Abstract
Borrelia burgdorferi outer surface protein (Osp) A has been used as a Lyme disease vaccine that blocks transmission: OspA antibodies of immune hosts enter ticks during blood feeding and destroy spirochetes before transmission to the host can occur. B. burgdorferi produce OspA in the gut of unfed Ixodes scapularis ticks, and many spirochetes repress OspA production during the feeding process. This preferential expression suggests that OspA may have an important function in the vector. Here we show that OspA mediates spirochete attachment to the tick gut by binding to an I. scapularis protein. The binding domains reside in the central region and COOH-terminus of OspA. OspA also binds to itself, suggesting that spirochete-spirochete interactions may further facilitate adherence in the gut. OspA-mediated attachment in the tick provides a possible mechanism for how stage-specific protein expression can contribute to pathogenesis during the B. burgdorferi natural cycle.
Introduction
Lyme disease is the most common arthropod-borne illness in the United States (1, 2). Outer surface protein (Osp) A elicits immunity against Borrelia burgdorferi infection and is being used as a human vaccine (3–6). OspA antibody blocks spirochete transmission to the vertebrate host by binding to B. burgdorferi within the gut of engorging Ixodes scapularis ticks (7–10). Phase III clinical trials demonstrate that vaccination with OspA provides 80–90% protection against Lyme disease, and vaccine failures are generally associated with low antibody responses to OspA in vaccine recipients (5, 6).
The temporal expression of ospA during the B. burgdorferi life cycle implies that OspA has a function within the arthropod host. Uninfected larval ticks acquire spirochetes while feeding on infected mice. B. burgdorferi produce OspA soon after leaving the vertebrate host and entering I. scapularis, suggesting an important role for OspA in vector colonization (7–10). Within nymphal ticks, spirochetes abundantly express ospA and remain in close association with the tick gut. When nymphs engorge on a vertebrate host, B. burgdorferi in the gut rapidly multiply, and 70–80% of the organisms clear OspA from their surface (7).
B. burgdorferi continues to repress ospA in the mammalian host. ospA mRNA is not readily detectable after tick-borne transmission of spirochetes to mice but can sometimes be found up to 14 days after syringe challenge of mice with OspA-producing B. burgdorferi (11–15). ospA is also not usually expressed during human infection. However, some individuals may develop humoral and cellular OspA responses in early-stage disease (16–19), and immune responses to OspA have been associated with chronic Lyme arthritis, indicating that this antigen is present in some patients (20, 21). The selective expression of ospA when B. burgdorferi goes into and resides within I. scapularis and the subsequent general downregulation of ospA when spirochetes exit the tick gut suggest that OspA may play an important role in B. burgdorferi colonization of the arthropod vector.
Methods
B. burgdorferi and I. scapularis: culture and cultivation
A clonal isolate of B. burgdorferi N40 that is infectious and pathogenic in mice, and represents a prototypic B. burgdorferi sensu stricto organism, was used throughout these studies (22). Spirochetes were cultivated in Barbour-Stoenner-Kelly (BSK) II medium at 33°C (23). B. burgdorferi 46047, a naturally occurring B. burgdorferi sensu stricto isolate that lacks OspA (24), was also used in some spirochete-OspA binding studies.
Mated adult female I. scapularis were collected in the field. The egg mass was then laid in the laboratory. Hatched larvae were fed on uninfected C3H mice to produce pathogen-free nymphs. All tick rearing was performed in an incubator at 26°C with 85% relative humidity and a 12-hour light/dark photo period regimen.
ELISA
Protein binding to the tick gut extract.
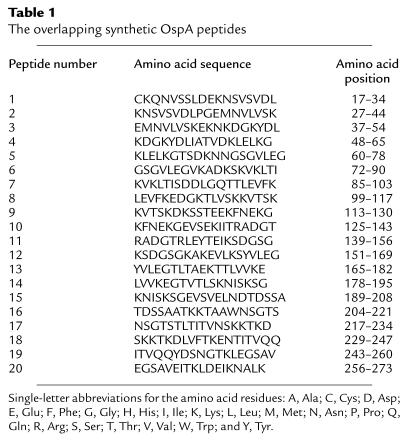
Recombinant OspAs from B. burgdorferi isolates 25015 and N40, and OspC from B. burgdorferi N40, were expressed and purified in their nonlipidated forms (25, 26, 27). OspA and OspC were expressed either without a fusion partner or as fusion proteins with glutathione transferase (after which the fusion partner was cleaved using thrombin), as described previously (15, 25, 26). OspA with mutations in specific amino acids, produced using site-directed mutagenesis (see later in this section), were also expressed as a fusion partner with glutathione transferase, and the fusion partner was subsequently removed with thrombin. Overlapping 18- or 19-mer OspA peptides were synthesized by A. Michel at the University of Mons, Belgium. Peptides were solubilized in DMSO and diluted in PBS. OspAs, OspA peptides, OspC, or BSA was labeled with FITC from Molecular Probes Inc. (Eugene, Oregon, USA). The extent of conjugation of FITC per molecule of protein was determined according to the manufacturer’s instructions. One milligram of each FITC-labeled protein represents 34 pmol of OspA, 43 pmol of OspC, and 15 pmol of BSA. One picomole of OspA, OspC, and BSA bound to 2, 2.4, and 6.8 pmol of FITC, respectively.
Guts from flat nymphal I. scapularis ticks were dissected in PBS and homogenized on ice with a Kontes microhomogenizer (VWR Scientific Products, West Chester, Pennsylvania, USA). Salivary glands from nymphal ticks were dissected in PBS and also homogenized on ice. One gut extract equivalent (0.5 μg) of protein or 0.5 μg of salivary gland protein extract per well was used to coat microtiter plates (ICN Biomedical Inc., Costa Mesa, California, USA). Protein concentrations were determined using the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories Inc., Hercules, California, USA). In some studies, the tick gut extract (TGE) in PBS was incubated with a combination of protease inhibitors (Sigma protease inhibitor cocktail, catalog no. P 8340; Sigma Chemical Co., St. Louis, Missouri, USA) to ensure that the potential release of proteases from the intestine would not degrade a putative receptor. The Sigma cocktail contains inhibitors with broad specificity for the inactivation of serine, cysteine, aspartic proteases, and aminopeptidases. One hundred microliters of each extract (5 μg/mL) in PBS was used to coat the wells. As controls, plates were coated (100 μL/well) with 10 μg/mL of BSA, gelatin, or FCS in a similar fashion. Plates were incubated overnight at 4°C, and tightly covered with cellophane to prevent evaporation. Plates were then washed three time with PBS with 0.05% Tween 20 (PBS-Tween-20). Nonspecific sites were blocked by incubating the TGE-coated wells with 15% normal FBS for 2 hours at 37°C. Plates were then incubated with 100 μL of FITC-labeled OspA, OspC, or BSA protein (10 μg/mL) or OspA peptide (5 μg/mL) at 37°C for 1 hour. A dose-response curve was also performed using 100 μL of FITC-labeled OspA, OspC, and BSA at 5, 10, 20, and 40 μg/mL. The plates were washed three times with PBS-Tween-20. Binding was detected using anti-FITC IgG-horseradish peroxidase (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA) as a secondary reagent and TMB microwell peroxidase substrate (KPL, Gaithersburg, Maryland, USA) was used for color development. The OD was read at 450 nm at either 5 or 15 minutes. In most of the studies, the OD was measured at 15 minutes, at which time the highest recorded values usually ranged between 0.5 and 1.0. In some studies (see Figures 1b and 3a), the OD values were recorded at 5 minutes because the values obtained with high concentrations of OspA approached the upper limits of detection of the assay.
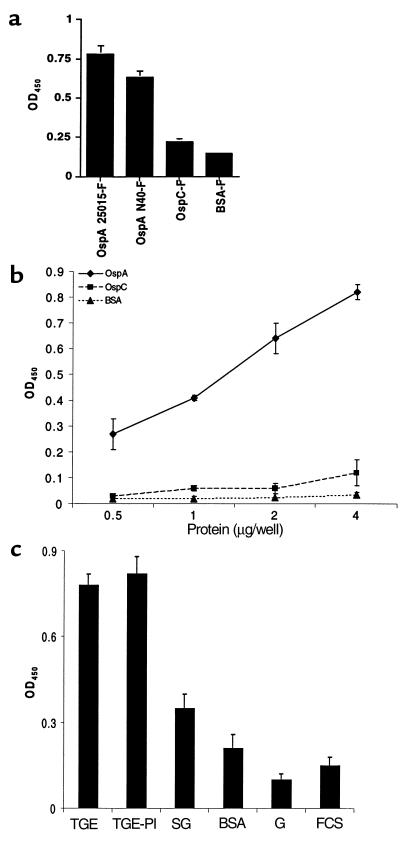
Figure 1.
OspA binds to an I. scapularis TGE. (a) FITC-labeled (-F) OspA from B. burgdorferi 25015 or N40, OspC from B. burgdorferi N40, and BSA were used to probe TGE-coated wells. Bars represent the OD450 at 15 minutes (mean ± SD) from three experiments. (b) Dose-response curves are presented for FITC-labeled OspA-N40, OspC, and BSA binding to TGE. The OD450 was measured at 5 minutes (mean ± SD) from three experiments. (c) FITC-labeled OspA from B. burgdorferi N40 was used to probe TGE-coated, tick salivary gland extract–coated (SG), BSA-coated, gelatin-coated (G), and FCS-coated (FCS) wells. In some studies, TGE was first treated with a protease inhibitor cocktail (TGE-PI) before incubation with OspA. Bars represent the OD450 at 15 minutes (mean ± SD) from three experiments.
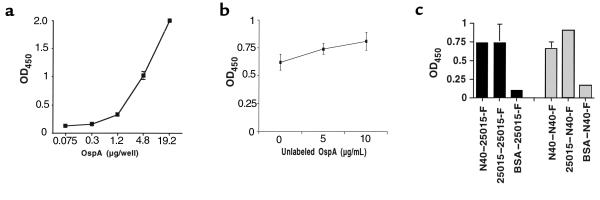
Figure 3.
Effect of increasing OspA on OspA-TGE binding, and OspA-OspA binding. (a) OspA binding to TGE is not saturable. OD was measured at 5 minutes. (b) Effect of excess unlabeled OspA on the binding of labeled OspA to TGE. TGE was probed with FITC-labeled OspA and increasing amounts of unlabeled OspA. These data represent the mean ± SD from three experiments. OD values were measured at 15 minutes. (c) Binding of OspA with OspA. OspA from B. burgdorferi N40, B. burgdorferi 25015, or BSA was probed with FITC-labeled OspA (25015-F, black bars; or N40-F, gray bars). Bars represent the mean ± SD from three experiments. OD values were measured at 15 minutes.
Spirochete binding to TGE.
To examine B. burgdorferi binding to the tick gut, the prepared wells containing TGE were incubated for 4 hours at 30°C with 107 B. burgdorferi N40 per well in PBS-Tween 20 supplemented with 5% FBS. Control wells were coated (100 μL/well) with BSA (10 μg/mL) or FCS (10 μg/mL). Unbound spirochetes were washed away using PBS-Tween. Then a mouse mAb (mAb H9724) directed against FlaB (28) was added to the wells at a dilution of 1:25 and incubated for 1 hour at 30°C. Anti-mouse IgG-HRP (Sigma Chemical Co.) was used as secondary reagent at a dilution of 1:2,000. TMB microwell peroxidase substrate (KPL) was used for color development, and the OD was read at 450 nm. In some experiments, spirochetes were incubated in the presence of 50 μg/well OspA.
Additional studies were performed in an identical fashion, except that PBS was used instead of PBS-Tween, in order to minimize permeabilization of the outer membrane. In these studies, a murine B. burgdorferi antisera was used to detect spirochetes.
OspA-OspA binding.
Microtiter wells were coated overnight at 4°C with 10 μg/well of OspA from B. burgdorferi N40, OspA from B. burgdorferi 25015, or BSA in PBS. Nonspecific sites were blocked with 15% FBS, and the plates were probed with 100 μL of 10 μg/mL FITC-labeled B. burgdorferi N40 OspA or B. burgdorferi 25015 OspA for 1 hour at 37°C.
Site-directed mutagenesis
The results suggest that conserved amino acids between B. burgdorferi in the amino acid regions OspA85–103 or OspA229–247 (see Figure 6d) may facilitate binding. Therefore, six OspAs, designated M1, M2, M3, M4, M5, and M6, which carry unique mutations in peptide sequence OspA85–103 or OspA229–247 (Table 1), were created by site-directed mutagenesis. The amino acid number and sequence in single-letter abbreviation in wild-type B. burgdorferi N40 and mutant OspAs are as follows, and encompass all the conserved amino acids in these regions:
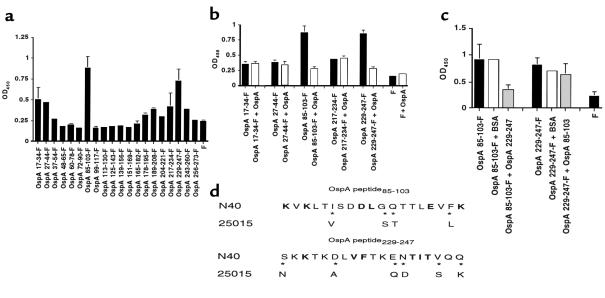
Figure 6.
OspA epitopes involved in binding. (a) Relative binding of overlapping OspA peptides (100 μL of 5 μg/mL peptide) to TGE. The background binding of FITC (F) to TGE has been indicated. The mean ± SD of three experiments is shown. (b) Inhibition of labeled OspA peptide binding to TGE by unlabeled OspA. TGE was probed with 100 μL of FITC-labeled OspA peptide (5 μg/mL) in the absence (black bars) or presence (white bars) of 250 μg/mL of unlabeled OspA. The mean ± SD of two experiments using OspA from B. burgdorferi N40 is shown. (c) OspA229–247 and peptide OspA85–103 bind to the same site, or effectively inhibit binding of a second site, in the tick gut. TGE was probed with 20 μg/mL of FITC-labeled OspA85–103 or OspA229–247 in the absence (black bars) or presence of competitor, 1 mg/mL of unlabeled OspA229–247 or OspA85–103, respectively (gray bars). Control studies used BSA as a competitor (white bars). The mean ± SD of six experiments is shown. (d) Amino acid polymorphism in OspA85–103 and OspA229–247. Comparison of the B. burgdorferi N40 and B. burgdorferi 25015 sequences (variable amino acids are indicated by asterisks). Seventy-nine OspA sequences available in GenBank were aligned, and the strictly conserved residues are presented in bold face. Single-letter abbreviations for the amino acid residues are indicated in Table 1.
Table 1.
The overlapping synthetic OspA peptides
M1 (KVK85-87 to ΔEVE), M2 (DL93-94 to ΔAR), M3 (EVFK100-103 to ΔAVFA), M4 (K231 to ΔA), M5 (VF236-237 to ΔGS), M6 (TIT242-244 to ΔANA).
A pGEX-2T–based vector was used to express wild-type or mutant OspAs. Mutant OspAs were generated using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, California, USA). The following mutagenic primers were used, and the substitution of codons or nucleotides is indicated by underlining and boldfaced type, respectively. All the mutations were confirmed by sequencing, and wild-type or mutant OspAs were expressed, purified, and labeled with FITC for binding studies as described previously.
M1 (5′cttgaaggcgtaaaagctgacaaaagtgaagtagaattaacaatttctgacgatc3′ and 5′gatcgtcagaaattgttaattctacttcacttttgtcagcttttacgccttcaag3′) M2 (5′ gtaaagtaaaattaacaatttctgacgctcgaggtcaaaccacacttg3′and 5′caagtgtggtttgacctcgagcgtcagaaattgttaattttactttac3’) M3 (5′ggtcaaaccacacttgcagttttcgcagaagatggcaaaacac3′ and 5′gtgttttgccatcttctgcgaaaactgcaagtgtggtttgacc3′) M4 (5′caactttaacaattactgtaaacagtaaagcaactaaagaccttgtgtttacaaaag3′ and 5′cttttgtaaacacaaggtcttttgttgctttactgtttacagtaattgttaaagttg3′)< M5 (5′ctgtaaacagtaaaaaaactaaagaccttgggtctacaaaagaaaacacaattacag3′ and 5′ctgtaattgtgttttcttttgtagacccaaggtctttagtttttttactgtttacag3′) M6 (5′gaccttgtgtttacaaaagaaaacgcaaatgcagtacaacaatacgac3′ and 5′gtcgtattgttgtactgcatttgcgttttcttttgtaaacacaaggtc3′).
Confocal microscopy and immunofluorescence
Five to ten midguts were dissected out from nymphal ticks in 100 μL of PBS. The organs were cut into two pieces and placed on silylated glass slides (PGC Scientific, Gaithersburg, Maryland, USA) to enhance attachment. Slides were washed twice with PBS, incubated with PBS-Tween 20 with 5% FBS for 30 minutes at room temperature, and then incubated for 1 hour at room temperature with FITC-labeled OspA, OspC, or BSA (50 μL of 100 μg/mL of FITC-labeled protein). Samples were subsequently stained with propidium iodide (50 μL of a 10 μg/mL solution) for 3 minutes at room temperature, washed three times with PBS-Tween-20, and mounted in glycerol for examination. The tissues were viewed using a Zeiss LSM 510 scanning laser confocal microscope equipped with an argon/krypton laser (Carl Zeiss Inc., Thornwood, New York, USA).
For immunofluorescence, B. burgdorferi (107 spirochetes/mL) were suspended in PBS, and 10-μL aliquots were placed on silylated glass slides and allowed to air dry. Each slide was then washed three times with PBS and probed with FITC-labeled OspA, OspC, or BSA (50 μL of 100 μg/mL of FITC-labeled protein). Using a conventional Zeiss Axioskop fluorescence microscope, spirochetes in ten microscope fields (100× objective) were examined.
Tick gut: fractionation, and treatment with lipase, glycosidase, or trypsin
Centricon concentrator tubes (Amicon Inc., Beverly, Massachusetts, USA) with different molecular mass cutoffs (10, 30, 50, and 100 kDa) were used to separate TGE into different-sized fractions. Equal amounts of protein (0.5 μg) from the filtrate and retentate were used to coat microtiter wells that were then probed with labeled OspA as described for ELISA.
TGE was prepared as described, and equal aliquots were incubated with either heat-inactivated (95°C for 10 minutes) or active lipase or glycosidases as follows: wheatgerm lipase at 10 U/mL (Sigma Chemical Co.) for 1 hour at 37°C; N-glycosidase F, endo-α-N-acetygalactosaminidase, α-2-3,6,8-neuraminidase, β-1,4-galactosidase, and β-N-acetylglucosaminidase treatment according to a glycoprotein deglycosylation kit (Calbiochem-Novabiochem Corp., San Diego, California, USA). An equal aliquot of TGE was also incubated with trypsin for 1 hour at 37°C at 10 μg/mL (Sigma Chemical Co.) in the presence or absence of 20 μg/mL soybean trypsin inhibitor (Sigma Chemical Co.). After enzyme incubation, the TGE with inactivated enzyme was coated onto microtiter plates and probed with labeled OspA as described for ELISA. In some studies, 0.5 μg of TGE was first used to coat the microtiter plate wells, and then the plates were washed with PBS and the nonspecific sites were blocked by incubating with 15% FBS for 2 hours at 37°C. Then the wells were incubated with trypsin for 1 hour at 37°C (with and without presence of soybean trypsin inhibitor). Plates were then probed with labeled OspA.
Results
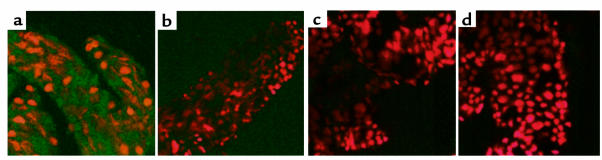
OspA adherence to a TGE was examined by ELISA. Binding of recombinant FITC-labeled OspA from B. burgdorferi N40, a prototypic B. burgdorferi sensu stricto isolate, and B. burgdorferi 25015, an isolate that has an OspA with variability in the epitopes involved in protective immunity (27), was assessed. Both OspAs readily bound to the TGE (Figure 1a). In addition, OspA bound to TGE in a dose-dependent manner (Figure 1b). Other proteins, such as BSA or B. burgdorferi OspC adhered poorly to the TGE (Figure 1, a and b). The binding appeared to be specific because OspA bound only weakly to a tick salivary gland extract and did not bind to BSA, gelatin, or antigens in FCS (Figure 1c). Furthermore, initial treatment of the TGE with a combination of proteases did not influence OspA-TGE adherence (Figure 1c). OspA also adhered directly to intact unfixed tick gut tissue, as shown by confocal microscopy (Figure 2). In contrast FITC-labeled OspC, FITC-labeled BSA, and FITC did not bind to the tick gut (Figure 2).
Figure 2.
OspA directly binds to the I. scapularis gut. Direct binding of FITC-labeled OspA (a) to the intact unfixed tick gut was detected using confocal microscopy. FITC-labeled OspC (b), FITC-labeled BSA (c), and FITC (d) were used as controls. After probing the tick gut with FITC-labeled protein (shown in green), the tissues were stained with propidium iodide to localize the nuclei of the gut cells (shown in red). The FITC and propidium iodide images were examined at ×630 and presented as a single image for clarity.
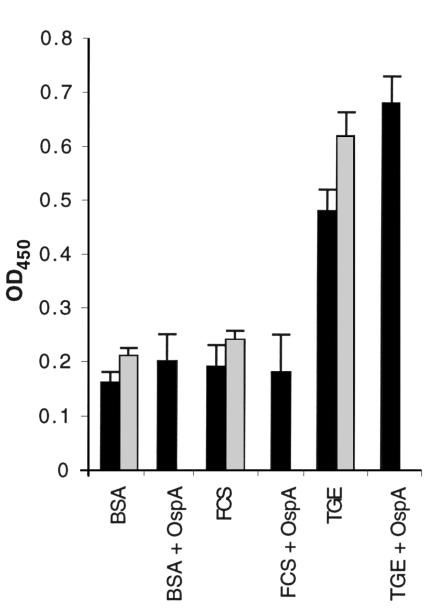
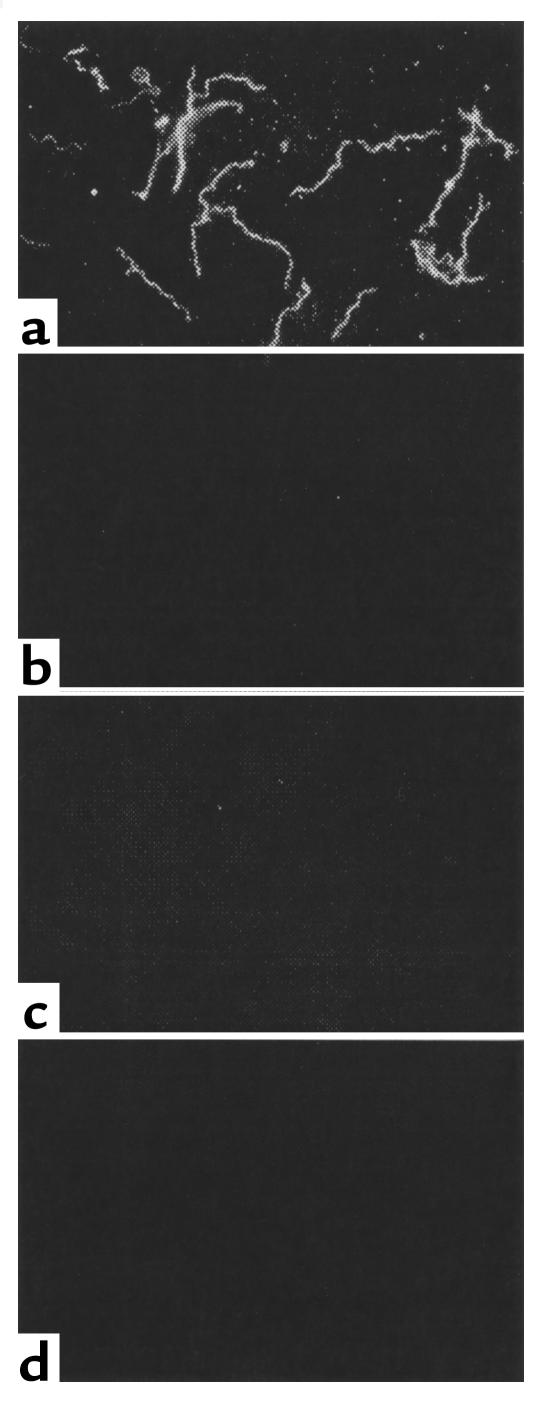
The influence of increasing concentrations of OspA on OspA attachment to TGE was then evaluated. The binding of FITC-labeled OspA to the TGE was augmented when increasing amounts of labeled protein were added and could not be saturated (Figure 3a). In Figure 3a, the OD was recorded at 5 minutes because the value for 19.2 μg/well OspA reached the upper limits of detection. At this interval, the OD for 1.2 μg/well OspA was only 0.3; however, this value increases to 0.74 when the plates are incubated for 15 minutes, consistent with the data in Figure 1. A similar pattern, with lower OD values, was apparent when the amount of immobilized TGE was reduced from 0.5 μg/well to 0.5 ng/well, and binding was no longer detectable when 0.5 pg/well of TGE was used to coat the plates (data not shown). The inability to saturate OspA binding could be due to a ubiquitous tick gut receptor or to OspA-OspA interactions. The binding of labeled OspA to the TGE did not decrease in the presence of excess unlabeled OspA, suggesting that OspA bound to OspA (Figure 3b). OspA-OspA interactions were therefore directly examined. Labeled OspAs from B. burgdorferi 25015 or N40 specifically bound to unlabeled OspAs (Figure 3c). Studies then assessed this phenomenon using whole B. burgdorferi and the TGE. As expected, spirochetes adhered to the TGE-coated, but not to BSA- or FCS-coated, wells (Figure 4). Spirochete suspended in PBS could be detected with the B. burgdorferi antisera, whereas bacteria suspended in PBS-Tween were also identified with the FlaB mAb. The addition of OspA enhanced B. burgdorferi binding to the TGE, consistent with the data on OspA attachment to the TGE (Figure 4). In contrast, the addition of OspA did not result in adherence of B. burgdorferi to BSA or FCS (Figure 4). OspA binding to B. burgdorferi N40 was then directly demonstrated by immunofluorescence (Figure 5). OspA did not effectively bind to B. burgdorferi 46047 that lack OspA, further suggesting that the binding was specific for OspA (Figure 5). These data demonstrated that B. burgdorferi OspA bound to itself as well as to the tick gut.
Figure 4.
B. burgdorferi N40 binding to tick gut extract is enhanced in the presence of OspA. BSA-, FCS-, and TGE-coated wells were incubated with 107 spirochetes per well in the absence or presence of OspA (example, TGE + OspA). Spirochetes suspended in PBS-Tween were probed with a FlaB mAb (black bars). Spirochetes suspended in PBS were probed with B. burgdorferi antisera (gray bars). Bars represent the mean ± SD from three experiments.
Figure 5.
OspA directly binds to B. burgdorferi. FITC-labeled OspA (a) was used to directly probe B. burgdorferi N40. FITC-labeled OspC (b) and FITC-labeled BSA (c) were used as controls to probe B. burgdorferi N40. FITC-labeled OspA was also used to probe B. burgdorferi 46047, an isolate that lacks OspA (d).
The OspA epitope or epitopes that bind TGE were then mapped using 20 overlapping peptides (Table 1). The first 16 amino acids of OspA, which encode a signal sequence, were not included in the studies. OspA85–103 and OspA229–247 strongly bound to the TGE, and several other peptides (OspA17–34, OspA27–44, and OspA217–234) demonstrated moderate binding (Figure 6a). These peptides did not bind to BSA- or serum-coated wells (data not shown). OspA from B. burgdorferi N40 competed for binding with OspA85–103 and OspA229–247 but not with the other three peptides, suggesting that OspA85–103 and OspA229–247 contained functional binding sites (Figure 6b). Similar results were obtained when OspA from B. burgdorferi 25015 was used for competition (data not shown). To test whether OspA85–103 and OspA229–247 adhered to the same TGE site, competition studies were performed using FITC-labeled OspA85–103 or OspA229–247 and excess unlabeled OspA85–103 or OspA229–247. OspA229–247 strongly competed for binding with OspA85–103 but not vice versa (Figure 6c). This suggests either that (a) the two peptides bind to the same site in the tick gut but that OspA229–247 binds with a much higher affinity than OspA85–103 or that (b) OspA229–247 can effectively block the binding at a second site.
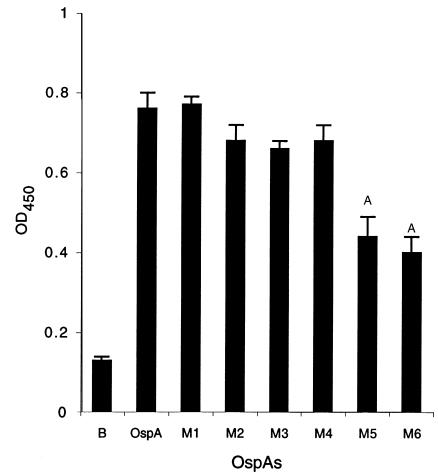
OspAs from B. burgdorferi N40 and 25015 were both able to bind to TGE. The OspA85–103 and OspA229–247 sequences of these spirochetes were therefore compared. Conserved amino acid positions within these regions of B. burgdorferi N40 and 25015 may possibly facilitate OspA adherence to the tick gut (Figure 6d). Site-directed mutagenesis was accordingly used to generate OspAs with amino acid substitutions in these regions, for use in TGE binding assays. Six mutant OspAs, three with substitutions in OspA85–103 and three with alterations in OspA229–247, were created that encompass all the conserved amino acids between B. burgdorferi species (see Methods and Figure 6d). OspA mutants M5 and M6, with replacements in amino acids 236–7 or 242–4 of OspA229–247, respectively, demonstrated significantly less (P < 0.001) binding to TGE than did authentic OspA (Figure 7).
Figure 7.
Binding of OspAs with mutations within amino acids 85–103 or 229–247 to TGE. Site-directed mutagenesis was performed to generate OspAs, designated M1–M6 with substitution in amino acids that are conserved between B. burgdorferi species within region 85–103 and 229–247 (Figure 6d). M1–M6 contain the following amino acid replacements: M1 (KVK85–87 to ΔEVE), M2 (DL93–94 to ΔAR), M3 (EVFK100–103 to ΔAVFA), M4 (K231 to ΔA), M5 (VF236–237 to ΔGS), and M6 (TIT242–244 to ΔANA). Binding of OspA and M1–M6 to TGE is depicted. B represents binding of OspA to BSA (control). M1–M6 showed a similar degree of binding to BSA (data not shown). Bars represent the mean ± SD from four experiments. AP < 0.001.
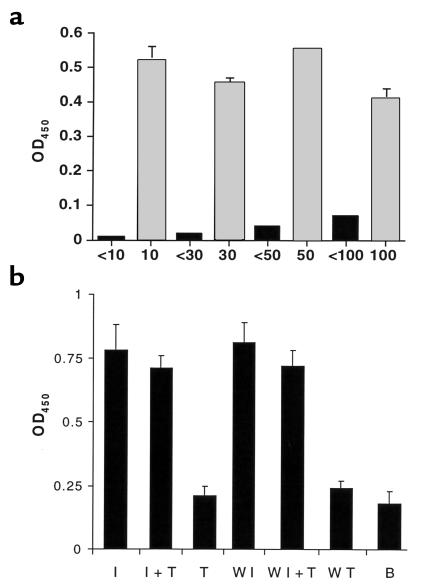
The OspA receptor in the tick was then partially characterized. To determine the approximate molecular mass of the ligand, size-separated fractions of the TGE were used in ELISA. A ligand or ligand complex greater than 100 kDa exhibited binding activity (Figure 8a). To understand the biochemical nature of the OspA binding moiety, the TGE was treated with wheatgerm lipases, a variety of glycosidases (N-glycosidase F, endo-—N-acetygalactosaminidase, α2-3,6,8-neuraminidase, β1,4-galactosidase, and β-N-acetylglucosaminidase), or trypsin. Heat-denatured enzyme (in the case of lipases or glycosidases) or proteolytic enzyme in the presence of specific inhibitor (soybean trypsin inhibitor) served as controls. Lipase or glycosidases did not affect OspA binding to the TGE (data not shown). In contrast, pretreatment of the TGE with trypsin markedly diminished OspA adherence (Figure 8b). A similar reduction in OspA binding was also observed when the TGE immobilized on microtiter wells was treated with trypsin (Figure 8b, lanes marked W). OspA therefore bound to a high molecular mass protein, or protein receptor complex, in the TGE.
Figure 8.
Characterization of the tick gut receptor that binds OspA. (a) Binding of OspA to different-sized TGE fractions. Tick gut extracts were subjected to size fractionation using Centricon concentrator tubes with molecular mass cutoffs of either 10, 30, 50, or 100 kDa. In each separate fractionation, black and gray bars represent the separated TGE containing the smaller and larger material, respectively. The means ± SD from two experiments are shown. (b) Effect of trypsin treatment on OspA binding to TGE. TGE was treated with soybean trypsin inhibitor (I), soybean trypsin inhibitor and trypsin (I + T), or trypsin (T). The trypsin-treated and control TGEs were then used to coat microtiter wells and probed with labeled OspA. The OD of the binding of labeled OspA to BSA is also shown as a control (B). In an additional series of experiments, marked “W,” TGE-coated microtiter wells were treated with soybean trypsin inhibitor, soybean trypsin inhibitor and trypsin, or trypsin. Bars represent the mean ± SD from three experiments.
Discussion
These data demonstrate that B. burgdorferi OspA specifically binds to a protein or proteins in the gut of I. scapularis. Selective ospA expression as the spirochete enters larval ticks may allow B. burgdorferi to adhere to and colonize the vector. Similarly, repression of ospA during tick feeding may facilitate B. burgdorferi detachment from the gut and migration to the salivary glands and ultimately the vertebrate host. The weak binding of OspA to tick salivary glands (Figure 1b) suggests that the tick receptor that binds OspA may be present, at least at some level, in this tissue as well. However, spirochetes that migrate from the tick gut to the salivary gland rarely, if ever, express ospA (7, 9, 10). Therefore, any degree of OspA-salivary gland binding is not likely to be functionally relevant. These studies also indicate that OspA-OspA interactions may increase spirochete adherence in the gut through self-binding, particularly at high B. burgdorferi density, and this phenomenon may partially explain the reported in vivo aggregation of B. burgdorferi in the tick gut or on cell lines in vitro (29–33). The downregulation of OspA by spirochetes during the bloodmeal may also facilitate dissemination to the tick salivary glands and the host’s dermis by preventing the bacteria from clumping to one another during transmission.
The crystal structure of OspA has recently been elucidated and demonstrates that OspA229–247 extends from β-strand 19 to 20 in the COOH-terminal barrel sheet (34). Interestingly, Phe-237 and Ile-243, within OspA229–247, are associated with a hydrophobic cavity suggested to be a ligand-binding site, and protein folding brings residues 236–7 and 242–4 into close proximity (34). The site-directed mutagenesis studies (Figure 7) further suggest that this region of OspA229–247 is important in OspA-TGE binding because substitutions in amino acids 236–7 or 242–4 significantly reduced the binding to OspA to TGE. The reduced degree of binding could be due to the replacement of amino acids that are directly involved in attachment, or in the alteration of the structure of the putative hydrophobic cavity that is the probable ligand binding domain. The fact that OspA molecules are capable of adhering to each other must also be taken into consideration in the analysis of the mutagenesis studies. The OspA mutants M5 and M6 may have even greater defects in attachment to the tick gut because OspA-OspA binding may mask a further decrease in OspA-tick gut adherence. Nevertheless, the significant decrease in binding of M5 and M6, with substitutions in amino acids 236–7 and 242–4, further demonstrates that the attachment of OspA to the tick gut is specific and, at least in part, mediated by the putative binding pocket in the COOH-terminus of the protein.
The role of OspA during mammalian infection is not yet known; however, one report has suggested that OspA may facilitate spirochete interactions with endothelium (35). Given that ospA is not generally expressed during human and murine infection, it is possible that OspA does not have an important role in the vertebrate host. Alternatively, reports have demonstrated ephemeral OspA antibody and T-cell responses during early-stage Lyme disease, and OspA responses have been identified during chronic antibiotic-resistant Lyme arthritis, suggesting that OspA may sometimes be present during infection (16–21). One speculation from these efforts is that OspA may play a role in persistent B. burgdorferi colonization of synovial tissue. Identification of the I. scapularis ligand(s) that binds to OspA in the tick may provide clues to additional ligands in the mammalian host that could also potentially interact with this lipoprotein.
B. burgdorferi has exquisitely adapted to survive in both the arthropod vector and vertebrate host, and we show that OspA is a spirochete antigen that facilitates adherence in I. scapularis. In the mammalian host, several spirochete antigens are involved in attachment to the host. For example, decorin-binding protein (Dbp) A mediates that binding of B. burgdorferi to decorin, and BBK32 promotes the binding of spirochetes to fibronectin (36–38). In I. scapularis, B. burgdorferi resides in specific locations during distinct intervals of infection, including the gut of unfed ticks and the salivary glands of engorging tick, and it is possible that different spirochete antigens assist in the colonization of different tissues within the vector. The present studies with OspA were performed at 37°C, whereas the ambient temperature in flat ticks is lower (20°C), and the temperature in engorging ticks and mammals is closer to 37°C; therefore, it is likely that temperature and other host factors can further influence specific B. burgdorferi-vector/vertebrate interactions. Understanding the function of B. burgdorferi genes that are selectively expressed throughout the spirochete life cycle, and the molecular basis of vector–B. burgdorferi interactions, will expedite new approaches to the control of Lyme disease and provide a model for other arthropod-borne infections.
Acknowledgments
We thank P. Voet and M. Vasil for assistance and A. van der Ende and A.P. van Dam for reviewing the manuscript.
This work was supported by grants from NIH, the Arthritis Foundation, the American Heart Association, the Eshe and Mathers Foundations, the Community Foundation of Greater New Haven,and a gift from SmithKline Beecham Biologicals. E. Fikrig is the recipient of a Burroughs Wellcome Clinical Scientist Award in Translational Research.
References
- 1.Nadelman RB, Wormser GP. Lyme borreliosis. Lancet. 1998;352:557–565. doi: 10.1016/S0140-6736(98)01146-5. [DOI] [PubMed] [Google Scholar]
- 2.Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- 3.Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 4.Schaible UE, et al. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci USA. 1990;87:3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sigal LH, et al. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 6.Steere AC, et al. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 7.de Silva AM, Telford SR, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Silva AM, Fish D, Burkot TR, Zhang Y, Fikrig E. OspA antibodies inhibit the acquisition of Borrelia burgdorferi by Ixodes ticks. Infect Immun. 1997;65:3146–3150. doi: 10.1128/iai.65.8.3146-3150.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman JL, et al. Plasminogen is required for efficient dissemination of Borrelia burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 10.Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montgomery RR, Malawista SE, Feen KJ, Bockenstedt LK. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gern L, Schaible UE, Simon MM. Mode of inoculation of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strains of mice. J Infect Dis. 1993;167:971–975. doi: 10.1093/infdis/167.4.971. [DOI] [PubMed] [Google Scholar]
- 13.Schaible UE, et al. Distinct patterns of protective antibodies are generated against Borrelia burgdorferi in mice experimentally inoculated with high and low doses of antigen. Immunol Lett. 1993;36:219–226. doi: 10.1016/0165-2478(93)90056-8. [DOI] [PubMed] [Google Scholar]
- 14.de Silva AM, et al. Immune evasion by tick-borne and host-adapted Borrelia burgdorferi. J Infect Dis. 1998;177:395–400. doi: 10.1086/514200. [DOI] [PubMed] [Google Scholar]
- 15.Fikrig E, et al. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol Microbiol. 1999;31:281–290. doi: 10.1046/j.1365-2958.1999.01171.x. [DOI] [PubMed] [Google Scholar]
- 16.Kalish RA, Leong JM, Steere AC. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi in Lyme disease. Infect Immun. 1995;63:2228–2235. doi: 10.1128/iai.63.6.2228-2235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schutzer SE, et al. Simultaneous expression of Borrelia OspA and OspC and IgM response in cerebrospinal fluid in early neurologic Lyme disease. J Clin Invest. 1997;100:763–767. doi: 10.1172/JCI119589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schutzer SE, Coyle PK, Dunn JJ, Luft BJ, Brunner M. Early and specific antibody responses to OspA in Lyme disease. J Clin Invest. 1994;94:454–457. doi: 10.1172/JCI117346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akin E, McHugh GL, Flavell RA, Fikrig E, Steere AC. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect Immun. 1999;67:173–181. doi: 10.1128/iai.67.1.173-181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalish R, Leong JM, Steere AC. Association of treatment-resistant chronic Lyme arthritis with antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross DM, et al. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 22.Barthold SW, Fikrig E, Bockenstedt LK, Persing DH. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson JF, et al. Novel Borrelia burgdorferi isolates from Ixodes scapularis and Ixodes dentatus ticks feeding on humans. J Clin Microbiol. 1996;34:524–529. doi: 10.1128/jcm.34.3.524-529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bockenstedt LK, Fikrig E, Barthold SW, Flavell RA, Kantor FS. Identification of a Borrelia burgdorferi OspA T cell epitope that promotes anti-OspA IgG in mice. J Immunol. 1996;157:5496–5502. [PubMed] [Google Scholar]
- 26.Bockenstedt LK, et al. Borrelia burgdorferi strain-specific Osp C–mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fikrig E, et al. Borrelia burgdorferi strain 25015: characterization of outer surface protein A and vaccination against infection. J Immunol. 1992;148:2256–2260. [PubMed] [Google Scholar]
- 28.Sadziene A, Thomas DD, Bundoc VG, Holt SC, Barbour AG. A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization. J Clin Invest. 1991;88:82–92. doi: 10.1172/JCI115308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgdorfer W, et al. Lyme disease: a tickborne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 30.Kurtti TJ, Munderloh UG, Krueger DE, Johnson RC, Schwan TG. Adhesion to and invasion of cultured tick (Acarina: Ixodidae) cells by Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) and maintenance of infectivity. J Med Entomol. 1993;30:586–596. doi: 10.1093/jmedent/30.3.586. [DOI] [PubMed] [Google Scholar]
- 31.Thomas DD, Comstock LE. Interaction of Lyme disease spirochetes with cultured eukaryotic cells. Infect Immun. 1989;57:1324–1326. doi: 10.1128/iai.57.4.1324-1326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Monco JC, Fernandez-Villar B, Benach JL. Adherence of the Lyme disease spirochete to glial cells and cells of glial origin. J Infect Dis. 1989;160:497–506. doi: 10.1093/infdis/160.3.497. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Monco JC, Benach JL. Lyme neuroborreliosis. Ann Neurol. 1995;37:691–702. doi: 10.1002/ana.410370602. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Dunn JJ, Luft BJ, Lawson CL. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc Natl Acad Sci USA. 1997;94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Comstock LE, Fikrig E, Shoberg RJ, Flavell RA, Thomas DD. A monoclonal antibody to OspA inhibits association of Borrelia burgdorferi with human endothelial cells. Infect Immun. 1993;61:423–431. doi: 10.1128/iai.61.2.423-431.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo BP, Brown EL, Dorward DW, Rosenberg LC, Hook M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol. 1998;30:711–723. doi: 10.1046/j.1365-2958.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 37.Guo B, Norris SJ, Rosenberg LC, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Probert WS, Johnson BJ. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]