Abstract
Although many previous investigations have studied how mercury compounds cause cell death, sub-cytotoxic levels may affect mechanisms essential for the proper development of the nervous system. The present study investigates whether low doses of methylmercury (MeHg) and mercury chloride (HgCl2) can modulate the activity of JAK/STAT signaling, a pathway that promotes gliogenesis. We report that sub-cytotoxic doses of MeHg enhance ciliary neurotrophic factor (CNTF) evoked STAT3 phosphorylation in human SH-SY5Y neuroblastoma and mouse cortical neural progenitor cells (NPCs). This effect is specific for MeHg, since HgCl2 fails to enhance JAK/STAT signaling. Exposing NPCs to these low doses of MeHg (30-300 nM) enhances CNTF-induced expression of STAT3-target genes such as glial fibrillary acidic protein (GFAP) and suppressors of cytokine signaling 3 (SOCS3), and increases the proportion of cells expressing GFAP following two days of differentiation. Higher, near-cytotoxic concentrations of MeHg and HgCl2 inhibit STAT3 phosphorylation and lead to increased production of superoxide. Lower concentrations of MeHg effective in enhancing JAK/STAT signaling (30 nM) do not result in a detectable increase in superoxide nor increased expression of the oxidant-responsive genes, heme oxygenase 1, heat shock protein A5 and sirtuin 1. These findings suggest that low concentrations of MeHg inappropriately enhance STAT3 phosphorylation and glial differentiation, and that the mechanism causing this enhancement is distinct from the reactive oxygen species -associated cell death observed at higher concentrations of MeHg and HgCl2.
Keywords: methylmercury, GFAP, neural progenitor cells, STAT3, gliogenesis, developmental neurotoxicity
1. Introduction
Despite a growing appreciation of the dangers posed by low levels of methylmercury, the pertinent effects on brain development are unclear. Much of our understanding of methylmercury's (MeHg) toxic effects comes from observations of relatively high dose exposures, notably in the cases of widespread poisonings in Minimata, Japan, and in Iraq (Gilbert and Grant-Webster, 1995; Harada, 1978; Marsh et al., 1980). Even recently, most in vitro investigations have employed doses that model the profound developmental neurotoxicity found in these severe cases of poisoning. This has established a large body of evidence linking micromolar concentrations of MeHg to elevated levels of reactive oxygen species (ROS) and subsequent cell-death in cells of central nervous system origin (Kaur et al., 2009; 2010; Kaur et al., 2008; Kim et al., 2005; Lu et al., 2011; Rush et al., 2012; Shanker et al., 2004; Shanker and Aschner, 2003; Shanker et al., 2005; Yin et al., 2007). In contrast, epidemiological studies have correlated exposure to much lower levels of MeHg with general disturbances of cognition in domains such as language, perception, attention, and motor coordination (Grandjean et al., 1997; Kjellstrom et al., 1986-1989; NRC, 2000; Oken et al., 2005; Sanfeliu et al., 2003; Trasande et al., 2005). Similar effects have been observed in rodent models of in utero exposure to MeHg (Montgomery et al., 2008; Onishchenko et al., 2008; Onishchenko et al., 2007; Stringari et al., 2006).
At these lower doses, the relevant toxic mechanisms of MeHg are less clear. Alterations in neural progenitor biology and imbalances in their signaling are both hypothesized to underlie developmental disorders that negatively affect cognition (Barone et al., 2000; Deverman and Patterson, 2009; Meyer et al., 2006; Patterson, 2009). Experiments examining CNS histology have produced evidence for altered glial cell distributions and astrocyte hypertrophy in absence of cell death (Barone et al., 1998; Kakita et al., 2002; Kakita et al., 2003; Vicente, 2004). Moreover, several studies suggest that MeHg interacts with cell signaling-cascades involved in astrocyte differentiation (Buzanska et al., 2009; Li et al., 2007; Tamm et al., 2008). Despite these observations, the effects of Hg-compounds on glial differentiation of neural progenitors are currently not well understood.
Neural precursor cells (NPC) differentiate into astrocytes during the third trimester of human development, corresponding with the perinatal and early postnatal developmental stage in rodents (Coluccia et al., 2007; Rowitch, 2004; Weir et al., 1984). Astrocyte induction is promoted by activation of the JAK/STAT signaling pathway (Bonni et al., 1997; Fan, 2005; Li and Grumet, 2007), which is stimulated when ligand cytokines bind to receptor complexes that contain the gp130 transmembrane protein, resulting in the activation of cytoplasmic Janus kinases (JAKs). JAKs then phosphorylate signal transducers and activators of transcription (STATs), which dimerize/multimerize and translocate to the nucleus where they bind to consensus regions in the promoters of a variety of target genes (Brierley and Fish, 2005; Heinrich et al., 1998) that include glial-fibrilary acid protein (GFAP), a marker for astrocyte-like cells (Maier et al., 2002).
Previously, Halvorsen and colleagues have shown JAK/STAT signaling is inhibited by relatively high doses of mercury chloride and cadmium, and that this involves increased oxidative stress (Kaur et al., 2005; Monroe and Halvorsen, 2006a; b; 2009). However, the role of JAK/STAT in MeHg toxicity, especially at very low MeHg levels, as well as potential associated oxidative stress, and their relative contributions to disrupting glial progenitor differentiation remain unknown.
The present study investigates the effect of different doses of MeHg and HgCl2 on STAT3 signaling and expression of relevant target-genes in vitro, employing two cell types often used as models for nervous system development. We demonstrate that low doses of MeHg increase STAT3 phosphorylation induced by ciliary neurotrophic factor (CNTF) treatment and enhance the differentiation of NPCs into GFAP expressing glial cells. Additionally, we show this occurs at doses where increases in ROS are absent or negligible.
2. Materials and Methods
2.1. Cell Culture
2.1.1. Cell lines
The human SH-SY5Y neuroblastoma cell line (Biedler et al., 1973), a gift from Dr. R. Nishi (UVM), was grown in Liebowitz (L)15-CO2 media (Sigma, St. Louis, MO) containing penicillin, streptomycin, and additional glucose and supplemented with 10% (vol./vol.) fetal calf serum (Atlanta Biologicals, Lawrenceville, GA). Mouse neural progenitor cells (NPC) were obtained from newborn C57/BL6 mouse cerebral cortex using standard methods (Jacqueline et al., 2006), and grown in Neurobasal medium (Invitrogen, Grand Isle, NY) containing penicillin, streptomycin, and additional glucose, EGF (20 ng/mL), bFGF (20 ng/mL) and B27. For treatments, SH-SY5Y cells were plated at 1.25 ×106 cells/mL in multi-well tissue culture plates (BD Biosciences, San Jose, CA) and allowed to recover 24 h. NPCs were dissociated from neurospheres (NeuroCult kit; Stemcell Technologies, Vancouver, BC), plated onto poly- [D] lysine/laminin coated plates at 150,000 cells/mL and were then expanded 24 h to a density of roughly ∼250 cells/mm2 or 70-80% confluence.
2.1.2. Treatments
Prior to drug exposure, cells were washed and placed in either serum free L15-CO2 media (SH-SY5Y) or Neurobasal medium without EGF/bFGF (NPC) for 1 h before treatment with different concentrations of toxicant in the same media conditions for 5 h. Methylmercury chloride and mercury (II) chloride (Sigma) were made as 100 mM stocks in DMSO (Fisher, Pittsburg, PA), and were diluted in DMSO such that the final concentration of DMSO for all cell culture conditions and controls = 0.1%. For western blot, qRT2PCR, and fluorescence microscopy experiments, this was followed by stimulation with rat recombinant CNTF (final concentration= 20 ng/mL, a gift from Dr. R. Nishi), which was dissolved in Modified Puck's Glucose (MPG) and added directly to the well without any washing steps. Cells were harvested after 30 min for western blot analysis of STAT3 phosphorylation, after 12 h for qRT2PCR analysis of mRNA levels, and after 48 h for GFAP immunocytochemistry and western blots.
2.2. MTT Cell Viability Assay
Cell survival was determined by the well established MTT (3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide, Sigma) assay (Mosmann, 1983). Briefly, MTT in PBS was added to the cell culture media after 5 h incubation with toxicant (final concentration = 0.35 mg/mL) and plates were returned to the incubator for 2 h. Formazan crystals were then solubilized, measured by absorbance at 570 nm and viability was calculated as (absorbance of toxicant treated – absorbance of blank) / (absorbance of vehicle treated– absorbance of blank) * 100%.
2.3. Western Blots
After treatment with mercury and CNTF, cells were washed with ice-cold PBS and solubilized in cold lysis buffer, (150 mM NaCl, 50 mM Tris, 1% P-40) containing freshly added protease/phosphatase inhibitors (5 μg/mL aproponin, leupeptin and pepstatin. 0.1 mM sodium orthovanadate, 0.5 mM phenylmethyl sulfonyl fluoride). This was followed by the addition of a 6X SDS Buffer (50 mM Tris, pH 6.8, 10% glycerol, 1.25% beta-mercaptoethanol, 0.1% bromophenol blue), after which samples were boiled for 5 min and stored at -20°C. Proteins were separated by standard SDS-polyacrylamide electrophoresis and blotted onto PVDF membranes (Bio-Rad). Membranes were blocked by incubation in Aquablock (East Coast Bio, North Berwick, ME) and incubated in primary antibodies (diluted in Aquablock containing 0.1% Tween-20, 0.02% sodium azide) specific for either Y705 phosphorylated STAT3 (M9C6, 1:1000, Cell Signaling Technology), total STAT3 (124H6, 1:1000, Cell Signaling Technology), GFAP (RPCA-GFAP, 1:2000 Encore Biotech, Alachua, FL), or beta-actin (“JCA20” 1:400, Santa Cruz Biotechnology).
Bound antibodies were detected by incubation with either an Alexa 680 conjugated goat anti-mouse antibody (1:5000, Invitrogen) or an IR Dye 800 conjugated donkey anti-rabbit antibody (1:5000 Rockland Inc., Gilbertsville, PA). Blots were visualized using an Odyssey Imager (Li-Cor, Lincoln, NE). Individual bands were scored for integrated density less background using the Odyssey imaging software. For phospho-STAT experiments values were normalized to total STAT3 levels or to β actin levels. GFAP values were normalized to β actin.
2.4. qRT2PCR Measurements
Quantitative real-time reverse transcriptase polymerase chain reaction (qRT2PCR) was used to obtain measurements of relative mRNA abundance. Total RNA was prepared from cells grown in 6-well plates by an acid guanidinium column-based method (RNeasy, QIA-shredder, Qiagen), followed by synthesis of cDNA using oligo dT20 and SuperscriptIII reverse transcriptase (Invitrogen). The quality of the cDNA was assured using an established qRT2PCR protocol employing primers to amplify beta-actin cDNA, with the requirement that 0.2 μg of starting cDNA result in threshold detection of the beta-actin signal below 20 cycles of amplification. The SYBR-green qRT2PCR method was used for measuring gene expression and levels were determined by the deltaCt method, using beta-actin as a housekeeping gene and were expressed in ppm relative to actin. The following primers were supplied by Operon (Huntsville, AL):
β-Actin (+) 5′-TATTGGCAACGAGCGGTTCC-3′
β-Actin (-) 5′-GGCATAGAGGTCTTTACGGATGTC-3′
βIII-tubulin (+) 5′-TTCTGGTGGACTTGGAACCTGG-3′
βIII-tubulin (-) 5′-TTCCGCACGACATCTAGGACTG-3′
GFAP (+) 5′-CGCTCAATGCTGGCTTCAAG-3′
GFAP (-) 5′-AAAGTTGTCCCTCTCCACCTCC-3′
Nestin (+) 5′-GCTGGAACAGAGATTGGAAGGC-3′
Nestin (-) 5′-TAGACCCTGCTTCTCCTGCTCC-3′
SOCS3 (+) 5′-CCCCCAGAAGAGCCTATTACA-3′
SOCS3 (-) 5′-ACGGTCTTCCGACAGAGATG-3′
Sirt1 (+) 5′-GATGACGATGACAGAACGTCACA-3′
Sirt1 (-) 5′-GGATCGGTGCCAATCATGAG-3′ (Prozorovski et al., 2008)
HO-1 (+) 5′-TACACATCCAAGCCGAGAAT-3′
HO-1 (-) 5′-GTTCCTCTGTCAGCATCACC-3′(Kraft et al., 2004)
HPSA5 (+) 5′-ACACTTGGTATTGAAACTGTGGGAG-3′
HPSA5 (-) 5′-GATTGTCTTTTGTTAGGGGTCGTTC-3′
2.5. Immunofluorescence
NPCs grown as described above were fixed for 20 min in PBS containing 4% (w/v) paraformaldehyde and 15% saturated picric acid. GFAP immunoreactivity was determined by overnight incubation in primary antibody (1:2000, Encor, Gainesville, FL), followed by incubation for 2 h in Cy-3 labeled anti-rabbit antibody (1:5000, Jackson Immunoresearch Laboratories West Grove, PA) and 15 min 1:500 Hoechst 34580 (Invitrogen). Labeled cells were visualized using a C1 Eclipse confocal microscope (Nikon USA, Melville, NY). Three random fields of view (each representing an 620 μm × 620 μm area) were photographed from each condition and cell counts were performed using ImageJ software. The data were normalized to account for differences in label intensity between experimental replicates collected at different times.
2.6. Nitroblue tetrazolium assay
NBT is a yellow water-soluble salt that reacts with superoxide to generate a blue insoluble formazan, providing a spectrophotometric assay of superoxide and related ROS (Mukherjee et al., 2005; Zhang et al., 2007). Cells on 24-well plates were serum (SH-SY5Y) or growth factor (NPC) starved for 1 h prior to being incubated with toxicants for 5 h as described above. Menadione (2-methyl-1,4-naphthoquinone), a positive control for superoxide production (Criddle et al., 2006), was added 20 min before NBT. Cells were washed in HBSS Hank's Buffered Salt Solution (HBSS), and incubated in 1.2 mg/mL Nitroblue tetrazolium (Sigma) in HBSS at 37°C for 45 min, washed, fixed in 100% methanol for 20 min at RT, air dried for 3 min, and lysed by adding 214 μL 2M KOH and 186 μL DMSO. Absorbance was read on a Synergy H3 (Biotek) at 630 nM and normalized to viability estimated from cell counting after fixation.
2.7. Statistical analysis
The statistical analyses of the data were performed using GraphPad Prism (Graph Pad Software Inc., Sand Diego, CA), unless otherwise stated experiments were conducted in at least independent triplicate and results are given and means +/- standard error of the mean. Where appropriate, differences between groups were analyzed with One-way ANOVA, followed by Bonferroni's post-hoc test. Statistical significance was accepted as p < 0.05. LC50 values were calculated from Prism curve-fits using a four-parameter logistic function. Values given are mean +/- SEM unless otherwise stated.
3. Results
3.1. Methylmercury is more toxic than mercury chloride in SH-SY5Y cells and NPCs
We selected the SH-SY5Y neuroblastoma cell line since it has been used extensively for investigations of metal toxicity on nervous system derived cells (Chen et al., 2008; Kaur et al., 2005; Petroni et al., 2011; Posser et al., 2010; Toimela and Tahti, 2004). NPCs represent a non-transformed cell type that can differentiate into either neurons, astrocytes or oligodendrocytes, the three main cell types of the nervous system (Alvarez-Buylla et al., 2001; Götz and Huttner, 2005; Guillemot, 2007), making them highly suitable to developmental neurotoxicity investigations (Breier et al., 2010; Breier et al., 2008; Moors et al., 2009; Tamm et al., 2006; Theunissen et al., 2010; Watanabe et al., 2009).
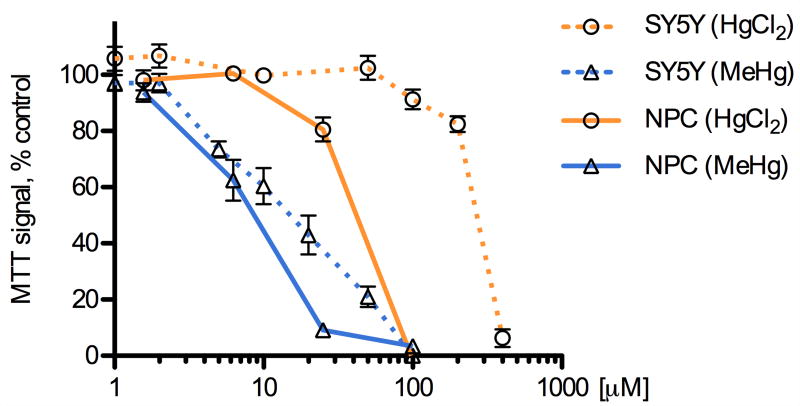
We compared the potency of methylmercury (MeHg) and mercury chloride (HgCl2) on the viability of the two cell types using a methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay of cell survival, which has been used previously to characterize the cytotoxicity of mercury compounds (Buzanska et al., 2009; Monroe and Halvorsen, 2006b; Xu et al., 2010). Mouse NPCs and human SH-SY5Y neuroblastoma cells were exposed to HgCl2 or MeHg for 5 h and dose-dependent effects on cell viability were measured (Fig. 1, average of triplicate wells, 3-7 independent determinations). Non-Linear regression curve fits were used to determine the LC50 values for MeHg and HgCl2. These determinations indicated that NPCs are significantly more sensitive than SH-SY5Y cells to both MeHg (LC50: 6.5 μM vs. 19.8 μM) and HgCl2 (LC50: 53.4 μM vs. 268 μM), with MeHg being between three- to five-fold more toxic than HgCl2. These data are in agreement with previous studies that compared these mercury species (Franco et al., 2009; Kim et al., 2007; Monnet-Tschudi et al., 1996; Mundy et al., 2010; Toimela and Tahti, 2004), and that compared NPCs to SH-SY5Y or other tumor lines (Li et al., 2007; Sanfeliu et al., 2001; Tamm et al., 2006; Watanabe et al., 2009).
Fig. 1.
MeHg and HgCl2 cause dose-dependent decreases in cell viability. SH-SY5Y cells and NPCs were exposed to various concentrations of methylmercury (MeHg), mercury chloride (HgCl2) or vehicle (DMSO) for 5 h, followed by an MTT assay to determine mitochondrial activity as a proxy for cell viability. MeHg was more potent than HgCl2 at eliciting cell death in both cell types and NPCs were more sensitive than SH-SY5Y cells to the toxic effects of either compound. Results are given as mean +/- SEM, vehicle control = 100%, from at least 3 independent replicates per condition.
Microscopic examination of the cells showed morphological correlates of toxicity (vacuolization, loss of cellular processes) at doses corresponding to the LC50 values established by the MTT assay. In addition, calceinAM/ethidium homodimer co-labeling was performed and results confirmed the relative differences in toxicity revealed by the MTT assay (data not shown).
3.2. Methylmercury enhances CNTF evoked STAT3 phosphorylation in SH-SY5Y cells and NPCs
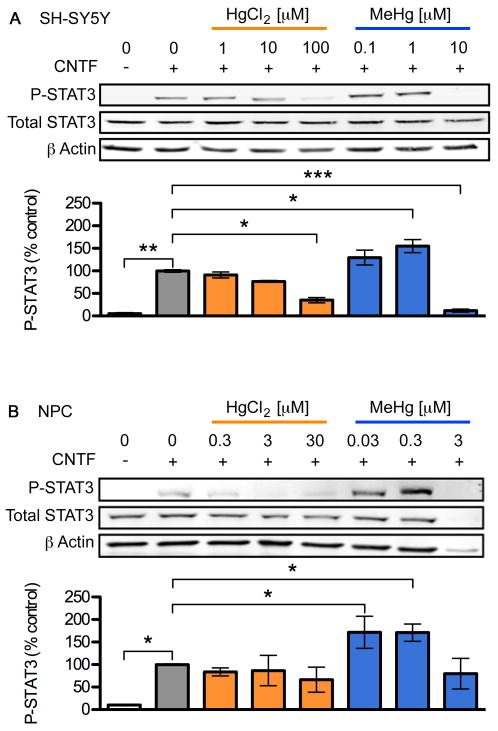
Previous reports indicate that JAK/STAT signaling is inhibited by various toxic metal species including HgCl2 (Monroe and Halvorsen, 2006a; b). To determine whether MeHg affects JAK/STAT in a similar manner to HgCl2 we quantified the effect of both toxicants on CNTF-mediated activation of STAT3 using a similar paradigm (Kaur et al., 2005; Monroe and Halvorsen, 2006a; b). We focused on STAT3 because of its crucial role in the generation of astrocytes in later stages of neurogenesis (Bauer et al., 2007). Using phospho-STAT3 specific antibodies we determined levels of STAT3 phosphorylated at amino acid Y705 30 min after addition of CNTF, corresponding to the time when maximal activation occurs (Kaur et al., 2002). Treatment with MeHg or HgCl2 alone did not elicit STAT3 Y705 phosphorylation, in either cell-type at any of the doses tested (data not shown). Treatment with 20 ng/mL CNTF alone for 30 min elicited a significant increase in STAT3 phosphorylation in both cell types (about 20-fold in SH-SY5Y and 10-fold in NPCs, Fig. 2). Unexpectedly, low concentrations of MeHg, but not HgCl2, further enhanced CNTF-induced STAT3 phosphorylation in both cell types to about 155% in SH-SY5Y and 170% in NPCs (3-5 independent replicates, p < 0.05). However, the maximum enhancement of phosphorylation was observed at different MeHg concentrations for the two cell types: approximately 1 μM for SH-SY5Y cells and 100 nM for NPCs (Fig. 2, and data not shown). Higher concentrations of MeHg and HgCl2 reduced STAT3 phosphorylation in both cell types, as had been previously shown for HgCl2 in a neuroblastoma cell line (Monroe and Halvorsen, 2006b).
Fig. 2.
Low dose MeHg enhances CNTF-evoked STAT3-phosphorylation. SH-SY5Y cells (A) and NPCs (B) were stimulated with 20 ng/mL CNTF for 30 min following 5 h toxicant exposure. P-Y705 STAT3, Total STAT3, and β actin levels in whole cell lysates were determined using western blots (upper three rows in each panel). The extent of STAT3 phosphorylation was quantified by densitometry, normalized to total STAT3 level and standardized to the value observed in cells treated with CNTF alone (bar graphs). HgCl2 inhibited STAT3 phosphorylation by CNTF whereas STAT3 phosphorylation was significantly enhanced 155-170% by ⎕ ⎕ ⎕ ⎕ ⎕̣ ⎕ ⎕ ⎕ ⎕ ⎕̣ ⎕ ⎕ MeHg and inhibited at higher doses. Data are mean +/- SEM from at least 3 independent replicates per condition. * = p < 0.05, ** = p < 0.01 significantly different from positive control by ANOVA and post hoc Bonferroni's test.
3.3. Methylmercury enhances CNTF evoked JAK/STAT target gene expression in NPCs
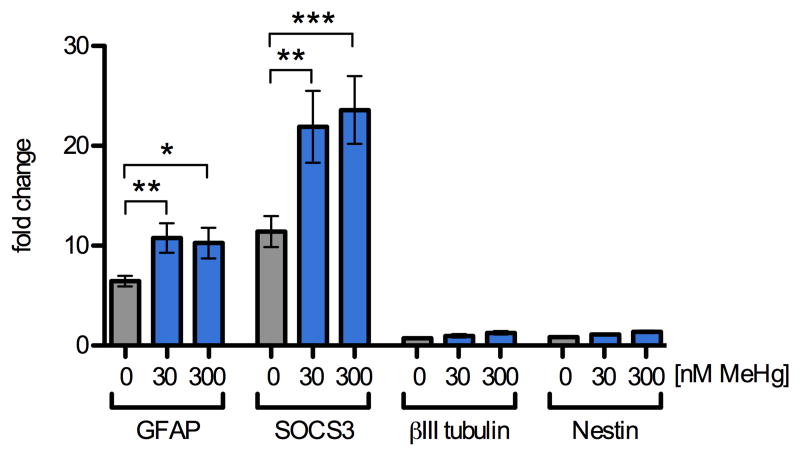
Because transient activation of signaling pathways can lead to lasting changes in gene expression, we determined whether MeHg-enhanced STAT3 phosphorylation correlated with increases in the mRNA expression of JAK/STAT target and lineage marker genes using quantitative reverse transcription real-time polymerase chain reaction (qRT2PCR). GFAP expression is commonly used as a marker for astrocytes, although immature and uncommitted NPCs can express GFAP under some conditions. The promoter of this target gene contains a STAT-response element, and GFAP expression is upregulated upon commitment to astrocyte-lineage (Freeman, 2010; Zerlin et al., 1995). We focused our investigations on NPCs since these cells showed changes in JAK/STAT target genes GFAP and SOCS3 (Cao et al., 2006; Krebs and Hilton, 2000), following 12 h CNTF treatment (Fig. 3), while these mRNAs were not detectable in SY5Y cells. GFAP mRNA was increased 6-fold by CNTF treatment alone and the presence of 30 and 300 nM MeHg further enhanced CNTF-induced GFAP-expression to 167% and 158% in mRNA abundance over stimulation with CNTF alone (6 independent replicates, p < 0.01 and p < 0.05). SOCS3 mRNA increased 11-fold after 12 h treatment with CNTF alone and the presence of 30 and 300 nM MeHg further enhanced CNTF-induced SOCS3 expression to 192% and 206% over stimulation with CNTF alone (6 independent replicates, p < 0.01 and p < 0.001). The levels of betaIII-tubulin mRNA (a marker for immature neurons) and nestin mRNA (a marker for non-differentiated NPCs) were not affected by MeHg and CNTF together or independently, suggesting that the effect of low doses of MeHg on GFAP and SOCS3 expression is selective and correlates in extent with the observed increase in STAT3 phosphorylation.
Fig. 3.
MeHg enhances STAT3 target gene expression. Levels of mRNA transcripts were assessed by qRT2PCR in NPCs pretreated for 5 h with 0, 30, or 300 nM MeHg followed by stimulation with CNTF (20 ng/mL) or vehicle for 12 h. Expression levels were normalized to beta-actin expression and standardized to expression levels observed in vehicle treated samples that were not exposed to CNTF. In vehicle treated cultures, CNTF alone caused a 6-fold and 11-fold increase in GFAP and SOCS3 mRNAs, respectively. Exposure to 30 and 300 nM MeHg in the presence of CNTF led to a further increase in GFAP and SOCS3 mRNA expression levels to approximately 10- and 22-fold, respectively. The expression levels of βIII-tubulin, a marker for immature neurons, and Nestin, a marker for uncommitted progenitors, were not significantly altered by CNTF or MeHg treatment. Results are mean fold-change from untreated control +/- SEM from at least 3 independent replicates per condition. * = p < 0.05, ** = p < 0.01 significantly different from positive control by ANOVA and post hoc Bonferroni's test. No changes in any of the genes were observed when NPC were treated with MeHg alone (not shown).
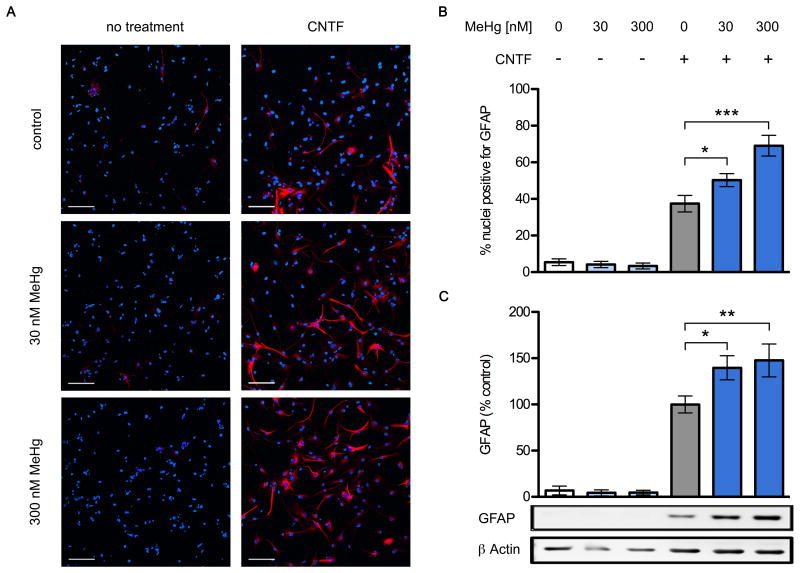
Exposing NPCs for 2 days to MeHg and CNTF also resulted in increased expression of GFAP protein. NPC cultures treated with CNTF alone contained 4.5 times more GFAP-immunoreactive cells than control cultures (Fig. 4 A,B) and the added presence of 30 nM and 300 nM MeHg enhanced the effect to 134% and 160% over the numbers seen in CNTF alone (6 independent replicates, p < 0.05 and p < 0.001). The cellular processes of GFAP-positive cells grown in the presence of CNTF and MeHg exhibited an elaborate branching pattern reminiscent of astrocytes, while cells grown without the toxicant had thinner and less complex processes. NPC cultures grown without CNTF contained only few weakly GFAP+ cells, and exposure to MeHg alone had no noticeable effect. The average number of nuclei per condition was unaffected by any of the treatments (data not shown). In addition, NPC cultures exposed to CNTF alone for 2 days and analyzed by western blot contained about 15-fold higher levels of GFAP than control cultures (Fig. 4 C) and the added presence of 30 nM and 300 nM MeHg further enhanced GFAP protein levels to 139% and 148% over the levels seen with CNTF alone (6 independent replicates, p < 0.05 and p < 0.01).
(COLOR FOR WEB) Fig. 4.
Chronic exposure to low level MeHg enhances glial differentiation. NPCs were grown for two days with or without 20 ng/mL CNTF, in the presence of 30 or 300 nM MeHg or vehicle. Cultures were fixed and GFAP expressing cells were identified using immunofluorescence. Representative images (A), GFAP: red, Hoechst 34580: blue. CNTF increased the proportion of GFAP+ cells, and this was enhanced in the 30nM and 300nM conditions. Also note the more elaborate branching of stained cellular processes in the presence of MeHg (scale bar = 100 μm). Percentage of nuclei expressing GFAP (B), 6 independent experiments, mean +/- SEM. * = p < 0.05, ** = p < 0.01, *** = p < 0.001 significantly different from positive control by ANOVA w/Bonferroni's test. GFAP and β actin protein levels in whole cell lysates were determined using western blots (C). GFAP was quantified by densitometry, normalized to β actin levels, and expressed as percent change from positive control. MeHg significantly increased GFAP protein content in the CNTF-treated conditions, reaching 139 and 148% at doses of 30nM and 300nM, respectively. No effects on GFAP levels were observed in cells not treated with CNTF, regardless of MeHg treatment. Data are mean +/- SEM from 6 independent experiments. * = p < 0.05, ** = p < 0.01 significantly different from positive control by ANOVA w/Bonferroni's test.
3.4. Low concentrations of MeHg fail to increase superoxide in SH-SY5Y and NPC
Superoxide, O2·-, is a ROS essential to normal cell physiology is that is generated from numerous sources by one-electron reduction of O2. When produced in excessive amounts, superoxide is able to react and form a cascade of more harmful ROS species such as hydrogen peroxide, peroxynitrite, and hydroxyl radicals (Buettner, 2011; Farina et al., 2011). Elevated production of superoxide as been associated with methylmercury toxicity in cell culture (Naganuma et al., 1998; Sarafian et al., 1994; Shanker et al., 2004; Shanker and Aschner, 2003), and in vivo (Mori et al., 2007). HgCl2 toxicity correlates with enhanced superoxide production in organ cultured arteries (Furieri et al., 2011) and its derivative, peroxynitrite mediates JAK/STAT inhibition by HgCl2 (Kaur et al., 2005; Monroe and Halvorsen, 2006a; b; 2009).
These reports led us to question the involvement of ROS in our system. Accordingly, we quantified superoxide abundance resulting from 5 h toxicant exposure using a nitroblue tetrazolium assay (Fig. 5, avg. of three measurements per condition, 3-6 independent experiments). Menadione was added to untreated cells for 20 min as a positive control. As expected, higher and cytotoxic concentrations of MeHg and HgCl2 significantly increased superoxide production in both SH-SY5Y (p < 0.05 at 100 μM HgCl2, 10 μM MeHg, Fig. 5 A) and NPCs (p < 0.05 at 100 μM HgCl2, 3-10 μM MeHg, Fig. 5 B), with MeHg being more effective than HgCl2 at generating superoxide. Importantly, MeHg enhanced superoxide production only at concentrations of more than 10-fold higher than those that lead to increased STAT3 phosphorylation by CNTF.
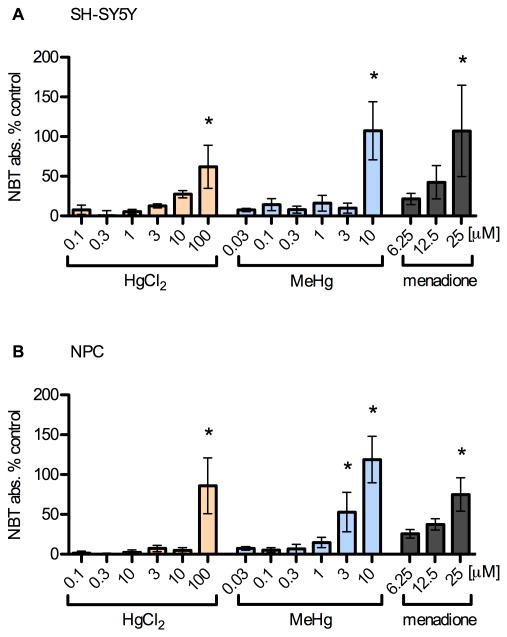
Fig. 5.
Mercury species increase superoxide production at high concentrations. SH-SY5Y (A) and NPC (B) cells were incubated with toxicants for 5 h as previously described. Conversion of NBT to formazan by superoxide was determined by absorbance normalized to cell viability. Results are mean % change from vehicle control +/- SEM from 4 independent experiments. * = p < 0.05 significantly different from positive control by ANOVA w/Bonferroni's test. High doses of MeHg (< 3 μM) and HgCl2 (100 μM) lead to significant increases in NBT signal as did the positive control, menadione (25 μM). Superoxide production was not increased in the dose range of the enhanced STAT3 effect in either cell type.
These observations demonstrate that the significant levels of superoxide generated in cells after exposure to near cytotoxic levels of MeHg are not involved in enhancing CNTF signaling, in fact these high levels likely inhibit STAT3 phosphorylation (Kaur et al., 2005; Monroe and Halvorsen, 2006b; 2009). As was shown in these studies, direct ROS stimulation with hydrogen peroxide inhibited CNTF-evoked STAT3 phosphorylation in SH-SY5Y cells and NPC (data not shown).
3.5. Oxidant-sensitive genes do not change in response to 30 nM MeHg
To extend the NBT assay results and rule out ROS involvement at a later time point, we assessed oxidative-stress at 12 h by quantifying mRNA transcripts of genes in ROS-response pathways. Nuclear factor E2-related factor 2 (Nrf2) is a transcription factor involved in cellular responses to oxidative stress, electrophile challenge, and diminished reducing capacity (Nguyen et al., 2009). By binding to antioxidant response elements (AREs), Nrf2 enhances transcription of genes involved in maintaining redox homeostasis.
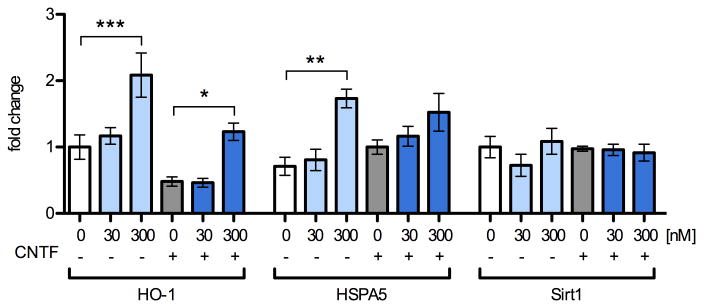
We quantified levels of the ARE-containing genes, heme oxygenase 1 (HO-1) and heat shock protein A5 (HSPA5) in the same NPC cDNA samples as in Fig. 3 by qRT2PCR (Fig. 6, 5 independent replicates). When compared to their respective controls, none of these genes responded to 30 nM MeHg treatment, indicating a lack of ROS response. HO-1 and HSPA5 transcripts were increased by 300 nM MeHg treatment, with HO-1 significantly upregulated 2.1-fold (p < 0.001) and 2.5-fold (p < 0.05) in the presence and absence of CNTF, respectively. HSPA5 was enhanced 1.9-fold (p < 0.01) by 300 nM in the no-CNTF condition only.
Fig. 6.
Oxidant-response gene-expression is unaffected by treatment with 30 nM MeHg and increases subtly with 300 nM. Levels of mRNA transcripts were assessed by qRT2PCR in NPCs pretreated for 5 h with 0, 30, or 300 nM MeHg with or without subsequent stimulation with CNTF (20 ng/mL) or vehicle for 12 h. Expression levels were normalized to beta-actin expression and expressed in fold-change from vehicle treated samples that were not exposed to CNTF. 300 nM MeHg increased HO-1 expression 2.1 and 2.6 –fold in control and CNTF-treated conditions, respectively. HSPA5 was significantly increased 1.9–fold when cells were treated with 300 nM in the absence of CNTF. Sirt1 expression was not changed under any of the conditions tested. Data are +/- SEM n=5. * = p < 0.05, ** = p < 0.01, *** = p < 0.001 significantly different from positive control by ANOVA and post hoc Bonferroni's test.
Nrf2 mediates a ubiquitous cellular response to oxidative challenge; however, NPC fate may be especially sensitive to redox status. Sirtuin 1 (Sirt1) is a protein deacetylase whose transcriptional regulation governs redox-dependent fate choices in NPC (Prozorovski et al., 2008). We found that Sirt1 expression did not change under any of the treatment conditions. Together, these results suggest that oxidative changes occur at higher concentrations of MeHg than those that affect STAT3 activity.
4. Discussion
Understanding the risks of exposure to low levels of MeHg hinges on identifying critical molecular and cellular targets of MeHg in the developing nervous system. The JAK/STAT pathway is involved in several stages of neurodevelopment and studies in cell lines have shown that environmental toxicants, including mercury chloride, inhibit cytokine-evoked JAK2 and STAT3 phosphorylation in a manner involving ROS (Kaur et al., 2005; Monroe and Halvorsen, 2006a; b; 2009). Despite MeHg's importance as a pollutant, it was not tested in these studies; therefore, we employed both human neuroblastoma cells and primary NPCs to test effects of MeHg on STAT3 phosphorylation using a similar design. The main observation of the present study is that exposing NPCs to MeHg levels around 30-fold below cytotoxic concentrations enhances CNTF-evoked STAT3 phosphorylation, and also enhances CNTF-induced expression of JAK/STAT responsive genes, notably the astrocyte marker gene GFAP. Low levels of HgCl2 have little effect on CNTF-evoked STAT3 phosphorylation, and higher levels of both mercury compounds inhibit STAT3 phosphorylation and increase ROS, consistent with the studies cited above. STAT3 phosphorylation is enhanced in NPCs at about five to ten times lower levels of MeHg than in SH-SY5Y cells, paralleling the enhanced sensitivity of NPCs to MeHg observed previously (Li et al., 2007; Sanfeliu et al., 2001; Tamm et al., 2006; Watanabe et al., 2009).
It is of interest to note that the low concentrations of MeHg found effective in enhancing CNTF signaling are likely within the range recently suggested by Aschner to reflect biologically relevant sub-threshold toxic levels, e.g. between 1-10 ngHg/mg protein (Aschner, 2012). Astrocytes treated for 24 h with 1 μM MeHg were shown to contain ∼86 ngHg/mg protein (Shapiro and Chan, 2008) and in our NPC system effects on are seen at 5, 12 and 48 h, using 30-fold less MeHg.
4.1. Lack of ROS at low MeHg concentration
Much of MeHg's toxicity is attributed to ROS; therefore, we investigated whether ROS was also involved in the effect on STAT3. We observed that nanomolar concentrations of MeHg which enhance JAK/STAT signaling are neither cytotoxic nor do they lead to a detectable increase in superoxide at 5 h. Furthermore, 30 nM MeHg failed to induce the expression of genes responsive to oxidative stress, such as HSPA5 (Qian et al., 2005; Zhang et al., 2012) and HO-1 (Lee et al., 2008; Ni et al., 2011). In addition, if ROS alone was capable of activating STAT3 signaling, as has been shown previously in other systems (Kim et al., 2008; Simon et al., 1998; Yu et al., 2006), enhanced STAT3 phosphorylation would have been observed without CNTF treatment, however this was not the case.
As was elegantly illustrated by Prozorovski and colleges (2008), slight changes to redox state can impact NPC fate decisions and the deacetylase Sirt1 has a central role in this process. The authors showed subtly oxidizing conditions led to increased astrocyte differentiation in E17.5 mouse NPC, with a prerequisite 6-fold upregulation of Sirt1 expression. We did not find changes in Sirt1 expression under any of the conditions tested, arguing against this pathway's involvement in MeHg's effects on glial differentiation.
It is clear from many studies that ROS mediates the cytotoxic effects of micromolar concentrations of MeHg (Kaur et al., 2009; 2010; Kaur et al., 2008; Kim et al., 2005; Lu et al., 2011; Naganuma et al., 1998; Rush et al., 2012; Shanker et al., 2004; Shanker and Aschner, 2003; Shanker et al., 2005; Yin et al., 2007), and some evidence suggests oxidative-stress can develop at nanomolar concentrations. For instance, 400 nM for 4 h caused ROS in a myogenic cell line (Usuki et al., 2011) and Li and colleagues (2007) observed ∼130% increases in DCF signal following 6 min, 1h, and 24 h 20 nM MeHg in O2A cells, a lineage-restricted form of NPC.
The evidence for ROS at nanomolar doses in SH-SY5Y or NPC is scant and inconsistent. For instance, SH-SY5Y cells exposed to 50 nM MeHg for 24-48 h showed 190-250% increased ROS signal, decreased glutathione content and tau fibril formation that was reversed by antioxidant treatment (Petroni et al., 2011), although no changes in ROS signal were seen in a similar study after 48 h exposure to 1.4 μM MeHg (Kim et al., 2005). In mouse E14.5 NPCs MeHg cytotoxicity was observed in the 0.1 μM to 1 μM range after 48 h, and was ameliorated by antioxidants (Watanabe et al., 2009). However, in NPC lines engineered from mouse embryonic stem cells, cytotoxicity took 10-14 days to develop at these concentrations (Visan et al., 2012; Vojnits et al., 2012).
To our knowledge, the present study is the first to directly evaluate ROS resulting from MeHg in cells shown to form neurospheres, a defining criterion for NPC (Reynolds and Weiss, 1996). Although sensitivity to MeHg appears highly dependent on the experimental context and specific cell culture conditions, our results are nonetheless consistent with the majority of in vitro studies, which show that the acute effects of non-cytotoxic nanomolar concentrations do not involve oxidative-stress.
4.2. Relevance to in vivo glial development
The basic design of the present study, exposing postnatal day 2 cortical NPCs to MeHg and CNTF, a JAK/STAT activating cytokine, models acute exposure to MeHg at a stage when neurogenesis is mostly complete and glial cells are being generated from NPCs, corresponding to the third trimester in humans (Alvarez-Buylla et al., 2001; Dobbing and Sands, 1979; Kessaris et al., 2008; Levison and Goldman, 1993). Activation of the JAK/STAT pathway by cytokines is crucial in this process (Bonni et al., 1997; Fan, 2005; Freeman, 2010; He et al., 2005; Li and Grumet, 2007). This integral role for JAK/STAT is supported by studies showing that inhibition of this pathway results in a reduction (Cao et al., 2006; Koblar et al., 1998; Nakashima et al., 1999), while activation leads to an increase in the number of differentiated astrocytes (Barnabé-Heider et al., 2005). The observation that cytokine-evoked JAK/STAT signaling can be enhanced by 5 h treatment with 30 nM concentrations of MeHg suggests that such levels might be reached in vivo in absence of overt cell death or gross histological disturbances in the developing nervous system. The same low doses of MeHg that enhance CNTF signaling have no measurable effect on signaling in the absence of CNTF, emphasizing the importance of the context provided by ongoing physiological cellular signaling when considering this and similar toxic mechanisms of MeHg in the developing brain.
Our data extend the findings of several previous studies. Immunohistochemistry has demonstrated increased numbers of BrdU labeled GFAP-expressing astrocytes in rodents exposed to MeHg during gliogenesis (Barone et al., 1998; Kakita et al., 2002; Kakita et al., 2003; Kakita et al., 2000). These results are consistent with our in vitro data, which suggest MeHg is affecting bona fide NPCs, which were not distinguished from committed progenitors in these studies. Increased GFAP expression in response to MeHg has also been observed previously in immature astrocyte-like cells in vitro (Monnet-Tschudi et al., 1996), however effects on gliogenic NPC were not clearly delineated in E16-derived aggregating cultures which received MeHg following several days of differentiation. Another report showed increases in GFAP expression in mature cerebellar astrocytes at MeHg concentrations that elicited cell death (Toimela and Tähti, 1995). A number of additional in vitro studies examining effects of MeHg on NPC differentiation have either not studied glial fate markers (Fujimura et al., 2008; Moors et al., 2009; Stummann et al., 2008; Tamm et al., 2006; Tamm et al., 2008; Xu et al., 2010), or did not examine JAK/STAT signaling (Buzanska et al., 2009; Li et al., 2007).
4.3. Concluding Remarks
The current study, together with the previous observations cited above support the hypothesis that inappropriate enhancement of cytokine-evoked JAK/STAT signaling mediates some of the deleterious effects of exposure to low doses of MeHg, especially during the suggested vulnerable period of gliogenesis (Rice and Barone, 2000; Wakabayashi et al., 1995). It is intriguing to speculate that enhanced cytokine-evoked JAK/STAT signaling leads to the generation of supernumerary or ectopic astrocytes and so contributes to the genesis of cognitive and behavioral problems observed in children exposed to low levels of MeHg given the hypothesized involvement of cytokine-imbalance in several developmental disorders (Meyer et al., 2006; Patterson, 2009; 2011). Future studies will incorporate detailed in vivo investigations to test the validity of our working hypothesis and to elucidate the underlying mechanisms.
We modeled developmental MeHg toxicity in postnatal day 2-derived mouse cortical neural precursor cells.
Our main finding is that exposing precursor cells to MeHg levels around 30-fold below cytotoxic concentrations enhanced CNTF-evoked STAT3 phosphorylation, STAT3 target-gene expression, and glial differentiation.
Low levels of HgCl2 did not show a similar effect.
The mechanism does not involve large increases in superoxide anion production associated with oxidative stress and cell-death.
Acknowledgments
Funding: National Institutes of Health/National Institute of Environmental Health Sciences RO1 ES 015550 awarded to MDR. The UVM COBRE facilities are supported by 5P20RR016435.
We would like to acknowledge the gracious contributions of B. Cuniff, C. Mahapatra, E. Zelazny, G. Engel, J. Spees, J. Dewitt, J. Dao, J. Knight, K. Carstens, N. Heintz, S. Otto, S. Clark, S. Sammut, S. White, T. Buttolph, and V. Ochoa.
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–93. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Aschner M. Considerations on methylmercury (MeHg) treatments in in vitro studies. Neurotoxicology. 2012;33:512–3. doi: 10.1016/j.neuro.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabé-Heider F, Wasylnka JA, Fernandes KJL, Porsche C, Sendtner M, Kaplan DR, et al. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron. 2005;48:253–65. doi: 10.1016/j.neuron.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Barone S, Haykal-Coates N, Parran DK, Tilson HA. Gestational exposure to methylmercury alters the developmental pattern of trk-like immunoreactivity in the rat brain and results in cortical dysmorphology. Brain Res Dev Brain Res. 1998;109:13–31. doi: 10.1016/s0165-3806(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Barone S, Jr, Das KP, Lassiter TL, White LD. Vulnerable processes of nervous system development: a review of markers and methods. NeuroToxicology. 2000;21:15–36. [PubMed] [Google Scholar]
- Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Rev Neurosci. 2007;8:221–32. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- Biedler JL, Helson L, Spengler BA. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Research. 1973;33:2643–52. [PubMed] [Google Scholar]
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–83. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- Breier JM, Gassmann K, Kayser R, Stegeman H, Groot DD, Fritsche E, et al. Neural progenitor cells as models for high-throughput screens of developmental neurotoxicity: State of the science. Neurotoxicology and Teratology. 2010;32:4–15. doi: 10.1016/j.ntt.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Breier JM, Radio NM, Mundy WR, Shafer TJ. Development of a high-throughput screening assay for chemical effects on proliferation and viability of immortalized human neural progenitor cells. Toxicol Sci. 2008;105:119–33. doi: 10.1093/toxsci/kfn115. [DOI] [PubMed] [Google Scholar]
- Brierley MM, Fish EN. Stats: multifaceted regulators of transcription. J Interferon Cytokine Res. 2005;25:733–44. doi: 10.1089/jir.2005.25.733. [DOI] [PubMed] [Google Scholar]
- Buettner GR. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anticancer Agents Med Chem. 2011;11:341–6. doi: 10.2174/187152011795677544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzanska L, Sypecka J, Molteni SN, Compagnoni A, Hogberg HT, Del Torchio R, et al. A Human Stem Cell Based Model For Identifying Adverse Effects Of Organic And Inorganic Chemicals On The Developing Nervous System. Stem Cells. 2009;27:2591–601. doi: 10.1002/stem.179. [DOI] [PubMed] [Google Scholar]
- Cao F, Hata R, Zhu P, Ma YJ, Tanaka J, Hanakawa Y, et al. Overexpression of SOCS3 inhibits astrogliogenesis and promotes maintenance of neural stem cells. Journal of Neurochemistry. 2006;98:459–70. doi: 10.1111/j.1471-4159.2006.03890.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Huang S. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic Biol Med. 2008;45:1035–44. doi: 10.1016/j.freeradbiomed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Coluccia A, Borracci P, Giustino A, Sakamoto M, Carratu M. Effects of low dose methylmercury administration during the postnatal brain growth spurt in rats. Neurotoxicology and Teratology. 2007;29:282–7. doi: 10.1016/j.ntt.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Criddle DN, Gillies S, Baumgartner-Wilson HK, Jaffar M, Chinje EC, Passmore S, et al. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem. 2006;281:40485–92. doi: 10.1074/jbc.M607704200. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Fan G. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development. 2005;132:3345–56. doi: 10.1242/dev.01912. [DOI] [PubMed] [Google Scholar]
- Farina M, Aschner M, Rocha JBT. Oxidative stress in MeHg-induced neurotoxicity. Toxicology and Applied Pharmacology. 2011:1–13. doi: 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, et al. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radical Biology and Medicine. 2009;47:449–57. doi: 10.1016/j.freeradbiomed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Freeman MR. Specification and Morphogenesis of Astrocytes. Science. 2010;330:774–8. doi: 10.1126/science.1190928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Usuki F, Sawada M, Rostene W, Godefroy D, Takashima A. Methylmercury exposure downregulates the expression of Racl, leads to neuritic degeneration and ultimately apoptosis in cerebrocortical neurons. NeuroToxicology. 2008;26 doi: 10.1016/j.neuro.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Furieri LB, Galán M, Avendaño MS, García-Redondo AB, Aguado A, Martínez S, et al. Endothelial dysfunction of rat coronary arteries after exposure to low concentrations of mercury is dependent on reactive oxygen species. Br J Pharmacol. 2011;162:1819–31. doi: 10.1111/j.1476-5381.2011.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SG, Grant-Webster KS. Neurobehavioral effects of developmental methylmercury exposure. Environ Health Perspect. 1995;103(Suppl 6):135–42. doi: 10.1289/ehp.95103s6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicology and Teratology. 1997;19:417–28. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Guillemot F. Cell fate specification in the mammalian telencephalon. Prog Neurobiol. 2007;83:37–52. doi: 10.1016/j.pneurobio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Harada M. Congenital Minamata disease: intrauterine methylmercury poisoning. Teratology. 1978;18:285–8. doi: 10.1002/tera.1420180216. [DOI] [PubMed] [Google Scholar]
- He F, Ge W, Martinowich K, Becker-Catania S, Coskun V, Zhu W, et al. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nature neuroscience. 2005;8:616–25. doi: 10.1038/nn1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacqueline GA, Kasia T, Laura PKK, Laurie DC, Shelley S. Neural Stem Cell Culture: Neurosphere generation, microscopical analysis and cryopreservation. 2006 [Google Scholar]
- Kakita A, Inenaga C, Sakamoto M, Takahashi H. Neuronal migration disturbance and consequent cytoarchitecture in the cerebral cortex following transplacental administration of methylmercury. Acta Neuropathol. 2002;104:409–17. doi: 10.1007/s00401-002-0571-3. [DOI] [PubMed] [Google Scholar]
- Kakita A, Inenaga C, Sakamoto M, Takahashi H. Disruption of postnatal progenitor migration and consequent abnormal pattern of glial distribution in the cerebrum following administration of methylmercury. J Neuropathol Exp Neurol. 2003;62:835–47. doi: 10.1093/jnen/62.8.835. [DOI] [PubMed] [Google Scholar]
- Kakita A, Wakabayashi K, Su M, Yoneoka Y, Sakamoto M, Ikuta F, et al. Intrauterine methylmercury intoxication. Consequence of the inherent brain lesions and cognitive dysfunction in maturity. Brain Res. 2000;877:322–30. doi: 10.1016/s0006-8993(00)02717-7. [DOI] [PubMed] [Google Scholar]
- Kaur N, Lu B, Monroe RK, Ward SM, Halvorsen SW. Inducers of oxidative stress block ciliary neurotrophic factor activation of Jak/STAT signaling in neurons. Journal of Neurochemistry. 2005;92:1521–30. doi: 10.1111/j.1471-4159.2004.02990.x. [DOI] [PubMed] [Google Scholar]
- Kaur N, Wohlhueter AL, Halvorsen SW. Activation and inactivation of signal transducers and activators of transcription by ciliary neurotrophic factor in neuroblastoma cells. Cell Signal. 2002;14:419–29. doi: 10.1016/s0898-6568(01)00280-7. [DOI] [PubMed] [Google Scholar]
- Kaur P, Evje L, Aschner M, Syversen T. The in vitro effects of selenomethionine on methylmercury-induced neurotoxicity. Toxicology in Vitro. 2009;23:378–85. doi: 10.1016/j.tiv.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Kaur P, Evje L, Aschner M, Syversen T. The in vitro effects of Trolox on methylmercury-induced neurotoxicity. Toxicology. 2010;276:73–8. doi: 10.1016/j.tox.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Kaur P, Schulz K, Heggland I, Aschner M, Syversen T. The use of fluorescence for detecting MeHg-induced ROS in cell cultures. Toxicology in Vitro. 2008;22:1392–8. doi: 10.1016/j.tiv.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Pringle N, Richardson WD. Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Philos Trans R Soc Lond, B, Biol Sci. 2008;363:71–85. doi: 10.1098/rstb.2006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Cho IH, Kim JE, Shin YJ, Jeon JH, Kim Y, et al. Ethyl pyruvate has an anti-inflammatory effect by inhibiting ROS-dependent STAT signaling in activated microglia. Free Radic Biol Med. 2008;45:950–63. doi: 10.1016/j.freeradbiomed.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim Y, Kim M, Ryu J. The inhibitory mechanism of methylmercury on differentiation of human neuroblastoma cells. Toxicology. 2007;234:1–9. doi: 10.1016/j.tox.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Chai YG, Ryu JC. Selenoprotein W as molecular target of methylmercury in human neuronal cells is down-regulated by GSH depletion. Biochem Biophys Res Commun. 2005;330:1095–102. doi: 10.1016/j.bbrc.2005.03.080. [DOI] [PubMed] [Google Scholar]
- Kjellstrom T, Kennedy P, Wallis S, Mantel C, Stewart A, Friberg L. National Swedish Environmental Protection Board Report. Solna: SWE; 1986-1989. Physical and Mental Development of Children with Prenatal Exposure to Mercury From Fish: Stage I+II. [Google Scholar]
- Koblar SA, Turnley AM, Classon BJ, Reid KL, Ware CB, Cheema SS, et al. Neural precursor differentiation into astrocytes requires signaling through the leukemia inhibitory factor receptor. Proc Natl Acad Sci U S A. 1998;95:3178–81. doi: 10.1073/pnas.95.6.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–12. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signaling. J Cell Sci. 2000;113(Pt 16):2813–9. doi: 10.1242/jcs.113.16.2813. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Cho HS, Park E, Kim S, Lee SY, Kim CS, et al. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology. 2008;250:109–15. doi: 10.1016/j.tox.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–12. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Li H, Grumet M. BMP and LIF signaling coordinately regulate lineage restriction of radial glia in the developing forebrain. Glia. 2007;55:24–35. doi: 10.1002/glia.20434. [DOI] [PubMed] [Google Scholar]
- Li Z, Dong T, Pröschel C, Noble M. Chemically Diverse Toxicants Converge on Fyn and c-Cbl to Disrupt Precursor Cell Function. Plos Biol. 2007;5:e35. doi: 10.1371/journal.pbio.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TH, Hsieh SY, Yen CC, Wu HC, Chen KL, Hung DZ, et al. Involvement of oxidative stress-mediated ERK1/2 and p38 activation regulated mitochondria-dependent apoptotic signals in methylmercury-induced neuronal cell injury. Toxicology Letters. 2011:1–10. doi: 10.1016/j.toxlet.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Maier J, Kincaid C, Pagenstecher A, Campbell IL. Regulation of signal transducer and activator of transcription and suppressor of cytokine-signaling gene expression in the brain of mice with astrocyte-targeted production of interleukin-12 or experimental autoimmune encephalomyelitis. The American journal of pathology. 2002;160:271–88. doi: 10.1016/S0002-9440(10)64371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DO, Myers GJ, Clarkson TW, Amin-Zaki L, Tikriti S, Majeed MA. Fetal methylmercury poisoning: clinical and toxicological data on 29 cases. Ann Neurol. 1980;7:348–53. doi: 10.1002/ana.410070412. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006;20:378–88. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Monnet-Tschudi F, Zurich MG, Honegger P. Comparison of the developmental effects of two mercury compounds on glial cells and neurons in aggregate cultures of rat telencephalon. Brain Research. 1996;741:52–9. doi: 10.1016/s0006-8993(96)00895-5. [DOI] [PubMed] [Google Scholar]
- Monroe RK, Halvorsen SW. Cadmium blocks receptor-mediated Jak/STAT signaling in neurons by oxidative stress. Free Radic Biol Med. 2006a;41:493–502. doi: 10.1016/j.freeradbiomed.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Monroe RK, Halvorsen SW. Mercury abolishes neurotrophic factor-stimulated Jak-STAT signaling in nerve cells by oxidative stress. Toxicol Sci. 2006b;94:129–38. doi: 10.1093/toxsci/kfl073. [DOI] [PubMed] [Google Scholar]
- Monroe RK, Halvorsen SW. Environmental toxicants inhibit neuronal Jak tyrosine kinase by mitochondrial disruption. NeuroToxicology. 2009;30:589–98. doi: 10.1016/j.neuro.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Montgomery K, Mackey J, Thuett K, Ginestra S, Bizon J, Abbott L. Chronic, low-dose prenatal exposure to methylmercury impairs motor and mnemonic function in adult C57/B6 mice. Behavioural Brain Research. 2008;191:55–61. doi: 10.1016/j.bbr.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Moors M, Rockel TD, Abel J, Cline JE, Gassmann K, Schreiber T, et al. Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing. Environ Health Perspect. 2009;117:1131–8. doi: 10.1289/ehp.0800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N, Yasutake A, Hirayama K. Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity. Arch Toxicol. 2007;81:769–76. doi: 10.1007/s00204-007-0209-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mukherjee TK, Mukhopadhyay S, Hoidal JR. The role of reactive oxygen species in TNFalpha-dependent expression of the receptor for advanced glycation end products in human umbilical vein endothelial cells. Biochim Biophys Acta. 2005;1744:213–23. doi: 10.1016/j.bbamcr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Mundy WR, Radio NM, Freudenrich TM. Neuronal models for evaluation of proliferation in vitro using high content screening. Toxicology. 2010;270:121–30. doi: 10.1016/j.tox.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Naganuma A, Miura K, Tanaka-Kagawa T, Kitahara J, Seko Y, Toyoda H, et al. Overexpression of manganese-superoxide dismutase prevents methylmercury toxicity in HeLa cells. Life Sci. 1998;62:PL157–61. doi: 10.1016/s0024-3205(98)00037-x. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, et al. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:5429–34. doi: 10.1523/JNEUROSCI.19-13-05429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–5. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Li X, Yin Z, Sidoryk-Węgrzynowicz M, Jiang H, Farina M, et al. Comparative study on the response of rat primary astrocytes and microglia to methylmercury toxicity. Glia. 2011;59:810–20. doi: 10.1002/glia.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. National Research Council: Toxicological Effects of Methylmercury. Washington, DC: 2000. [Google Scholar]
- Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, et al. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environmental health perspectives. 2005;113:1376–80. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishchenko N, Karpova N, Sabri F, Castrn E, Ceccatelli S. Long-lasting depression-like behavior and epigenetic changes of BDNF gene expression induced by perinatal exposure to methylmercury. Journal of Neurochemistry. 2008;106:1378–87. doi: 10.1111/j.1471-4159.2008.05484.x. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Tamm C, Vahter M, Hökfelt T, Johnson JA, Johnson DA, et al. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol Sci. 2007;97:428–37. doi: 10.1093/toxsci/kfl199. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behavioural Brain Research. 2009;204:313–21. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection and immune involvement in autism. Trends in molecular medicine. 2011;17:389–94. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroni D, Tsai J, Agrawal K, Mondal D, George W. Low-dose methylmercury-induced oxidative stress, cytotoxicity, and tau-hyperphosphorylation in human neuroblastoma (SH-SY5Y) cells. Environ Toxicol. 2011 doi: 10.1002/tox.20672. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Posser T, Dunkley PR, Dickson PW, Franco JL. Human neuroblastoma cells transfected with tyrosine hydroxylase gain increased resistance to methylmercury-induced cell death. Toxicology in vitro : an international journal published in association with BIBRA. 2010;24:1498–503. doi: 10.1016/j.tiv.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schröter F, Ninnemann O, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nature. 2008;10:385–94. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- Qian Y, Zheng Y, Ramos KS, Tiffany-Castiglioni E. The Involvement of Copper Transporter in Lead-induced Oxidative Stress in Astroglia. Neurochem Res. 2005;30:429–38. doi: 10.1007/s11064-005-2677-1. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowitch DH. Glial specification in the vertebrate neural tube. Nature reviews Neuroscience. 2004;5:409–19. doi: 10.1038/nrn1389. [DOI] [PubMed] [Google Scholar]
- Rush T, Liu X, Nowakowski AB, Petering DH, Lobner D. Glutathione-mediated neuroprotection against methylmercury neurotoxicity in cortical culture is dependent on MRP1. NeuroToxicology. 2012;33:476–81. doi: 10.1016/j.neuro.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Sanfeliu C, Sebastia J, Cristofol R, Rodriguez-Farre E. Neurotoxicity of organomercurial compounds. Neurotoxicity research. 2003;5:283–305. doi: 10.1007/BF03033386. [DOI] [PubMed] [Google Scholar]
- Sanfeliu C, Sebastià J, Ki SU. Methylmercury neurotoxicity in cultures of human neurons, astrocytes, neuroblastoma cells. NeuroToxicology. 2001;22:317–27. doi: 10.1016/s0161-813x(01)00015-8. [DOI] [PubMed] [Google Scholar]
- Sarafian TA, Vartavarian L, Kane DJ, Bredesen DE, Verity MA. bcl-2 expression decreases methyl mercury-induced free-radical generation and cell killing in a neural cell line. Toxicol Lett. 1994;74:149–55. doi: 10.1016/0378-4274(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Shanker G, Aschner JL, Syversen T, Aschner M. Free radical formation in cerebral cortical astrocytes in culture induced by methylmercury. Brain Res Mol Brain Res. 2004;128:48–57. doi: 10.1016/j.molbrainres.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Shanker G, Aschner M. Methylmercury-induced reactive oxygen species formation in neonatal cerebral astrocytic cultures is attenuated by antioxidants. Brain Res Mol Brain Res. 2003;110:85–91. doi: 10.1016/s0169-328x(02)00642-3. [DOI] [PubMed] [Google Scholar]
- Shanker G, Syversen T, Aschner J, Aschner M. Modulatory effect of glutathione status and antioxidants on methylmercury-induced free radical formation in primary cultures of cerebral astrocytes. Molecular Brain Research. 2005;137:11–22. doi: 10.1016/j.molbrainres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Chan HM. Characterization of demethylation of methylmercury in cultured astrocytes. Chemosphere. 2008;74:112–8. doi: 10.1016/j.chemosphere.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. The American journal of physiology. 1998;275:C1640–52. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- Stringari J, Meotti FC, Souza DO, Santos ARS, Farina M. Postnatal methylmercury exposure induces hyperlocomotor activity and cerebellar oxidative stress in mice: dependence on the neurodevelopmental period. Neurochem Res. 2006;31:563–9. doi: 10.1007/s11064-006-9051-9. [DOI] [PubMed] [Google Scholar]
- Stummann T, Hareng L, Bremer S. Hazard assessment of methylmercury toxicity to neuronal induction in embryogenesis using human embryonic stem cells. Toxicology. 2008;10 doi: 10.1016/j.tox.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Tamm C, Duckworth J, Hermanson O, Ceccatelli S. High susceptibility of neural stem cells to methylmercury toxicity: effects on cell survival and neuronal differentiation. Journal of Neurochemistry. 2006;97:69–78. doi: 10.1111/j.1471-4159.2006.03718.x. [DOI] [PubMed] [Google Scholar]
- Tamm C, Duckworth JK, Hermanson O, Ceccatelli S. Methylmercury inhibits differentiation of rat neural stem cells via Notch signalling. Neuroreport. 2008;19:339–43. doi: 10.1097/WNR.0b013e3282f50ca4. [DOI] [PubMed] [Google Scholar]
- Theunissen PT, Schulpen SH, van Dartel DA, Hermsen SA, van Schooten FJ, Piersma AH. An abbreviated protocol for multilineage neural differentiation of murine embryonic stem cells and its perturbation by methyl mercury. Reproductive toxicology. 2010;29:383–92. doi: 10.1016/j.reprotox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Toimela T, Tahti H. Mitochondrial viability and apoptosis induced by aluminum, mercuric mercury and methylmercury in cell lines of neural origin. Arch Toxicol. 2004;78:565–74. doi: 10.1007/s00204-004-0575-y. [DOI] [PubMed] [Google Scholar]
- Toimela TA, Tähti H. Effects of mercury, methylmercury and aluminium on glial fibrillary acidic protein expression in rat cerebellar astrocyte cultures. Toxicol In Vitro. 1995;9:317–25. doi: 10.1016/0887-2333(95)00002-p. [DOI] [PubMed] [Google Scholar]
- Trasande L, Landrigan PJ, Schechter C. Public health and economic consequences of methyl mercury toxicity to the developing brain. Environmental health perspectives. 2005;113:590–6. doi: 10.1289/ehp.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuki F, Yamashita A, Fujimura M. Post-transcriptional Defects of Antioxidant Selenoenzymes Cause Oxidative Stress under Methylmercury Exposure. Journal of Biological Chemistry. 2011;286:6641–9. doi: 10.1074/jbc.M110.168872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente T. Cerebrospinal Fluid S100B Increases Reversibly in Neonates of Methyl Mercury-Intoxicated Pregnant Rats. NeuroToxicology. 2004;25:771–7. doi: 10.1016/j.neuro.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Visan A, Hayess K, Sittner D, Pohl EE, Riebeling C, Slawik B, et al. Neural differentiation of mouse embryonic stem cells as a tool to assess developmental neurotoxicity in vitro. Neurotoxicology. 2012;33:1135–46. doi: 10.1016/j.neuro.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Vojnits K, Ensenat-Waser R, Gaspar JA, Meganathan K, Jagtap S, Hescheler J, et al. A tanscriptomics study to elucidate the toxicological mechanism of methylmercury chloride in a human stem cell based in vitro test. Curr Med Chem. 2012;19:6224–32. [PubMed] [Google Scholar]
- Wakabayashi K, Kakita A, Sakamoto M, Su M, Iwanaga K, Ikuta F. Variability of brain lesions in rats administered methylmercury at various postnatal development phases. Brain Research. 1995;705:267–72. doi: 10.1016/0006-8993(95)01208-7. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Nakamachi T, Ogawa T, Naganuma A, Nakamura M, Shioda S, et al. Characterization of antioxidant protection of cultured neural progenitor cells (NPC) against methylmercury (MeHg) toxicity. The Journal of toxicological sciences. 2009;34:315–25. doi: 10.2131/jts.34.315. [DOI] [PubMed] [Google Scholar]
- Weir MD, Patel AJ, Hunt A, Thomas DG. Developmental changes in the amount of glial fibrillary acidic protein in three regions of the rat brain. Brain Research. 1984;317:147–54. doi: 10.1016/0165-3806(84)90092-0. [DOI] [PubMed] [Google Scholar]
- Xu M, Yan C, Tian Y, Yuan X, Shen X. Effects of low level of methylmercury on proliferation of cortical progenitor cells. Brain Research. 2010:1–9. doi: 10.1016/j.brainres.2010.08.069. [DOI] [PubMed] [Google Scholar]
- Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JB, Souza DO, et al. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Research. 2007;1131:1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Zhi JL, Cui Y, Tang EH, Sun SN, Feng JQ, et al. Role of the JAK-STAT pathway in protection of hydrogen peroxide preconditioning against apoptosis induced by oxidative stress in PC12 cells. Apoptosis. 2006;11:931–41. doi: 10.1007/s10495-006-6578-9. [DOI] [PubMed] [Google Scholar]
- Zerlin M, Levison SW, Goldman JE. Early patterns of migration, morphogenesis, and intermediate filament expression of subventricular zone cells in the postnatal rat forebrain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:7238–49. doi: 10.1523/JNEUROSCI.15-11-07238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gajate C, Yu LP, Fang YX, Mollinedo F. Mitochondrial-derived ROS in edelfosine-induced apoptosis in yeasts and tumor cells. Acta Pharmacologica Sinica. 2007;28:888–94. doi: 10.1111/j.1745-7254.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu R, Liu W, Wu Y, Qian H, Zhao X, et al. Hormetic Effects of Acute Methylmercury Exposure on GRP78 Expression in Rat Brain Cortex. Dose-Response. 2012;11:109–20. doi: 10.2203/dose-response.11-055.Rongzhu. [DOI] [PMC free article] [PubMed] [Google Scholar]