Abstract
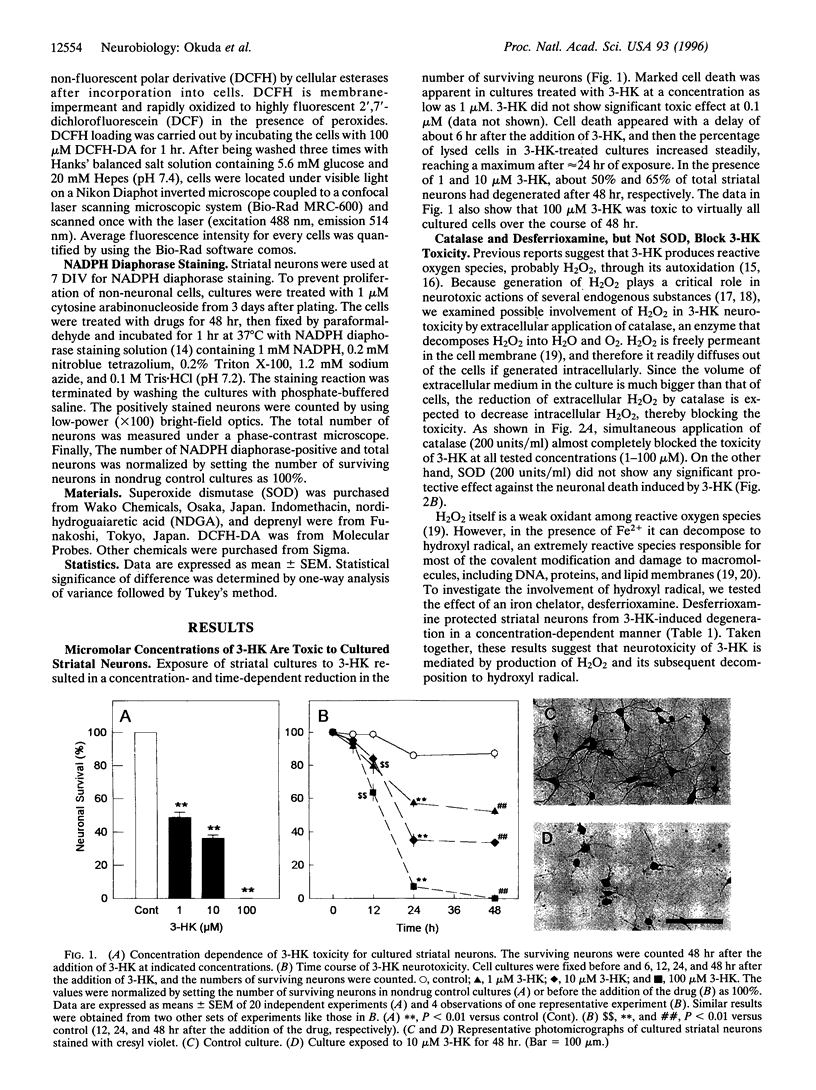
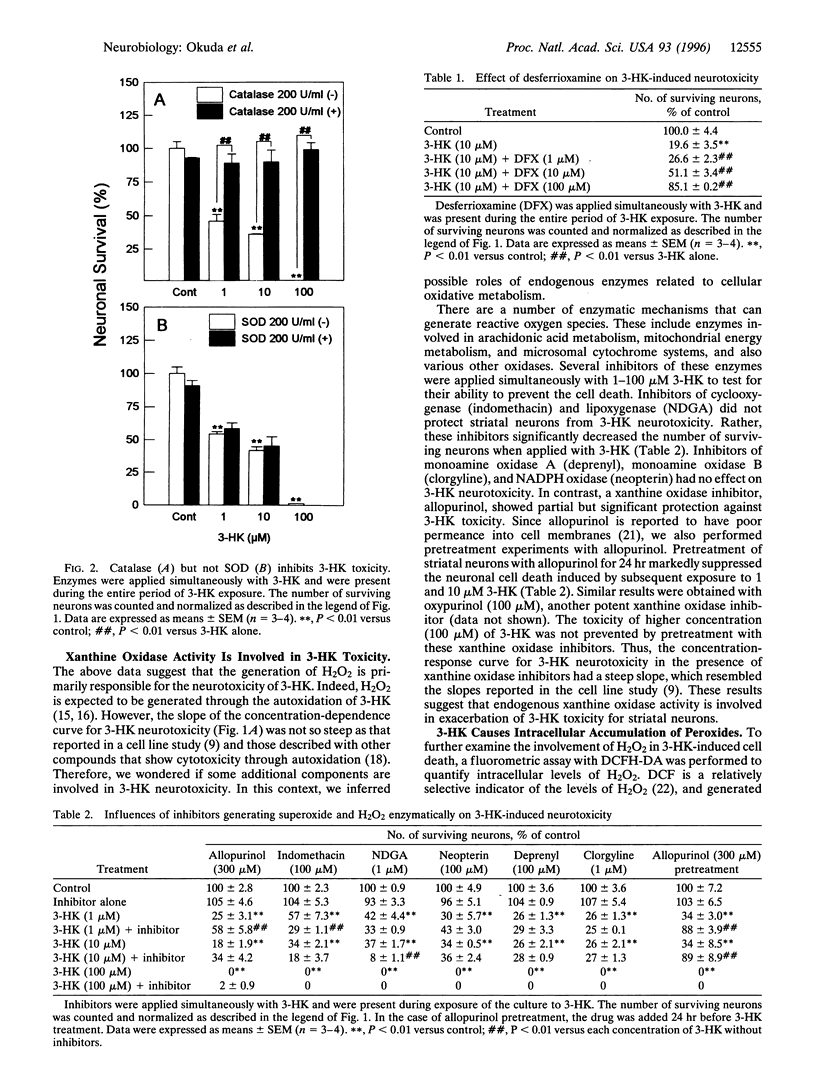
3-Hydroxykynurenine (3-HK) is a tryptophan metabolite whose level in the brain is markedly elevated under several pathological conditions, including Huntington disease and human immunodeficiency virus infection. Here we demonstrate that micromolar concentrations (1-100 microM) of 3-HK cause cell death in primary neuronal cultures prepared from rat striatum. The neurotoxicity of 3-HK was blocked by catalase and desferrioxamine but not by superoxide dismutase, indicating that the generation of hydrogen peroxide and hydroxyl radical is involved in the toxicity. Measurement of peroxide levels revealed that 3-HK caused intracellular accumulation of peroxide, which was largely attenuated by application of catalase. The peroxide accumulation and cell death caused by 1-10 microM 3-HK were also blocked by pretreatment with allopurinol or oxypurinol, suggesting that endogenous xanthine oxidase activity is involved in exacerbation of 3-HK neurotoxicity. Furthermore, NADPH diaphorase-containing neurons were spared from toxicity of these concentrations of 3-HK, a finding reminiscent of the pathological characteristics of several neurodegenerative disorders such as Huntington disease. These results suggest that 3-HK at pathologically relevant concentrations renders neuronal cells subject to oxidative stress leading to cell death, and therefore that this endogenous compound should be regarded as an important factor in pathogenesis of neurodegenerative disorders.
Full text
PDF
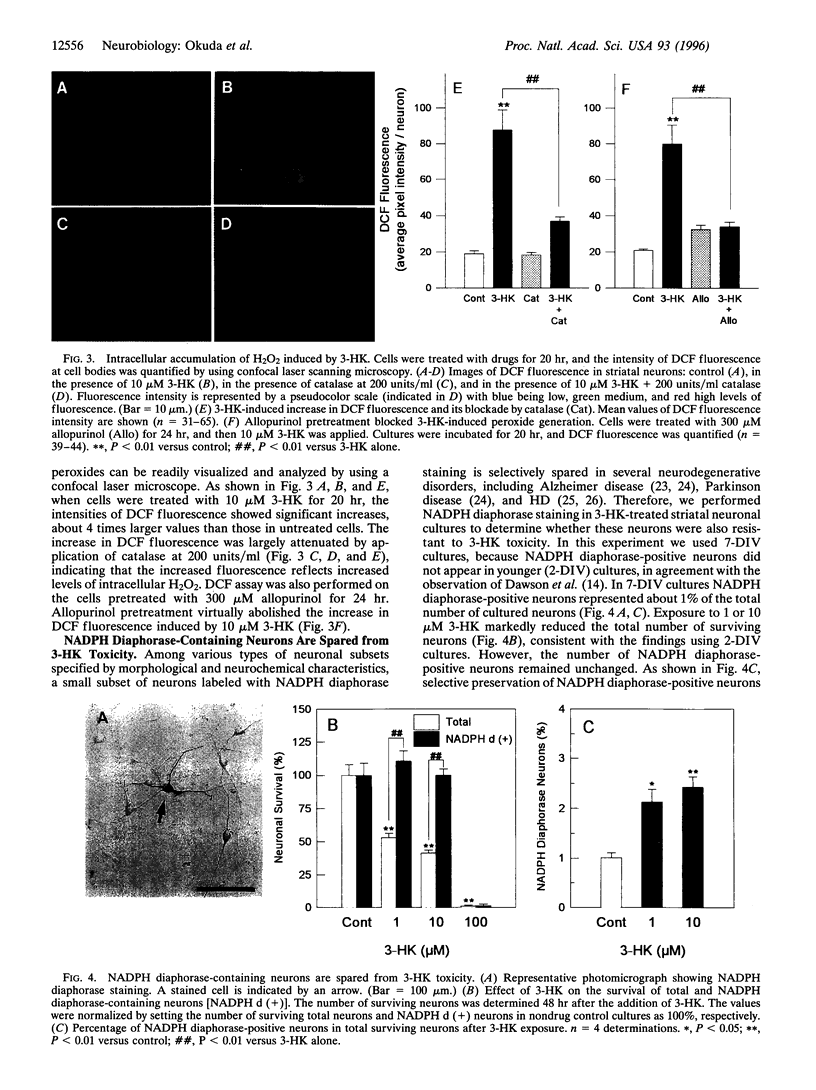


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal M. F., Kowall N. W., Ellison D. W., Mazurek M. F., Swartz K. J., Martin J. B. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986 May 8;321(6066):168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- Behl C., Davis J. B., Lesley R., Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994 Jun 17;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Brady D. R., Carey R. G., Mufson E. J. Reduced nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) profiles in the amygdala of human and New World monkey (Saimiri sciureus). Brain Res. 1992 Apr 17;577(2):236–248. doi: 10.1016/0006-8993(92)90279-i. [DOI] [PubMed] [Google Scholar]
- Ceballos G., Tuttle J. B., Rubio R. Differential distribution of purine metabolizing enzymes between glia and neurons. J Neurochem. 1994 Mar;62(3):1144–1153. doi: 10.1046/j.1471-4159.1994.62031144.x. [DOI] [PubMed] [Google Scholar]
- Chevion M., Navok T., Glaser G., Mager J. The chemistry of favism-inducing compounds. The properties of isouramil and divicine and their reaction with glutathione. Eur J Biochem. 1982 Oct;127(2):405–409. doi: 10.1111/j.1432-1033.1982.tb06886.x. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993 Oct 29;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Dawbarn D., De Quidt M. E., Emson P. C. Survival of basal ganglia neuropeptide Y-somatostatin neurones in Huntington's disease. Brain Res. 1985 Aug 12;340(2):251–260. doi: 10.1016/0006-8993(85)90921-7. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., Bartley D. A., Uhl G. R., Snyder S. H. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993 Jun;13(6):2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens J. A., Stern A., Trenkner E. Mechanism of kainate toxicity to cerebellar neurons in vitro is analogous to reperfusion tissue injury. J Neurochem. 1987 Oct;49(4):1222–1228. doi: 10.1111/j.1471-4159.1987.tb10014.x. [DOI] [PubMed] [Google Scholar]
- Eastman C. L., Guilarte T. R. Cytotoxicity of 3-hydroxykynurenine in a neuronal hybrid cell line. Brain Res. 1989 Aug 28;495(2):225–231. doi: 10.1016/0006-8993(89)90216-3. [DOI] [PubMed] [Google Scholar]
- Eastman C. L., Guilarte T. R., Lever J. R. Uptake of 3-hydroxykynurenine measured in rat brain slices and in a neuronal cell line. Brain Res. 1992 Jul 3;584(1-2):110–116. doi: 10.1016/0006-8993(92)90883-b. [DOI] [PubMed] [Google Scholar]
- Eastman C. L., Guilarte T. R. The role of hydrogen peroxide in the in vitro cytotoxicity of 3-hydroxykynurenine. Neurochem Res. 1990 Nov;15(11):1101–1107. doi: 10.1007/BF01101711. [DOI] [PubMed] [Google Scholar]
- Ferrante R. J., Kowall N. W., Beal M. F., Richardson E. P., Jr, Bird E. D., Martin J. B. Selective sparing of a class of striatal neurons in Huntington's disease. Science. 1985 Nov 1;230(4725):561–563. doi: 10.1126/science.2931802. [DOI] [PubMed] [Google Scholar]
- Fukui S., Schwarcz R., Rapoport S. I., Takada Y., Smith Q. R. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991 Jun;56(6):2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Goodman Y., Mattson M. P. Secreted forms of beta-amyloid precursor protein protect hippocampal neurons against amyloid beta-peptide-induced oxidative injury. Exp Neurol. 1994 Jul;128(1):1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- Guidetti P., Eastman C. L., Schwarcz R. Metabolism of [5-3H]kynurenine in the rat brain in vivo: evidence for the existence of a functional kynurenine pathway. J Neurochem. 1995 Dec;65(6):2621–2632. doi: 10.1046/j.1471-4159.1995.65062621.x. [DOI] [PubMed] [Google Scholar]
- Hiraku Y., Inoue S., Oikawa S., Yamamoto K., Tada S., Nishino K., Kawanishi S. Metal-mediated oxidative damage to cellular and isolated DNA by certain tryptophan metabolites. Carcinogenesis. 1995 Feb;16(2):349–356. doi: 10.1093/carcin/16.2.349. [DOI] [PubMed] [Google Scholar]
- Ishii T., Iwahashi H., Sugata R., Kido R. Formation of hydroxanthommatin-derived radical in the oxidation of 3-hydroxykynurenine. Arch Biochem Biophys. 1992 May 1;294(2):616–622. doi: 10.1016/0003-9861(92)90733-d. [DOI] [PubMed] [Google Scholar]
- Moorhouse P. C., Grootveld M., Halliwell B., Quinlan J. G., Gutteridge J. M. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett. 1987 Mar 9;213(1):23–28. doi: 10.1016/0014-5793(87)81458-8. [DOI] [PubMed] [Google Scholar]
- Mufson E. J., Brandabur M. M. Sparing of NADPH-diaphorase striatal neurons in Parkinson's and Alzheimer's diseases. Neuroreport. 1994 Feb 24;5(6):705–708. doi: 10.1097/00001756-199402000-00011. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Matson W. R., Beal M. F., Myers R. H., Bird E. D., Milbury P., Saso S. Kynurenine pathway abnormalities in Parkinson's disease. Neurology. 1992 Sep;42(9):1702–1706. doi: 10.1212/wnl.42.9.1702. [DOI] [PubMed] [Google Scholar]
- Okuda S., Saito H., Katsuki H. Arachidonic acid: toxic and trophic effects on cultured hippocampal neurons. Neuroscience. 1994 Dec;63(3):691–699. doi: 10.1016/0306-4522(94)90515-0. [DOI] [PubMed] [Google Scholar]
- Pearson S. J., Reynolds G. P. Determination of 3-hydroxykynurenine in human brain and plasma by high-performance liquid chromatography with electrochemical detection. Increased concentrations in hepatic encephalopathy. J Chromatogr. 1991 Apr 19;565(1-2):436–440. doi: 10.1016/0378-4347(91)80406-3. [DOI] [PubMed] [Google Scholar]
- Pearson S. J., Reynolds G. P. Increased brain concentrations of a neurotoxin, 3-hydroxykynurenine, in Huntington's disease. Neurosci Lett. 1992 Sep 14;144(1-2):199–201. doi: 10.1016/0304-3940(92)90749-w. [DOI] [PubMed] [Google Scholar]
- Reynolds G. P., Pearson S. J., Halket J., Sandler M. Brain quinolinic acid in Huntington's disease. J Neurochem. 1988 Jun;50(6):1959–1960. doi: 10.1111/j.1471-4159.1988.tb02503.x. [DOI] [PubMed] [Google Scholar]
- Reynolds G. P., Pearson S. J. Increased brain 3-hydroxykynurenine in Huntington's disease. Lancet. 1989 Oct 21;2(8669):979–980. doi: 10.1016/s0140-6736(89)90987-2. [DOI] [PubMed] [Google Scholar]
- Reynolds G. P., Pearson S. J. Neurochemical-clinical correlates in Huntington's disease--applications of brain banking techniques. J Neural Transm Suppl. 1993;39:207–214. [PubMed] [Google Scholar]
- Rosenberg P. A. Catecholamine toxicity in cerebral cortex in dissociated cell culture. J Neurosci. 1988 Aug;8(8):2887–2894. doi: 10.1523/JNEUROSCI.08-08-02887.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall J. A., Ischiropoulos H. Evaluation of 2',7'-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993 May;302(2):348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- Sardar A. M., Bell J. E., Reynolds G. P. Increased concentrations of the neurotoxin 3-hydroxykynurenine in the frontal cortex of HIV-1-positive patients. J Neurochem. 1995 Feb;64(2):932–935. doi: 10.1046/j.1471-4159.1995.64020932.x. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Whetsell W. O., Jr, Mangano R. M. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983 Jan 21;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Johansson O., Hökfelt T., Skirboll L., Elde R. P., Terenius L., Kimmel J., Goldstein M. NADPH-diaphorase: a selective histochemical marker for striatal neurons containing both somatostatin- and avian pancreatic polypeptide (APP)-like immunoreactivities. J Comp Neurol. 1983 Jul 1;217(3):252–263. doi: 10.1002/cne.902170303. [DOI] [PubMed] [Google Scholar]