Abstract
The ability to conduct validated analyses of glutathione (GSH)-adducts and their metabolites is critically important in order to establish whether they play a role in cellular biochemical or pathophysiological processes. The use of stable isotope dilution (SID) methodology in combination with liquid chromatography–tandem mass spectrometry (LC-MS/MS) provides the highest bioanalytical specificity possible for such analyses. Quantitative studies normally require the high sensitivity that can be obtained by the use of multiple reaction monitoring (MRM)/MS rather than the much less sensitive but more specific full scanning methodology. The method employs a parent ion corresponding to the intact molecule together with a prominent product ion that obtained by collision induced dissociation. Using SID LC-MRM/MS, analytes must have the same relative LC retention time to the heavy isotope internal standard established during the validation procedure, the correct parent ion and the correct product ion. This level of specificity cannot be attained with any other bioanalytical technique employed for biomarker analysis. This review will describe the application of SID LC-MR/MS methodology for the analysis of GSH-adducts and their metabolites. It will also discuss potential future directions for the use of this methodology for rigorous determination of their utility as disease and exposure biomarkers.
Keywords: glutathione-adducts, stable isotopes, LC-MS, MRM, mercapturic acids, leukotrienes
Introduction
GSH is a tripeptide (γ-L3Glutamyl-L3cysteinylglycine) with the chemical structure (2S)-2-amino-5-[[(2R)-1-(carboxymethylamino)-1-oxo-3-sulfanylpropan-2-yl]amino]-5-oxo-pentanoic acid (Blair, 2006). The analysis of endogenous GSH-adducts and their metabolites is often very challenging due to the target analyte’s presence in the biofluid of interest. There is also a fundamental uncertainty as to whether the endogenous signal is actually the analyte of interest and not some interfering substance with similar physicochemical properties. To best distinguish between chemical background peaks and the target analyte, assays have to be conducted with the maximal specificity and sensitivity possible using stable isotope analogs as internal standards. Gas chromatography- and LC-MS/MS are the two most widely used instrument platforms to employ SID methodology. LC-MS/MS is more applicable to the analysis of GSH-adducts and their metabolites than gas chromatography-MS/MS and is also inherently easier to use for rigorous validation. Hence, the present review will focus on the use of LC-MS/MS methodology. However, the underlying principles relating to the specificity of SID-based approaches are relevant to both techniques.
Quantitative studies often require the most sensitive means of detection possible. The MS platform and analysis mode best suited for a particular analysis needs to be determined empirically and will depend on the molecule and matrix involved. In general, a triple quadrupole (TQ) operated in the multiple reaction monitoring (MRM)/MS mode will have adequate sensitivity and selectivity when coupled to LC. In this mode of operation, a precursor ion is resolved in Q1 of the TQ, fragmented by collision-induced dissociation (CID) in Q2, and the resultant product ion is resolved in Q3. Under optimal operating conditions, the precursor to product ion is monitored many times per second, resulting in extremely reproducible chromatographic peak shape and intensity. In this way, a heavy isotope-labeled standard is used in SID LC-MRM/MS to establish the presence of an endogenous analyte using both the LC retention time and MS/MS mass selection of the TQ instrument. This level of specificity cannot be attained with any other bioanalytical technique employed for endogenous metabolites.
An authentic isotope labeled analog of a compound is identical to the endogenous molecule except for mass. The use of structural analogs as internal standards, rather than authentic isotope labeled analogs, is undesirable because they will have different retention times and ionization properties compared with the analyte of interest. Therefore, differential ionization can occur between an analyte and a structural analog in the source of the mass spectrometer. This difference arises in part from suppression of ionization by constituents present in the biofluid that is being analyzed and can lead to significant imprecision during quantitative analyses (King et al., 2000). Unfortunately, suppression effects can vary with chromatographic retention time and with biofluid samples from different individuals. It is therefore impossible to standardize the amount of suppression occurring within any particular sample. The ideal control offered by an authentic isotope labeled internal standard is not always possible because for many biomarkers only deuterated and structural analogs are available. Deuterated forms of a compound are not perfect internal standards, since there is a small but significant separation of the deuterium analog internal standards and their corresponding endogenous protium forms during chromatography. This slight difference in retention times during chromatography can result in differential suppression or enhancement of ionization and affect the quality of the analytical data. Structural analogs are even less representative of the endogenous compound, since in addition to differences in LC retention time, the structural analog can show different absorptive loss. Selective binding to active sites on glassware or other surfaces can occur during extraction and chromatography, leading to significant analyte loss. Whereas a structural analog might not account for this loss, an isotope-labeled internal standard has identical physicochemical properties, and is therefore lost at the exact rate as the endogenous analyte. Because of this feature of stable isotope analogs, they may act as a carrier, preventing the loss of trace amounts of analyte during extraction and analyses (Oe et al., 2006). This is an often overlooked benefit offered by the isotope-labeled internal standard. Finally, variability introduced during compound isolation can be fully controlled by an authentic isotope labeled standard. In many cases, compound enrichment is required to improve analytical performance, as was found with the difficult amyloid β-peptides (Oe et al., 2006) and DNA-adducts (Mangal et al., 2009), which required immunoaffinity purification.
Role of Glutathione in Cellular Biochemistry
Intracellular GSH provides one of the major defenses against oxidative stress in mammalian cells. During oxidative stress, reduced GSH is converted to GSSG. GSH is the most abundant small molecule thiol in cells with concentrations in the millimolar range (Blair, 2006). In contrast, concentrations of GSSG in mammalian cells are usually two orders of magnitude lower (Schafer and Buettner, 2001). The high abundance of GSH and low abundance of GSSG helps to maintain cells under a reducing environment and prevents oxidative damage to cellular macromolecules. Hydrogen peroxide and lipid hydroperoxides undergo GSH peroxidase-mediated reduction to water and lipid hydroxides, respectively with GSH providing the reducing equivalents (Arthur, 2000; Blair, 2008). GSH also readily forms adducts with a great variety of both exogenously- and endogenously-derived electrophilic reactive intermediates (Fig. 1) (Blair, 2006; Doss and Baillie, 2006). Formation of the GSH-adducts is generally facilitated by GSH S-transferases (GSTs) and is normally considered to represent a detoxification of the relevant reactive intermediate. The resulting GSH-adducts are then exported from cells by ATP binding cassette transporters and/or non-ATP binding cassette transporters (Awasthi et al., 2007; Deeley et al., 2006). GSH/GSSG homeostasis plays an important role in maintaining cellular redox status (Schafer and Buettner, 2001). Changes in the half-cell reduction potential of the 2GSH/GSSG couple correlate with the biological status of the cell. Therefore, determinations of the reduction potential can be used to more fully understand the redox biochemistry that results from oxidative stress. The redox potential of the 2GSH/GSSG couple can be readily obtained from the Nernst equation: Eh = Eo + RT/nF × ln [(GSSG)/(GSH)2] with lower (more negative) redox potential representing more reducing conditions. According to this equation, the cellular redox potential is a second-order function of GSH concentration, which means that a change in concentration of GSH even without a change in GSH/GSSG ratio could alter the cellular redox status.
Figure 1.
Formation of GSH-adducts and small molecule biomarkers.
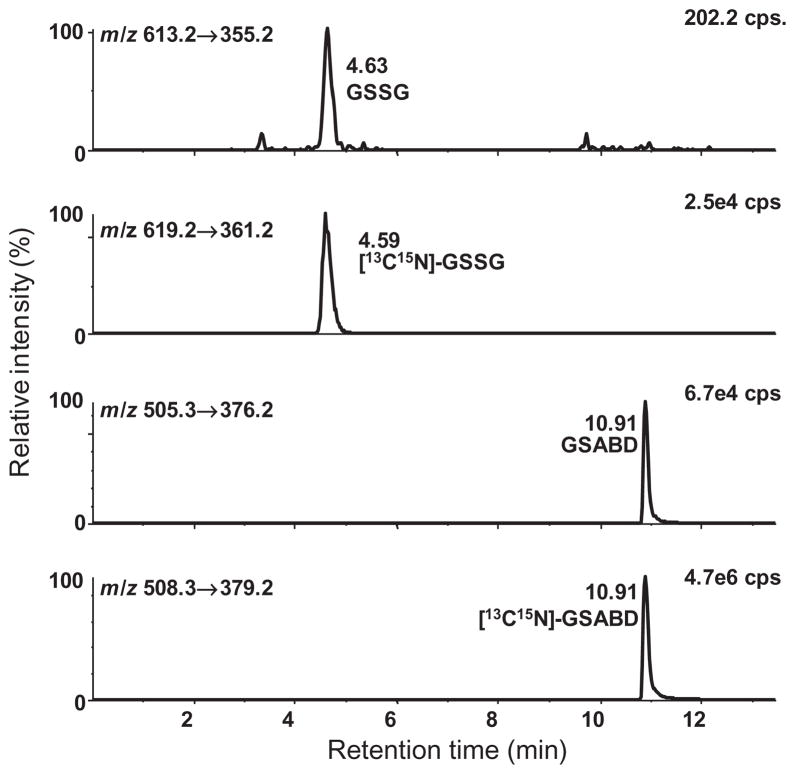
Determinations of the cellular redox state require methodology for accurately determining the intracellular concentrations of both GSSG and GSH. Many different methods have been published for the quantitative analysis of GSH and GSSG. Despite the large variety of methods that are available for GSH and GSSG analysis, many of them still have limitations. It is well known that GSH oxidation is a serious problem, which can lead to overestimation of GSSG, particularly in cell lysates where redox cycling with abundant protein thiols can readily occur. This stimulated the development of a SID LC-MRM/MS method for the simultaneous quantitation of cytosolic GSH and GSSG (Fig. 2) (Zhu et al., 2008). The method utilizes 4-fluoro-7-sulfamoylbenzofuran as a thiolderivatizing reagent that can rapidly and completely derivatize GSH. This fast and efficient derivatization was essential for preventing oxidation and enabling reliable measurements of GSSG to be made in the presence of a large excess of GSSG. The method was validated and shown to give parallel GSH standard curves when GSSG was added in increasing amounts to cell lysates and buffer samples (Zhu et al., 2008). This parallelism experiment is illustrative of a general method to determine whether a SID LC-MRM/MS method is quantifying the correct endogenous analyte or some interfering substance. The assay was able to detect subtle changes in the redox potentials of two macrophage cell lines with different phenotypes when they were treated with the endogenous reactive electrophile, 4-oxo-2(E)-nonenal (ONE) (Zhu et al., 2008). It is currently being employed to examine the redox status of epithelial cells from different animal models and circulating lymphocytes from different human disease states. Elaboration of the SID LC-MRM/MS biomarker methodology for GSH and GSSG to other important cellular thiols such as homocysteine, cysteine and coenzyme A, together with their corresponding disulfides is currently under development. This will then provide a comprehensive method to monitor thiol biomarkers of oxidative stress to complement other biomarkers of oxidative stress such as the isoprostanes (Milne et al., 2008).
Figure 2.
SID LC-MRM/MS chromatograms of lower limit of quantification samples with 0.0005 mM GSSG and 0.005 mM GSH. MRM chromatograms for GSABD (m/z 505.3 → 376.2), GSSG (m/z 613.2 → 355.2), [13C2, 15N1]-GSABD (m/z 508.3 → 379.2) and [13C4, 15N2]-GSSG (m/z 619.2 → 361.2). Reprinted with permission from Zhu et al. (2008).
Mechanisms of Endogenous GSH-adduct Formation
As noted above, intracellular GSH is present at concentrations that range from 3–4 mM (Blair, 2006). Most of the GSH is found in the cytosol (85–90%), with the remainder being found in the mitochondria, nuclear matrix, and peroxisomes (Lu, 2000). This means that intracellular reactions with endogenous electrophiles occur with a large molar excess of GSH. With the exception of bile, which contains up to 10 mM GSH (Griffith, 1999), extracellular concentrations of GSH are much lower. For example, GSH concentrations in plasma are in the range of 2–20 μM (Jones, 2002). This enormous concentration difference coupled with the presence of numerous intracellular GSTs facilitates the detoxification of endogenous reactive metabolites through the formation of GSH-adducts within the cellular milieu (Blair, 2006).
Mammalian GSTs are a family of enzymes with more than 20 human cytosolic forms and five that are membrane bound (Dourado et al., 2008; Hayes et al., 2005). Mutations in specific GSTs have weak associations with increased in hepatocellular and lung cancer risk with (Hosgood et al., 2007; White et al., 2008). Their central importance in detoxification arises from a unique capacity to form adducts with both endogenous and exogenous reactive intermediates (Rinaldi et al., 2002). GSTs also display GSH peroxidase activity and can thus help protect cells from oxidative damage (Coles and Kadlubar, 2005; Sharma et al., 2004). Microsomal GST1 (MGST1) is unusual in that it can be activated by a large variety of electrophilic reactive intermediates (Rinaldi et al., 2002). It is a member of a superfamily named membrane-associated proteins in eicosanoid and glutathione metabolism (MAPEG) (Jakobsson et al., 2000; Lam and Austen, 2002; Murakami and Kudo, 2006). The MAPEG family includes six human proteins: 5-lipoxygenase (LOX)-activating protein, leukotriene (LT) C4 synthase, MGST1, MGST2, MGST3 and prostaglandin-E synthase, earlier known as microsomal glutathione transferase 1-like-1 (MGST1-L-1). Members of the MAPEG superfamily are involved in the transformation of reactive lipid intermediates to bioactive eicosanoids (Fig. 3) or unreactive products such as lipid alcohols or GSH-adducts. MGSTs 2 and 3 have both been shown to act as GSH peroxidases as well as LTC4 synthases (Jakobsson et al., 2000).
Figure 3.
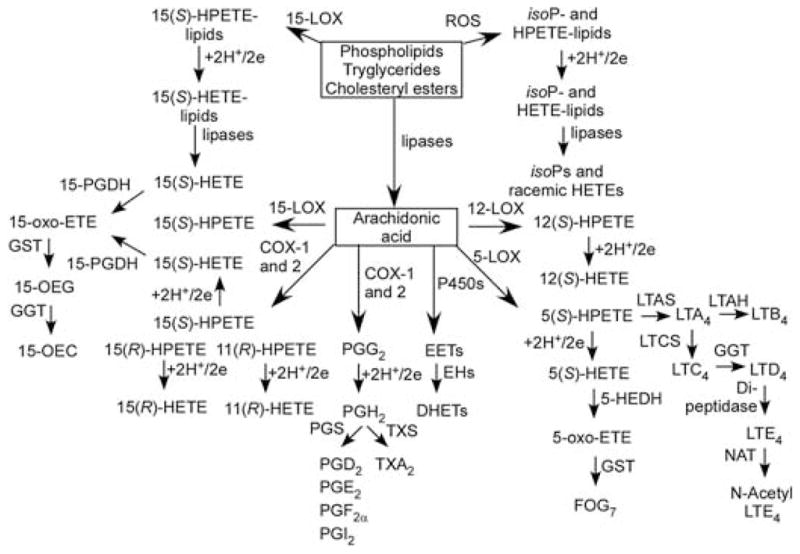
Formation of eicosanoids and eicosanoid-derived GSH-adducts. Abbreviations: 5-HEDH, 5-hydroxyeicosanoid dehydrogenase; EH, epoxide hydrolase; FLAP, 5- lipoxygenase activating protein; FOG-7, 5-oxo-eicosatetraenoic acid GSH-adduct, LTAH, leukotriene A4 hydrolase; LTAS, leukotriene A4 synthase; PGS, prostaglandin synthase; TX, thromboxane; TXS, thromboxane synthase.
Many years ago 15-oxo-ETE was identified as a metabolite from 15-hydroxyprostaglandin dehydrogenase (PDGH)-mediated metabolism of 15(S)-HETE (Fig. 3) (Bergholte et al., 1987). It was subsequently shown to arise from 15-PGDH-mediated metabolism of cyclooxygenase (COX)-2- and 15-LOX-derived 15(S)-HETE (Lee et al., 2007; Wei et al., 2009). It is noteworthy that 15-PGDH is down-regulated in colon cancer tissue (Backlund et al., 2005), suggesting a potential role for 15-oxo-ETE in the inhibition of carcinogenesis. Recent studies have established that 15-oxo-ETE can inhibit endothelial cell proliferation in vitro and that it rapidly forms a GSH-adduct, 15-OEG (Fig. 3), although the biological relevance of this finding remains to be evaluated (Wei et al., 2009).
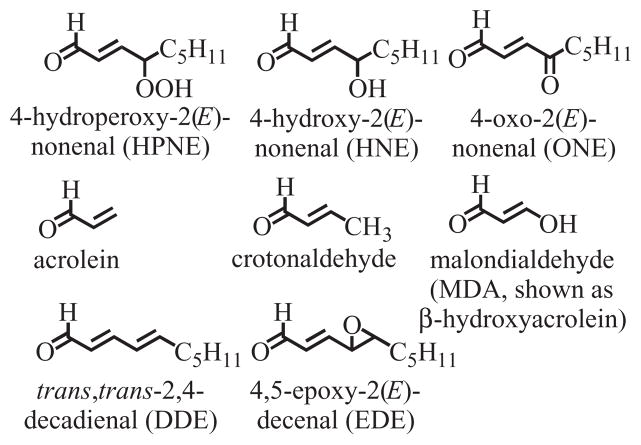
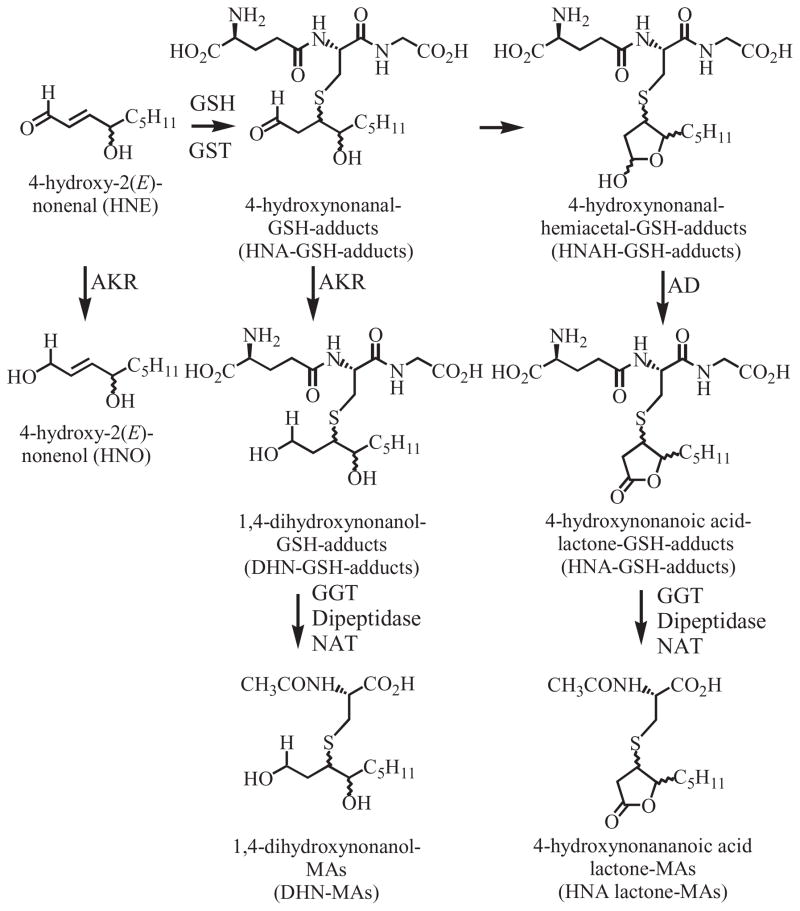
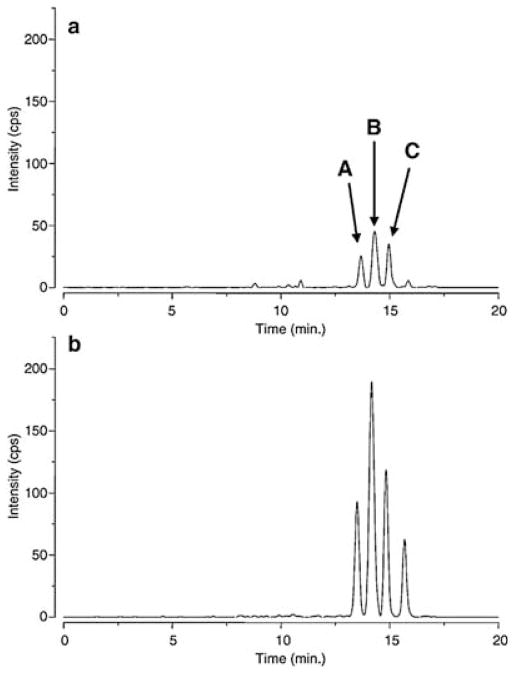
Lipid peroxidation can occur non-enzymatically through the action of ROS on polyunsaturated fatty acids or enzymatically by the action of COXs and LOXs (Blair, 2001, 2005, 2008; Lee and Blair, 2001). Decomposition of the resulting lipid hydroperoxides results in the formation of HPNE, an α,β-unsaturated aldehyde that is then either oxidized to ONE or reduced to HNE (Fig. 4) (Lee et al., 2001; Schneider et al., 2001). There is also evidence that other unsaturated aldehydes such as acrolein, crotonaldehyde, malondialdehyde (MDA, shown as β-hydroxyacrolein), trans,trans-2,4-decadienal (DDE) and 4,5-epoxy-2(E)-decenal (EDE), are also formed by the decomposition of lipid hydroperoxides (Chung et al., 2003; Minko et al., 2009) (Fig. 4). Base propenals, which are formed by ROS-mediated oxidation of the sugar backbone of DNA, contain the α,β-unsaturated aldehyde MDA (Zhou et al., 2005). All of these reactive α,β-unsaturated aldehyde bifunctional electrophiles are detoxified by GST-mediated metabolism to form GSH-adducts. HNE-GSH-adducts (Fig. 5) exist as a mixture of eight potential hemiacetal diastereomers (Uchida, 2003) four of which can be separated by LC-MS analysis (Fig. 6) (Völkel et al., 2005).
Figure 4.
Lipid hydroperoxide-derived bifunctional electrophiles that can form GSH-adducts.
Figure 5.
Metabolism of HNE to GSH-adducts and MA derivatives. AD, aldehyde dehydrogenase.
Figure 6.
HNE-GSH determination (MRM m/z 464.3 → 308.1) in rat liver tissue (a) of a vehicle-treated control rat and (b) 5 h after a single ip dose (15 mg/kg) of Fe(III)NTA. Reprinted with permission from Völkel et al. (2005).
Catechol estrogens are formed as a consequence of cytochrome P-450 1A1, 1B1, 1A2, and 3A4-mediated metabolism of estradiol and estrone. Redox cycling to the corresponding 2,3- and 3,4-quinones can then readily occur in the absence of further metabolism by catechol O-methyl transferases, uridine diphosphate glucuronosyltransferases, or sulfotransferases (Blair, 2009; Bolton and Thatcher, 2008). The estrogen quinones readily form endogenous GSH-adducts that can act as biomarkers of quinone formation (Bolton et al., 2000).
Metabolism of Endogenous GSH-adducts
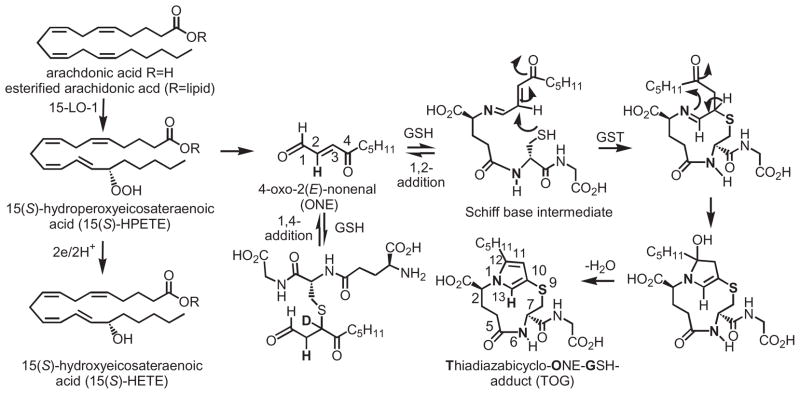
GSH-adducts are secreted from cells by various transporters (Awasthi et al., 2007; Deeley et al., 2006) and then metabolized by GGT, an enzyme that resides on the extracellular surface of the plasma membrane and is present in plasma (Lee and Jacobs, 2009). This results in the formation of a cysteinylglycine adduct, as exemplified in the case of LTC4, which is converted to LTD4 by GGT (Fig. 3). LTD4 then undergoes dipeptidase-mediated metabolism to LTE4, and NAT-mediated metabolism to N-acetyl-LTE4. Mercapturic acid (MA) derivatives (N-acetyl-cysteine adducts) can be used as biomarkers of formation of the originally formed endogenous GSH-adducts (Blair, 2006). HNE-derived GSH-adducts contain a terminal aldehyde moiety that can cyclize to form a hemicacetal. Therefore, both reductive metabolism of the aldehyde to give 1,4-dihydroxynonanol (DHN) and oxidative metabolism to give 4-hydroxynonanoic acid (HNA) lactone can occur (Fig. 5). The first biosynthetic pathway is mediated by aldoketo reductases (AKRs) of the 1C family (Burczynski et al., 2001), whereas the latter pathway involves the action of aldehyde dehydrogenase (Alary et al., 2003). The resulting DHN–GSH-adduct and HNA–lactone–GSH-adduct, together with their GGT, dipeptidase, and NAT-mediated MA metabolites can also potentially serve as biomarkers of HNE–GSH-adduct formation. The lipid hydroperoxide-derived thiadiazabicyclo–ONE–GSH-adduct (TOG) is formed through a novel GST-mediated reaction (Fig. 7) (Jian et al., 2007). TOG does not contain a free aldehyde and, in addition, the glutamate residue is relatively inaccessible to hydrolases. As a consequence, TOG is not a substrate for GGTs or AKRs (Blair IA, unpublished) and so it can provide a specific biomarker of lipid hydroperoxide-derived ONE formation.
Figure 7.
Formation of TOG from a lipid hydroperoxide [15(S)-HPETE].
Analysis of Endogenous GSH-adducts
Positive ion LC-MRM/MS has been used extensively to detect GSH-adduct biomarkers in cell culture and perfused organs. Analyses are performed either in the full scanning more or by using constant neutral loss scanning for 129 Da (γ-glutamyl moiety) to detect GSH-derived metabolites followed by CID and MS/MS analysis of MH+ Although this technique has primarily been used to detect drug-derived GSH-adducts (Levsen et al., 2005) it can be very useful for endogenous adducts as well. Negative ion ESI methodology in combination with precursor ion scanning of m/z 272 (M-H-H2S) can provide a complementary method for detecting GSH-adducts in cellular incubations (Dieckhaus et al., 2005). It proved to be particularly useful for detecting HNE-derived GSH-adducts. Recently, stable isotope dilution methodology was used in combination with an isotope pattern-dependent scanning method was applied to the data acquisition of GSH-adducts from drug-derived reactive metabolites (Ma et al., 2008). Recorded full-scan MS and MS/MS data sets were further processed with neutral loss filtering, product ion filtering and extracted ion chromatographic analysis to search for protonated molecules and MS/MS spectra of GSH-adducts. This approach was very effective in detecting low levels of drug-derived GSH-adducts, regardless of their fragmentation patterns. It clearly also has potential utility for the characterization of endogenous GSH-adducts.
There is increasing interest in the use of LC-MRM/MS-based methodology for the analysis of endogenous GSH-adducts as biomarkers of oxidative stress. Analysis of GSH-adducts has been found to provide insight into the pathological role of 4-hydroxy-2(E)-nonenal (Carini et al., 2004). HNE-GSH-adducts have also been used as biomarkers for monitoring oxidative stress in animal models (Völkel et al., 2005) and in Alzheimer’s disease (Völkel et al., 2006) (Fig. 8). Intriguingly (as noted above) 13(S)-hydroperoxyeicosatetraenoic acid, a product of 15-LOX-mediated metabolism of linoleic acid, was found to undergo enantioselective oxidative decomposition to 4(S)-HNE (Schneider et al., 2001). This suggests that by monitoring the individual HNE-GSH-adduct diastereomers, it could be possible to distinguish enzymatic (chiral) pathways of lipid peroxidation from those occurring non-enzymatically (racemic) through ROS-mediated pathways.
Figure 8.
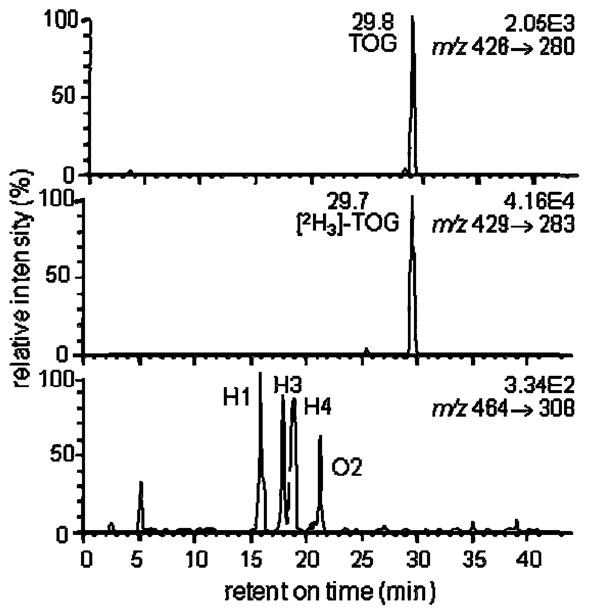
Quantitative analysis of TOG and HNE-GSH-adducts after adding 10 μM t-butyl-hydroperoxide and 500 μM FeII to EA.hy 926 endothelial cells. The upper chromatogram shows the MRM signal for endogenously generated TOG m/z 426 (MH+) → m/z 280. The center chromatogram shows the MRM signal for the [2H3]-TOG internal standard m/z 429 (MH+) → m/z 283 and the lower channel shows the MRM signal for endogenously generated HNE-GSH-adducts and ONO-GSH-adducts m/z 464 (MH+) → m/z 308 (MH+-C9H16O2). The concentration of intracellular TOG and the HNE-GSH-adduct corresponded to 8.6 and 0.5 μM, respectively, as determined from a standard curve constructed in blank cell lysate buffer. Reprinted with permission from Jian et al. (2007).
SID LC-MRM/MS methodology has been developed for the analysis of TOG and HNE–GSH-adducts in cellular models of oxidative stress (Fig. 9). Using SID LC-MRM/MS, it was possible to show that the lipid hydroperoxide 15(S)-HPETE was detoxified more rapidly and produced less TOG when it was formed in the cytosol of a macrophage cell line compared with 15(S)-HPETE formed on intact lipids (Zhu et al., 2009). Furthermore, [13C9]-TOG was formed from 15-LOX-mediated metabolism of exogenous [13C20]-arachidonic acid added to the cells, providing additional speciffcity for the analytical procedure. It will be interesting to analyze the residual aldehyde that is left on the lipid after the loss of ONE to determine whether it survives unchanged or whether it forms a GSH-adduct in the lipid bilayer. This could have particular importance for cell–cell signaling (Hazen, 2008). Other ONE-like bifunctional electrophiles can be formed from the homolytic decomposition of lipid hydroperoxides. For example, dioxododecenoic acid (Lee et al., 2005) and dioxooctenoic acid (Jian et al., 2005b) contain the carboxylate terminus from linoleic acid- and arachidonic-derived lipid hydroperoxides, respectively. Both dioxododecenoic acid and dioxooctenoic acid also form TOG-like GSH-adducts (Jian et al., 2005a). Therefore, in the future it might be possible to use these GSH-adduct biomarkers to identify the particular polyunsaturated fatty acid-derived lipid hydroperoxides that are involved in the induction of intracellular oxidative stress. GSH-adducts could also potentially be formed on intact lipids following loss of ONE from the ω-terminus of the esterified polyunsaturated fatty acid lipid hydroperoxide.
Figure 9.
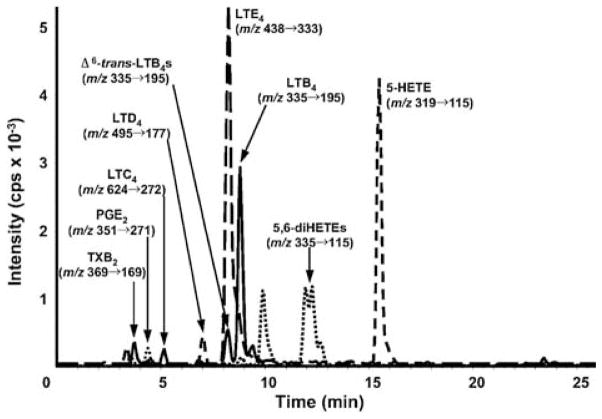
LC/MS/MS profile of LTC4, its metabolites, and selected eicosanoids in mouse peritoneal lavage. Mice were treated with 1 mg zymosan for 2 h, and eicosanoids analyzed by SID LC-MRM/MS, monitoring the specific m/z transitions indicated. Retention times of deuterated internal standards (coincided with their cognate metabolites. Reprinted with permission from Zarini et al. (2009).
LTC4 and oxo-ETE-derived GSH-adducts (OEGs) can also be analyzed using SID LC-MRM/MS methodology (Blair, 2006; Lee et al., 2007; Murphy and Zarini, 2002; Wei et al., 2009). Many of these studies have been restricted to cell culture models because of the extensive metabolism of the GSH-adducts that occurs in vivo as described above. However, Zarini et al. were able to quantify LTC4 by SID LC-MRM/MS in peritoneal lavage fluid during zymosan-induced peritonitis using bone marrow transplants with transgenic mice deficient in key enzymes of LT synthesis (Fig. 9). Their results demonstrated the potential relevance of transcellular exchange of LTA4 for the synthesis of LTs mediating biological activities during inflammatory events in vivo (Zarini et al., 2009).
Endogenous catechol estrogens are readily oxidized to give o-quinones (Bolton and Thatcher, 2008). The half-life of 2-hydroxyestrone-o-quinone is only 47 s, whereas 4-hydroxyestrone-o-quinone is longer lived with a half-life of 12 min (Bolton and Shen, 1996). In the presence of GSH, 4-hydroxyestrone-o-quinone is efficiently trapped as a GSH-adduct, which can be readily analyzed by LC-MS (Chandrasena et al., 2008). The formation of catecholamine–GSH-adducts was inferred from studies in which 5-S-cysteinyl derivatives of dopamine, DOPA, and DOPAC were synthesized and used as reference compounds in HPLC analyses of extracts from various brain regions of humans and other mammalian species (Shen et al., 1996). All three metabolites were detected in the brains of all the species studied. These findings suggested autoxidation of catechols to quinones had occurred in a similar manner to that described above for catechol estrogens and that GST-mediated GSH addition had occurred at C-5 to give a dopamine–GSH-adduct. Recently, off-line LC separation followed by MS analysis was employed to show that GSH-adducts were formed from the reaction of GSH with aminochrome, a dopamine-derived quinone (Zhou and Lim, 2009).
Analysis of Endogenous Cysteinylglycine- and Cysteine-adducts
LTD4 is one of the most potent cysteinylglycine-adducts that is formed in vivo. Therefore, it is surprising that there are so few descriptions on the use of LC-MRM/MS methodology for the analysis of LTD4. This is probably because the bis-carboxylate cysteinylglycine-adduct does not ionize very well under conventional ESI conditions. Furthermore, because of the high potency, of LTD4, it is generally present in only trace amounts in many biological fluids. In spite of these difficulties Zarini et al. (2009) were able to detect very small amounts of LTD4 in mouse peritoneal fluid (Fig. 9). Similarly, there are no reported studies on the analysis of 15-OEC (Fig. 3) in biological fluids using LC-MRM/MS. In contrast, the mono-carboxylate containing cysteine-adduct, LTE4, can be readily analyzed in complex biological matrices such as mouse peritoneal fluid and urine (Armstrong et al., 2009; Hardy et al., 2005; Hishinuma et al., 2001; Mizugaki et al., 1999; Nakamura et al., 2001; Stanke-Labesque et al., 2008; Suzuki et al., 2000; Zarini et al., 2009). The signal for LTE4 in mouse peritoneal fluid was several orders of magnitude more intense than that for LTD4 (Fig. 9) (Zarini et al., 2009). Immunoaffinity purification coupled with SID LC-MRM/MS analysis provided impressive chromatograms for urinary LTE4 with minimal ion suppression (Fig. 10) (Armstrong et al., 2009). This method provides a gold standard by which the specificity of all other LTE4 assays can be evaluated. This revealed that an enzyme immunoassay for LTE4 overestimated the amount that was present in urine by over 20-fold (639 pg/mg creatine to 5685 pg/mg creatine) when compared with the LC-MRM/MS assay (29 pg/mg creatinine to 145 pg/mg creatine). This highlights the importance of using SID LC-MRM/MS methodology for the analysis of endogenous cysteine-adducts.
Figure 10.
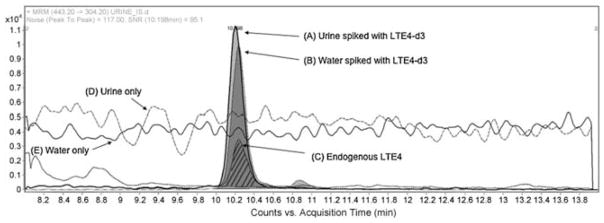
Evaluation of ion suppression on response of LTE4 and LTE4-d3 in urine and water. Injection experiments: extracted ion chromatogram (EIC) of MRM transitions for LTE4-d3 (m/z 443.2 → 304.3) spiked at 200 pg/mL in a human urine sample (A) and water (B), and MRM transition of endogenous LTE4 (m/z 440.2 → 301.3) in a human urine sample (C, hatched lines). Data demonstrate that the response of internal standard is not affected by ion suppression. Data was collected in positive electrospray ionization mode. The peak area for urine and water spiked with LTE4-d3 was 115754 and 107672, respectively. Post-column infusion experiments: LTE4 was infused and the 440.2 → 301.2 transition was monitored during injection of urine (D) and water (E) samples. Data demonstrate the absence of ion suppression in a urine matrix. Reprinted with permission from Armstrong et al. (2009).
Analysis of Endogenous MA Metabolites
MAs have been used as biomarkers of exposure to many exogenously derived chemicals (Anders, 2008; Haufroid and Lison, 2005; Hecht, 2002; Keum et al., 2005; Tang, 2003) and endogenously derived bioactive substances (Blair, 2006; Pombrio et al., 2001; Sidell et al., 2001; Stevens and Maier, 2008). For example, DHN-MAs are the major urinary metabolites of HNE (Fig. 5), which in turn is an important product of lipid peroxidation (Fig. 4). The urinary excretion of DHN-MA from (S)-HNE was found to be significantly lower in rats (9.8 ± 0.4%) than that observed after the (R)-enantiomer (27.3 ± 6.6%) was injected into rats (Gueraud et al., 2005). In vitro studies using rat liver cytosolic incubations revealed that this was due to differences in the intermediate pathways involved in their metabolism (Fig. 5). An enantioselective LC-MRM/MS assay has not been developed for the analysis of DHN-MAs. However, using an achiral SID LC-MRM/MS method, it was possible to show that the urinary excretion of DHN-MA increased significantly after BrCCl3 administration to rats compared with vehicle-treated animals (Peiro et al., 2005). This suggested the DHN-MA could be a useful biomarker of lipid peroxidation-induced oxidative stress. Urinary excretion of DHN-MA was also increased when a heme supplemented diet was given to human subjects, suggesting that the diet caused an increase in lipid peroxidation (Pierre et al., 2006).
Crotonaldehyde, which is thought to be a product of lipid peroxidation, is also present in many foods and beverages, ambient air and tobacco smoke. Two MA metabolites, 3-hydroxy-1-methylpropylmercapturic acid (HMPMA) and 2-carboxyl-l-methylethylmercapturic acid (CMEMA), were excreted in rat urine after subcutaneous injection of crotonaldehyde (Scherer et al., 2007). This stimulated the development of a SID LC-MRM/MS method for determination of HMPMA and CMEMA in human urine. It was found that cigarette smokers excreted about 3–5-fold more HMPMA, and only slightly elevated amounts of CMEMA, in their urine compared with nonsmokers. In smokers, significant correlations were also between the urinary excretion levels of HMPMA (but not CMEMA) and several markers of exposure for smoking, including the daily cigarette consumption, carbon monoxide in exhaled breath, salivary cotinine and nicotine plus five of its major metabolites in urine. Smoking cessation or switching from smoking conventional cigarettes to experimental cigarettes with lower crotonaldehyde delivery led to significant reductions of urinary HMPMA excretion, but not CMEMA excretion. Thus, HMPMA is a potentially useful biomarker for smoking-related exposure to crotonaldehyde (Scherer et al., 2007). This method could also form the basis for determining inter-individual exposure to crotonaldehyde from endogenous pathways of lipid peroxidation.
More futuristically, it is reasonable to consider conducting metabolomic profiling for a number of MA derivatives in order to provide insight into inter-individual exposure to reactive metabolites. With this concept in mind the Völkel group performed a control study using urine samples from 30 healthy male and female human subjects were collected at intervals of 8 h twice a day for three consecutive days (Wagner et al., 2007). Using LC-MRM/MS methodology in combination with a column-switching tool for the analysis of the MA pattern, samples were screened for time and gender differences, the most common confounders. The results suggested that it could be possible to use of metabolic profiling of MAs for the detection of disease or toxicity markers in the future. The use of SID methodology using [13C]-labeled internal standards coupled with improved sensitivity of detection could provide important insight into various disease processes as well as providing exposure biomarkers to different environmental chemicals.
Summary and Future Directions
Modern TQ instrumentation can conduct up to 200 MRM transitions every 2 s, which opens up the possibility of conducting multiple quantitative analyses of MAs as pioneered by the Völkel group (Wagner et al., 2007) together with rigorous SID LC-MS-based methodology. Sensitivity in the ESI/MS mode can be further improved by the use of nanoliter and low microliter per minute flow rates coupled with robust new column technologies such as fused core stationary phases (Abrahim et al., 2010). The improved sensitivity will make it possible to reduce the amount of individual urine samples that are used. This will preserve important samples such as those obtained from longitudinal studies as well as reducing potential interferences from the urine samples. New high-sensitivity TQ instrumentation will make it possible to conduct simultaneous quantification of free and oxidized thiols in circulating lymphocytes in order to assess inter-individual differences in oxidative stress (Zhu et al., 2008) and how this is related to endogenous urinary MA excretion. Additional increases in sensitivity can also be obtained using pre-ionized derivatives (Blair, 2009). This could prove to be particularly useful in the analysis of urinary MAs, making it possible to rigorously evaluate the role of endogenous pathways of MA formation in different disease states. The use of appropriate [13C]-labeled standards in SID LC-MRM/MS assays is critically important and is an active area of research within the field. This is because the [13C]-labeled standard will define the scope and accuracy of an assay. Without the ability to conduct rigorous bioanalytical validation of the MA assays, subsequent costly clinical studies will not attain adequate sensitivity and specificity to distinguish different disease states.
Acknowledgments
We acknowledge the support of NIH grants UO1ES16004, RO1CA091016, RO0130038, and P30ES0130508.
Abbreviations
- APCI
atmospheric pressure chemical ionization
- API
atmospheric pressure ionization
- CID
collision-induced dissociation
- COX
cyclooxygenase
- DDE
trans,trans-2,4-decadienal
- ECAPCI
electron capture atmospheric pressure chemical ionization
- DHN
dihydroxynonanol
- EDE
4,5-epoxy-2(E)-decenal
- ESI
electrospray ionization
- ETE
eicosatetraenoic acid
- GGT
γ-glutamyltranspeptidase
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione-S-transferase
- LC
liquid chromatography
- LT
leukotriene
- HETE
hydroxyeicosatetraenoic acid
- HNA
hydroxynonanoic acid
- HPETE
hydroperoxyeicosatetraenoic acid
- HPNE
4-hydroperoxy-2(E)-nonenal
- HNE
4-hydroxy-2(E)-nonenal
- HSD
hydroxysteroid dehydrogenase
- LOX
lipoxygenase
- MA
mercapturic acid
- MDA
malondialdehyde
- MRM
multiple reaction monitoring
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- NAT
N-acetyl transferase
- OEC
oxoeicosatetraenoic acid cysteinylglycine-adduct
- OEG
oxoeicosatetraenoic acid glutathione-adduct
- ONE
4-oxo-2(E)-nonenal
- PG
prostaglandin
- ROS
reactive oxygenspecies
- SID
stableisotopedilution
- TOG
thiadiazabicylco-4-oxo-2(E)-nonenalglutathione-adduct
- TQ
triple quadrupole
References
- Abrahim A, Al-Sayah M, Skrdla P, Bereznitski Y, Chen Y, Wu N. Practical comparison of 2.7 micron fused-core silica particles and porous sub-2 micron particles for fast separations in pharmaceutical process development. Journal of Pharmaceutical and Biomedical Analysis. 2010;51:131–137. doi: 10.1016/j.jpba.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Alary J, Gueraud F, Cravedi JP. Fate of 4-hydroxynonenal in vivo: disposition and metabolic pathways. Molecular Aspects of Medicine. 2003;24:177–187. doi: 10.1016/s0098-2997(03)00012-8. [DOI] [PubMed] [Google Scholar]
- Anders MW. Chemical toxicology of reactive intermediates formed by the glutathione-dependent bioactivation of halogen-containing compounds. Chemical Research in Toxicology. 2008;21:145–159. doi: 10.1021/tx700202w. [DOI] [PubMed] [Google Scholar]
- Armstrong M, Liu AH, Harbeck R, Reisdorph R, Rabinovitch N, Reisdorph N. Leukotriene-E4 in human urine: comparison of on-line purification and liquid chromatography–tandem mass spectrometry to affinity purification followed by enzyme immunoassay. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences. 2009;877:3169–3174. doi: 10.1016/j.jchromb.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JR. The glutathione peroxidases. Cellular and Molecular Life Sciences. 2000;57:1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi YC, Sharma R, Yadav S, Dwivedi S, Sharma A, Awasthi S. The non-ABC drug transporter RLIP76 (RALBP-1) plays a major role in the mechanisms of drug resistance. Current Drug Metabolism. 2007;8:315–323. doi: 10.2174/138920007780655414. [DOI] [PubMed] [Google Scholar]
- Backlund MG, Mann JR, Holla VR, Buchanan FG, Tai HH, Musiek ES, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. Journal of Biological Chemistry. 2005;280:3217–3223. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergholte JM, Soberman RJ, Hayes R, Murphy RC, Okita RT. Oxidation of 15-hydroxyeicosatetraenoic acid and other hydroxy fatty acids by lung prostaglandin dehydrogenase. Archives of Biochemistry and Biophysics. 1987;257:444–450. doi: 10.1016/0003-9861(87)90589-3. [DOI] [PubMed] [Google Scholar]
- Blair IA. Lipid hydroperoxide-mediated DNA damage. Experimental Gerontology. 2001;36:1473–1481. doi: 10.1016/s0531-5565(01)00133-4. [DOI] [PubMed] [Google Scholar]
- Blair IA. The role of oxidative stress in cancer and toxicology. In: Caprioli RM, Gross ML, editors. Encyclopedia of Mass Spectrometry. Elsevier; Amsterdam: 2005. pp. 283–307. [Google Scholar]
- Blair IA. Endogenous glutathione adducts. Current Drug Metabolism. 2006;7:853–872. doi: 10.2174/138920006779010601. [DOI] [PubMed] [Google Scholar]
- Blair IA. DNA-adducts with lipid peroxidation products. Journal of Biological Chemistry. 2008;283:15545–15549. doi: 10.1074/jbc.R700051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair IA. Analysis of estrogens in serum and plasma from postmenopausal women: past, present, and future. Steroids. 2009 doi: 10.1016/j.steroids.2010.01.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Shen L. p-Quinone methides are the major decomposition products of catechol estrogen o-quinones. Carcinogenesis. 1996;17:925–929. doi: 10.1093/carcin/17.5.925. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Thatcher GR. Potential mechanisms of estrogen quinone carcinogenesis. Chemical Research in Toxicology. 2008;21:93–101. doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chemical Research in Toxicology. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- Burczynski ME, Sridhar GR, Palackal NT, Penning TM. The reactive oxygen species—and Michael acceptor-inducible human aldoketo reductase AKR1C1 reduces the alpha, beta-unsaturated aldehyde 4-hydroxy-2-nonenal to 1,4-dihydroxy-2-nonene. Journal of Biological Chemistry. 2001;276:2890–2897. doi: 10.1074/jbc.M006655200. [DOI] [PubMed] [Google Scholar]
- Carini M, Aldini G, Facino RM. Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrometry Reviews. 2004;23:281–305. doi: 10.1002/mas.10076. [DOI] [PubMed] [Google Scholar]
- Chandrasena RE, Edirisinghe PD, Bolton JL, Thatcher GR. Problematic detoxification of estrogen quinones by NAD(P)H-dependent quinone oxidoreductase and glutathione-S-transferase. Chemical Research in Toxicology. 2008;21:1324–1329. doi: 10.1021/tx8000797. [DOI] [PubMed] [Google Scholar]
- Chung FL, Pan J, Choudhury S, Roy R, Hu W, Tang MS. Formation of trans-4-hydroxy-2-nonenal- and other enal-derived cyclic DNA adducts from omega-3 and omega-6 polyunsaturated fatty acids and their roles in DNA repair and human p53 gene mutation. Mutation Research. 2003;531:25–36. doi: 10.1016/j.mrfmmm.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Coles BF, Kadlubar FF. Human alpha class glutathione S-transferases: genetic polymorphism, expression, and susceptibility to disease. Methods in Enzymology. 2005;401:9–42. doi: 10.1016/S0076-6879(05)01002-5. [DOI] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiological Reviews. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- Dieckhaus CM, Fernandez-Metzler CL, King R, Krolikowski PH, Baillie TA. Negative ion tandem mass spectrometry for the detection of glutathione conjugates. Chemical Research in Toxicology. 2005;18:630–638. doi: 10.1021/tx049741u. [DOI] [PubMed] [Google Scholar]
- Doss GA, Baillie TA. Addressing metabolic activation as an integral component of drug design. Drug Metabolism Reviews. 2006;38:641–649. doi: 10.1080/03602530600959466. [DOI] [PubMed] [Google Scholar]
- Dourado DF, Fernandes PA, Ramos MJ. Mammalian cytosolic glutathione transferases. Current Protein and Peptide Science. 2008;9:325–337. doi: 10.2174/138920308785132677. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radical Biology and Medicine. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Gueraud F, Crouzet F, Alary J, Rao D, Debrauwer L, Laurent F, et al. Enantioselective metabolism of (R)- and (S)-4-hydroxy-2-nonenal in rat. Biofactors. 2005;24:97–104. doi: 10.1002/biof.5520240111. [DOI] [PubMed] [Google Scholar]
- Hardy G, Boizel R, Bessard J, Cracowski JL, Bessard G, Halimi S, et al. Urinary leukotriene E4 excretion is increased in type 1 diabetic patients: a quantification by liquid chromatography–tandem mass spectrometry. Prostaglandins and Other Lipid Mediators. 2005;78:291–299. doi: 10.1016/j.prostaglandins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Haufroid V, Lison D. Mercapturic acids revisited as biomarkers of exposure to reactive chemicals in occupational toxicology: a minireview. International Archives of Occupational and Environmental Health. 2005;78:343–354. doi: 10.1007/s00420-005-0620-z. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annual Review of Pharmacology and Toxicology. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. Journal of Biological Chemistry. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- Hishinuma T, Suzuki N, Aiba S, Tagami H, Mizugaki M. Increased urinary leukotriene E4 excretion in patients with atopic dermatitis. British Journal of Dermatology. 2001;144:19–23. doi: 10.1046/j.1365-2133.2001.03947.x. [DOI] [PubMed] [Google Scholar]
- Hosgood HD, III, Berndt SI, Lan Q. GST genotypes and lung cancer susceptibility in Asian populations with indoor air pollution exposures: a meta-analysis. Mutation Research. 2007;636:134–143. doi: 10.1016/j.mrrev.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson PJ, Morgenstern R, Mancini J, Ford-Hutchinson A, Persson B. Membrane-associated proteins in eicosanoid and glutathione metabolism (MAPEG). A widespread protein superfamily. American Journal of Respiratory Critical Care Medicine. 2000;161:S20–S24. doi: 10.1164/ajrccm.161.supplement_1.ltta-5. [DOI] [PubMed] [Google Scholar]
- Jian W, Arora JS, Oe T, Shuvaev VV, Blair IA. Induction of endothelial cell apoptosis by lipid hydroperoxide-derived bifunctional electrophiles. Free Radical Biology and Medicine. 2005a;39:1162–1176. doi: 10.1016/j.freeradbiomed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Jian W, Lee SH, Arora JS, Silva Elipe MV, Blair IA. Unexpected formation of etheno-2′-deoxyguanosine adducts from 5(S)-hydroperoxyeicosa-tetraenoic acid: evidence for a bis-hydroperoxide intermediate. Chemical Research in Toxicology. 2005b;18:599–610. doi: 10.1021/tx049693d. [DOI] [PubMed] [Google Scholar]
- Jian W, Lee SH, Mesaros C, Oe T, Silva Elipe MV, Blair IA. A novel 4-oxo-2(E)-nonenal-derived endogenous thiadiazabicyclo glutathione adduct formed during cellular oxidative stress. Chemical Research in Toxicology. 2007;20:1008–1018. doi: 10.1021/tx700001t. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods in Enzymology. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- Keum YS, Jeong WS, Kong AN. Chemopreventive functions of isothio-cyanates. Drug News Perspectives. 2005;18:445–451. doi: 10.1358/dnp.2005.18.7.939350. [DOI] [PubMed] [Google Scholar]
- King R, Bonfiglio R, Fernandez-Metzler C, Miller-Stein C, Olah T. Mechanistic investigation of ionization suppression in electrospray ionization. Journal of the American Society of Mass Spectrometry. 2000;11:942–950. doi: 10.1016/S1044-0305(00)00163-X. [DOI] [PubMed] [Google Scholar]
- Lam BK, Austen KF. Leukotriene C4 synthase: a pivotal enzyme in cellular biosynthesis of the cysteinyl leukotrienes. Prostaglandins and Other Lipid Mediators. 2002;68–69:511–520. doi: 10.1016/s0090-6980(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Lee DH, Jacobs DR., Jr Is serum gamma-glutamyltransferase a marker of exposure to various environmental pollutants? Free Radical Research. 2009;43:533–537. doi: 10.1080/10715760902893324. [DOI] [PubMed] [Google Scholar]
- Lee SH, Blair IA. Oxidative DNA damage and cardiovascular disease. Trends in Cardiovascular Medicine. 2001;11:148–155. doi: 10.1016/s1050-1738(01)00094-9. [DOI] [PubMed] [Google Scholar]
- Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- Lee SH, Silva Elipe MV, Arora JS, Blair IA. Dioxododecenoic acid: a lipid hydroperoxide-derived bifunctional electrophile responsible for etheno DNA adduct formation. Chemical Research in Toxicology. 2005;18:566–578. doi: 10.1021/tx049716o. [DOI] [PubMed] [Google Scholar]
- Lee SH, Rangiah K, Williams MV, Wehr AY, Dubois RN, Blair IA. Cyclo-oxygenase-2-mediated metabolism of arachidonic acid to 15-oxo-eicosatetraenoic acid by rat intestinal epithelial cells. Chemical Research in Toxicology. 2007;20:665–1675. doi: 10.1021/tx700130p. [DOI] [PubMed] [Google Scholar]
- Levsen K, Schiebel HM, Behnke B, Dotzer R, Dreher W, Elend M, et al. Structure elucidation of phase II metabolites by tandem mass spectrometry: an overview. Journal of Chromatography A. 2005;1067:55–72. doi: 10.1016/j.chroma.2004.08.165. [DOI] [PubMed] [Google Scholar]
- Lu SC. Regulation of glutathione synthesis. Current Topics in Cellular Regulation. 2000;36:95–116. doi: 10.1016/s0070-2137(01)80004-2. [DOI] [PubMed] [Google Scholar]
- Ma L, Wen B, Ruan Q, Zhu M. Rapid screening of glutathione-trapped reactive metabolites by linear ion trap mass spectrometry with isotope pattern-dependent scanning and postacquisition data mining. Chemical Research in Toxicology. 2008;21:1477–1483. doi: 10.1021/tx8001115. [DOI] [PubMed] [Google Scholar]
- Mangal D, Vudathala DK, Park JH, Lee SH, Penning TM, Blair IA. Analysis of 7,8-dihydro-8-oxo-2′-deoxyguanosine in cellular DNA during oxidative stress. Chemical Research in Toxicology. 2009;22:788–797. doi: 10.1021/tx800343c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne GL, Yin H, Morrow JD. Human biochemistry of the isoprostane pathway. Journal of Biological Chemistry. 2008;283:15533–15537. doi: 10.1074/jbc.R700047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minko IG, Kozekov ID, Harris TM, Rizzo CJ, Lloyd RS, Stone MP. Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha, beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chemical Research in Toxicology. 2009;22:759–778. doi: 10.1021/tx9000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizugaki M, Hishinuma T, Suzuki N. Determination of leukotriene E4 in human urine using liquid chromatography–tandem mass spectrometry. Journal of Chromatography B Analytical Technologies in the Biomedical and Life Sciences. 1999;729:279–285. doi: 10.1016/s0378-4347(99)00174-7. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kudo I. Prostaglandin E synthase: a novel drug target for inflammation and cancer. Current Pharmaceutical Design. 2006;12:943–954. doi: 10.2174/138161206776055912. [DOI] [PubMed] [Google Scholar]
- Murphy RC, Zarini S. Glutathione adducts of oxyeicosanoids. Prostaglandins and Other Lipid Mediators. 2002;68–69:471–482. doi: 10.1016/s0090-6980(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Hishinuma T, Suzuki N, Chiba S, Tsukamoto H, Takabatake M, et al. Difference in urinary 11-dehydro TXB2 and LTE4 excretion in patients with rheumatoid arthritis. Prostaglandins Leukotrienes and Essential Fatty Acids. 2001;65:301–306. doi: 10.1054/plef.2001.0329. [DOI] [PubMed] [Google Scholar]
- Oe T, Ackermann BL, Inoue K, Berna MJ, Garner CO, Gelfanova V, et al. Quantitative analysis of amyloid beta peptides in cerebrospinal fluid of Alzheimer’s disease patients by immunoaffinity purification and stable isotope dilution liquid chromatography/negative electrospray ionization tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2006;20:3723–3735. doi: 10.1002/rcm.2787. [DOI] [PubMed] [Google Scholar]
- Peiro G, Alary J, Cravedi JP, Rathahao E, Steghens JP, Gueraud F. Dihydroxynonene mercapturic acid, a urinary metabolite of 4-hydroxynonenal, as a biomarker of lipid peroxidation. Biofactors. 2005;24:89–96. doi: 10.1002/biof.5520240110. [DOI] [PubMed] [Google Scholar]
- Pierre F, Peiro G, Tache S, Cross AJ, Bingham SA, Gasc N, et al. New marker of colon cancer risk associated with heme intake: 1,4-dihydroxynonane mercapturic acid. Cancer Epidemiology Biomarkers and Prevention. 2006;15:2274–2279. doi: 10.1158/1055-9965.EPI-06-0085. [DOI] [PubMed] [Google Scholar]
- Pombrio JM, Giangreco A, Li L, Wempe MF, Anders MW, Sweet DH, et al. Mercapturic acids (N-acetylcysteine S-conjugates) as endogenous substrates for the renal organic anion transporter-1. Molecular Pharmacology. 2001;60:1091–1099. doi: 10.1124/mol.60.5.1091. [DOI] [PubMed] [Google Scholar]
- Rinaldi R, Eliasson E, Swedmark S, Morgenstern R. Reactive intermediates and the dynamics of glutathione transferases. Drug Metabolism and Disposition. 2002;30:1053–1058. doi: 10.1124/dmd.30.10.1053. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radical Biology and Medicine. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Scherer G, Urban M, Hagedorn HW, Feng S, Kinser RD, Sarkar M, et al. Determination of two mercapturic acids related to crotonaldehyde in human urine: influence of smoking. Human and Experimental Toxicology. 2007;26:37–47. doi: 10.1177/0960327107073829. [DOI] [PubMed] [Google Scholar]
- Schneider C, Tallman KA, Porter NA, Brash AR. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. Journal of Biological Chemistry. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxidants and Redox Signaling. 2004;6:289–300. doi: 10.1089/152308604322899350. [DOI] [PubMed] [Google Scholar]
- Shen XM, Xia B, Wrona MZ, Dryhurst G. Synthesis, redox properties, in vivo formation, and neurobehavioral effects of N-acetylcysteinyl conjugates of dopamine: possible metabolites of relevance to Parkinson’s disease. Chemical Research in Toxicology. 1996;9:1117–1126. doi: 10.1021/tx960052v. [DOI] [PubMed] [Google Scholar]
- Sidell KR, Amamath V, Montine TJ. Dopamine thioethers in neurode-generation. Current Topics in Medicinal Chemistry. 2001;1:519–527. doi: 10.2174/1568026013394705. [DOI] [PubMed] [Google Scholar]
- Stanke-Labesque F, Pofelski J, Moreau-Gaudry A, Bessard G, Bonaz B. Urinary leukotriene E4 excretion: a biomarker of inflammatory bowel disease activity. Inflammatory Bowel Diseases. 2008;14:769–774. doi: 10.1002/ibd.20403. [DOI] [PubMed] [Google Scholar]
- Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Molecular Nutrition and Food Research. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Hishinuma T, Abe F, Omata K, Ito S, Sugiyama M, et al. Difference in urinary LTE4 and 11-dehydro-TXB2 excretion in asthmatic patients. Prostaglandins and Other Lipid Mediators. 2000;62:395–403. doi: 10.1016/s0090-6980(00)00091-5. [DOI] [PubMed] [Google Scholar]
- Tang W. The metabolism of diclofenac—enzymology and toxicology perspectives. Current Drug Metabolism. 2003;4:319–329. doi: 10.2174/1389200033489398. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Progress in Lipid Research. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Völkel W, varez-Sanchez R, Weick I, Mally A, Dekant W, Pahler A. Glutathione conjugates of 4-hydroxy-2(E)-nonenal as biomarkers of hepatic oxidative stress-induced lipid peroxidation in rats. Free Radical Biology and Medicine. 2005;38:1526–1536. doi: 10.1016/j.freeradbiomed.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Völkel W, Sicilia T, Pahler A, Gsell W, Tatschner T, Jellinger K, et al. Increased brain levels of 4-hydroxy-2-nonenal glutathione conjugates in severe Alzheimer’s disease. Neurochemistry International. 2006;48:679–686. doi: 10.1016/j.neuint.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Wagner S, Scholz K, Sieber M, Kellert M, Völkel W. Tools in metabonomics: an integrated validation approach for LC-MS metabolic profiling of mercapturic acids in human urine. Analytical Chemistry. 2007;79:2918–2926. doi: 10.1021/ac062153w. [DOI] [PubMed] [Google Scholar]
- Wei C, Zhu P, Shah SJ, Blair IA. 15-Oxo-eicosatetraenoic acid, a metabolite of macrophage 15-hydroxyprostaglandin dehydrogenase that inhibits endothelial cell proliferation. Molecular Pharmacology. 2009;76:516–529. doi: 10.1124/mol.109.057489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DL, Li D, Nurgalieva Z, El-Serag HB. Genetic variants of glutathione S-transferase as possible risk factors for hepatocellular carcinoma: a HuGE systematic review and meta-analysis. American Journal of Epidemiology. 2008;167:377–389. doi: 10.1093/aje/kwm315. [DOI] [PubMed] [Google Scholar]
- Zarini S, Gijon MA, Ransome AE, Murphy RC, Sala A. Transcellular biosynthesis of cysteinyl leukotrienes in vivo during mouse peritoneal inflammation. Proceedings of the National Academy of Sciences USA. 2009;106:8296–8301. doi: 10.1073/pnas.0903851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Taghizadeh K, Dedon PC. Chemical and biological evidence for base propenals as the major source of the endogenous M1dG adduct in cellular DNA. Journal of Biological Chemistry. 2005;280:25377–25382. doi: 10.1074/jbc.M503079200. [DOI] [PubMed] [Google Scholar]
- Zhou ZD, Lim TM. Roles of glutathione (GSH) in dopamine (DA) oxidation studied by improved tandem HPLC plus ESI-MS. Neurochemical Research. 2009;34:316–326. doi: 10.1007/s11064-008-9778-6. [DOI] [PubMed] [Google Scholar]
- Zhu P, Oe T, Blair IA. Determination of cellular redox status by stable isotope dilution liquid chromatography/mass spectrometry analysis of glutathione and glutathione disulfide. Rapid Communications in Mass Spectrometry. 2008;22:432–440. doi: 10.1002/rcm.3380. [DOI] [PubMed] [Google Scholar]
- Zhu P, Jian W, Blair IA. A 4-oxo-2(E)-nonenal-derived glutathione adduct from 15-lipoxygenase-1-mediated oxidation of cytosolic and esterified arachidonic acid. Free Radical Biology and Medicine. 2009;47:953–961. doi: 10.1016/j.freeradbiomed.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]