Abstract
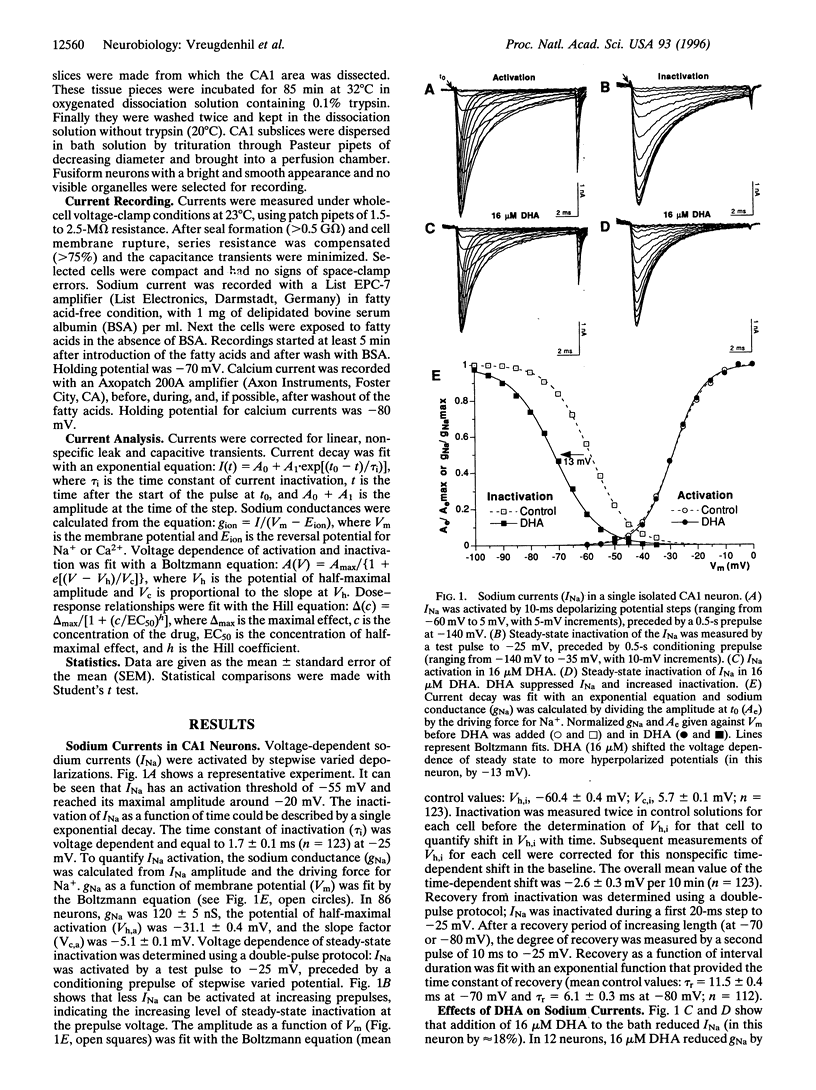
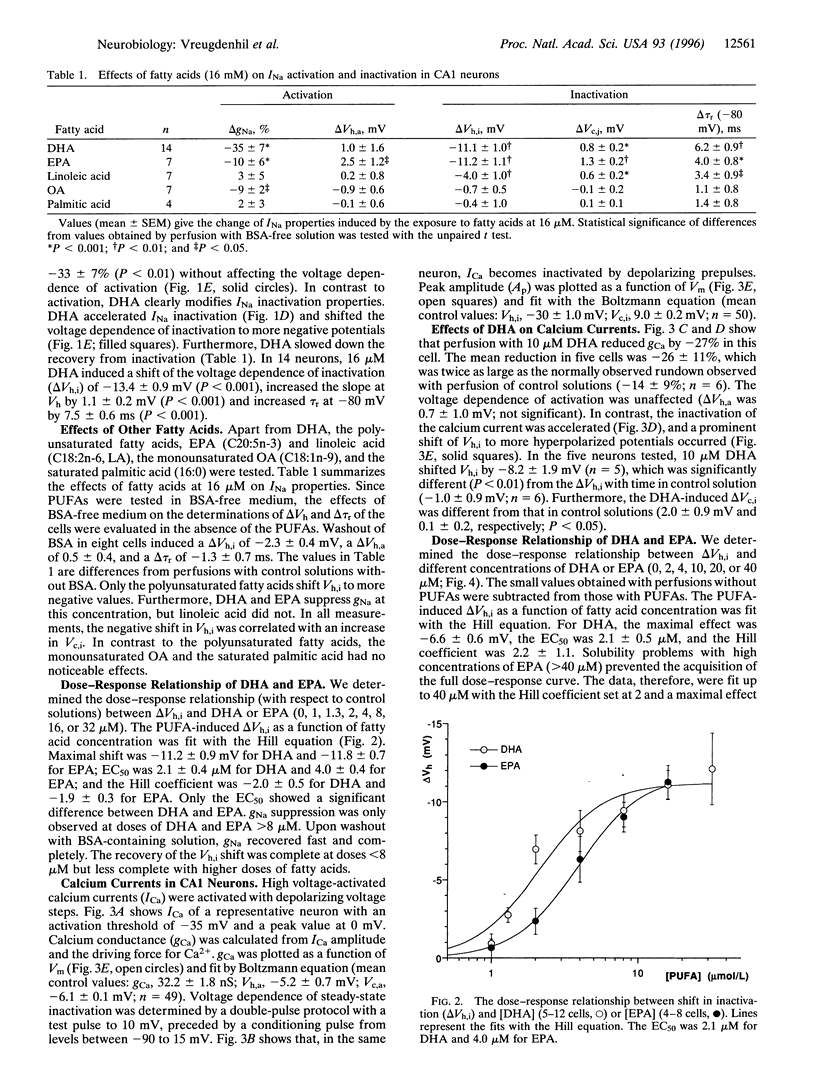
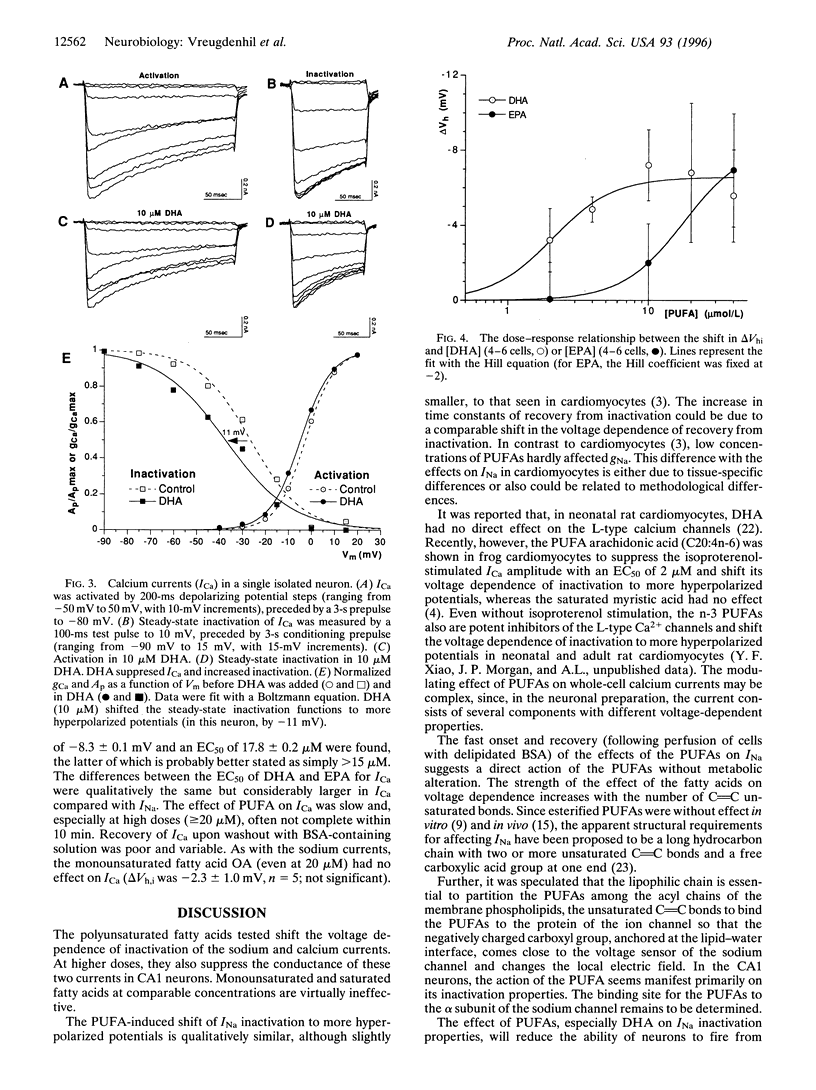
Recent evidence indicates that long-chain polyunsaturated fatty acids (PUFAs) can prevent cardiac arrhythmias by a reduction of cardiomyocyte excitability. This was shown to be due to a modulation of the voltage-dependent inactivation of both sodium (INa) and calcium (ICa) currents. To establish whether PUFAs also regulate neuronal excitability, the effects of PUFAs on INa and ICa were assessed in CA1 neurons freshly isolated from the rat hippocampus. Extracellular application of PUFAs produced a concentration-dependent shift of the voltage dependence of inactivation of both INa and ICa to more hyperpolarized potentials. Consequently, they accelerated the inactivation and retarded the recovery from inactivation. The EC50 for the shift of the INa steady-state inactivation curve was 2.1 +/- 0.4 microM for docosahexaenoic acid (DHA) and 4 +/- 0.4 microM for eicosapentaenoic acid (EPA). The EC50 for the shift on the ICa inactivation curve was 2.1 +/- 0.4 for DHA and > 15 microM for EPA. Additionally, DHA and EPA suppressed both INa and ICa amplitude at concentrations > 10 microM. PUFAs did not affect the voltage dependence of activation. The monounsaturated oleic acid and the saturated palmitic acid were virtually ineffective. The combined effects of the PUFAs on INa and ICa may reduce neuronal excitability and may exert anticonvulsive effects in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P., Cohen C. J., Tsien R. W. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983 May;81(5):613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman G. E., Hallaq H., Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr M. L., Fehily A. M., Gilbert J. F., Rogers S., Holliday R. M., Sweetnam P. M., Elwood P. C., Deadman N. M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989 Sep 30;2(8666):757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- Connor W. E., Neuringer M., Reisbick S. Essential fatty acids: the importance of n-3 fatty acids in the retina and brain. Nutr Rev. 1992 Apr;50(4 ):21–29. doi: 10.1111/j.1753-4887.1992.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Sebben M., Haynes L., Pin J. P., Bockaert J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988 Nov 3;336(6194):68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- Elliott P. Action of antiepileptic and anaesthetic drugs on Na- and Ca-spikes in mammalian non-myelinated axons. Eur J Pharmacol. 1990 Jan 10;175(2):155–163. doi: 10.1016/0014-2999(90)90226-v. [DOI] [PubMed] [Google Scholar]
- Hamano H., Nabekura J., Nishikawa M., Ogawa T. Docosahexaenoic acid reduces GABA response in substantia nigra neuron of rat. J Neurophysiol. 1996 Mar;75(3):1264–1270. doi: 10.1152/jn.1996.75.3.1264. [DOI] [PubMed] [Google Scholar]
- Hock C. E., Beck L. D., Bodine R. C., Reibel D. K. Influence of dietary n-3 fatty acids on myocardial ischemia and reperfusion. Am J Physiol. 1990 Nov;259(5 Pt 2):H1518–H1526. doi: 10.1152/ajpheart.1990.259.5.H1518. [DOI] [PubMed] [Google Scholar]
- Honoré E., Barhanin J., Attali B., Lesage F., Lazdunski M. External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1937–1941. doi: 10.1073/pnas.91.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. X., Leaf A. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. X., Leaf A. Evidence that free polyunsaturated fatty acids modify Na+ channels by directly binding to the channel proteins. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3542–3546. doi: 10.1073/pnas.93.8.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. X., Leaf A. Protective effects of free polyunsaturated fatty acids on arrhythmias induced by lysophosphatidylcholine or palmitoylcarnitine in neonatal rat cardiac myocytes. Eur J Pharmacol. 1996 Feb 15;297(1-2):97–106. doi: 10.1016/0014-2999(95)00701-6. [DOI] [PubMed] [Google Scholar]
- Kang J. X., Xiao Y. F., Leaf A. Free, long-chain, polyunsaturated fatty acids reduce membrane electrical excitability in neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3997–4001. doi: 10.1073/pnas.92.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Sladek C. D., Aguado-Velasco C., Mathiasen J. R. Arachidonic acid activation of a new family of K+ channels in cultured rat neuronal cells. J Physiol. 1995 May 1;484(Pt 3):643–660. doi: 10.1113/jphysiol.1995.sp020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limousin P., Pollak P., Benazzouz A., Hoffmann D., Le Bas J. F., Broussolle E., Perret J. E., Benabid A. L. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995 Jan 14;345(8942):91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- McLennan P. L., Abeywardena M. Y., Charnock J. S. Influence of dietary lipids on arrhythmias and infarction after coronary artery ligation in rats. Can J Physiol Pharmacol. 1985 Nov;63(11):1411–1417. doi: 10.1139/y85-232. [DOI] [PubMed] [Google Scholar]
- Meves H. Modulation of ion channels by arachidonic acid. Prog Neurobiol. 1994 Jun;43(2):175–186. doi: 10.1016/0301-0082(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Kimura S., Akaike N. Facilitatory effect of docosahexaenoic acid on N-methyl-D-aspartate response in pyramidal neurones of rat cerebral cortex. J Physiol. 1994 Feb 15;475(1):83–93. doi: 10.1113/jphysiol.1994.sp020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway R. W., Singer J. J., Walsh J. V., Jr Direct regulation of ion channels by fatty acids. Trends Neurosci. 1991 Mar;14(3):96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- Petit-Jacques J., Hartzell H. C. Effect of arachidonic acid on the L-type calcium current in frog cardiac myocytes. J Physiol. 1996 May 15;493(Pt 1):67–81. doi: 10.1113/jphysiol.1996.sp021365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poling J. S., Karanian J. W., Salem N., Jr, Vicini S. Time- and voltage-dependent block of delayed rectifier potassium channels by docosahexaenoic acid. Mol Pharmacol. 1995 Feb;47(2):381–390. [PubMed] [Google Scholar]
- Robinson P. J., Rapoport S. I. Kinetics of protein binding determine rates of uptake of drugs by brain. Am J Physiol. 1986 Dec;251(6 Pt 2):R1212–R1220. doi: 10.1152/ajpregu.1986.251.6.R1212. [DOI] [PubMed] [Google Scholar]
- Rogawski M. A., Porter R. J. Antiepileptic drugs: pharmacological mechanisms and clinical efficacy with consideration of promising developmental stage compounds. Pharmacol Rev. 1990 Sep;42(3):223–286. [PubMed] [Google Scholar]
- Schwarz J. R., Grigat G. Phenytoin and carbamazepine: potential- and frequency-dependent block of Na currents in mammalian myelinated nerve fibers. Epilepsia. 1989 May-Jun;30(3):286–294. doi: 10.1111/j.1528-1157.1989.tb05300.x. [DOI] [PubMed] [Google Scholar]
- Siscovick D. S., Raghunathan T. E., King I., Weinmann S., Wicklund K. G., Albright J., Bovbjerg V., Arbogast P., Smith H., Kushi L. H. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA. 1995 Nov 1;274(17):1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- Uauy R., Peirano P., Hoffman D., Mena P., Birch D., Birch E. Role of essential fatty acids in the function of the developing nervous system. Lipids. 1996 Mar;31 (Suppl):S167–S176. doi: 10.1007/BF02637071. [DOI] [PubMed] [Google Scholar]
- Voskuyl R. A., Dingemanse J., Danhof M. Determination of the threshold for convulsions by direct cortical stimulation. Epilepsy Res. 1989 Mar-Apr;3(2):120–129. doi: 10.1016/0920-1211(89)90039-9. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M., Wadman W. J. Enhancement of calcium currents in rat hippocampal CA1 neurons induced by kindling epileptogenesis. Neuroscience. 1992 Jul;49(2):373–381. doi: 10.1016/0306-4522(92)90103-9. [DOI] [PubMed] [Google Scholar]
- Xiao Y. F., Kang J. X., Morgan J. P., Leaf A. Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda S., Carasso R. L., Mostofsky D. I. Essential fatty acid preparation (SR-3) raises the seizure threshold in rats. Eur J Pharmacol. 1994 Mar 11;254(1-2):193–198. doi: 10.1016/0014-2999(94)90387-5. [DOI] [PubMed] [Google Scholar]
- de Lorgeril M., Renaud S., Mamelle N., Salen P., Martin J. L., Monjaud I., Guidollet J., Touboul P., Delaye J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994 Jun 11;343(8911):1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]


