals received a single injection of 1.5 mg/kg s.c. (rats) or 3.0 mg/kg i.p. (mice) of SKF 83959, administered at a volume of 1 ml/kg or 5 ml/kg respectively.
Western Blot
Ninety minutes following SKF 83959 administration brains were removed and tissue from PFC dissected. This time point was chosen based on previous findings of maximal changes in BDNF expression and GSK-3β phosphorylation following 1–2 hours of SKF 83959 treatment in cultured striatal (Hasbi et al., 2009) or cortical neurons (Yu et al., 2008). Tissue was suspended in a hypotonic buffer solution and 15–40 micrograms of protein incubated in sample buffer for 3 minutes at 95° C. Samples were separated by SDS-PAGE on a tris-glycine gel and electroblotted onto PVDF transfer membrane. Membranes were blocked and incubated overnight at 4° C with primary antibody to CaMKIIα, Akt, pAkt Ser473, pAkt Thr308, GSK-α/β, pGSK-α/β Ser21/9 (Cell Signalling), pCaMKIIα Thr286 (Pierce Antibodies), TrkB (BD Bioscience), BDNF, GAD67, GAPDH, iNOS (Abcam). Membranes were washed in TBS-Tween and incubated for 2 hours at room temperature with secondary antibody (Bio-Rad Laboratories, Hercules, CA, USA). Antibody labelling of proteins was detected with enhanced chemiluminescence (Mandel Scientific) and signal intensity quantified using Zeiss AxioVision4 software. All samples for each experiment were run in tandem at every stage of the Western Blot protocol and developed on the same film.
Statistical Analysis
Values are reported as mean ± s.e.m. Data was collected by densitometry and the main dependent variable was Grey x area of band, expressed as a percent of saline-treated controls. Comparisons of means for protein expression in rat PFC were performed by Student’s t test (two-tailed, unpaired). Comparisons of means for protein expression in mouse PFC were performed by ANOVA followed by Bonferroni post-hoc test. Statistical significance set at P<0.05 and computations were performed using the SPSS/PC+ statistical package.
Results
Effect of dopamine D5R activation on BDNF signalling in PFC
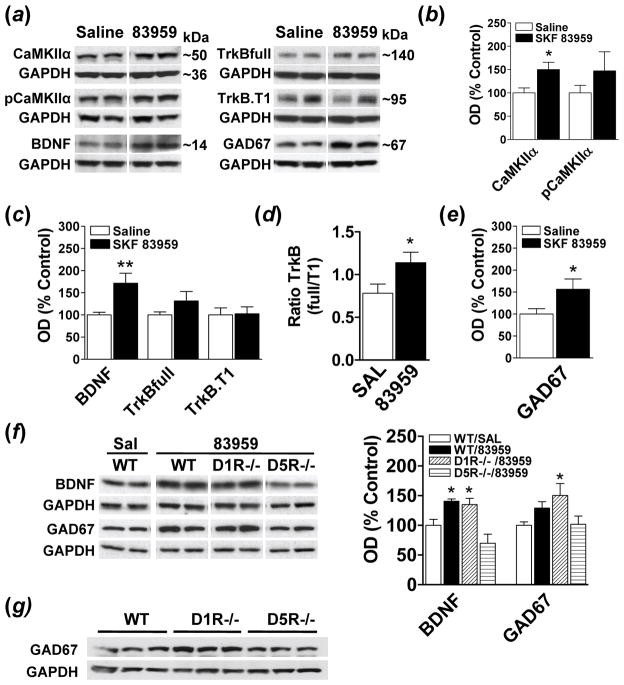
Activation of CaMKIIα has been linked to BDNF expression in brain (Zhou et al., 2006) and we have previously documented increased D1-D2 receptor heteromer-induced pCaMKIIα and BDNF expression in striatum following SKF 83959 (Hasbi et al., 2009; Ng et al., 2010). To elucidate the effects of SKF 83959 in regulating BDNF signalling in rat PFC, we examined the expression of total and phosphorylated CaMKIIα, BDNF and its receptor, tropomyosin receptor kinase B (TrkB), in this region following an injection of SKF 83959 (Fig. 1a–d). SKF 83959 induced a significant increase in total CaMKIIα expression {t(15)=−2.6, P<0.02} (Fig. 1b), however the expression of pCaMKIIα showed high levels of variability and did not differ from wildtype controls. SKF 83959 also induced a ~70% increase in the expression of BDNF in PFC {t(15)= −3.1, P<0.008} (Fig. 1c).
Fig. 1.
Increased BDNF signalling by the D5R in rodent PFC. (a) Representative blots depicting the effects of a single injection of the PLC-lined dopamine agonist SKF 83959 on CaMKIIα, pCaMKIIα, BDNF, TrkBfull, TrkB.T1, and GAD67 in rat PFC 90 minutes post injection (n=8–9 rats/group). GAPDH was used as a loading control. (b) SKF 83959 (1.5 mg/kg) increased total expression levels of CaMKIIα but did not alter phosphorylation of CaMKIIα at Thr286. (c) Expression of BDNF was elevated following SKF 83959, with no changes in the total expression levels of TrkBfull and TrkB.T1. (d) An increase in the ratio of TrkBfull:TrkB.T1, an index of receptor signalling, was evident following SKF 83959 administration. (e) SKF 83959 increased GAD67 expression in rat PFC. (f) Increased BDNF and GAD67 expression in D1R−/− mice, but not D5R−/− mice, following SKF 83959 (3 mg/kg) administration (n=6–7 mice/group). (g) Increased basal GAD67 expression in D1R−/− mice. Representative blots are also shown. Bars shown represent means ± s.e.m. and, with the exception of the TrkBfull:TrkB.T1 ratio, are expressed as a percentage of saline-treated controls. *P<0.05, ** P<0.01.
The physiological actions of BDNF are mediated largely by the TrkB receptor, which exists as both functional full length (TrkBfull) and truncated non-signalling (TrkB.T1) isoforms. SKF 83959 did not increase the expression of TrkBfull or TrkB.T1 in rat PFC (Fig. 1c). However, changes in the relative proportion of TrkBfull and TrkB.T1 would potentially have significant effects on BDNF-induced signalling, with a higher TrkBfull:TrkB.T1 ratio being indicative of heightened signalling. SKF 83959 increased the TrkB ratio {t(15)= −2.2, P<0.05} (Fig. 1d) indicating that this agonist may function to heighten BDNF signalling in rat PFC by increasing BDNF and the relative abundance of TrkBfull. As BDNF induces GAD67 expression in cortical neurons in vivo (Cotrufo et al., 2003), we assessed whether increased BDNF signalling by SKF 83959 was associated with a parallel upregulation of GAD67. SKF 83959 induced a significant increase in GAD67 expression in rat PFC {t(14)= −2.2, P<0.05} (Fig. 1e).
To determine whether the effects of SKF 83959 on BDNF and GAD67 levels were mediated by the D5R or the D1-D2 receptor heteromer, we examined protein expression in PFC of D1R−/− or D5R−/− mice following SKF 83959 (Fig. 1f). ANOVA revealed a significant effect of Experimental Group on BDNF and GAD67 expression {BDNF, F(3, 21)=9.4, P<0.001: GAD67, F(3, 23)=3.4, P<0.04}. SKF 83959 significantly increased BDNF levels in PFC of wildtype mice {P<0.02} and of D1R−/− mice {P<0.04}, but had no effect in D5R−/− mice, signifying that these effects were mediated by the D5R. Consistent with this, there were no changes in GAD67 expression in PFC of D5R−/− mice with a trend towards increased GAD67 expression in wildtype mice {P<0.07}. A significant increase (~50%) in GAD67 levels in D1R−/− mice {P<0.02} was also evident, although the ~30% elevation in basal GAD67 levels observed in the D1R−/− mice compared to wildtype (Fig. 1g), likely contributed to this increase. No differences in basal BDNF levels between wildtype mice and either gene-deleted strain were apparent (data not shown). Together these findings indicate that BDNF signalling in rodent PFC is regulated by the D5R.
Effect of dopamine D5R activation on Akt signalling in PFC
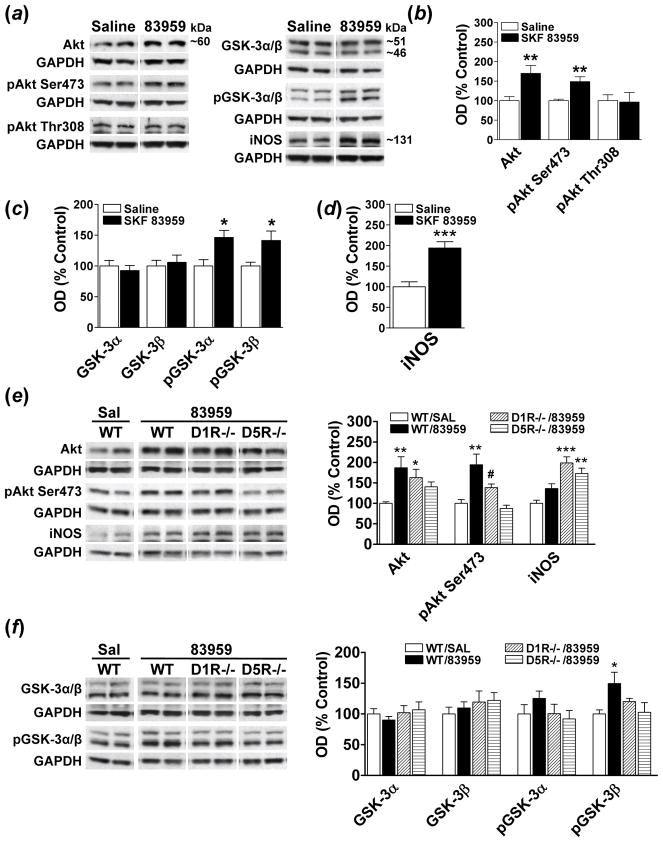
Akt signalling and iNOS expression has been shown to be increased following SKF 83959 treatment in cultured cortical neurons (Yu et al., 2008). Therefore, we determined whether SKF 83959 would exert similar effects in vivo in rat PFC (Fig. 2). Akt activity is regulated by phosphorylation at two regulatory residues, Ser473 and Thr308. SKF 83959 elevated total Akt expression in rat PFC and increased phosphorylation of Akt at Ser473 by ~50% {t(14)= −3.9, P<0.002}, with no change at Thr308 (Fig. 2b). Two substrates of Akt are GSK-3α and GSK-3β, are constitutively active kinases that are inhibited via phosphorylation at Ser21 (GSK-3α) and Ser9 (GSK-3β). Although no changes in total GSK-3α and GSK-3β levels were observed following SKF 83959 (Fig. 2c), there was an elevation in the phosphorylation and inactivation, of both kinases {GSK-3α, t(14)= −2.8, P<0.02: pGSK-3β, t(15)= −2.6, P<0.02}. These changes were paralleled by increased expression of iNOS (Fig. 2d), a protein pivotal to NO signalling in brain.
Fig. 2.
Regulation of Akt-GSK-3 signalling by the D5R in rodent PFC. (a) Representative blots depicting the effects of SKF 83959 on proteins associated with Akt-GSK-3 signalling in rat PFC 90 minutes post injection (n=8–9 rats/group). GAPDH was used as a loading control. (b) SKF 83959 (1.5 mg/kg) increased expression of Akt and phosphorylation of Akt at Ser473 with no effect on pAkt Thr308. (c) Phosphorylation levels of GSK-3α and GSK-3β were elevated following SKF 83959. (d) Expression of iNOS, a downstream target of GSK-3β, was also increased by SKF 83959. (e) Enhanced Akt expression in D1R−/− mice, but not D5R−/− mice, following SKF 83959 (3 mg/kg) administration. Phosphorylation of Akt at Ser 473 was also significantly higher in D1R−/− mice than in D5R −/− mice. iNOS levels were elevated in response to SKF 83959 in both gene-deleted strains. (f) Phosphorylation of GSK-3β was elevated in wiltype mice but not in D1R−/− or D5R−/− mice following SKF 83959 (n=6–7 mice/group). Representative blots are also shown. Bars shown represent means ± s.e.m. and are expressed as a percentage of saline-treated controls. *P<0.05, ** P<0.01, *** P<0.001 compared to saline-treated controls, # P<0.05 compared to D1R−/− mice.
To asses the contribution of the D5R or D1-D2 receptor heteromer in Akt-GSK-3 signalling in PFC, we repeated the experiments in D1R−/− and D5R−/− mice (Fig. 2e). Basal levels of Akt, pAkt Ser473, iNOS, GSK-3α/β and pGSK-3α/β did not differ between wildtype mice and either D1R−/− or D5R−/− mice. ANOVA revealed a significant effect of Experimental Treatment on the total and phosphorylated levels of Akt at Ser473 {Akt, F(3, 21)=3.7, P<0.03; pAkt Ser473, F(3, 21)=10.7, P<0.001}. SKF 83959 increased expression of Akt in PFC of wildtype mice {P<0.01} and D1R−/− mice {P<0.04}, but not D5R−/− mice. Phosphorylation of Akt at Ser473 was also elevated in the PFC of wildtype mice {P<0.01}. An approximate 38% increase in the phosphorylation of Akt at Ser473 was seen in D1R−/− mice, and although this was not significantly higher than the wildtype mice {P<0.07}, it was significantly greater than the D5R−/− mice {P<0.04}. SKF 83959 had no effect on pAkt Ser473 levels in D5R−/− mice.
Analysis of the expression and phosphorylation of GSK-3α/β by ANOVA revealed a significant effect of Experimental Treatment on pGSK-3β, but not pGSK-3α levels in mouse PFC {F(3, 21)=3.1, P<0.05}. Compared to GSK-3β, GSK-3α levels appeared to be much lower in the mouse PFC, a possible reason as to why the observed elevation in pGSK-3α in mouse PFC was modest and insignificant. Consistent with the effects of SKF 83959 on pAkt Ser473, there was also increased pGSK-3β levels in wildtype mice {P<0.03}. A modest elevation (~20%) of pGSK-3β in D1R−/− mice was also observed, with no change in pGSK-3β levels in D5R−/− mice. Increased iNOS expression was evident in both D1R−/− mice {P<0.001} and D5R−/− mice {P<0.01} following SKF 83959, {Experimental Treatment: F(3, 22)=12.4, P<0.001}. These findings suggest that while the D1-D2 heteromer and D5R both contribute to Akt-GSK-3 signalling in rodent PFC, the D5R appears to play a more preponderant role.
Discussion
In the present study we identified a novel physiological function for the D5R as a regulator of BDNF and Akt signalling in PFC. Specifically, following administration of SKF 83959, we observed enhanced expression of BDNF and the full length signalling isoform of its receptor TrkB, relative to its non-signalling counterpart, indicative of increased BDNF signalling. These changes were coincident with increased GAD67 expression, a protein whose downregulation has been linked to prefrontal cortical GABAergic dysfunction intrinsic to schizophrenia. Activation of the D5R also enhanced Akt signalling as evidenced by increased total expression of Akt and phosphorylation at Ser473, a regulatory residue linked to activation of Akt kinase activity. This activation of Akt paralleled an increase in the phosphorylation and deactivation of GSK-3α and GSK-3β, downstream substrates of Akt. Finally, we showed a significant elevation in the expression of iNOS, a protein whose expression has been linked to the activation of GSK-3β (Yu et al., 2008).
An involvement for BDNF signalling in the etiology of neuropsychiatric disorders has been demonstrated, however some reports specifically suggest that increasing BDNF signalling in the PFC is therapeutic. For example, while the downregulation of BDNF expression in rat PFC enhanced the reinforcing effect of cocaine (Sadri-Vakili et al., 2010), infusion of BDNF into this region suppressed cocaine seeking through a TrkB receptor-dependent mechanism (Whitfield et al., 2011). An intra-PFC infusion of BDNF additionally normalized cocaine-induced neuroadaptations affecting glutamate function in NAc (Berglind et al., 2009). Deficits in the levels of BDNF mRNA and protein expression in the PFC have also been reported in schizophrenia patients (Hashimoto et al., 2005; Weickert et al., 2003), findings of particular relevance as a correlation between BDNF signalling and reduced expression of proteins thought to be involved in the prefrontal cortical GABAergic dysfunction characteristic of schizophrenia, namely GAD67 and reelin, have been shown (Hashimoto et al., 2005; Pillai and Mahadik, 2008). Indeed, in one study examining heterozygous reeler mice, an animal model of schizophrenia, normalization of GAD67, mature BDNF and TrkBfull levels in frontal cortex and hippocampus was associated with improved cognitive functioning (Kutiyanawalla et al., 2011).
Accumulating evidence implicates abnormal GSK-3 signalling in schizophrenia and mood disorders (Beaulieu, 2012; Emamian et al., 2004), and it is now known that the therapeutic effects of various psychiatric drugs are mediated, at least in part, by their inhibition of GSK-3 activity. In rodent PFC, for example, antipsychotics, mood stabilizers and antidepressants all increased Akt signalling (Emamian et al., 2004; Sutton and Rushlow, 2011), resulting in the inhibition of GSK-3α and GSK-3β (Alimohamad et al., 2005; Sutton and Rushlow, 2011). Interestingly, the effects of D5R activation on Akt phosphorylation in PFC reported herein more closely mimicked that of mood stabilizers and antidepressants, increasing phosphorylation solely at the Ser473 site (Sutton and Rushlow, 2011). Although the mechanism by which the D5R phosphorylates Akt at Ser473 remains unknown, it has been shown that BDNF can induce Akt phosphorylation at Ser473 in cortical neuronal culture (Yoshii and Constantine-Paton, 2007), suggesting that increased Akt signalling may be secondary to BDNF-induced activation of TrkB.
In conclusion the present findings highlight a novel physiological role for the dopamine D5R in PFC, linking receptor activation to enhanced BDNF and Akt signalling, with the subsequent inactivation of GSK-3β. This ability of the D5R to increase BDNF signalling in PFC, as well regulate the activity of Akt in a manner similar to mood stabilizers and antidepressants, indicates that selective activation of this receptor may be efficacious in treating both neuropsychiatric and mood disorders. We therefore suggest that further research into the role of dopamine D5R in PFC as a therapeutic target in psychiatric disease is warranted, with the development of D5R-selective therapeutic agents.
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse (to S.R.G. and B.F.O.) and a CIHR Postdoctoral Fellowship (to M.L.P.). S.R.G. holds a Canada Research Chair in Molecular Neuroscience.
Footnotes
Statement of Interest: None.
References
- Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biological Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. Journal of Psychiatry and Neuroscience. 2012;37:7–16. doi: 10.1503/jpn.110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, et al. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. Journal of Neuroscience. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrufo T, Viegi A, Berardi N, Bozzi Y, et al. Effects of neurotrophins on synaptic protein expression in the visual cortex of dark-reared rats. Journal of Neuroscience. 2003;23:3566–3571. doi: 10.1523/JNEUROSCI.23-09-03566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, et al. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nature Genetics. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, et al. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, et al. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proceedings of the National Academy of Sciences U S A. 2009;106:21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, et al. Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. Journal of Neuroscience. 2005;25:372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LQ, Goswami S, Cai G, Zhen X, et al. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. Journal of Neurochemistry. 2003;85:378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Kutiyanawalla A, Promsote W, Terry A, Pillai A. Cysteamine treatment ameliorates alterations in GAD67 expression and spatial memory in heterozygous reeler mice. International Journal of Neuropsychopharmacology. 2011;22:1–14. doi: 10.1017/S1461145711001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, et al. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. Journal Biological Chemistry. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Luedtke RR, Griffin SA, Conroy SS, Jin X, et al. Immunoblot and immunohistochemical comparison of murine monoclonal antibodies specific for the rat D1a and D1b dopamine receptor subtypes. Journal of Neuroimmunology. 1999;101:170–187. doi: 10.1016/s0165-5728(99)00142-3. [DOI] [PubMed] [Google Scholar]
- Ng J, Rashid AJ, So CH, O’Dowd BF, et al. Activation of calcium/calmodulin-dependent protein kinase IIalpha in the striatum by the heteromeric D1-D2 dopamine receptor complex. Neuroscience. 2010;165:535–541. doi: 10.1016/j.neuroscience.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Mahadik SP. Increased truncated TrkB receptor expression and decreased BDNF/TrkB signaling in the frontal cortex of reeler mouse model of schizophrenia. Schizophrenia Research. 2008;100:325–333. doi: 10.1016/j.schres.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, et al. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proceedings of the National Academy of Sciences U S A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. Journal of Neuroscience. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, et al. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Molecular Pharmacology. 2009;75:447–453. doi: 10.1124/mol.108.053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmanian S, Najafi SM, Rafipour M, Arjomand MR, et al. Regulation of GSK-3beta and beta-Catenin by Galphaq in HEK293T cells. Biochemical and Biophysical Research Communications. 2010;395:577–582. doi: 10.1016/j.bbrc.2010.04.087. [DOI] [PubMed] [Google Scholar]
- So CH, Verma V, Alijaniaram M, Cheng R, et al. Calcium signaling by dopamine D5 receptor and D5-D2 receptor hetero-oligomers occurs by a mechanism distinct from that for dopamine D1-D2 receptor hetero-oligomers. Molecular Pharmacology. 2009;75:843–854. doi: 10.1124/mol.108.051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton LP, Rushlow WJ. The effects of neuropsychiatric drugs on glycogen synthase kinase-3 signaling. Neuroscience. 2011;199:116–124. doi: 10.1016/j.neuroscience.2011.09.056. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, et al. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Molecular Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- Whitfield TW, Jr, Shi X, Sun WL, McGinty JF. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. Journal of Neuroscience. 2011;31:834–842. doi: 10.1523/JNEUROSCI.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nature Neuroscience. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- Yu Y, Wang JR, Sun PH, Guo Y, et al. Neuroprotective effects of atypical D1 receptor agonist SKF83959 are mediated via D1 receptor-dependent inhibition of glycogen synthase kinase-3 beta and a receptor-independent anti-oxidative action. Journal of Neurochemistry. 2008;104:946–956. doi: 10.1111/j.1471-4159.2007.05062.x. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao WN, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]