Abstract
Background
Despite the high prevalence of HIV in adults (11 %) in Malawi, testing among surgical patients is not routine. We examined the feasibility of universal opt-out HIV testing and counseling (HTC) on the surgical wards of Kamuzu Central Hospital in Lilongwe, Malawi, and sought to further delineate the role of HIV in surgical presentation and outcome.
Methods
We reviewed HTC and surgical admission records from May to October 2011 and compared these data to data collected prospectively on patients admitted from November 2011 through April 2012, after universal HTC implementation.
Results
Prior to universal HTC, 270 of the 2,606 (10.4 %) surgical admissions were tested; 13 % were HIV-infected. After universal HTC implementation, HTC counselors reviewed 1,961 of the 2,488 admissions (79 %): 310 (16 %) had known status (157 seropositive, 153 seronegative) and 1,651 had unknown status (81 %). Among those with unknown status, 97 % (1,598, of 64 % of all admissions) accepted testing, of whom 9 % were found to be HIV-infected. Patients with longer lengths of stay (LOS) (mean = 11 vs. 5 days, p <0.01) and those who underwent surgical intervention (odds ratio [OR] 2.5; confidence interval [CI] 2.0–3.1) were more likely to have a known status on discharge. HIV was more prevalence in patients with infection and genital/anal warts or ulcers and lower in trauma patients. HIV-positive patients received less surgical intervention (OR 0.69; CI 0.52–0.90), but there was no association between HIV status and length of stay or mortality.
Conclusions
Universal opt-out HTC on the surgical wards was well accepted and increased the proportion of patients tested. High HIV prevalence in this setting merits implementation of universal HTC.
Introduction
The HIV epidemic continues to plague southern Africa with a third of all HIV-related deaths occurring in that region [1]. HIV is the leading cause of mortality and morbidity in Malawi [2]. With widespread availability of highly active antiretroviral therapy (HAART) beginning in 2004 [3], estimates of HIV prevalence in Malawi’s general adult population has decreased from 15 % in 2000 to the present level of 11 % [4–6], and AIDS-associated mortality has decreased [7]. Inadequate provision of HIV testing and counseling (HTC) and late ART initiation are two reasons there has not been further reduction in HIV prevalence/ incidence and subsequent HIV-related mortality and morbidity in country [6]. To address this, the Malawi Ministry of Health has mandated that universal opt-out HTC be provided in all health-care settings [8].
Despite these efforts, HTC is not a part of routine care for surgical patients. At Kamuzu Central Hospital (KCH), a tertiary-care referral hospital in Lilongwe, Malawi, surgical patients comprise 25 % of inpatients, with ~5,000 admissions a year. The hospital serves a catchment of over 5 million from the capital city of Lilongwe and referrals from the country’s entire central region. Surgical patients most often present with non-HIV-related diagnoses and may not enter a clinical setting. Implementing universal opt-out HTC on the surgical wards could promote earlier HIV diagnosis.
Currently, suboptimal HIV testing in this population has resulted in a dearth of knowledge about the prevalence and epidemiology of HIV among surgical patients in southern Africa. Prevalence of HIV in surgical inpatients (32–39 %) is thought to be significantly higher than the estimates of HIV in the general population [9–11]. Of 445 patients admitted to the surgical wards in Blantyre, Malawi, over two 2-week periods in 1999 and 2000, 36 % were seropositive overall, with a 52 % prevalence rate in patients with deep infections [9]. In Durban, South Africa, HIV prevalence was 39 % in a cohort of 350 surgical patients admitted over 6 weeks, and HIV-positive patients had more surgical interventions overall, largely due to surgical management of infections [11]. Despite the high HIV prevalence, outcomes were similar for HIV-seropositive and -seronegative patients [10–13].
In developed countries, except for slower wound healing in seropositive patients with anorectal disease, morbidity and mortality among seropositve and seronegative HIV patients have also been shown to be similar [13–16]. However, small sample sizes with only short-duration provision of HTC limits our understanding of current HIV epidemiology among surgical patients in southern Africa, particularly given that most assessments occurred before the widespread availability of ART.
To this end, we implemented universal HTC on the surgical wards of KCH. We assessed the feasibility of ongoing testing using the existing public infrastructure and estimated the current inpatient HIV prevalence in the setting of widespread ART availability. We also prospectively gathered data on patients’ clinical course and hospital outcome to investigate differences among HIV-seropositive and seronegative patients.
Methods
Study setting and population
All patients admitted to the KCH adult (aged ≥ 13 years) surgical wards from November 2011 through April 2012 were eligible for inclusion in the study. Patients under 13 years old were not eligible due to a need for guardian consent for HTC according to Malawi Ministry of Health guidelines.
Study design
We conducted a prospective cohort study of patients admitted from November 1, 2011 through April 30, 2012, after initiation of universal opt-out HTC. We collected clinical course and outcome data through May 14, 2012. In addition, we performed a retrospective review of HTC records and adult surgical ward admission records over the 6-month period prior to our study (May through October 2011) to determine the proportion of patients tested at baseline.
Universal HTC implementation
We used a universal opt-out HTC approach consistent with Malawi HTC guidelines [8]. The opt-out method of testing is a method advocated by WHO and the Malawi Ministry of Health that requires the client to actively decline HIV testing. All patients are approached by the HTC counselor, provided with pretest counseling regarding risks, benefits, and outcomes, and undergo testing unless they specifically decline. Testing is not mandatory or coercive, and patients who opt-out are entitled to equal surgical care. For the purposes of better understanding the cultural barriers to testing in our study, patients who opted-out were asked to provide their reason for declining testing if they felt comfortable doing so.
Prior to HTC implementation at KCH, a trained HTC counselor staffed the HTC program but HTC was done only when ordered by a clinician. Counseling was available from 8 am to 4 pm, Monday through Friday only. After implementation, an additional counselor was placed on the wards to be dedicated to the surgical patients and worked Monday through Saturday. All counselors were oriented in procedures and participated in sensitization activities designed to improve their knowledge and skills regarding opt-out HTC in surgical patients.
Due to the acute nature of many surgical conditions, patients generally were tested on the surgical wards on admission day 2 or later to prevent interfering with stabilization of the patient. Earlier HIV testing occurred by order of a clinician only to guide medical care if a patient presented in extremis.
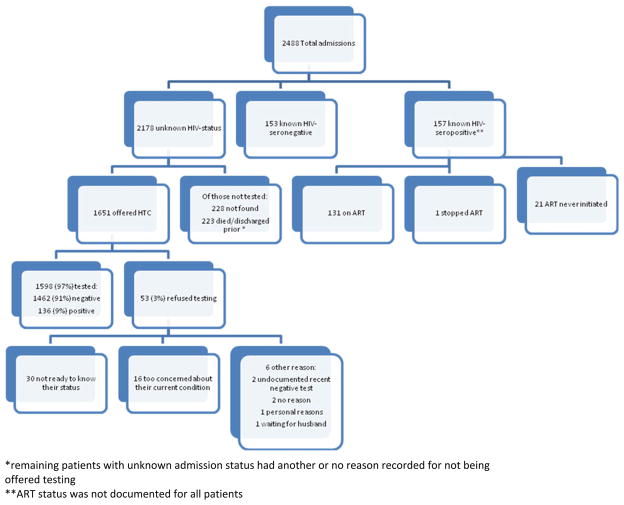
A study clerk identified all admitted patients in the nurse logbook. HTC counselors found patients on the ward and verified HIV and ART status. All patients with an unknown HIV status or a negative HIV test result older than 3 months were counseled by a certified HTC counselor and offered HIV testing. We followed the Malawi serial testing algorithm using rapid testing (Determine HIV 1/2 as a screening test and Unigold HIV 1/2 as a confirmatory test) [8]. Non-ambulatory patients were offered bedside testing which was the standard of care prior to universal HTC implementation. Ambulatory patients could choose between bedside testing and testing in a private room Fig. 1.
Fig. 1.
Admission HIV status and universal HTC results. Flowchart of all admissions, their initial HIV and ART status, and testing results for those who had an unknown status on admission. * Remaining patients with unknown admission status had another or no reason recorded for not being offered testing. ** ART status was not documented for all patients
HIV test results were recorded in the HTC register, the surgical chart, and the patient’s health passport (a hand-held medical record) to ensure communication of the result to health-care providers. Counselors completed a prewritten CD4 order form for all newly reactive patients and gave this to the ward nurse to order a CD4 count. Newly reactive patients were referred for follow-up at an HIV care center for further evaluation after discharge.
Clinical course and outcome data collection
Using a data extraction form, the study clerk and the HTC counselor collected data on admission HIV status, admission ART status, inpatient HIV test results, CD4 counts when available, admission diagnosis and vital signs, surgical procedures, full blood count (FBC) and transfusions, hospital length of stay (LOS), and outcome (death or discharge).
Data analyses
Data extraction forms were prenumbered to give each admission a unique identifier. Data were entered into Microsoft’s Access 2007 and analyzed using Stata 11.2. Duplicates were removed and outlier variables were verified manually for consistency.
Patients with known and unknown HIV status at final outcome and HIV-seropositive and -seronegative patients were compared with respect to clinical course and outcome variables collected. Categorical variables were compared using Pearson’s χ2 test or Fisher’s exact test. Continuous variables were compared with the 2-sample t test. Odds ratios (OR), confidence intervals (CI), and adjusted odds ratios (AOR) for death and HIV status were determined using a logistic regression model controlling for age and sex.
Ethics
Ethics approval was obtained for this study from the Malawi National Health Science Research Committee and the University of North Carolina Medical School institutional review board.
Results
During the 6-month period prior to universal opt-out HTC implementation, 270 patients of a total of 2,606 admissions (10 %) on the male (195/1,755) and female (75/851) adult surgical wards were tested. Thirty-six of the tested patients (13 %) were reactive (10 % of males, 21 % of females).
Patient population following HTC implementation
Over 6 months, 2,488 surgical admissions occurred: 53 % were elective and 47 % were emergencies. Admitted patients’ age ranged from 13 to 102 years (mean = 40, median = 36) and 68 % were male. The most common indications for surgical admission were trauma/burn (28 %), abdominal complaints (acute abdomen, bowel obstruction, hernia, non-acute abdominal pain) (19 %), infection (abscess, infected wound, sepsis, cellulitis, osteomyelitis, septic joint, surgical site infection, pyomyositis) (12 %), and tumor (known cancer, soft tissue mass, other tumor) (9 %).
A total of 1,908 of the 2,488 (77 %) surgical admission patients had a known HIV status at final outcome, and their age ranged from 13 to 102 years (mean = 41 ± 17.6 -years), with a male:female ratio of 1.9:1 (Table 1). 54 % were emergency admissions, 39 % had surgical intervention, and 96 % survived to discharge. Hospital LOS ranged from 0 to 87 days (mean = 10.6 ± 10.5 days, median = 8 days). The most common indications for surgical admission were trauma/burn (30 %), abdominal complaints (22 %), infection (14 %), and tumor (12 %).
Table 1.
Comparison of patients with unknown HIV status with those with known status
| Unknown status (n = 580) | Known status (n = 1,908) | P value | |
|---|---|---|---|
| Age (years) [mean (SD)] | 37.8 (16.7) | 41.0 (17.6) | <0.01 |
| Sex [n (%)] | |||
| Male | 430 (74 %) | 1253 (81 %) | |
| Female | 149 (26 %) | 655 (19 %) | <0.01 |
| Admission type [n (%)] | |||
| Elective/direct | 245 (42 %) | 873 (46 %) | |
| Emergency | 335 (58 %) | 1,035 (54 %) | 0.136 |
| Trauma | 240 (72 %) | 581 (56 %) | |
| Other emergency | 95 (28 %) | 454 (44 %) | <0.01 |
| Outcome [n (%)] | |||
| Discharge | 544 (97 %) | 1,762 (96 %) | |
| Death | 19 (3 %) | 71 (4 %) | 0.59 |
| Length of stay (days) [mean (SD)] | 5.1 (6.8) | 10.6 (10.6) | <0.01 |
| Surgical intervention [n (%)] | 115 (20 %) | 720 (39 %) | <0.01 |
Universal HTC implementation
The number of patients tested for HIV increased with the introduction of universal HTC. The HTC counselor saw 1,961/2,488 (79 %) of all admissions, of whom 157 (8 %) were known to be HIV-positive, with 130/157 (83 %) on ART; 153/1,961 (8 %) were known to be HIV-negative; and 1,651/1,961 (84 %) had unknown status and were offered testing. Among all admitted patients, 1,598 (64 %) received testing during their hospital admission and 1,908 (77 %) had known HIV status by the time of discharge or death. The patients with an unknown status on admission who did not receive testing were either not found on the wards after three attempts by the counselor, had been discharged, or died prior to the HIV counselor’s daily rounds.
Of those offered testing, 1,598 (97 %) accepted testing with only 53 patients (3 %) refusing. The most common reason for refusal was not being prepared to know one’s HIV status (n = 30), followed by being too concerned about their current condition to consider testing at that time (n = 16). No patients cited the bedside nature of the testing as a reason for refusal. Of the newly tested patients, 136/1,598 (9 %) were reactive. CD4 counts were ordered for 124 (91 %) of the newly reactive patients, but the result was recorded in the charts for only 25 (20 %) of those patients. Thirteen (52 %) of these were eligible for ART.
Compared to those without known status at final outcome, factors associated with having a known HIV status at the time of discharge or death included a longer LOS and receiving surgical intervention (Table 1). Patients missed by the counselor (unknown status at discharge or death) had a shorter mean LOS compared to those with a known status (mean LOS = 5.1 vs. 10.6 days, p <0.01). Patients who had surgical intervention were over twice as likely to have a known their status when discharged (OR 2.5; CI 2.0–3.1). On the other hand, admission on a Saturday, which meant testing would not be offered until admission day 3 or later due to the lack of a counselor on Sundays, was not associated with an increased chance of being missed (OR 1.2; CI 0.9–1.7). A larger proportion of males were missed, and missed patients on average were younger. Trauma patients were also more likely to be missed than non-trauma patients after adjusting for age and sex (AOR 1.4; CI 1.2–1.8). Otherwise, the range of admission diagnoses between the two groups was similar. Also, there was no significant difference in admission type (emergency vs. elective) or outcome (death or discharge) between those with known status and those with unknown status by the end of their hospital stay.
HIV epidemiology and correlation with outcome
HIV prevalence among patients with a known status at discharge or death was 15 % and was lower in males than in females (14 vs. 18 %, p <0.01) (Table 2). HIV-positive patients were younger than HIV-negative patients (mean age = 37 vs. 42 years, p <0.01). HIV-positive males were slightly older than HIV-positive females (mean age = 38 vs. 36 years, p = 0.04).
Table 2.
Comparison of HIV-positive with HIV-negative patients
| HIV-positive (N = 293, 15 %) | HIV-negative (N = 1,615, 85 %) | P value | |
|---|---|---|---|
| Age (years) [mean (SD)] | 36.6 (11.4) | 41.8 (18.3) | <0.01 |
| Sex [n (%)] | |||
| Male | 173 (59 %) | 1,080 (67 %) | |
| Female | 120 (41 %) | 535 (33 %) | <0.01 |
| Admission type [n (%)] | |||
| Elective/direct | 139 (47 %) | 734 (45 %) | |
| Emergency | 154(53 %) | 881 (55 %) | 0.53 |
| Trauma | 74 (48 %) | 507 (58 %) | |
| Other emergency | 80 (52 %) | 374 (42 %) | 0.03 |
| Outcome [n (%)] | |||
| Discharge | 273 (97 %) | 1489 (96 %) | |
| Death | 9 (3 %) | 62 (4 %) | 0.52 |
| Length of stay (days) [mean (SD)] | 9.9 (9.3) | 10.8 (10.8) | 0.20 |
| Surgical intervention [n (%)] | 90 (32 %) | 630 (40 %) | <0.01 |
HIV was more prevalent in non-trauma patients (16.4 %) than in trauma patients (12.7 %) (AOR 1.47; CI 1.10–1.98) when adjusted for sex and age. Specifically, admission diagnosis of infection was associated with increased odds of being HIV-positive (AOR 1.59; CI 1.15–2.20) as were genital/anal warts or ulcers (AOR 22.13; CI 6.12–79.98). However, seropositive patients were not more likely to require operative intervention (I&D, debridement, or amputation) for their infection when compared to seronegative patients (AOR 0.61; CI 0.32–1.16). In fact, HIV-positive patients received surgical intervention less often overall than HIV-negative patients (AOR 0.69; CI 0.52–0.90), and there was no significant association between HIV status and admission type, LOS, or mortality (p >0.05).
Discussion
Our study answers three important HIV-related questions regarding a surgical inpatient cohort in a resource-limited setting in southern Africa. Implementing universal HTC on surgical wards using existing infrastructure is feasible. The estimated adult HIV prevalence on the surgical wards was 15 %, higher than national estimates. Lastly, we showed that there was no significant correlation of HIV status with increased mortality or LOS.
Ward-based, universal opt-out HTC is an effective and patient-acceptable way to increase HTC. The significant increase in the number of patients tested and the low HTC refusal rate during our study period suggest this model could be transferred to other similar settings. However, some challenges remain in creating an optimal testing system. We were unable to capture 100 % of the patients, in part due to early deaths and short hospital stays. Additionally, the methods used to assign beds within the wards made it difficult to locate all admitted patients.
Overall HIV prevalence in our cohort (15 %) was lower than that estimated by previous studies in southern Africa [9–11]. Our ongoing testing approach allowed us to have a longer study period and a larger sample size, thus strengthening our ability to draw conclusions from the data collected. Unfortunately, we were unable to test all consecutive admissions. The population missed by our counselors, largely young male trauma patients, appeared to have a lower HIV prevalence such that our HIV estimate may be inflated. Alternatively, if refusals and early deaths represented a higher risk group for HIV, our estimate may be modestly underestimated given that these were a small proportion of the overall admissions. Regardless, our finding of lower prevalence of HIV than that found in previous studies in Malawi is realistic given the successful ART program and the reduction in adult HIV prevalence over time [7]. In addition, South Africa and the southern region of Malawi are known to have a higher HIV prevalence than Malawi’s central region, where we conducted the study, which further explains the difference [6]. Still, given the high prevalence on the surgical wards and our ability to identify a significant number of newly tested seropositive patients, we would recommend universal HTC in this setting and other similar settings.
Furthermore, ART has been shown to dramatically reduce HIV transmission [17], and mathematical models have shown that universal HIV testing and immediate ART initiation could greatly reduce HIV incidence and mortality within 10 years [18, 19]. Universal HTC provides an opportunity for early enrollment of patients in HIV care as well as inpatient initiation of ART for patients with a longer LOS, a program that we are currently implementing on the KCH wards. Our strategy of universal opt-out HTC with early ART initiation could significantly impact the fight for the elimination of the HIV epidemic.
Universal HTC is beneficial not only to patients because it allows for earlier detection and care, but also to surgeons because it identifies HIV-infected individuals prior to surgery. A surgeon’s risk of HIV seroconversion during their career increases with higher HIV prevalence in the patient population [20]. Despite a low rate of occupational exposure, the relative seroconversion risk for African surgeons is estimated to be up to 15 times greater than the risk in Western countries [21]. With universal testing of surgical patients, if exposure occurs, surgeons will be able to immediately begin postexposure prophylaxis as opposed to having to deal with the logistics of patient consent and testing following the incident [22, 23].
We found that admission diagnoses of infection and genital/anal warts or ulcers were associated with increased odds of an HIV-positive status when compared to other diagnoses. This finding is consistent with that of past studies [9–11]; however, in our population we found a lower rate of operative interventions overall in HIV-seropositive patients compared to HIV-seronegative patients. As with any resource-limited setting, the admission diagnosis is often based on clinical findings because of the lack of imaging, tissue diagnosis, and lab tests. However, we feel that the recorded diagnoses are representative of what would be recorded in other resource-poor settings.
With respect to infection rates and drainage and debridement procedures, diagnosis of cutaneous abscesses was suggestive of advanced disease in the Soweto population, though the study did not specify CD4 counts [10]. An immunocompromised state in these patients could be responsible for the greater need for surgical intervention. Patients in our cohort may have higher CD4 counts. 83 % of admitted patients with a previous diagnosis of HIV were on ART and only 52 % of the newly reactive patients with CD4 counts were eligible for ART. Also, given that the majority of our patients presented due to trauma, our patient population was otherwise healthy, with fewer patients meeting the criteria for an immunocompromised state. We do not believe that surgical resources are being withheld from HIV-seropositive patients in this environment since testing was not always conducted before surgery and clinician-ordered HTC was not common prior to routine HTC implementation. In addition, despite differences in the number of surgical interventions, we found no difference in mortality between HIV-seropositive and -seronegative patients.
Finally, similar to other published studies, HIV status did not significantly affect hospital LOS or the outcome of death or discharge in our cohort. Unfortunately, due to limited CD4 count data, we were unable to stratify CD4 counts to detect significant differences in clinical course and death rates. Patients with a lower CD4 count have been shown to have a higher complication rate when CD4 counts drop below 50 and poorer wound healing after anorectal surgery, but we are unable to support or refute this with our study data [13, 14, 16]. Also, we did not have the ability to follow outcome past hospital discharge. It is possible that some deaths occurred shortly after discharge. Lastly, in addition to clinical management, there are likely many socioeconomic and cultural factors that affect a patient’s LOS in this type of setting. However, all patients, regardless of serostatus, are subject to these same issues. Previous studies had similar limitations and had too few deaths to support the significance of their findings [9–11, 14]. Our larger sample size provides 80 % power to detect a 7 % difference in mortality. Thus, preliminary findings of previous studies are supported by our data: overall HIV status was not predictive of significantly increased in-hospital mortality or longer length of stay.
In conclusion, HIV prevalence remains higher in the inpatient surgical setting compared to general population estimates. HIV serostatus does not appear to influence clinical course or outcome except that HIV-seropositive patients had fewer surgical interventions in our study. The similar hospital outcomes between seropositive and seronegative patients and the low death rates overall suggest that HIV-seropositive patients are benefiting from improved ART access given that the vast majority of patients presenting with a known seropositive status were already enrolled in HIV care and on ART. Our findings support implementation of ongoing universal opt-out HTC on inpatient surgical wards in similar resource-poor settings as a way to identify seropositive patients at earlier disease stages, a practice that can benefit both patients and surgeons and have an impact on the HIV epidemic.
Contributor Information
Bryce E. Haac, University of North Carolina School of Medicine, Chapel Hill, NC, USA
Anthony G. Charles, Email: anthchar@med.unc.edu, Department of Surgery, University of North Carolina School of Medicine, Chapel Hill, NC, USA. UNC Project-Malawi, Lilongwe, Malawi
Mitch Matoga, UNC Project-Malawi, Lilongwe, Malawi.
Sylvia M. LaCourse, Division of Allergy and Infectious Diseases, University of Washington, Seattle, WA, USA
Dominic Nonsa, The Lighthouse Trust, Lilongwe, Malawi.
Mina Hosseinipour, UNC Project-Malawi, Lilongwe, Malawi. Center for Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, NC, USA.
References
- 1.USAID. [Accessed 1 May 2013];HIV/AIDS Health Profile Southern Africa. 2011 Available at http://transition.usaid.gov/our_work/global_health/aids/Countries/africa/southernafrica_profile.pdf.
- 2.Bowie C. The burden of disease in Malawi. Malawi Med J. 2006;18(3):103–110. [PMC free article] [PubMed] [Google Scholar]
- 3.Harries AD, Makombe SD, Libamba E, et al. Why did the scale-up of HIV treatment work? A case example from Malawi. J Aquir Immune Defic Syndr. 2011;57:S64–S67. doi: 10.1097/QAI.0b013e31821f6bab. [DOI] [PubMed] [Google Scholar]
- 4.National Statistical Office (NSO) (Malawi) and ORC Macro (Calverton, MD, USA) [Accessed 6 May 2013];Malawi Demographic and Health Survey 2000. 2001 Available at www.measuredhs.com/pubs/pdf/FR123/FR123.pdf.
- 5.National Statistical Office (NSO) (Malawi) and ORC Macro (Calverton, MD, USA) [Accessed 6 May 2013];Malawi Demographic and Health Survey 2010. 2011 Available at www.measuredhs.com/pubs/pdf/FR247/FR247.pdf.
- 6.UNAIDS. [Accessed 6 May 2013];Malawi HIV and AIDS Monitoring and Evaluation Report 2008–2009. 2010 Available at http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2010countries/malawi_2010_country_progress_report_en.pdf.
- 7.Jahn A, Floyd S, Crampin AC, et al. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet. 2008;371(9624):1603–1611. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malawi Ministry of Health. [Accessed 6 May 2013];Guidelines for HIV Testing and Counseling (HTC) (3). 2009 Available at http://www.hivunitmohmw.org/Main/HIVTesting.
- 9.Lewis DK, Callaghan M, Phiri K, et al. Prevalence and indicators of HIV and AIDS among adults admitted to medical and surgical wards in Blantyre, Malawi. Trans R Soc Trop Med Hyg. 2003;97(1):91–96. doi: 10.1016/s0035-9203(03)90035-6. [DOI] [PubMed] [Google Scholar]
- 10.Martinson NA, Omar T, Gray GE, et al. High rates of HIV in surgical patients in Soweto, South Africa: impact on resource utilisation and recommendations for HIV testing. Trans R Soc Trop Med Hyg. 2007;101(2):176–182. doi: 10.1016/j.trstmh.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Cacala SR, Mafana E, Thomson SR, et al. Prevalence of HIV status and CD4 counts in a surgical cohort: their relationship to clinical outcome. Ann R Coll Surg Eng. 2006;88(1):46–51. doi: 10.1308/003588406X83050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhagwanjee S, Muckart D, Jeena PM, et al. Does HIV status influence the outcome of patients admitted to a surgical intensive care unit? A prospective double blind study. BMJ. 1997;314(7087):1081–1084. doi: 10.1136/bmj.314.7087.1077a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madiba TE, Muckart DJ, Thomson SR. Human immunodeficiency disease: how should it affect surgical decision making? World J Surg. 2009;33(5):899–909. doi: 10.1007/s00268-009-9969-6. [DOI] [PubMed] [Google Scholar]
- 14.Horberg MA, Hurley LB, Klein DB, et al. Surgical outcomes in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Arch Surg. 2006;141(12):1238–1245. doi: 10.1001/archsurg.141.12.1238. [DOI] [PubMed] [Google Scholar]
- 15.Wakeman R, Johnson CD, Wastell C. Surgical procedures in patients at risk of human immunodeficiency virus infection. J R Soc Med. 1990;83(5):315–318. doi: 10.1177/014107689008300513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consten EC, Slors FJ, Noten HJ, et al. Anorectal surgery in human immunodeficiency virus-infected patients. Clinical outcome in relation to immune status. Dis Colon Rectum. 1995;38(11):1169–1175. doi: 10.1007/BF02048332. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378(9787):256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 20.Lin EY, Brunicardi FC. HIV infection and surgeons. World J Surg. 1994;18(5):753–757. doi: 10.1007/BF00298922. [DOI] [PubMed] [Google Scholar]
- 21.Consten EC, van Lanschot JJ, Henny PC, et al. A prospective study on the risk of exposure to HIV during surgery in Zambia. AIDS. 1995;9(6):585–588. doi: 10.1097/00002030-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 22.McGowan DR, Norris JM, Smith MD, et al. Routine testing for HIV in patients undergoing elective surgery. Lancet. 2012;380(9846):e5. doi: 10.1016/S0140-6736(12)61537-2. [DOI] [PubMed] [Google Scholar]
- 23.Olapade-Olaopa EO, Salami MA, Afolabi AO. HIV/AIDS and the surgeon. Afr J Med Med Sci. 2006;35(Suppl):77–83. [PubMed] [Google Scholar]