Abstract
It has been known for decades that urinary potassium excretion varies with a circadian pattern. In this review, we consider the historical evidence for this phenomenon and present an overview of recent developments in the field. Extensive evidence from the latter part of the last century clearly demonstrates that circadian potassium excretion does not depend on endogenous aldosterone. Of note is the recent discovery that the expression of several renal potassium transporters varies with a circadian pattern that appears to be consistent with substantial clinical data regarding daily fluctuations in urinary potassium levels. We propose the circadian clock mechanism as a key regulator of renal potassium transporters, and consequently renal potassium excretion. Further investigation into the mechanism of regulation of renal potassium transport by the circadian clock is warranted in order to increase our understanding of the clinical relevance of circadian rhythms to potassium homeostasis.
Historical Perspective on Circadian Regulation of Potassium Balance
Circadian patterns in renal electrolyte excretion in humans have been known for many years. Mills in his encyclopedic review in 1966 described sodium, potassium, water and numerous other substances exhibiting circadian excretion1. Renal function, and a vast array of physiologic processes including sleep, physical activity, secretion of hormones, intermediate metabolism and the metabolism and excretion of drugs, exhibit circadian rhythms. By definition a circadian process exhibits oscillation under constant conditions (i.e. in the absence of a light/dark cycle). Circadian rhythms exhibit a peak (maximum) and minimum magnitude approximately 12 hours apart, and they are driven by a circadian pacemaker in the suprachiasmiatic nucleus (SCN) of the hypothalamus (the central clock). The intrinsic 24 hour cycle of the clock is synchronized by the ambient light/dark cycle and also by feeding cues. The central clock, in turn, entrains pacemakers in individual cells of various organs throughout the body, termed peripheral clocks. The rhythm displays an approximate 24 hour cycle length in the absence of external entraining signals, hence the term originated by F. Halberg, “circa diem”, meaning “approximately one day” 1. The circadian cycle for potassium is validated by its demonstration in the absence of changes in other factors that modify potassium excretion. The magnitude of potassium excretion at a given time results from the summed effects of a number of controlling pathways, including that of the circadian rhythm.
Circadian rhythms are not unique to humans or other mammals. Quite the contrary, they are found from bacteria to multicellular organisms, plant as well as animal, in each case being driven by an oscillator. This review focuses on the circadian rhythm of potassium by the mammalian kidney. The rhythm is considered an important component of the complex mechanism which enables the kidney to maintain the total body potassium within a limited range despite wide variations in potassium intake. In other words, it is a component of the system responsible for potassium homeostasis.
Homeostatic control of potassium excretion was for many years attributed to a reactive system, that is, a system which reacts to the magnitude of potassium intake by regulating the magnitude of excretion 2. A negative feedback system with two kaliuretic components, the plasma concentrations of potassium and aldosterone, was considered to be the unique and sufficient mechanism for the homeostatic regulation of potassium excretion. According to this paradigm, oral intake of potassium, when absorbed, increased the plasma potassium concentration. This had a dual effect: a direct stimulation of distal tubular potassium secretion and a stimulation of the secretion of aldosterone, the adrenal steroid hormone possessing potent antinatriuretic as well as purportedly kaliuretic properties. Both plasma potassium and aldosterone acted to stimulate potassium secretion by the principal cells of the distal nephron and collecting duct. This active potassium secretion added significantly to the moiety of the filtered potassium that reached the distal nephron, potentially destined for urinary potassium excretion. Discovery of distal nephron potassium reabsorption added a second primary active transport component3. This reabsorption depended on a hydrogen for potassium exchange by a luminal membrane H, K-ATPase in intercalated cells and was activated during reduced potassium intake. Anotherreactive component was introduced with the proposal and demonstration of a splanchnic reflex control of potassium excretion 2, 4, 5. Current views indicate this reflex is initiated in response to local changes in the potassium concentration in gut, hepatic portal vein, or liver. It is thought to act through two pathways. One involves vagal nerve afferent signals to the central nervous system (CNS) that initiate efferent signals from CNS to kidney 4, 6; a second acts through a direct stimulation of a kaliuretic “gut factor” 4, 7. This reflex constitutes a feed forward control system: signals initiating the increased excretion are not altered by the excretion itself.
Moore-Ede 8 noted that circadian cyclic excretion constituted a “predictive” homeostatic system in that it anticipated and enhanced the capacity of the nephron to perform its reactive response at the times of the 24 hour cycle when intake was greatest. Thus, peak circadian excretion of sodium and potassium in humans occurs in the day and in nocturnal rodents at night.
Circadian rhythms of potassium and of sodium have been identified in humans, the squirrel monkey, rats, and mice 4, 9. Studies on the underlying genetic and molecular mechanisms of the circadian system in renal cells have been almost entirely carried out on mice. Notably, the elegant and persuasive study of Sarelius and Greenway demonstrated the absence of circadian cyclic potassium excretion in sheep10. Within the 24 hour cycle the circadian rhythm can -produce wide swings in excretion. In the rat, for example, the ratio of peak to minimum excretion lies in the ranges of 4:1 to 10:1 (reviewed by Rabinowitz4); in humans on normal potassium diet, ratios on the order of 5:1 are found11. Thus the ratio between the normal intraday excretion peak and minimum may exceed changes in 24 hour excretion produced by large alterations of dietary potassium intake, making it a potent control system.
Some mechanism of transmitting CNS clock activity to the oscillators in renal tubule cells must exist or the two would have independent oscillation patterns. Two possible pathways, neural and humoral, exist. Mills noted the likelihood of a humoral pathway on the basis of circadian rhythmic excretion in a transplanted human kidney1. More recently Guo et al. provided direct evidence for a humoral pathway in rats using parabiosis studies12, but the humoral substance or substances that carry the message is unknown and constitutes a major unsolved mystery. Recent and ongoing studies have primarily focused on the nature of the oscillator system in renal cells responsible for circadian variations in transport. It is reasonable to assume this system includes cell receptor(s) of CNS signal(s), intracellular messengers and effectors, and membrane transporters, although a coherent picture of this intracellular system has not yet emerged.
We note some of the complexities of this problem as they relate to circadian cycles of potassium and sodium. In all species studied to date in which these rhythms occur, peak excretion of potassium and sodium overlap and occur at virtually the same time in the 24 hour cycle. It is thought that the peak in sodium excretion corresponds to a reduction in sodium reabsorption and a peak in potassium corresponds to an increase in potassium secretion.
An experiment in rats to determine if the secretion of potassium by the amiloride sensitive principal cells was responsible for the peak in potassium excretion provided strong support for this assertion 13. Amiloride given at the time of peak potassium and sodium excretion decreased the peak excretion of potassium by 80%. Simultaneously, sodium, which is absorbed by the principal cells concurrently with potassium secretion, showed a 3.7 fold increase in excretion. At the time of minimum circadian excretion it was shown that both potassium secretion and sodium reabsorption by the amiloride sensitive principal cells was a small fraction of the rates at the time of peak excretion. This finding localized the majority of peak potassium circadian excretion to an amiloride-sensitive mechanism consistent with the action of amiloride on the CCD potassium secretion, but also showed that amiloride-sensitive sodium absorption persisted during maximal natriuresis. Additional evidence for the independence of nephron sites generating the sodium and potassium cycles included an earlier onset for the potassium peak14 and different resynchronization patterns for the urinary rhythms of sodium and potassium in rats after light-dark shifts15.
These results raised the question as to which cells in the nephron had decreased sodium reabsorption at the time of peak sodium excretion. They also raised a fundamental general question. If more than one cell type was involved in the generation of the cycles of different ions and of water, one had to ask whether intracellular tubular clocks were responding to different signals from the CNS oscillator, each signal directed to the control of a specific substance and a specific cell. The alternative was that there was a single messenger substance originating in the CNS. In that case it would be differences in response by the intracellular circadian clock system of different cell types that produced diverse circadian rhythms (both in respect to time of peak excretion and in response to reactive control signals). In this context we note that experiments in rats showed that with a large step increase in potassium intake the cyclic patterns of both potassium and sodium were unchanged but the magnitude of the peak and minimum of the potassium cyclic excretion were elevated while those of sodium remained unchanged. Likewise with a large step increase in sodium intake, the cyclic patterns of both ions and the magnitude of the potassium excretion peak and minimum were unchanged, while the magnitude of the sodium peak and minimum were greatly elevated16. These findings clearly indicated that there are separate cell types as well as a difference in the transport mechanisms responding to the CNS circadian entrainment for these ions. These observations call attention to a major unresolved question pertinent to a full understanding of circadian rhythms in renal excretion, not only of potassium, but of other ions and of water, substances whose homeostasis is essential to the maintenance of life.
One may exclude roles for changes in the plasma concentration of potassium or aldosterone in the determination of the time of peak excretion, the pattern, and the magnitude of cycle peak of potassium on the basis of studies in humans, squirrel monkey and rats. Several groups have convincingly shown that that adrenalectomy in rats did not alter the potassium circadian cyclic excretion pattern14, 17, 18. Further studies by Rutledge and Rabinowitz16 showed the changes in the magnitude of peak and minimum excretion produced by step increases in the intake of either potassium or sodium were virtually identical in adrenal-intact rats, adrenalectomized rats, and adrenalectomized rats given a constant infusion of aldosterone. Moore-Ede et al. found potassium cycles unchanged in the adrenalectomized squirrel monkey19. Studies in the squirrel monkey20 and in rats21, 22 indicated cyclic changes in potassium excretion were not dependent on changes in plasma potassium concentration. However a correlation between plasma potassium and cyclic potassium excretion was observed in humans11.
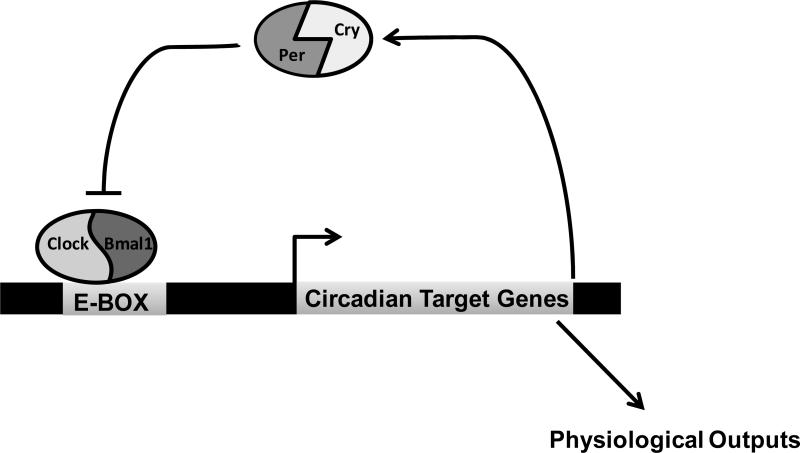
Over time the concept of a circadian clock or pacemaker in the central nervous system has evolved from initial evidence of loss of circadian rhythms following lesions in the hypothalamus to what is now recognized as a complex system of neural feedback loops based on identified genomic origins. More recently the same clock components and genetic basis have been identified in renal tubular cells. Indeed, the discovery and cloning of several core circadian clock genes in the late part of the 20th century (for review see Loudon et al. 23) ushered in an important era in understanding the molecular basis of circadian rhythms. The most thorough studies have been done in the brain and liver and have yielded a basic model for a transcriptional feedback loop involving key clock proteins (Figure 1). The mechanism of the circadian clock in the kidney is currently under investigation9, 24, 25.
Figure 1. Transcriptional Regulation and Feedback Control in the Circadian Clock.
In the positive loop, Bmal1 and Clock heterodimerize to drive transcription of Period (Per) and Cryptochrome (Cry). In the negative loop, Per/Cry inhibit the action of Bmal1/Clock, thereby inhibiting their own expression. This transcriptional mechanism is responsible for the regulation of many circadian target genes, which vary in a tissue specific manner. In the kidney, these targets almost certainly include a variety of potassium transporters.
Substantial advances in knowledge, as described in the following text, have been made, yet basic questions remain unresolved regarding the circadian excretion of potassium and other substances that play key roles in homeostasis.
Mechanism of Circadian Fluctuations in Potassium
Insights from Animal Models
Examination of how the circadian clock regulates renal potassium transporters is still in its infancy. Studies in rodents have demonstrated that several potassium transporters in the SCN appear to be under circadian control. The voltage gated K,3.1 and K,3.2 potassium channels and the Na, K ATPase were shown to function in the circadian regulation of SCN neuron spontaneous firing rates. Calcium-activated potassium channels have a role in the circadian regulation of spontaneous SCN neuron firing rates and also contribute to circadian locomotor activity and physiological rhythms (reviewed in Ko et al.26). Deletion of the BK gene Kcnma1 impaired physiological output rhythms of SCN neuron spontaneous firing rates in mice. This apparent coordinate regulation of K channels and subsequent neuron excitability by the circadian clock is one possible mechanism by which the central clock might contribute to the control of output rhythms.
The role of circadian rhythms in pathophysiological states is an active area of investigation. Sudden cardiac death resulting from ventricular arrhythmias is responsible for the majority of deaths from heart disease, globally. Whether death results from a heritable defect or an acquired disease, this mortality exhibits a circadian variation. A recent report demonstrated that cardiac repolarization and arrhythmogenesis are governed by circadian rhythms27. Jeyaraj and colleagues specifically identified the clock-dependent transcription factor Klf15 as a critical factor for regulation of a potassium channel interaction protein, KChIP2. KChIP2 modulates an outward rectifier potassium channel. Mice overexpressing the transcriptional regulator Kruppel-like factor 15 (Klf15) or lacking Klf15 displayed loss of rhythmic QT variation, abnormal repolarization and an increased sensitivity to induced ventricular arrhythmias. The molecular explanation for this phenomenon may involve these authors’ novel observation that potassium channel activity in the heart and QT interval exhibit circadian rhythmicity.
Gene Regulation by the Renal Circadian Clock. Whereas the circadian variation in the function of potassium transporters in the SCN and the heart has been directly studied, no such studies are yet available for potassium channels in the kidney. However, Firsov and colleagues shed significant light on this issue by microdissecting DCT/CNT (distal convoluted tubule and connecting segment) and CCD (cortical collecting duct) segments from mice every four hours for a 24 hr period to evaluate mRNA expression. Microarray analysis of expressed genes yielded the first characterization of the circadian transcriptome of these nephron segments28. For the purpose of this review, the published list of clock-controlled genes (defined by a change of at least 1.8 fold between two points) was examined for potassium transporters, channels, and ancillary subunits. The results are intriguing but have not yet been independently confirmed. Table 1 lists 28 individual potassium transport related genes that exhibit a circadian pattern of expression in either the DCT/CNT, CCD or both. The table identifies the nephron segments in which the mRNA displayed circadian expression and the acrophase (time of peak expression) is listed in zeitgeiber time (ZT), which refers to the hours that have elapsed since “lights on.” Thus, ZT0 would indicate 7 am in mice maintained on a 7am-7pm light:dark cycle. Among the listed genes are those which encode major elements in distal tubular potassium transport, including the H, K-ATPase catalytic subunits (Atp4a and Atp12a) to the beta subunit of the BK channel (Kcnmb1). This list of genes also includes several voltage gated K channels as well as inward rectifiers. Of note, the K channel associated with Long QT syndrome, Kvlqt1 (Kcnq1) exhibited circadian expression in the DCT/CNT but not in the CCD and showed a peak expression (acrophase) at ZT16.
Table 1.
| Gene Name1 | Gene Function | Acrophase2 | |
|---|---|---|---|
| CCD | DCT/CNT | ||
| Atp12a | HKalpha 2 | 4 | 8 |
| Atp1a1 | Na,K alpha 1 | 4 | 4 |
| Atp1a2 | Na,K alpha 2 | 8 | n/a |
| Atp1b2 | Na, K beta 2 | 12 | 20 |
| Atp4a | HKalpha1 | 0 | n/a |
| Kcna1 | voltage gated shaker family | 8 | n/a |
| Kcnab2 | Kv beta 2voltage gated shaker family, beta subunit | 8 | 12 |
| Kcnd2 | Kv 4.2 voltage gated, shal family | 4 | n/a |
| Kcnd3 | Kv4.3 voltage gated Sha1 related | 8 | 0 |
| Kcne4 | voltage gated Isk family | 20 | n/a |
| Kcnip1 | Kv channel interacting protein | n/a | 20 |
| Kcnj15 | Kir 1.3 inward rectifier | 12 | 12 |
| Kcnj3 | Kir 3.1 inward rectifier | n/a | 12 |
| Kcnj8 | Kir 6.1 inward rectifier | n/a | 0 |
| Kcnk6 | K2p6.1 inward rectifier | n/a | 16 |
| Kcnmb1 | BK channel beta subunit Ca activated | n/a | 16 |
| Kcnn4 | IK1, small conductance, Ca activated | n/a | 16 |
| Kcnq1 | Kvlqt1 slow delayed rectifier, voltage gated | n/a | 16 |
| Kcnq3 | Kv7.3 voltage gated | n/a | 0 |
| Kcnq4 | Kv7.4 voltage gated | n/a | 8 |
| Kcnq5 | Kv 7.5 voltage gated | 8 | 12 |
| Kcns3 | Kv 9.3 voltage gated | 4 | n/a |
| Kctd12b | tetramerization domain containing | 0 | n/a |
| Kctd14 | tetramerization domain containing | 8 | n/a |
| Kctd15 | tetramerization domain containing | 20 | 12 |
| Kctd5 | tetramerization domain containing | 8 | n/a |
| Kctd8 | tetramerization domain containing | n/a | 8 |
| Slc24a3 | NCKX3 Na/K/Ca exchanger | 8 | n/a |
Genes identified as clock-controlled in 28.
Acrophase: the time of peak expression where time 0 equals lights on
A major goal of the investigation of the circadian variation in renal tubular gene expression is the identification of those tubular transporters which are responsible for the circadian potassium cycle. In this respect there are two genes (Kcnj15 and Atp1a1) which merit attention among those genes whose expression exhibits a circadian rhythm (Table 1). Not only are they unique in being expressed in a circadian fashion in both the DCT/CNT and CCD but they also have the same acrophase at ZT12, which is the onset of the mouse's active period, during which urinary potassium levels are the highest. Kcnj15 encodes Kir1.3, an inward rectifying K channel that is expressed in the developing kidney29. Atp1a1 encodes the catalytic alpha subunit of the Na, K-ATPase; this result is consistent with the demonstrated circadian variation in Na, K-ATPase activity in the cortex of the rat kidney30. ROMK exhibited apparent circadian expression at the level of mRNA, as reported31, and it was postulated that this finding could contribute to the mechanism underlying the well-established observation that urine potassium excretion varies with the daily cycle.
We suggest these genes and the transport components they express may therefore be important elements in generating the potassium circadian cycle. Their identification indicates why the study of gene expression now constitutes a major pathway towards completing our understanding of potassium homeostasis.
In a follow up study, Firsov and colleagues examined the whole kidney circadian transcriptome9 which differed from that identified in microdissected DCT/CNT and CCD. Although no potassium transporters met the statistical test for exhibiting circadian expression in the whole kidney samples, the transcription factor Klf12 was identified as a putative clock-controlled gene in this study. This is an especially intriguing result given the identification of a KLF family member, Klf15, as a transcriptional regulator responsible for the circadian control of a critical potassium channel in the heart, as discussed above. It remains to be determined if Klf12 mediates similar effects on potassium channels in the kidney.
A recent report examined the circadian expression of Atp12a32. This gene encodes the HKα2 subunit, the catalytic portion of the “colonic” H, K-ATPase, which is also expressed in the kidney (for review 33). As noted in Table 1, this gene underwent a greater than 1.8 fold difference in expression between time points, even though this result did not reach statistical significance in the original study28. Crambert and colleagues examined serum potassium and urinary potassium excretion in Atp12a (HKα2) null mice during the active period separately from the rest period in mice. Whereas WT serum potassium was not significantly different between the day and night periods, HKα2 KO mice appeared to exhibit a decrease in serum potassium during the rest period. This result correlated with the authors’ observation that HKα2 KO mice excreted more urinary potassium during the rest period compared to WT mice. While these results are intriguing, this study raises as many questions as it answers. This study suggests a role for HKα2 in maintaining circadian potassium homeostasis; additional studies in H, K-ATPase KO animals will be necessary to improve our understanding of the role these transporters play in circadian potassium handling.
Aldosterone: Aldosterone and potassium balance have long been linked, with hyperaldosteronism frequently exhibiting hypokalemia 34. Aldosterone levels fluctuate with an apparent circadian rhythm, and peak during sleep in humans 35. Aldosterone levels may be directly controlled by the circadian clock. Plasma aldosterone levels follow a circadian pattern in rats 36 and mice 9. Interestingly, the circadian pattern in mice is disrupted in mice lacking the functional Clock protein 9, even though the 24 hr mean aldosterone values were not significantly different. Given the circadian rhythm of urinary potassium excretion, a connection between it and aldosterone was logical 36. However, as discussed above, studies in adrenalectomized animals have clearly demonstrated that the circadian excretion of potassium is unchanged in the absence of endogenous aldosterone (reviewed by Rabinowitz4). Importantly, aldosterone has been linked to the control of the asymmetric distribution of potassium between the extracellular and intracellular fluid compartments37 and it has been suggested that this distribution may have a circadian cyclic rhythm 38,14.
If the circadian oscillator plays a major role in determining the pattern of cyclic potassium excretion, then aberrant urine potassium profiles would be expected in circadian mutant mice. Indeed, in a recent report by Nikolaeva et al., the circadian profile for urinary potassium excretion is reported for the first time in WT mice and is compared to the rhythm in mice lacking the Clock protein (Clock KO)9. Consistent with reports in rats, squirrel monkeys, and humans, normal mice exhibit a circadian variation in urinary potassium excretion. Interestingly, Clock KO mice exhibited a decrease in the day/night difference in urinary K excretion (Supplemental Data in9.)
Interestingly, global loss of the renal potassium transporter, NKCC1 (Na-2Cl-K cotransporter resulted in an aberrant circadian blood pressure phenotype in mice 39. NKCC1 is expressed basolaterally in the IMCD, and is also found in extra glomerular mesangial cells and renin-producing juxtaglomerular cells of the afferent arteriole. Of note, NKCC has been proposed as the hepatoportal potassium sensor, and a regulator of urinary potassium excretion6. On a high salt diet, NKCC1 KO mice exhibited higher blood pressure in the night, during the active period39. These animals exhibited increased plasma aldosterone levels and also elevated plasma renin activity. The circadian potassium excretion profile in this mouse model could prove to be informative.
Clinical implications
Only relatively few studies have attempted to determine whether the circadian rhythm in potassium is characteristic of clinical problems and whether alterations in this rhythm may be involved in the etiology of conditions such as hypertension or abnormal fluid and electrolyte homeostasis. What little is known in this area is detailed below.
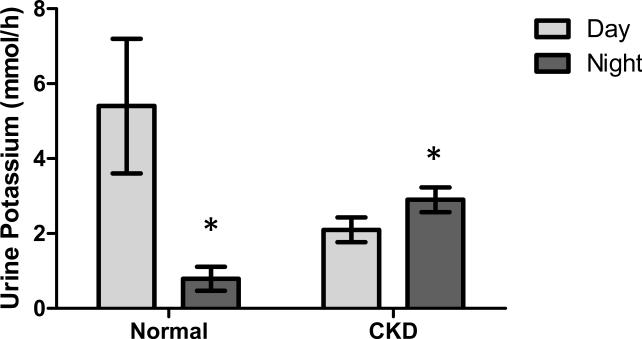
Loss of Circadian Urine Potassium Fluctuation in Disease. Agarwal investigated the day/night rhythm of urinary electrolyte secretion in 22 subjects with chronic kidney disease (CKD)40 and compared it to previously published results in normal subjects41. A comparison of these data can be seen in the replotted graph in Figure 2. As can be seen for normal, healthy individuals, the well established decrease in urinary potassium excretion at night is clearly present. However, CKD patients exhibited a reversal of the circadian potassium excretion, with a 39% increase in nighttime urine potassium levels. The mechanism of this effect is not known.
Figure 2. The Circadian Pattern of Urinary Potassium Excretion is Disrupted in Chronic Kidney Disease Patients.
Data from Agarwal40 and Koopman et al.41 were used to generate a comparison between day and night urinary potassium excretion in normal subjects compared to CKD subjects. *P<0.05.
Bankir et al. evaluated nighttime blood pressure and nocturnal dipping in several hundred subjects of African descent42. They show a positive association between nocturnal blood pressure dipping and the daytime urinary potassium excretion rate. Perucca and Bankir demonstrated that dietary potassium supplementation in rats increased urine volume and altered circadian sodium excretion patterns43. These more recent findings are consistent with previous meta-analysis findings showing that oral potassium supplementation was useful for lowering blood pressure44.
Potassium and Blood Pressure: Healthy individuals experience a “dip” in blood pressure during the night and the presence of “non-dipping” has been linked to increased risk for adverse cardiovascular events. Thus, maintaining a normal circadian pattern of blood pressure is critical to cardiovascular health. Many researchers have proposed that nighttime blood pressure is particularly important and may be a better predictor of outcomes and mortality than daytime blood pressure45,46. Potassium supplementation has been used as a therapy for hypertension (reviewed by Haddy et al. 47) and is especially effective in salt sensitive hypertension because it results in increased urinary salt excretion. Even in mostly normotensive volunteers, dietary supplementation with potassium chloride or potassium citrate had a blood pressure lowering effect 48. Given that renal salt handling has been linked to the regulation of the dipping phenotype (reviewed in Braschi and Naismith49), it follows that renal potassium handling may be critical as well. Indeed, several studies have shown that potassium supplementation leads to lowering of nocturnal blood pressure (reviewed in Vij and Peixoto50). Interestingly, even normotensive nondippers benefitted from increased potassium intake, which restored the dipping profile. All nondippers in one study were converted to dippers after three weeks of 80 mEq per day potassium intake, and this result was observed in the absence of an effect on daytime blood pressure51.
Increasing evidence demonstrates that renal sodium handling and the renal contribution to blood pressure are influenced by the circadian clock at the level of gene regulation9, 25, 28, 52. We are only beginning to understand how this same mechanism might apply to potassium transport in the kidney. On a molecular level, future studies designed to investigate the regulation of potassium transporters by the circadian clock should aid our understanding of the underlying mechanism of circadian potassium excretion. Additional clinical significance may be expected to unfold with a further understanding and recognition of the phenomenon of and mechanism underlying the potassium circadian rhythm.
Acknowledgements
This work was supported by DK085193 and University of Florida, Department of Medicine funds to MLG. The authors would like to thank Dr. Megan Greenlee for critical review of the manuscript and Jacob Richards for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mills JN. Human circadian rhythms. Physiol Rev. 1966;46:128–171. doi: 10.1152/physrev.1966.46.1.128. [DOI] [PubMed] [Google Scholar]
- 2.Youn JH, McDonough AA. Recent advances in understanding integrative control of potassium homeostasis. Annu Rev Physiol. 2009;71:381–401. doi: 10.1146/annurev.physiol.010908.163241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingo CS. Active proton secretion and potassium absorption in the rabbit outer medullary collecting duct. Functional evidence for proton-potassium- activated adenosine triphosphatase. J Clin Invest. 1989;84:361–365. doi: 10.1172/JCI114165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinowitz L. Aldosterone and potassium homeostasis. Kidney Int. 1996;49:1738–1742. doi: 10.1038/ki.1996.258. [DOI] [PubMed] [Google Scholar]
- 5.Greenlee M, Wingo CS, McDonough AA, Youn JH, Kone BC. Narrative review: Evolving concepts in potassium homeostasis and hypokalemia. Ann Intern Med. 2009;150:619–625. doi: 10.7326/0003-4819-150-9-200905050-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita H, Fujiki N, Miyahara T, Lee K, Tanaka K. Hepatoportal bumetanide-sensitive k(+)-sensor mechanism controls urinary k(+) excretion. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1134–1139. doi: 10.1152/ajpregu.2000.278.5.R1134. [DOI] [PubMed] [Google Scholar]
- 7.Lee FN, Oh G, McDonough AA, Youn JH. Evidence for gut factor in k+ homeostasis. Am J Physiol Renal Physiol. 2007;293:F541–547. doi: 10.1152/ajprenal.00427.2006. [DOI] [PubMed] [Google Scholar]
- 8.Moore-Ede MC. Physiology of the circadian timing system: Predictive versus reactive homeostasis. Am J Physiol. 1986;250:R737–752. doi: 10.1152/ajpregu.1986.250.5.R737. [DOI] [PubMed] [Google Scholar]
- 9.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, Bonny O, Firsov D. The circadian clock modulates renal sodium handling. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2011080842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarelius IH, Greenway RM. Rhythmic fluctuations in the urine composition of sheep: Separation of feed-dependent from other rhythms. Pflugers Arch. 1975;355:243–259. doi: 10.1007/BF00583687. [DOI] [PubMed] [Google Scholar]
- 11.Moore Ede MC, Brennan MF, Ball MR. Circadian variation of intercompartmental potassium fluxes in man. J Appl Physiol. 1975;38:163–170. doi: 10.1152/jappl.1975.38.1.163. [DOI] [PubMed] [Google Scholar]
- 12.Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci U S A. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton PL, Rabinowitz L. Amiloride effect on diurnal cyclic na and k excretion in rats. Am J Physiol. 1989;256:R510–517. doi: 10.1152/ajpregu.1989.256.2.R510. [DOI] [PubMed] [Google Scholar]
- 14.Rabinowitz L, Wydner CJ, Smith KM, Yamauchi H. Diurnal potassium excretory cycles in the rat. Am J Physiol. 1986;250:F930–941. doi: 10.1152/ajprenal.1986.250.5.F930. [DOI] [PubMed] [Google Scholar]
- 15.Poulis J, Roelfsema F, van der Heide D, Smeenk D. Resynchronization patterns for urinary rhythms in rats after light-dark shifts. Am J Physiol. 1985;249:R402–409. doi: 10.1152/ajpregu.1985.249.4.R402. [DOI] [PubMed] [Google Scholar]
- 16.Rutledge JC, Rabinowitz L. Kaliuretic regulatory factors in the rat. Am J Physiol. 1987;253:F1182–1196. doi: 10.1152/ajprenal.1987.253.6.F1182. [DOI] [PubMed] [Google Scholar]
- 17.Ikonomov OC, Stoynev AG, Vrabchev NC, Shisheva AC, Tarkolev NT. Circadian rhythms of food and 1% nacl intake, urine and electrolyte excretion, plasma renin activity and insulin concentration in adrenalectomized rats. Acta Physiol Hung. 1985;65:181–198. [PubMed] [Google Scholar]
- 18.Poulis JA, Roelfsema F, van der Heide D. Circadian urinary excretion rhythms in adrenalectomized rats. Am J Physiol. 1986;251:R441–449. doi: 10.1152/ajpregu.1986.251.3.R441. [DOI] [PubMed] [Google Scholar]
- 19.Moore-ede MC, Schmelzer WS, Kass DA, Herd JA. Cortisol-mediated synchrinization of circadian rhythm in urinary potassium excretion. Am J Physiol. 1977;233:R230–238. doi: 10.1152/ajpregu.1977.233.5.R230. [DOI] [PubMed] [Google Scholar]
- 20.Moore-Ede MC, Meguid MM, Fitzpatrick GF, Boyden CM, Ball MR. Circadian variation in response to potassium infusion. Clin Pharmacol Ther. 1978;23:218–227. doi: 10.1002/cpt1978232218. [DOI] [PubMed] [Google Scholar]
- 21.Rabinowitz L, Berlin R, Yamauchi H. Plasma potassium and diurnal cyclic potassium excretion in the rat. Am J Physiol. 1987;253:F1178–1181. doi: 10.1152/ajprenal.1987.253.6.F1178. [DOI] [PubMed] [Google Scholar]
- 22.Aizman RI, Rabinowitz L, Mayer-Harnisch C. Early effects of uninephrectomy on k homeostasis in unanesthetized rats. Am J Physiol. 1996;270:R434–442. doi: 10.1152/ajpregu.1996.270.2.R434. [DOI] [PubMed] [Google Scholar]
- 23.Loudon AS, Semikhodskii AG, Crosthwaite SK. A brief history of circadian time. Trends Genet. 2000;16:477–481. doi: 10.1016/s0168-9525(00)02122-3. [DOI] [PubMed] [Google Scholar]
- 24.Gumz ML, Cheng KY, Lynch IJ, Stow LR, Greenlee MM, Cain BD, Wingo CS. Regulation of alphaenac expression by the circadian clock protein period 1 in mpkccd(c14) cells. Biochim Biophys Acta. 2010;1799:622–629. doi: 10.1016/j.bbagrm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stow LR, Richards J, Cheng KY, Lynch IJ, Greenlee MM, Cain BD, Wingo CS, Gumz ML. The circadian protein per1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension. 2012 doi: 10.1161/HYPERTENSIONAHA.112.190892. Accepted for Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko GY, Shi L, Ko ML. Circadian regulation of ion channels and their functions. J Neurochem. 2009;110:1150–1169. doi: 10.1111/j.1471-4159.2009.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS, Jain MK. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–99. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A. 2009;106:16523–16528. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiery E, Gosset P, Damotte D, Delezoide AL, de Saint-Sauveur N, Vayssettes C, Creau N. Developmentally regulated expression of the murine ortholog of the potassium channel kir4.2 (kcnj15). Mech Dev. 2000;95:313–316. doi: 10.1016/s0925-4773(00)00364-6. [DOI] [PubMed] [Google Scholar]
- 30.Segura D, Eblen-Zajjur A, Proverbio F, Proverbio T, Carrera F, Caruso-Neves C, Marin R. A blood plasma inhibitor is responsible for circadian changes in rat renal na,k-atpase activity. Int J Biochem Cell Biol. 2004;36:2054–2065. doi: 10.1016/j.biocel.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Firsov D, Tokonami N, Bonny O. Role of the renal circadian timing system in maintaining water and electrolytes homeostasis. Mol Cell Endocrinol. 2012;349:51–55. doi: 10.1016/j.mce.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Salhi A, Centeno G, Firsov D, Crambert G. Circadian expression of h,katpase type 2 contributes to the stability of plasma k+ levels. FASEB J. 2012 doi: 10.1096/fj.11-199711. [DOI] [PubMed] [Google Scholar]
- 33.Gumz ML, Lynch IJ, Greenlee MM, Cain BD, Wingo CS. The renal h+-k+-atpases: Physiology, regulation, and structure. Am J Physiol Renal Physiol. 2010;298:F12–21. doi: 10.1152/ajprenal.90723.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan-Smith Z, Stewart PM. Inherited forms of mineralocorticoid hypertension. Curr Opin Endocrinol Diabetes Obes. 2011;18:177–185. doi: 10.1097/MED.0b013e3283469444. [DOI] [PubMed] [Google Scholar]
- 35.Fu L, Lee CC. The circadian clock: Pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 36.Hilfenhaus M. Circadian rhythm of the renin-angiotensin-aldosterone system in the rat. Arch Toxicol. 1976;36:305–316. doi: 10.1007/BF00340536. [DOI] [PubMed] [Google Scholar]
- 37.Young DB, Jackson TE. Effects of aldosterone on potassium distribution. Am J Physiol. 1982;243:R526–530. doi: 10.1152/ajpregu.1982.243.5.R526. [DOI] [PubMed] [Google Scholar]
- 38.Firsov D, Bonny O. Circadian regulation of renal function. Kidney Int. 2010;78:640–645. doi: 10.1038/ki.2010.227. [DOI] [PubMed] [Google Scholar]
- 39.Kim SM, Eisner C, Faulhaber-Walter R, Mizel D, Wall SM, Briggs JP, Schnermann J. Salt sensitivity of blood pressure in nkcc1-deficient mice. Am J Physiol Renal Physiol. 2008;295:F1230–1238. doi: 10.1152/ajprenal.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal R. Relationship between circadian blood pressure variation and circadian protein excretion in ckd. Am J Physiol Renal Physiol. 2007;293:F655–659. doi: 10.1152/ajprenal.00188.2007. [DOI] [PubMed] [Google Scholar]
- 41.Koopman MG, Koomen GC, Krediet RT, de Moor EA, Hoek FJ, Arisz L. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci (Lond) 1989;77:105–111. doi: 10.1042/cs0770105. [DOI] [PubMed] [Google Scholar]
- 42.Bankir L, Bochud M, Maillard M, Bovet P, Gabriel A, Burnier M. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in african subjects. Hypertension. 2008;51:891–898. doi: 10.1161/HYPERTENSIONAHA.107.105510. [DOI] [PubMed] [Google Scholar]
- 43.Perucca J, Bankir L. Dietary potassium supplementation increases urine volume and alters the circadian pattern of sodium excretion: Possible mechanism for its lowering effect on blood pressure. FASEB J. 2007 Abstract A510. [Google Scholar]
- 44.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA. 1997;277:1624–1632. doi: 10.1001/jama.1997.03540440058033. [DOI] [PubMed] [Google Scholar]
- 45.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. doi: 10.1161/HYPERTENSIONAHA.109.133900. [DOI] [PubMed] [Google Scholar]
- 46.Ernst ME. Nighttime blood pressure is the blood pressure. Pharmacotherapy. 2009;29:3–6. doi: 10.1592/phco.29.1.3. [DOI] [PubMed] [Google Scholar]
- 47.Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R546–552. doi: 10.1152/ajpregu.00491.2005. [DOI] [PubMed] [Google Scholar]
- 48.Braschi A, Naismith DJ. The effect of a dietary supplement of potassium chloride or potassium citrate on blood pressure in predominantly normotensive volunteers. Br J Nutr. 2008;99:1284–1292. doi: 10.1017/S0007114507864853. [DOI] [PubMed] [Google Scholar]
- 49.Stow LR, Gumz ML. The circadian clock in the kidney. J Am Soc Nephrol. 2011;22:598–604. doi: 10.1681/ASN.2010080803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vij R, Peixoto AJ. Management of nocturnal hypertension. Expert Rev Cardiovasc Ther. 2009;7:607–618. doi: 10.1586/erc.09.42. [DOI] [PubMed] [Google Scholar]
- 51.Wilson DK, Sica DA, Devens M, Nicholson SC. The influence of potassium intake on dipper and nondipper blood pressure status in an african-american adolescent population. Blood Press Monit. 1996;1:447–455. [PubMed] [Google Scholar]
- 52.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS. The circadian clock protein period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest. 2009;119:2423–2434. doi: 10.1172/JCI36908. [DOI] [PMC free article] [PubMed] [Google Scholar]