Abstract
Hearing loss is one of the most common disabilities, affecting approximately 10% of the population. Hair cells and spiral ganglion neurons are usually damaged in most cases of hearing loss. Currently, there is virtually no biological approach to replace damaged hearing cells. Recent developments in stem cell technology provide new opportunities for the treatment of deafness. Two major strategies have been investigated: differentiation of endogenous stem cells into new hair cells; and introduction of exogenous cells into the inner ear to substitute injured hearing neurons. Although there is still a learning curve in stem cell-based replacement, the probability exists to utilize personalized stem cells to eventually provide a novel intervention for patients with deafness in future clinical research trials.
Keywords: Biological-EAR, differentiation, epithelial-to-mesenchymal transition, hair cell, hearing, implantation, neurotrophins, regeneration, spiral ganglion, stem cell
The challenge in hearing regeneration & stem cell opportunity
It is reported that approximately two to three out of 1000 children are born with severe-to-profound hearing loss [1]. The US NIH estimates that approximately 15% (26 million) of Americans between the ages of 20 and 69 years have high-frequency hearing loss due to exposure to loud sounds or noise at work, or in leisure activities. There is an observed relationship between age and reported hearing impairment: 18% of 45–64-year-old Americans are hearing impaired, as well as 30% of Americans aged 65–74 years and 47% of Americans aged 75 years or older. Hearing impairment severely affects the daily activities of the patients of deafness. Therefore, the social and economic implications of hearing impairment are enormous, as is the size of the affected population.
Hearing impairment is divided into three major types: conductive hearing loss; sensorineural hearing loss; and mixed hearing loss. Conductive hearing loss is usually caused by external and/or middle ear malformation and/or dysfunction, which can generally be treated by surgical approaches and/or hearing aids. Sensorineural hearing loss is a result of dysfunction of the inner ear cochlea. This may involve loss of sensory hair cells and a secondary degeneration of spiral ganglion neurons (SGNs). In mixed hearing loss, both conductive and sensorineural hearing loss is present. While nonmammalian vertebrates can regenerate their hair cells throughout life, mammals do not possess such a capability as the mammalian progenitor cells that give origin to auditory hair cells are only produced during the embryonic and early post-natal stages. Usually, damage to adult mammalian hair cells and the associated nervous tissue is irreversible.
In clinics, cochlear implants have been applied to treat sensorineural hearing loss for more than two decades. The US NIH reported that approximately 219,000 patients had received cochlear implants worldwide, according to the report of the US FDA in December 2010 [101]. In cochlear implants, implanted electrodes convey the signals that are generated from outside sound to the central auditory system via surviving SGNs and associated auditory nerve fibers, thus bypassing the damaged sensory hair cells. It is notable that hearing through a cochlear implant is different from normal hearing; therefore, postoperative learning is critical to interpret the sound signals that are produced by cochlear implants. As cochlear implants convey auditory signals via surviving SGNs, the number of SGNs is essential to the efficacy of cochlear implants. Other hearing prostheses, such as auditory brainstem implants and auditory midbrain implants, have been developed in recent years [2–4]. However, there is virtually no biological approach to replace damaged sensory hair cells and SGNs.
Stem cells usually possess the capability of self-renewal and differentiation. Based on this ability, stem cells are categorized as either totipotent, pluripotent, multipotent or unipotent. During normal development, stem cells proliferate and differentiate to form tissues and organs. In adults, most of the stem cells exit the cell cycle, thus losing the ability to grow and generate new cells. In some of the adult mammalian tissues, such as bone marrow, hair follicles and intestine crypts, stem cells can re-enter the cell cycle and generate new cells in physiological and pathological conditions. In the adult nervous system, it was generally considered that neurons cannot be regenerated until neural stem cells (NSCs) were identified in mature mammalian brain tissue [5,6].
Embryonic stem (ES) cells are inner cell mass-derived pluripotent stem cells that are able to proliferate almost unlimitedly and differentiate into cells of the three germ layers: ectoderm, mesoderm and endoderm. After the first mouse ES cell line was established in 1981 [7] and human ES cell line in 1998 [8], ES cells have been cultured in vitro and induced into a variety of cell types, such as neurons [9,10], liver cells [11–13], cardiac cells [14,15] and insulin-secreting cells [16–18]. Therefore, ES cells offer a remarkable cell resource for replacement therapy in regenerative medicine. The application of ES cells in clinical research trials, however, may be hindered unless the ethical and immune rejection issues can be solved. Recent advances in induced pluripotent stem (iPS) cell technology provide the opportunity to generate personalized pluripotent stem cells from the individual’s own somatic cells, which are able to differentiate into cells of the three germ layers [19–21]. Various cell types that are generated from iPS cells can be potentially used to replace damaged cells in regenerative medicine [22,23].
Developments in stem cell technology bring new hope for the treatment of sensorineural hearing loss. One potential therapeutic approach is to replace damaged hair cells and SGNs with stem cell-derived cells. This stem cell-based cell replacement may be achieved by the following strategies: induction of local inner ear progenitors to re-enter the cell cycle and differentiate into new hearing cells; and transplantation of exogenous stem cells or stem cell-derived hearing cells into the inner ear.
Identification & activation of endogenous progenitors for hearing regeneration
One approach for substituting damaged hair cells and SGNs is via the proliferation and differentiation of resident progenitors. In this approach, significant attention has been paid to hair cell generation and promising results are emerging; therefore, the advances of hair cell regeneration will be reviewed in this section.
In nonmammalian vertebrates, damaged hair cells can be replaced by new hair cells throughout life, indicating that the inner ears of these species possess stem/progenitor cells that are able to self-renew and differentiate into new hair cells and supporting cells [24,25]. It is still undetermined whether there is a specialized reserve pool of distinct stem cells in adult vertebrate sensory epithelia. It is generally accepted that the most likely source of stem cells in the inner ear sensory epithelia is the supporting cells [26]. Supporting cells in the inner ear can generate new hair cells via either regenerative responses of dedifferentiation, proliferation and differentiation, or a direct phenotype conversion called transdifferentiation [26,27]. Additionally, we cannot rule out the possibility that some of the new hair cells are actually survivors that recover their morphology and function following insult [28].
With regard to the mammalian inner ear, it is reported that pluripotent stem cells exist in the adult mouse utricles [29]. These pluripotent stem cells can form spheres and differentiate into new hair-like cells in vitro. Subsequent studies indicate that sphere-forming cells are also found in the cochlea [30,31]. A study using postnatal rat cochleae indicates that postmitotic mammalian supporting cells retain the ability to proliferate and differentiate into new cells expressing hair cell proteins in vitro [32]. It is still controversial as to whether the mammalian sphere-forming cell is a specific type of stem cell or a subtype of the supporting cells [29]. Generally, supporting cells are considered to be the source of mammalian hair cell progenitors based on the following observations: new hair cells can be derived from supporting cells when hair cells are laser-ablated in the developing mouse inner ear [33]; and postnatal mouse supporting cells can proliferate and/or transdifferentiate into new hair cells in vitro [32].
In humans, while progenitor cells have been identified from fetal inner ears [34], study of hair cell progenitors is severely limited because it is virtually impossible to obtain inner ear tissues from normal humans owing to ethical considerations. Recent reports indicate that it is possible to collect discarded tissues from inner ear surgery [35,36]. Acoustic neuroma (vestibular schwannoma) is a benign primary intracranial tumor of the myelin-forming cells of the vestibulocochlear nerve. In a trans labyrinthine (TL) surgical approach for the treatment of acoustic neuroma, the utricle and semicircular canals have to be removed to provide access to the tumor [37]. Therefore, the discarded tissues could be collected from the TL surgery and the harvested cell material served as a human model to investigate whether the human inner ear possesses cells with progenitor properties. One study demonstrates that human utricular cells can be cultured in vitro for at least 25 passages. In addition, human utricular cells expressed genes and proteins that are usually observed in hair cell progenitors and stem cells, indicating that human inner ear sensory epithelial cells are able to present hair cell progenitor features in appropriate culture conditions [35].
Regardless of the source, there are experimental results suggesting that stem/progenitor cells indeed exist in the hair cell epithelium of not only nonmammalian inner ears but also mammalian vestibular (postnatal and adult) and cochlear (postnatal) systems [24,25,29,32,35,38]. The question is why adult mammalian progenitor cells cannot regenerate hair cells. The reason is still obscure but one possibility is that progenitors undergo certain genetic changes during maturation, thus losing the ability to self-renew and differentiate. The other possibility is that progenitors in the adult inner ear do not situate in the appropriate stem cell niche that they used to during embryonic stages. In other words, the mature microenvironment in the mammalian inner ear does not permit the progenitors to keep their identity and/or the ability to differentiate into new hair and supporting cells. Accordingly, identification of the genes critical for preservation of hair cell progenitor identity, the growth factors involved in the maintenance of stem cell niche and the mechanism associated with the activation of a hair cell progenitor will be fundamental to the understanding of the absence of hair cell regeneration in the adult mammalian inner ear.
The existence of progenitors in the adult mammalian inner ear leads to the possibility of therapeutic applications. In this approach, it is essential to understand the mechanism for the initiation of the regeneration machinery. Because regeneration of avian inner ear sensory epithelia is usually confined to the damaged region [24,25,39], hair cell loss is considered as the trigger that activates a regenerative response of supporting cells in nonmammalian vertebrates. Hair cell loss in adult mammals, however, is obviously not sufficient to trigger supporting cells to initiate regenerative responses, which may be one of the major causes of the severely limited regeneration ability [40,41]. In in vitro studies, sphere-forming cells have been identified from the adult mammalian inner ear sensory epithelium [29,30], indicating that a suspension culture condition with supplemented growth factors might be required for the activation or generation of hair cell progenitors. However, the underlying mechanism and the intra-cellular signaling pathways responsible for the induction of hair cell progenitor identity remain obscure. Recently, an epithelial-to-mesenchymal transition (EMT) has been hypothesized as a potential mechanism associated with the in vitro activation of the regeneration machinery for embryonic avian [38] and adult mammalian inner ear sensory epithelia [35,42].
In general, epithelial cells produce close cell–cell contacts and establish the apicobasal polarity via intercellular connections, such as tight junctions, adherens junctions and desmosomes [42–44]. During EMT, epithelial cells undergo remarkable phenotypic changes, such as the loss of intercellular junctions, the loss of the apicobasal polarity and the acquisition of mesenchymal features [44]. During EMT, epithelial markers, including tight junction proteins and E-cadherin, are downregulated, while the expression of mesenchymal markers such as fibronectin, N-cadherin and vimentin are upregulated to maintain a mesenchymal trait. In addition, F-actin is rearranged from an epithelial ‘actin ring’ pattern into a mesenchymal ‘stress fiber’ pattern. During development, EMT plays an important role in the formation of the body polarity and generation of organs and tissues. In adults, EMT is involved in pathological conditions, such as fibrosis, tissue repair and carcinoma progression. EMT has been reported to be associated with the proliferation of epithelial cell lines cultured in vitro [43,45]. In the culture of human ES cells, the cells underwent EMT to show mesenchymal properties while expressing pluripotent markers POU5F1 and NANOG [46]. When epithelial cells from pancreatic islets are cultured on 2D substrates in vitro, they dedifferentiate into mesenchymal-like cells, which can readily be expanded to large populations [47,48]. In the sensory system, mammalian inner ear sensory epithelial cells (mouse utricular cells [MUCs]) that are cultured on substrates gradually lose their epithelial features and acquire mesenchymal characteristics [42], which may contribute to changes in cell–cell contact and cell–matrix contact. For instance, MUCs changed their epithelial columnar shape and lost intercellular junctions when they grew flat on the 2D substrate. The F-actin of MUCs was arranged into a ‘stress fiber’ pattern. In parallel to the loss of the epithelial features, MUCs obtained mesenchymal properties, such as the expression of Fn1 (fibronectin), Cdh2 (N-cadherin) and Vim (vimentin). The data of morphological, genetic and protein expression indicate that adult mammalian sensory epithelial cells are able to undergo EMT when they are cultured on 2D substrates. Furthermore, MUCs acquired the features of inner ear progenitors, including the expression of several genes widely expressed during inner ear development [42]. This included the expression of three main candidates for the markers of prosensory domains in the otocyst during inner ear development [49]: Jag1 (Jagged1, the notch ligand); Lfng (Lunatic fringe, the notch regulator); and the secreted signaling molecule Bmp4. In addition, MUCs also expressed other prosensory cell genes and proteins, such as Isl1 (Islet 1), Notch1, Eya1, Numb, Cdkn1b (P27kip1), Six1, Dlx5, Pax8 and Hes1, suggesting that MUCs obtained the properties of inner ear prosensory cells. MUCs cultured on 2D substrates expressed several transcription factors that are usually shown in pluripotent stem cells, such as Pou5f1 (OCT4), Nanog (NANOG) and Sox2 (SOX2) [50,51], indicating that MUCs may acquire pluripotent features. In addition, Nes (Nestin), an intermediate filament expressed in stem/precursor cells, and Gfap (GFAP), commonly expressed in neural progenitors and astrocytes [52,53], were also detected in MUCs that had undergone EMT. These results may support the hypotheses that mesenchymal status may be associated with the acquisition and/or maintenance of pluripotency or multipotency, and that adult mammalian inner ear sensory epithelial cells can dedifferentiate into stem/progenitor cells via EMT. The establishment of such an in vitro dedifferentiation model for mammalian sensory epithelial cells might potentially be used to identify the molecules that are critical to the regulation of cell fate determination in future attempts towards cell-based auditory system regeneration.
Introduction of exogenous cells for hearing recovery
Although promising results are emerging in hair cell generation via the differentiation of ES, iPS and inner ear progenitor cells ex vivo [32,54,55], introduction of these new hair cells into the mammalian inner ear has rarely been reported. One of the major challenges is the microenvironment in the host organ of Corti, which is significantly different from the culture condition where new hair cells are generated in vitro. For example, the high potassium level in the host endolymph is toxic to the transplanted cells. A phalangeal scar is usually formed at the organ of Corti after hair cell damage. Therefore, removal of the phalangeal scar and integration of exogenous cells into the sensory epithelia are the major obstacles in hair cell transplantation. In addition, reconstruction of the 3D architecture of the complicated organ of Corti remains a major challenge. In the meantime, recent development in NSC biology stimulates significant advances in SGN replacement. Recent developments in transplantation aiming at SGN replacement in this section, including the donor cell types, surgical approach, survival, differentiation and integration of transplanted cells will now be discussed.
A number of cell types have been transplanted into the mammalian inner ear, including embryonic neuronal tissues, ES cells, iPS cells, NSCs, mesenchymal stem cells, inner ear stem cells and stem cell-derived neurons [56–58]. SGNs are bipolar glutamatergic neurons; therefore, the donor cells are ideally bipolar glutamatergic neurons or progenitors with the capability of differentiating into such neurons. During development, Neurog1 (Neurogenin 1) [59], Neurod1 (NeuroD1) [60], Ntf3 (NT-3) and Bdnf (BDNF) [61] are reported to be crucial for SGN generation. Overexpression of Neurogenin has led to neuronal differentiation in vitro [62,63]. Generally, induction of stem cells to differentiate into neural progenitors in vitro is encouraged prior to transplantation. In addition, supporting cell-derived inner ear progenitors, such as MUCs, may possess the ability to dedifferentiate into a more primitive state, such as otocyst-like cells expressing Islet1. It may be possible to guide these Islet1-expressing progenitor cells to become SGNs via the overexpression of genes critical for SGN development.
An ideal surgical approach is to obtain access to the SGN area with minimal trauma to the inner ear. Currently, a retroauricular approach is mostly used to expose the cochlea for the injection of exogenous cells into the perilymph in the scala vestibuli and scala tympani (ST), the endolymph in the scala media and the modiolus in the center of the cochleae [64–68]. In the scala vestibular approach, a cochleostomy is usually made at the lateral wall of the scala vestibuli to obtain access to perilymph and bony modiolus. Because of the tiny size of the cochlea and the proximity of the scala vestibuli and the scala media, the cochleostomy at the lateral wall of the scala vestibuli may disturb the stria vascularis located at the lateral wall of the scala media. In the scala media approach, the stria vascularis has to be traumatically damaged during the surgical approach, which may affect the blood supply to the cochlea and the maintenance of endocochlear potential. The ST approach is made by a round-window membrane penetration or a cochleostomy at the lateral wall of the ST, which provides access to the perilymph in the ST and the SGN area (Rosenthal’s canal [RC]) within the bony modiolus. To evaluate the potential disturbance of auditory function caused by the surgical approach, auditory brainstem responses (ABRs) to click and tone stimuli were investigated in mice injected with exogenous cells via the aforementioned three approaches [65]. It was reported that approximately 15 dB of hearing loss was observed in the mouse receiving small-size cochleostomy (less than 0.4 mm). However, ABR thresholds were 45 dB greater postoperatively in mice that received a large sized cochleostomy (0.8–1 mm) [65]. To evaluate the effect of surgical approach on inflammatory tissue response and survival of SGNs, exogenous cells were implanted into the inner ear via three approaches: a cochleostomy into the ST; direct access to RC via a localized fracture of the osseous spiral lamina; and direct access to the auditory nerve via a TL surgical approach. The results indicate that the ST approach is the best approach, which is demonstrated by the extent of the inflammatory tissue response (TL ≫ RC ≥ ST) and the survival of SGNs (ST > RC ≫ TL). In general, the ST approach causes the least damage to the inner ear and has been widely used in clinical cochlear implant surgery [4].
Few studies have investigated the survival rate of transplanted cells and the mechanism responsible for cell survival following transplantation into the inner ear. Brain-derived NSCs usually have a low survival rate following implantation into the inner ear. Approximately 0.05% of embryonic mouse brain-derived NSCs and 0.04–0.07% of adult mouse brain-derived NSCs survived following implantation into the adult mammalian inner ear [62,69]. On the other hand, implanted cells were oberved to survive better in newly-damaged inner ears [62,70], indicating that the damaged inner ear may release small molecules to support the survival of implanted cells. The low survival rate of implanted cells was observed in cell transplant therapy for other neurodegenerative diseases [71,72], which was considered to be related to apoptosis and the microenvironment at the implantation site. For example, in implantation of NSCs/progenitor cells into the spinal cord, it was found that apoptosis of implanted cells mostly occurred during the first day after implantation, and that microenvironment at the implantation sites may contribute to the low survival rate at a late stage [73].
In cell transplantation for the inner ear, the exogenous cells are transplanted into either perilymph or bony modiolus [65–67,74–76]. Perilymph is a physiological solution that is mainly composed of ions and glucose, with minimal growth factors and neurotrophic factors, which are required for the survival of implanted cells. In addition, the cochlea does not have a robust blood supply because it is perfused by a single end artery, the common cochlear artery. Therefore, the microenvironment at the implantation site in the cochlea is not capable of providing long-term nutrition to support the survival of implanted cells, which is a major limiting factor for the success of transplantation [56]. To enhance cell survival, exogenous neurotrophic factors can be supplied to the implanted inner ear. It has been found that NGF can enhance the survival of transplanted embryonic mouse ganglion neurons and inner ear-derived NSCs [75,77]. These observations were supported by a study in which a significant number of mouse ES cells survived in the inner ear of adult guinea pigs when BDNF and GDNF were supplied [63]. In addition, neural cografts, suggested to release growth factors, have been observed to enhance the survival of ES cells injected into the ST of adult guinea pigs [78]. These studies indicate that exogenous growth factors, such as neurotrophins, are required to support the survival of cells implanted into the inner ear. Due to the poor survival of implanted cells, uncontrolled cell growth, such as tumor and/or carcinoma formation, has not been observed in the inner ear transplantation.
While neural differentiation of implanted stem cells is reported in the transplantation into the mammalian inner ear, the mechanism of neuronal differentiation remains unclear. Generally, ES cells have the potential to automatically differentiate into neurons both in vitro and following implantation into the inner ear [63,70,78–80]. The rate of neuronal differentiation of ES cells following implantation into the inner ear, however, has rarely been characterized. In addition, implanted ES cells that have not adopted a neuronal fate may retain the potential to differentiate into cells of the three germ layers, which is clearly not desirable for an inner ear replacement therapy. Therefore, it is reasonable to guide ES cells to become neural progenitors in vitro prior to implantation into the inner ear. These neural progenitors are expected to possess limited proliferation and differentiation abilities while expressing NSC proteins such as Sox2 and Nestin.
Because SGNs are bipolar glutamatergic neurons, it is ideal to induce ES cells to adopt a glutamatergic fate in vitro prior to transplantation into the inner ear. Few studies have investigated glutamatergic neuronal differentiation of stem cells in inner ear transplantation [63,75]. Neurog1 and Neurog2 are found to be essential for the neuronal differentiation of ES cells and NSCs in SGN replacement [62,63]. In addition, neurotrophins that are critical for the generation and/or survival of SGNs have been investigated [75]. The results indicate the ability of neurotrophins to guide glutamatergic neuronal differentiation in vitro. However, the effect of neurotrophin on stimulating glutamatergic differentiation of stem cells has rarely been observed in vivo. Currently, inner ear-derived NSCs seem to be more ready to adopt a SGN-like glutamatergic neuronal fate [75]. Identification of molecular mechanisms responsible for glutamatergic neuronal differentiation of stem cells remains one of the major challenges in SGN replacement.
Few studies have tested the function of the cells following transplantation into the inner ear. The function of implanted cells depends on the integration of the exogenous cells, including the formation of a neural connection with the host auditory system. Coculture of exogenous neurons with hair cell explants indicates that it is possible for stem cell-derived neurons to form neural contacts with hair cells [81–83]. In an implantation study using embryonic neuronal tissue, the implanted embryonic neurons were found to extend neurites close to the SGNs located in the modiolus [68,84]. Neurite outgrowth was stimulated in the presence of exogenous NGF and chronic electrical stimulations [85]. However, this enhancement did not translate into functional significance based on electrically-evoked ABRs [85], possibly due to too few cells being integrated with the host tissue. In a recent study, human stem cell-derived neural progenitors were transplanted into gerbils that have been treated with ouabain to damage the SGNs [74]. Functional recovery was monitored weekly by measuring ABRs and distortion-product otoacoustic emissions. Approximately 30-dB sound pressure level improvements were recognized in animals implanted with human stem cell-derived neural progenitors [74], indicating the possibility of exogenous cells to recover the auditory function of deafened mammals. This report reveals the possibility of rebuilding the neural contacts between the implanted cells and host auditory system.
Conclusion
Recent advances in stem cell technology provide new approaches for the treatment of human hearing loss using stem cell-based replacement therapy. In hair cell replacement, it is generally accepted that supporting cells are the most likely source for hair cell progenitors in nonmammalian vertebrates, mammals and humans. The molecular mechanisms that are critical for the activation of hair cell progenitor identity and the proliferation and differentiation of hair cell progenitors remain undetermined. EMT and mesenchymal-to-epithelial transition have been proposed as the possible mechanisms that regulate the proliferation and differentiation of hair cell progenitors in vitro. In SGN replacement, stem cells and their derivatives have been implanted into mature mammalian inner ears. Neurogenin and neurotrophins are found to be critical for the generation of SGN-like glutamatergic neurons as a donor cell source. Other candidate genes that are fundamental to SGN development have not been tested. The ST approach is reported to cause minimal traumatic inner ear disorders. The hearing improvement in animals transplanted with human stem cell-derived neural progenitors suggests the possibility of hearing restoration via exogenous cell transplantation.
Future perspective
Developments in iPS cell technology [20,21,55,86] reveal the feasibility of using personalized human stem cell-derived neurons for the treatment of hearing loss in humans. Combined with implanted electrodes, as previously proposed [56], it would be possible to generate a Biological-EAR model, a perspective biomedical approach to treat hearing loss, to replace the function of the peripheral auditory system (Figure 1). This Biological-EAR consists of a microphone, a speech processor, a transmitter and an array of microelectrodes with iPS cell-derived neurons. In a Biological-EAR model, outside sound will be captured by a receiver, converted into electrical signals by a speech processor and conducted to implanted microelectrodes by a transmitter. The implanted microelectrodes will directly stimulate implanted iPS cell-derived neurons that have formed neural contacts with the cochlear nucleus in the brain. With the help of a Biological-EAR model, the auditory signals would be conveyed to the brain, thus bypassing the damaged hair cells and SGNs (Figure 1). Although there are critical challenges in this novel Biological-EAR model, such as tono-topic delivery of auditory frequency information to implanted cells and stimulation of implanted neurons to form precise topographic connections with the brainstem nucleus, this Biological-EAR model may provide a novel intervention option for the treatment of sensorineural hearing loss in the future. In the meantime, hair cell regeneration remains one of the most appealing approaches because an intact peripheral auditory system plays essential roles in the plasticity of the whole auditory system. Owing to the complexity of the architecture at the organ of Corti, induction of endogenous hair cell progenitors to re-enter the cell cycle and differentiate into sensory hair cells is currently one of the most intriguing approaches. Identification of the genes and intracellular pathways critical for the activation of progenitors is thus a major focus for research in this field.
Figure 1. Biological-EAR model.
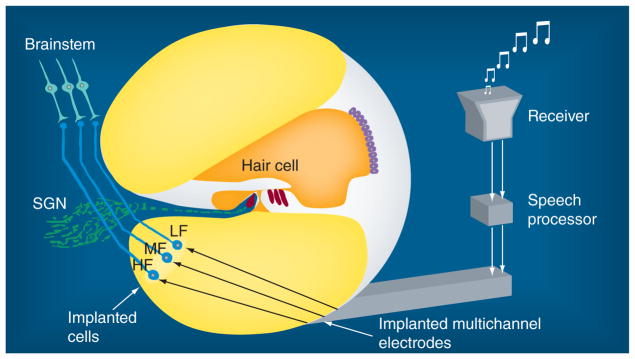
Stem cell-derived cells will replace the function of degenerated SGNs. The implanted electrodes will stimulate implanted stem cell-derived neurons to transfer auditory signals to the brainstem, thus bypassing the damaged hair cells and SGNs. The speech processor will decode the outside sound into frequency components of LFs, MFs and HFs. The implanted electrodes will be composed of multichannels that are responsible for the transduction of LFs, MFs and HFs. The implanted cells will be tonotopically organized to receive the auditory information at the corresponding frequencies, such as LFs, MFs and HFs. These implanted cells are expected to form precise topographic connections with the brainstem nucleus.
HF: High frequency; LF: Low frequency; MF: Medium frequency; SGN: Spiral ganglion neuron.
Modified with permission from [56].
Executive summary.
The challenge in hearing regeneration & stem cell opportunity
Developments in stem cell technology provide new opportunities for the treatment of hearing loss.
Identification & activation of endogenous progenitors for hearing regeneration
Stem/progenitor cells exist in the mammalian inner ear with the supporting cell population being the most likely source.
An epithelial–mesenchymal transition may be involved in the generation of prosensory-like cells from inner ear supporting cells in vitro.
Introduction of exogenous cells for hearing recovery
Exogenous growth factors, such as neurotrophins, are required for the survival of cells implanted into the adult mammalian inner ear.
The scala tympanic approach causes minimal trauma to the inner ear compared with other surgical approaches.
Future perspective
Exogenous cells have the potential to replace the function of damaged hearing cells.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This manuscript is supported by NIDCD /NIH (R03DC011597). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Belzner KA, Seal BC. Children with cochlear implants: a review of demographics and communication outcomes. Am Ann Deaf. 2009;154(3):311–333. doi: 10.1353/aad.0.0102. [DOI] [PubMed] [Google Scholar]
- 2.Colletti V, Shannon RV, Carner M, Veronese S, Colletti L. Progress in restoration of hearing with the auditory brainstem implant. Prog Brain Res. 2009;175:333–345. doi: 10.1016/S0079-6123(09)17523-4. [DOI] [PubMed] [Google Scholar]
- 3.Lim HH, Lenarz T, Joseph G, et al. Electrical stimulation of the midbrain for hearing restoration: insight into the functional organization of the human central auditory system. J Neurosci. 2007;27(49):13541–13551. doi: 10.1523/JNEUROSCI.3123-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mowry SE, Woodson E, Gantz BJ. New frontiers in cochlear implantation: acoustic plus electric hearing, hearing preservation, and more. Otolaryngol Clin North Am. 2012;45(1):187–203. doi: 10.1016/j.otc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 5▪.Clarke DL, Johansson CB, Wilbertz J, et al. Generalized potential of adult neural stem cells. Science. 2000;288(5471):1660–1663. doi: 10.1126/science.288.5471.1660. Determined the location and multipotency of adult neural stem cells. [DOI] [PubMed] [Google Scholar]
- 6▪.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. Reviewed the identification and location of mammalian neural stem cells. [DOI] [PubMed] [Google Scholar]
- 7.Martin GR, Knox L. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 9.Kriks S, Shim JW, Piao J, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480(7378):547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110(3):385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 11.Kuai XL, Cong XQ, Li XL, Xiao SD. Generation of hepatocytes from cultured mouse embryonic stem cells. Liver Transpl. 2003;9(10):1094–1099. doi: 10.1053/jlts.2003.50207. [DOI] [PubMed] [Google Scholar]
- 12.Lavon N, Yanuka O, Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004;72(5):230–238. doi: 10.1111/j.1432-0436.2004.07205002.x. [DOI] [PubMed] [Google Scholar]
- 13.Basma H, Soto-Gutierrez A, Yannam GR, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136(3):990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sachinidis A, Fleischmann BK, Kolossov E, Wartenberg M, Sauer H, Hescheler J. Cardiac specific differentiation of mouse embryonic stem cells. Cardiovasc Res. 2003;58(2):278–291. doi: 10.1016/s0008-6363(03)00248-7. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 16.Lavon N, Yanuka O, Benvenisty N. The effect of overexpression of Pdx1 and Foxa2 on the differentiation of human embryonic stem cells into pancreatic cells. Stem Cells. 2006;24(8):1923–1930. doi: 10.1634/stemcells.2005-0397. [DOI] [PubMed] [Google Scholar]
- 17.D’Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 18.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 19▪▪.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. Generation of mammalian induced pluripotent stem (iPS) cells via overexpression of four transcriptional factors. [DOI] [PubMed] [Google Scholar]
- 20▪▪.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. Generation of human iPS cells from somatic cells. [DOI] [PubMed] [Google Scholar]
- 21▪▪.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. Generation of human iPS cells from somatic cells. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan GJ, Hay DC, Park IH, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51(1):329–335. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yazawa M, Hsueh B, Jia XL, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471(7337):230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240(4860):1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 25.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240(4860):1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 26.Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51(6–7):633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- 27.Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;205(1):17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- 28.Gale JE, Meyers JR, Periasamy A, Corwin JT. Survival of bundleless hair cells and subsequent bundle replacement in the bullfrog’s saccule. J Neurobiol. 2002;50(2):81–92. doi: 10.1002/neu.10002. [DOI] [PubMed] [Google Scholar]
- 29▪.Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9(10):1293–1299. doi: 10.1038/nm925. Determined the presence of stem cells in the adult mammalian inner ear. [DOI] [PubMed] [Google Scholar]
- 30.Diensthuber M, Oshima K, Heller S. Stem/progenitor cells derived from the cochlear sensory epithelium give rise to spheres with distinct morphologies and features. J Assoc Res Otolaryngol. 2009;10(2):173–190. doi: 10.1007/s10162-009-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshima K, Grimm CM, Corrales CE, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8(1):18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪.White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–987. doi: 10.1038/nature04849. Determined the ability of purified supporting cells to transdifferentiate into hair-like cells. [DOI] [PubMed] [Google Scholar]
- 33.Kelley MW, Talreja DR, Corwin JT. Replacement of hair cells after laser microbeam irradiation in cultured organs of corti from embryonic and neonatal mice. J Neurosci. 1995;15(4):3013–3026. doi: 10.1523/JNEUROSCI.15-04-03013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen W, Johnson SL, Marcotti W, Andrews PW, Moore HD, Rivolta MN. Human fetal auditory stem cells can be expanded in vitro and differentiate into functional auditory neurons and hair cell-like cells. Stem Cells. 2009;27(5):1196–1204. doi: 10.1002/stem.62. [DOI] [PubMed] [Google Scholar]
- 35▪.Hu Z, Luo X, Zhang L, et al. Generation of human inner ear prosensory-like cells via epithelial-to-mesenchymal transition. Regen Med. 2012;7(5):663–673. doi: 10.2217/rme.12.53. Generation of prosensory-like cells from human inner ear tissues via epithelial-to-mesenchymal transition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rask-Andersen H, Bostrom M, Gerdin B, et al. Regeneration of human auditory nerve. In vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear Res. 2005;203(1–2):180–191. doi: 10.1016/j.heares.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Baguley DM, Jones S, Wilkins I, Axon PR, Moffat DA. The inhibitory effect of intravenous lidocaine infusion on tinnitus after translabyrinthine removal of vestibular schwannoma: a double-blind, placebo-controlled, crossover study. Otol Neurotol. 2005;26(2):169–176. doi: 10.1097/00129492-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Hu Z, Corwin JT. Inner ear hair cells produced in vitro by a mesenchymal-to-epithelial transition. Proc Natl Acad Sci USA. 2007;104(42):16675–16680. doi: 10.1073/pnas.0704576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warchol ME, Corwin JT. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J Neurosci. 1996;16(17):5466–5477. doi: 10.1523/JNEUROSCI.16-17-05466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259(5101):1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- 41.Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259(5101):1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Hu Z. Sensory epithelial cells acquire features of prosensory cells via epithelial to mesenchymal transition. Stem Cells Dev. 2012;21(10):1812–1821. doi: 10.1089/scd.2011.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joglekar MV, Hardikar AA. Epithelial-to-mesenchymal transition in pancreatic islet beta cells. Cell Cycle. 2010;9(20):4077–4079. doi: 10.4161/cc.9.20.13590. [DOI] [PubMed] [Google Scholar]
- 44.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Ulianich L, Garbi C, Treglia AS, et al. ER stress is associated with dedifferentiation and an epithelial-to-mesenchymal transition-like phenotype in PC Cl3 thyroid cells. J Cell Sci. 2008;121(Pt 4):477–486. doi: 10.1242/jcs.017202. [DOI] [PubMed] [Google Scholar]
- 46.Eastham AM, Spencer H, Soncin F, et al. Epithelial–mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67(23):11254–11262. doi: 10.1158/0008-5472.CAN-07-2253. [DOI] [PubMed] [Google Scholar]
- 47▪▪.Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306(5705):2261–2264. doi: 10.1126/science.1101968. Demonstrated the generation of islet precursors from human pancreata via a dedifferentiation process called epithelial-to-mesenchymal transition in vitro. [DOI] [PubMed] [Google Scholar]
- 48▪.Gallo R, Gambelli F, Gava B, et al. Generation and expansion of multipotent mesenchymal progenitor cells from cultured human pancreatic islets. Cell Death Differ. 2007;14(11):1860–1871. doi: 10.1038/sj.cdd.4402199. Determined the multipotency of mesenchymal stem cells derived from human pancreatic islets. [DOI] [PubMed] [Google Scholar]
- 49.Driver EC, Kelley MW. Specification of cell fate in the mammalian cochlea. Birth Defects Res C Embryo Today. 2009;87(3):212–221. doi: 10.1002/bdrc.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95(3):379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 51.Ellis P, Fagan BM, Magness ST, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26(2–4):148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 52.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7(11):1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 53.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 54.Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci USA. 2003;100(23):13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪.Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141(4):704–716. doi: 10.1016/j.cell.2010.03.035. Determined that iPS cell-derived otic progenitors were able to become hair-like cells in the presence of avian stromal cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hu Z, Ulfendahl M. Cell replacement therapy in the inner ear. Stem Cells Dev. 2006;15(3):449–459. doi: 10.1089/scd.2006.15.449. [DOI] [PubMed] [Google Scholar]
- 57.Coleman B, de Silva MG, Shepherd RK. Concise review: the potential of stem cells for auditory neuron generation and replacement. Stem Cells. 2007;25(11):2685–2694. doi: 10.1634/stemcells.2007-0393. [DOI] [PubMed] [Google Scholar]
- 58.Okano T, Kelley MW. Stem cell therapy for the inner ear: recent advances and future directions. Trends Amplif. 2012;16(1):4–18. doi: 10.1177/1084713812440336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1(2):129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim WY, Fritzsch B, Serls A, et al. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128(3):417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14(6):1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 62.Hu Z, Wei D, Johansson CB, et al. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 2005;302(1):40–47. doi: 10.1016/j.yexcr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 63▪.Reyes JH, O’Shea KS, Wys NL, et al. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J Neurosci. 2008;28(48):12622–12631. doi: 10.1523/JNEUROSCI.0563-08.2008. Determined that mouse embryonic stem cells were able to become auditory-like glutamatergic neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Backhouse S, Coleman B, Shepherd R. Surgical access to the mammalian cochlea for cell-based therapies. Exp Neurol. 2008;214(2):193–200. doi: 10.1016/j.expneurol.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bogaerts S, Douglas S, Corlette T, et al. Microsurgical access for cell injection into the mammalian cochlea. J Neurosci Methods. 2008;168(1):156–163. doi: 10.1016/j.jneumeth.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 66.Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti. J Neurobiol. 2006;66(13):1489–1500. doi: 10.1002/neu.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iguchi F, Nakagawa T, Tateya I, et al. Surgical techniques for cell transplantation into the mouse cochlea. Acta Otolaryngol Suppl. 2004;551:43–47. doi: 10.1080/03655230310016816. [DOI] [PubMed] [Google Scholar]
- 68.Hu Z, Olivius NP, Ulfendahl M. Survival of nervous tissue following xenograft implantation into the adult rat inner ear. Presented at. To Restore Hearing: the 36th Karolinska Institutet Nobel Conference.; Stockholm, Sweden. 9–13 June 2002. [Google Scholar]
- 69.Iguchi F, Nakagawa T, Tateya I, et al. Trophic support of mouse inner ear by neural stem cell transplantation. Neuroreport. 2003;14(1):77–80. doi: 10.1097/00001756-200301200-00015. [DOI] [PubMed] [Google Scholar]
- 70.Lang H, Schulte BA, Goddard JC, et al. Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury. J Assoc Res Otolaryngol. 2008;9(2):225–240. doi: 10.1007/s10162-008-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shukla S, Chaturvedi RK, Seth K, Roy NS, Agrawal AK. Enhanced survival and function of neural stem cells-derived dopaminergic neurons under influence of olfactory ensheathing cells in parkinsonian rats. J Neurochem. 2009;109(2):436–451. doi: 10.1111/j.1471-4159.2009.05983.x. [DOI] [PubMed] [Google Scholar]
- 72.Olstorn H, Moe MC, Roste GK, Bueters T, Langmoen IA. Transplantation of stem cells from the adult human brain to the adult rat brain. Neurosurgery. 2007;60(6):1089–1098. doi: 10.1227/01.NEU.0000255461.91892.0D. discussion 1098–1089. [DOI] [PubMed] [Google Scholar]
- 73.Mothe AJ, Kulbatski I, Parr A, Mohareb M, Tator CH. Adult spinal cord stem/progenitor cells transplanted as neurospheres preferentially differentiate into oligodendrocytes in the adult rat spinal cord. Cell Transplant. 2008;17(7):735–751. doi: 10.3727/096368908786516756. [DOI] [PubMed] [Google Scholar]
- 74▪▪.Chen W, Jongkamonwiwat N, Abbas L, et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490(7419):278–282. doi: 10.1038/nature11415. Investigated the hearing function following implantation of human embryonic stem cell-derived otic progenitors into the inner ear of adult mammals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L, Jiang H, Hu Z. Concentration-dependent effect of NGF on cell fate determination of neural progenitors. Stem Cells Dev. 2011;20:1723–1731. doi: 10.1089/scd.2010.0370. [DOI] [PubMed] [Google Scholar]
- 76.Praetorius M, Vicario I, Schimmang T. Efficient transfer of embryonic stem cells into the cochlea via a non-invasive vestibular route. Acta Otolaryngol. 2008;128(7):720–723. doi: 10.1080/00016480701714236. [DOI] [PubMed] [Google Scholar]
- 77.Hu Z, Ulfendahl M, Olivius NP. NGF stimulates extensive neurite outgrowth from implanted dorsal root ganglion neurons following transplantation into the adult rat inner ear. Neurobiol Dis. 2005;18(1):184–192. doi: 10.1016/j.nbd.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 78.Hu Z, Andang M, Ni D, Ulfendahl M. Neural cograft stimulates the survival and differentiation of embryonic stem cells in the adult mammalian auditory system. Brain Res. 2005;1051(1–2):137–144. doi: 10.1016/j.brainres.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 79.Coleman B, Hardman J, Coco A, et al. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Transplant. 2006;15(5):369–380. doi: 10.3727/000000006783981819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Regala C, Duan M, Zou J, Salminen M, Olivius P. Xenografted fetal dorsal root ganglion, embryonic stem cell and adult neural stem cell survival following implantation into the adult vestibulocochlear nerve. Exp Neurol. 2005;193(2):326–333. doi: 10.1016/j.expneurol.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 81.Martinez-Monedero R, Yi E, Oshima K, Glowatzki E, Edge AS. Differentiation of inner ear stem cells to functional sensory neurons. Dev Neurobiol. 2008;68(5):669–684. doi: 10.1002/dneu.20616. [DOI] [PubMed] [Google Scholar]
- 82.Shi F, Corrales CE, Liberman MC, Edge AS. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur J Neurosci. 2007;26(11):3016–3023. doi: 10.1111/j.1460-9568.2007.05909.x. [DOI] [PubMed] [Google Scholar]
- 83.Matsumoto M, Nakagawa T, Kojima K, Sakamoto T, Fujiyama F, Ito J. Potential of embryonic stem cell-derived neurons for synapse formation with auditory hair cells. J Neurosci Res. 2008;86(14):3075–3085. doi: 10.1002/jnr.21754. [DOI] [PubMed] [Google Scholar]
- 84.Hu Z, Ulfendahl M, Olivius NP. Survival of neuronal tissue following xenograft implantation into the adult rat inner ear. Exp Neurol. 2004;185(1):7–14. doi: 10.1016/j.expneurol.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 85.Hu Z, Ulfendahl M, Prieskorn DM, Olivius P, Miller JM. Functional evaluation of a cell replacement therapy in the inner ear. Otol Neurotol. 2009;30(4):551–558. doi: 10.1097/MAO.0b013e31819fe70a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nishimura K, Nakagawa T, Ono K, et al. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport. 2009;20(14):1250–1254. doi: 10.1097/WNR.0b013e32832ff287. [DOI] [PubMed] [Google Scholar]
Website
- 101.US NIDCD. Fact Sheet: Cochlear Implant. www.nidcd.nih.gov/staticresources/health/hearing/FactSheetCochlearImplant.pdf.


