Abstract
Angiogenesis is likely critical for the process of intramembranous osteogenesis; however the developmental relationship between blood vessels and bone mineralization is not well studied within intramembranous bones. Given its importance, changes in angiogenesis regulation are likely to contribute to evolutionarily and medically relevant craniofacial variation. We summarize what is known about the association between angiogenesis and intramembranous osteogenesis, supplementing with information from the better studied processes of endochondral ossification and distraction osteogenesis. Based on this review, we introduce a model of angiogenesis during early intramembranous osteogenesis as well as a series of null hypotheses to be tested. This model can serve as a basis of future research on the spatio-temporal association and regulatory interactions of mesenchymal, vascular, and bone cells, which will be required to illuminate the potential effects of angiogenesis dysregulation on craniofacial skeletal phenotypes.
Keywords: Craniofacial Development, Intramembranous Ossification, Vascular Invasion
Introduction
The development of the craniofacial skeleton is based on complex gene expression patterns, cell-cell interactions, and the functional pressures of a number of critically important organs. The coordinated development of multiple tissues is critical in craniofacial development, such that developmental dysregulation of one tissue can influence the development of another. This includes interactions between relatively independent developmental modules, such as the eye and the craniofacial skeleton (Kish et al., 2011), as well as more direct interactions necessary for basic morphogenesis (e.g., epithelial-mesenchymal). For example, bone mineralization during endochondral and intramembranous ossification is tightly coupled with blood vessel branching and growth (angiogenesis) (Marks and Odgren, 2002), making variation in angiogenesis a plausible basis for variation in osteogenesis. This includes evolutionarily relevant variation such as a reduction in facial bone size during human evolution. In addition, angiogenesis dysregulation may contribute to medically relevant conditions including the postnatal result of vasculopathies (Dietrich and Antoniades, 2012) and prenatally induced craniofacial dysmorphology such as midfacial hypoplasia and craniosynostosis (Percival, 2013). However, in order to determine the effect of variation in angiogenesis on craniofacial skeletal phenotype, the underlying mechanisms of their association must be identified.
Compared to endochondral long bone ossification, intramembranous ossification is poorly understood (Abzhanov et al., 2007; Karaplis, 2008), including the mechanisms that underlie known spatio-temporal associations between angiogenesis and intramembranous osteogenesis. The lack of fundamental information about the developmental interactions between blood vessels and mineralizing intramembranous bones contributes to our inadequate understanding of skull formation. Because of the relatively small number of studies that focus on angiogenesis and intramembranous bones, information obtained in the study of endochondral bones and distraction osteogenesis provide the major basis for the formulation of simple hypotheses about the role that angiogenesis plays during intramembranous bone formation.
Intramembranous bones, which include many craniofacial bones and the clavicle, ossify directly from preosteogenic condensations of multipotent mesenchymal cells while prechondrogenic condensations differentiate into a cartilage model before endochondral ossification occurs. A significant divergence in gene expression patterns within preosteogenic and prechondrogenic condensations (Eames and Helms, 2004), including Wnt expression (Karaplis, 2008) is associated with differences in mesenchymal cell fate. During the ossification of intramembranous condensations and endochondral cartilages, metabolic and morphogenetic differences are noted between these two modes of osteogenesis (Hall, 2005), but similar expression patterns of genes associated with cell differentiation suggest strong parallels in regulation of bone formation (Eames and Helms, 2004). Using what is known about endochondral osteogenesis to develop a model for intramembranous osteogenesis is logical, but these models must be tested.
This review focuses on what is known about the relationship between angiogenesis and osteogenesis in order to develop simple hypotheses about the role that angiogenesis plays during intramembranous bone formation. First, the association of vasculature with bone mesenchymal condensations and the process of intramembranous ossification are reviewed. Second, the potential regulatory basis of angiogenesis during intramembranous osteogenesis is discussed. Third, the reasons why vasculature is critical for intramembranous osteogenesis are summarized. Finally, a model of the association between angiogenesis and intramembranous osteogenesis is proposed. Specific hypotheses based on this model are highlighted and implications for medical and evolutionary questions are discussed.
Vasculature and Mesenchymal Condensations
Intramembranous bones and the cartilage anlages that mineralize during endochondral ossification are derived from condensations of multipotent mesenchymal cells. The mesenchymal precursors of the craniofacial complex arise either as cephalic neural crest cells from the neuroectoderm or cells from the paraxial mesoderm (Fig 1) (Jiang et al., 2002; Noden and Trainor, 2005; McBratney-Owen et al., 2008; Yoshida et al., 2008). Regardless of cellular origin, bones of the cranial base and caudal cranial vault ossify endochondrally, while bones of the face and rostral cranial vault ossify intramembranously (Fig 1) (Depew et al., 2002).
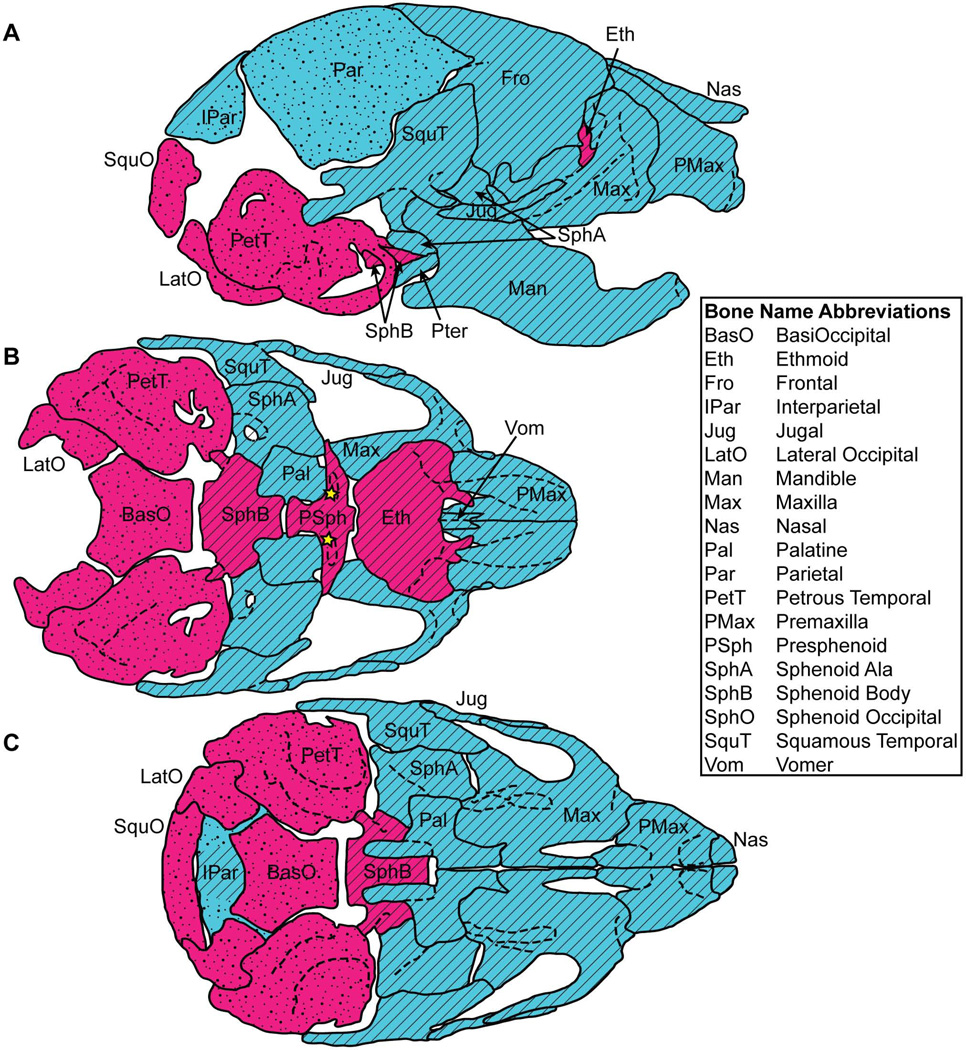
Figure 1.
Ossification type and cellular origin of postnatal day eight (P8) mouse craniofacial bones, as shown on (A) a right lateral view, (B) a superior interior view lacking calvaria, nasals, and a mandible, and (C) an interior view lacking a mandibles. Red: endochondral ossification; Blue: intramembranous ossification; Diagonal lines: neural crest derived cellular origin; Dots: mesoderm derived cellular origin. The stars identify small portions of medosderm derived bone within the mostly neural crest derived presphenoid. Ossification identification from (Depew et al., 2002). Cellular origin of cranial base from (McBratney-Owen et al., 2008). Calvarial cellular origin from (Jiang et al., 2002). Other cellular origin from (Noden and Trainor, 2005).
Mesoderm derived vasculature is found within populations of loose mesenchymal cells, including loose mesenchyme derived from neural crest cells (Yoshida et al., 2008). However, prechondrogenic and preosteogenic mesenchymal condensations formed from these populations do not display any vasculature from the time of their initial condensation until their ossification (Eames et al., 2003). A core region that includes the forming mesenchymal condensation and a thin layer of surrounding loose mesenchyme becomes avascular in chick (Drushel et al., 1985; Feinberg et al., 1986) and mouse limb buds (Eshkar-Oren et al., 2009). Avascular limb condensations differentiate into cartilage, while the surrounding vascularized region differentiates into muscle and other tissues. Avascularity may be necessary for aggregation of mesenchymal cells into dense homogeneous condensations (Yin and Pacifici, 2001) or to provide positional cues for chondrocyte differentiation (Caplan et al., 1983; Drushel et al., 1985) via reduction in factors present near vessels (Yin and Pacifici, 2001) or increased hypoxia (Eshkar-Oren et al., 2009). After the differentiation of prechondrogenic condensations into cartilage models, the resulting cartilage remains avascular through expression of an anti-angiogenic factor that prevents vascular invasion, although they may contain vascular canals (Kuettner et al., 1983).
Sites of intramembranous bone formation have also been associated with avascular zones, but are less well studied. An avascular zone surrounds chick mesenchymal scleral condensations at their initial formation (Jourdeuil and Franz-Odendaal, 2012), chick frontal bone mesenchymal condensations prior to their ossification (Thompson et al., 1989), chick mandibular condensations (Eames and Helms, 2004), and rat mandibular condensations prior to ossification (Zernik et al., 1990). Chick frontal bone mesenchymal condensations are surrounded by a thin avascular layer of loose mesenchyme (Thompson et al., 1989), similar to those seen in limb bud condensations. Even though few studies mention the association of vasculature and preosteogenic mesenchymal condensations, it is likely that avascular regions surround preosteogenic condensations in avian and mammalian species. However, even in the case of prechondrogenic condensations, the mechanism underlying the establishment of avascular zones remains unclear (Eshkar-Oren et al., 2009). Given the evidence of avascular zones surrounding avascular preosteogenic condensations, we postulate that the avascularity is necessary for condensation growth and subsequent intramembranous ossification, as is the case during differentiation and ossification of prechondrogenic condensations. Even if we are correct, it is unknown whether the same molecular signals lead to avascualrity in preosteogenic and prechondrogenic condensations.
Intramembranous Osteogenesis
Most information pertaining to intramembranous osteogenesis comes from research focusing on the frontal and parietal bones, whose mesenchymal condensations initiate at the supraorbital ridge. Initial ossification of these condensations occurs as presumptive bone cells rapidly proliferate and migrate outwards from the condensations. Studies utilizing DiI staining provide evidence that the bone primordial cells come from the condensations rather than being recruited from other mesenchymal populations surrounding the brain (Yoshida et al., 2008; Ting et al., 2009). Studies staining for osteopontin (Iseki et al., 1997), ALP expression (Ting et al., 2009), and BSP expression (Rice et al., 2000) collectively suggest a pattern of presumptive bone cells expanding outward from mesenchymal condensations, particularly towards the apex of the head. During the earliest embryonic days of vault bone osteogenesis and under normal regulatory conditions, these expanding condensations define the primary region of osteoblast differentiation and osteogenesis, as well as the early shape of the developing bone.
After this period of quick condensation expansion and initial mineralization, growth of vault bones toward each other is driven by proliferation and differentiation of preosteogenic mesenchymal cells at osteogenic fronts along fibrous sutures (Iseki et al., 1997; Liu et al., 1999; Rice et al., 2003), as earlier ossified portions thicken and form a trabecular structure (Yoshida et al., 2008). Differentiated osteoblasts first form bone spicules, which develop and eventually fuse together to form trabeculae, which become interconnected to form woven bone (Kanczler and Oreffo, 2008). As first described more than a century ago (e.g. Thoma, 1913; Murray, 1985) and supported by images from recent studies (Fig 2), the initial woven bone of flat intramembranous bones can be described as a bone lattice or network that is filled in as ossification progresses.
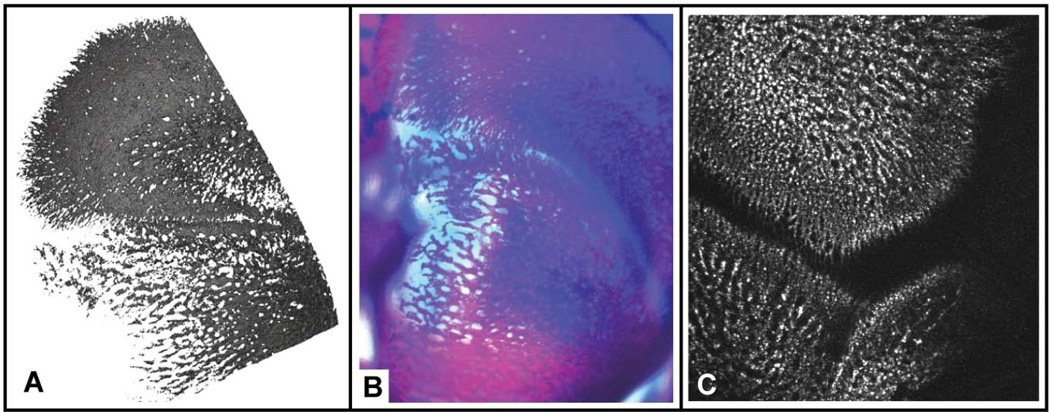
Figure 2.
High resolution images of perinatal mouse frontal and parietal bones produced by multiple imaging modalities. Note the lattice-like pattern at the edges of ossification, with bone filling in the gaps in older areas of bone. All images are an oblique dorsal view for which caudal is towards the bottom of the image and medial is to the left. A) Surface reconstruction around the medial coronal suture of a newborn mouse from an image produced at the High-Resolution X-ray Computed Tomography Facility at the University of Texas at Austin. The hard edges of the image represent the extent of the region of the bone that was imaged. B) Lightfield microscope image of the medial coronal suture of a whole-mount Ailzarin red/Alacian blue clear and stained E18.5 mouse. Image courtesy of Mizuho Kawasaki and Kazuhiko Kawasaki. C) Two photon laser scanning microscopy image of the lateral coronal suture of an E19.5 C57BL/6 mouse. The bones have been marked with calcein. Image produced by Kevin Flaherty and Patrick Drew at Penn State.
Distinct from endochondral ossification, where initial mineralization occurs within a cartilage model possessing the rough shape of the adult bone (Caplan et al., 1983; Colnot et al., 2004), the initial stage of intramembranous osteogenesis is mineralization of a collagenous framework produced by differentiating osteoblasts within a rapidly expanding population of mesenchymal cells. Only after the stage of mesenchymal cell expansion is complete, at a point when ossification is well underway in some parts of the bone, does vault bone growth begin to resemble more traditional descriptions of radial growth from a center (Lana-Elola et al., 2007; Yoshida et al., 2008).
Intramembranous ossification of non-vault bones (e.g., bones of the facial skeleton) is not well described. For example, initial ossification of the rat dentary is noted as an arch surrounding Meckel’s cartilage (Zernik et al., 1990). It is not known whether the cartilage plays a regulatory role in the ossification of the intramembranous dentary or serves as a morphological template. Whether cells from an initial mandibular mesenchymal condensation expand outward as ossification begins, as in vault bone condensations, is also unknown.
Angiogenesis and Intramembranous Ossification
The critical role of angiogenesis during endochondral long bone ossification has been well established and its importance during intramembranous ossification is generally assumed. We use research on the role of angiogenesis during endochondral osteogenesis and distraction osteogenesis to supplement current information on the role of angiogenesis during intramembranous ossification. While intusucceptive angiogenesis (Levin et al., 2007) may produce vessels associated with craniofacial bone development (De Spiegelaere et al., 2010), recent histological work continues to support the idea that sprouting angiogenesis, a process based on the extension of vessel sprouts outward from existing vasculature, is the primary form of angiogenesis associated with osteogenesis (Maes et al., 2010).
Craniofacial bones are known to be well vascularized (Brookes and Revell, 1998), but only a single study of chick frontal bone osteogenesis outlines the association of angiogenesis with intramembranous osteogenesis of a vault bone (Thompson et al., 1989). According to this study, during the developmental period just prior to initial ossification of the frontal bone, small bore capillaries move into the thin avascular layer of loose mesenchyme surrounding the mesenchymal condensation. These small vessels then invade the condensation at or near the site of initial ossification at the supraorbital ridge. After a short time, the earliest mineralized bone is associated with extensive internal and external vascularization, while the cascade of vascular invasion and ossification continues as a front of bone expansion moving outward in all directions. Within the earlier ossified and maturing bone of the chick frontal, the osteoblast layer is adjacent to large bore capillaries (Thompson et al., 1989).
This description suggests similarities in the pattern of vascular invasion of vault mesenchymal condensations and the cartilage model of long bones at the point of initial ossification. During endochondral ossification of a long bone, vasculature enters the avascular buffer layer of loose mesenchymal cells surrounding the mid-diaphysis of the cartilage model just before the initiation of angiogenesis and the mineralization of a bone collar within the perichondrium (Mackie et al., 2008; Takimoto et al., 2009; Nakamura et al., 2010). This vascular invasion of the perichondrium is necessary for osteoblast differentiation within it, its transformation into the periosteum, and for subsequent vascular invasion at the mid-diaphysis of the cartilage model by endothelial sprouts originating in the perichondrium (Zelzer et al., 2002; Colnot et al., 2004; Takimoto et al., 2009). Chondroclasts are found preceding the tips of invading capillaries during osteogenesis (Fig 3B), allowing endothelial cells to make their way through hypertrophic cartilage (Lewinson and Silbermann, 1992; Streeten and Brandi, 1990). In addition, capillary sprouts made of endothelial cells are found in close association with preosteoblasts during the first stages of endochondral osteogenesis (Maes et al., 2010). After ossification at the mid-diaphysis, the developmental cascade of chondrocyte hypertrophy, vascular invasion, osteoid formation, and calcification moves towards the ends of the bone (Caplan et al., 1983). After the formation of epiphyseal growth plates, the cascade continues postnatally as the basis for increasing long bone length, a process that has been well described (Fig 3A) (Kronenberg, 2003; Mackie et al., 2008; Amizuka et al., 2012). Although similarities between the vascular invasion into surrounding avascular loose mesenchyme and the association of invading vessels with mineralization exist between calvarial and long bone osteogenesis, fundamental differences in the osteogenesis of endochondral and intramembranous bones suggest the potential for differences in the role of angiogenesis during the two types of bone formation. These observations prompt our proposal of a distinct model of intramembranous ossification.
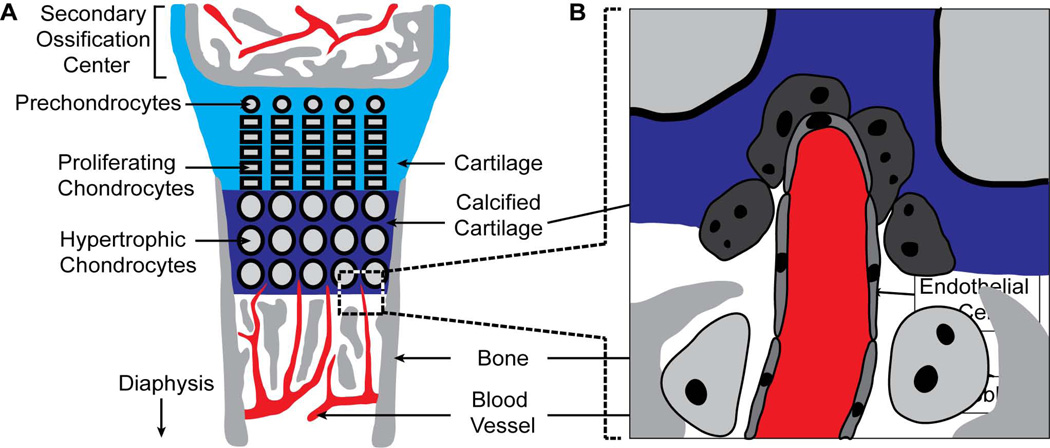
Figure 3.
Schematic of the epiphyseal ossification of endochondral long bones, with emphasis on the process of capillary growth into calcified epiphyseal cartilage and subsequent trabecular ossification. A) Chondrocytes differentiate from proliferating prechondrocytes within the growth plate. The chondrocytes are pushed toward the diaphysis by this continuous process and then enlarge under hypoxia, leading to mineralization of surrounding cartilage and the attraction of blood vessels required for bone formation. B) A magnified view of bracketed zone from ‘A’ showing capillaries, in association with chondroclasts, growing towards hypertrophic chondrocytes as a precursor to osteoblast activity and bone growth at the epiphysis. Figure adapted from (Streeten and Brandi, 1990; Lewinson and Silbermann, 1992; Bloom and Fawcett, 1994; Kronenberg, 2003).
Distraction Osteogenesis
Although fundamentally different from the initial osteogenesis of a normally expanding mesenchymal condensation, distraction osteogenesis, for which intramembranous ossification is the primary mode of ossification (Aronson et al., 1990; Delloye et al., 1990), provides another reference point for our model of intramembranous osteogenesis. During distraction osteogenesis, after the initial separation, a bone callus of rigid connective tissue forms in response to tissue inflammation. A central fibrous interzone made up of fibroblasts, chondrocyte-like cells, and cells of intermediate morphology forms when tensile forces are applied to the callus (Fig 4) (Choi et al., 2002; Al-Aql et al., 2008). Vascular in-growth appears on either side of the fibrous interzone within which osteoblasts begin laying down osteoid along collagen bundles, forming the zone of microcolumn (linear bone features) formation. In between the zone of microcolumn formation and the fibrous interzone is a zone of proliferating cells called the primary mineralization front, which also overlaps with the encroaching vasculature (Fig 4). Vascular sinuses formed by the vascular in-growth are the sites from which bone formation begins (Choi et al., 2002). After distraction ceases, the microcolumns of osteoid and bone grow towards each other, filling the fibrous interzone. Remodeling of the bony region is the last step in the process.
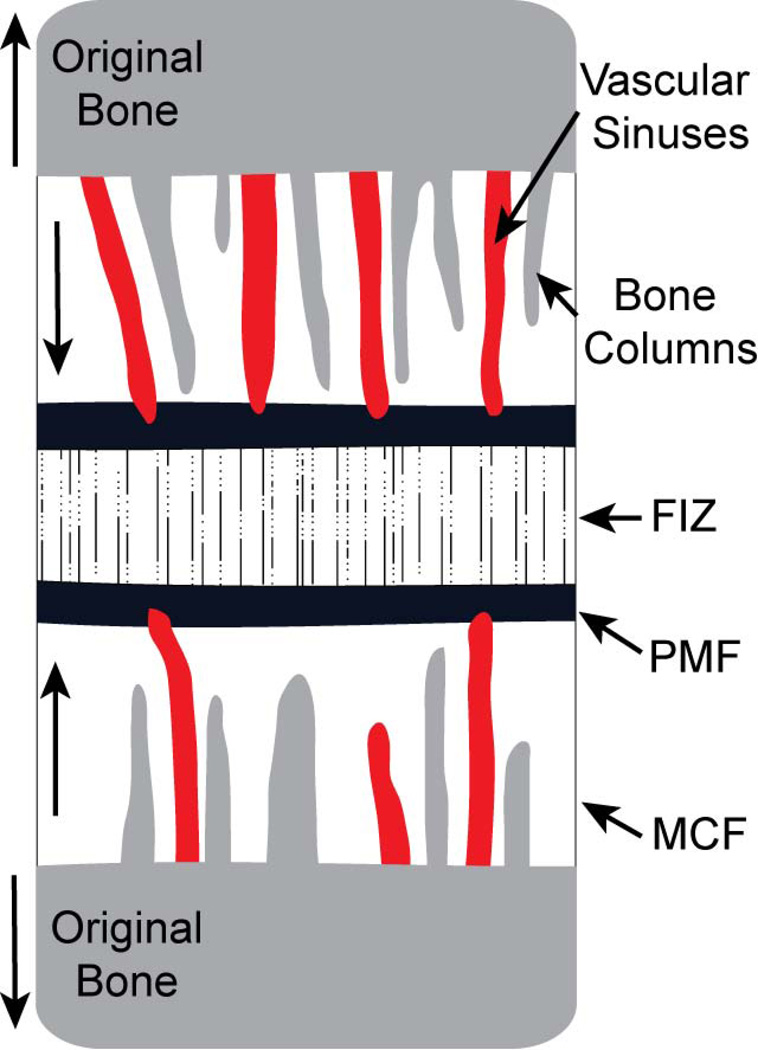
Figure 4.
Schematic of the cellular zones of distraction osteogenesis. The fibrous interzone (FIZ) forms first and is composed of a variety of cells, including osteoblasts that deposit osteoid along parallel collagen bundles. Zones of microcolumn formation (MCF) form on either side of the insult and include invading vascular sinuses and vessels originating from the cut bone. These vessels and microcolumns of mineralizing bone are parallel to tension applied during distraction. Between the FIZ and MCF is the primary mineralization front (PMF), which is a thin zone of high cellular proliferation. The MCF continues expanding as the portions of the original bone are pulled apart, while the FIZ remains a constant width.
During distraction osteogenesis, there is a significant increase in blood supply and rates of blood flow at sites of bone formation. Vessels of uniform diameter extend from the surfaces of the cut bone toward each other along the collagenous fibers, but do not enter the fibrous interzone (Aronson et al., 1990; Delloye et al., 1990; Aronson, 1994; Choi et al., 2000). Just ahead of the mineralization front in the fibrous interzone are parallel capillaries that have a close temporal and spatial relationship with sites of new mineralization at the distraction gap. After distraction ceases, these vessels aligned along collagen fibers grow towards each other and meet before the gap is completely filled with osteogenic tissue (Choi et al., 2002). In rats undergoing distraction osteogenesis, treatment with an angiogenic inhibitor led to non-union of the separated bones, a lack of both ossified bone and blood vessels between the original cut bone portions, and reduced expression of a number of genes, including those associated with osteogenesis. Additional experiments where mechanical tension was not introduced led to fibrous tissue lacking evidence of vasculature between the separated bones (Fang et al., 2005). This suggests that in addition to tension, the expression of angiogenic factors and subsequent vascular invasion towards the fibrous interzone are necessary for intramembranous ossification to occur during distraction osteogenesis.
Other studies have indicated that tension across sutures between growing intramembranous bones leads to an upregulation of certain gene products, including angiogenic and osteogenic fibroblast growth factor 2 (FGF-2), leading to the addition of bone at the sutural edge of vault bones (Yu et al., 2001; Opperman and Rawlins, 2005). This implies that distraction osteogenesis might be a good model for intramembranous ossification occurring at sutures and that the inhibition of angiogenesis at the sutures might reduce the rate of ossification.
Although not discussed in detail here, there are similar associations between blood vessels and bone formation during stress fracture healing (Wohl et al., 2009), another model of non-endochondral bone formation. A recent study includes evidence that osteogenesis is impaired when angiogenesis is inhibited (Tomlinson et al., 2013). Studies of stress fracture healing, although perhaps more similar to bone remodeling than bone formation, provide another valuable model where angiogenesis is necessary for osteoblast activity.
During distraction osteogenesis, angiogenesis is necessary for ossification and directly precedes, in time and space, the appearance of differentiated osteoblasts and mineralized bone, as it does at epiphyseal growth plates of endochondral long bones. This concordance suggests that angiogenesis likely precedes and is necessary for intramembranous bone osteogenesis. However, the initiation of intramembranous ossification in a developing head/organism and intramembranous bone formation by distraction osteogenesis may be fundamentally different processes.
Regulation of Intramembranous Angiogenesis
Because hypoxia of chondrocytes is the basis for gene expression associated with angiogenesis of endochondral cartilage models and because hypoxia promotes angiogenesis in many other contexts, it is typically assumed that hypoxia of avascular mesenchymal condensations is a major regulatory basis for angiogenesis associated with intramembranous osteogenesis. Vascular endothelial growth factor (VEGF) is an important regulator of angiogenesis in endochondral ossification from the earliest limb bud formation through long bone epiphyseal growth. VEGF expression in condensing limb bud mesenchyme helps to determine vascularization of regions around growing prechondrogenic condensations (Eshkar-Oren et al., 2009). Vessels are attracted towards cartilaginous bone models before their initial ossification by high levels of VEGF expression in the perichondrium and nearby mesenchyme (Towler, 2008; Zelzer et al., 2002; Takimoto et al., 2009). VEGF expression by hypertrophic chondrocytes is critical for vascular invasion and subsequent ossification at the initial site of mid-diaphyseal ossification (Zelzer et al., 2002) and in the epiphyseal growth plates (Gerber et al., 1999). Deletion of a single VEGF allele leads to embryonic lethality in mice, while the loss of certain isoforms leads to serious skeletal defects of postcranial and calvarial bones, associated with delayed vascular invasion (Zelzer et al., 2002). However, VEGF mediates not only angiogenesis, but chondrocyte differentiation, osteoblast differentiation, and osteoclast recruitment (Zelzer and Olsen, 2004; Dai and Rabie, 2007), revealing that its regulatory role during osteogenesis is complex.
Hypoxia inducible factor (HIF), commonly upregulated in regions of hypoxia (Pugh and Ratcliffe, 2003), but also upregulated via oxygen-independent mechanisms (Chun et al., 2002; Patel et al., 2010), is a promoter of VEGF expression and is known to directly regulate osteoblasts in endochondral bones (Towler, 2008; Rankin et al., 2011). Increased expression of HIFα in osteoblasts of early postnatal mice is associated with increased VEGF expression, increased vascular density in their long bones as well as increased long bone growth rate, leading to increased femur bone volume and trabecular number (Wang et al., 2007). However, it has been shown that other factors (Towler, 2008), including bone morphogenetic proteins (BMPs) (Langenfield and Langenfield, 2004; Towler, 2008) and fibroblast growth factor (FGFs) (Saadeh et al., 2000; Takai et al., 2007) can also upregulate VEGF expression associated with ossification (Kawaguchi et al., 2001; Liu et al., 2007). In addition, a number of genetic factors, independently respond to hypoxia in tissue culture, including angiopoietins, FGFs, and genes involved in matrix metabolism (Pugh and Ratcliffe, 2003). Producing pro-angiogenic factors is one way that osteoblast lineage cells can regulate the activity of endothelial and other vascular cells. Further experimental studies will be necessary to identify which genes expressed by osteoblasts during intramembranous osteogenesis play a role in regulating vascular cell migration and activity.
Factors not associated with hypoxia also play significant roles in angiogenesis associated with endochondral ossification. For example, a lack of vitamin D commonly associated with rickets leads to a lack of normal vascular invasion into hypertrophic epiphyseal cartilage as well as reduced bone growth and lower levels of mineralization (Hunter et al., 1991). While a higher than normal level of vitamin D is unlikely to promote angiogenesis towards non-hypoxic regions of cartilage, increased hypoxia cannot cause angiogenesis at the epiphysis in the absence of vitamin D.
It is likely that other non-genetic and genetic factors that are not associated with hypoxia are important for normal angiogenesis associated with osteogenesis. This is especially true of intramembranous ossification, for which the assumption of hypoxia driven angiogenesis, however logical or likely, remains untested. Local angiogenesis is likely critical for the intramembranous ossification of preosteogenic mesenchymal condensations, but this does not necessarily mean that hypoxia serves as the primary basis of the signals that lead to angiogenesis in this context. While hypoxic cartilage cells within cartilage bone models serve to upregulate angiogenesis during endochondral bone growth, is there a similarly thick mass of hypoxic cells near sites of initial intramembranous ossification that drives angiogenesis?
Expression comparisons of angiogenesis factors often associated with hypoxia between fetal pig forelimb and palate revealed that the HIF-α1 isoform was detected only in endochondral bones, while isoform HIF-α2 and HIF associated factors angiopoietin and VEGF were expressed in both intramembranous and endochondrally forming bone (De Spiegelaere et al., 2010). In addition to this potential difference in HIF expression, experimentally increased HIFα leads to increased vasculature of murine long bones while calvarial bones are reportedly unaffected (Wang et al., 2007). Many of the factors associated with upregulation of angiogenesis during endochondral osteogenesis are expressed during intramembranous osteogenesis, supporting the idea that angiogenesis is important during intramembranous bone formation. It also suggests that there are strong general similarities in the regulatory mechanisms of angiogenesis during both types of osteogenesis. While factors like VEGF and HIF are associated with hypoxic chondrocytes during endochondral bone growth, they are also known to be upregulated by oxygen-independent factors in certain contexts. This leaves open the possibility, however small, that hypoxia within mesenchymal condensations is not the primary basis of early angiogenesis of intramembranous bones. Future studies focused on gene regulation during intramembranous osteogenesis are required to test this common assumption and to determine the similarities between angiogenesis regulation that are associated with both forms of osteogenesis.
The Importance of Vascular Proximity for Osteogenesis
If angiogenesis within an expanding population of preosteogenic mesenchymal cells is necessary for intramembranous bone formation, as it is within endochondral cartilage models, blood vessels are likely to provide something to regions of mineralizing bone that would otherwise be missing. Therefore, a change in the spatial or temporal distribution of blood vessels via angiogenesis might modify the locations or speed of intramembranous osteogenesis. We explore the roles that proximity of vasculature to sites of bone formation might play as the basis for discussion about how dysregulation of angiogenesis might modify osteogenesis.
A major role of blood is to transport oxygen and carbon dioxide, which is mostly bound to the hemoglobin of erythrocytes (Thiriet, 2008). Given that angiogenesis often occurs in regions of hypoxia, the delivery of oxygen to hypoxic cells is assumed to be an important role of new capillary systems, but a proximate vascular network can also provide access to the variety of circulating electrolytes, proteins, gasses, lipids, pluripotent cells, and minerals, including the calcium and phosphate ions necessary for osteogenesis (Heaney, 2008). Access to oxygen and other factors necessary for increased cell activity associated with normal ossification (Shapiro et al., 1988) is provided by vasculature, while simultaneously serving as a sink for cellular waste products.
Access to the correct combination of circulating hormones is necessary for non-dysmorphic skeletal development (Karaplis, 2008). However, hormones that are integral for bone growth postnatally may not be necessary for initial ossification. For instance, mice lacking the receptor for growth hormone (GH), necessary for growth of limbs to expected lengths, do not display modified bone growth before three weeks of age (Sims et al., 2000). Other blood circulating factors including a variety of electrolytes are likely to play a role in the normal activity of cells local to sites of initial ossification, but it is unclear whether these factors are also available at sufficient concentrations in local extracellular fluid. Similarly, some blood circulating proteins (serum proteins) are likely to be utilized by osteoblasts in the production of the bone matrix, but many matrix proteins are likely produced locally.
Cell Precursor Origin
Vessels may also serve as the vehicle for preosteogenic cells to reach regions where ossification is occurring. A varied population of circulating osteogenic precursor cells have been identified and there is evidence that they contribute to bone formation at sites of tissue injury, including bone fractures (Pignolo and Kassem, 2011). It is unknown whether these circulating cells are the principal bone forming cells during fracture repair and normal bone remodeling (Parfitt, 2001; Eghbali-Fatourechi et al., 2007; Eriksen et al., 2007), although the results of some studies suggest that periosteum and bone marrow are the most significant sources of osteoprogenitors (Colnot, 2009; Colnot et al., 2012). Even if circulating cells are found to be a major source of osteoblasts during postnatal growth and development, they may not have the same significance during prenatal osteogenesis; as several sources of osteogenic cells for the earliest stages of bone mineralization have been proposed over the years.
During the earliest stages of endochondral bone mineralization, including bone collar formation and mid-diaphyseal ossification, it has been proposed that osteoblasts differentiate from local mesenchymal cells surrounding the mid-diaphysis (Caplan et al., 1983) and/or adjacent perichondrium (Kronenberg, 2003). As ossification progresses into the more internal parts of the bone and towards the future epiphyses, blood borne cells have been implicated as osteoblast precursors (Collin-Osdoby, 1994), although local chondrocytes (Boyde and Shapiro, 1987) and local endothelial cells have also been suggested as sources (Trueta, 1963; Hansen, 1993). Osteoblast origin may differ from site to site along with differences in the ability or likelihood of cells to de-differentiate or transform (Hall, 2005).
Recent advances in cellular staining have allowed researchers to identify the cellular populations from which osteoblasts are derived during initial ossification. Using X-gal staining on renal explants of mouse limb cartilage bone models, it was demonstrated that perichondrium of an explanted bone is the source of both cortical and trabecular osteoblasts in the earliest stages of endochondral ossification, although associated endothelial cells originate outside of the explanted bone (Colnot et al., 2004). This result was recently confirmed for mice in vivo (Maes et al., 2010). The perichondrium is the primary source of osteoblasts in this case and local endothelial cells appear to have a different origin than osteoblasts, but the possibility that some osteoblasts come from local chondrocytes remains.
During initial intramembranous ossification, it has been proposed that local mesenchymal cells differentiate into the osteogenic cells that produce woven bone, which is later remodeled (Collin-Osdoby, 1994). DiI staining of mouse frontal bone mesenchymal condensations at embryonic day 13.5 (E13.5) indicates that cells derived from this condensation populate the whole frontal bone domain at E17.5 and E18.5 (Yoshida et al., 2008). Therefore, the apical expansion of the presumptive bone from the supraorbital ridge utilizes cells from the original mesenchymal condensation rather than recruiting significant numbers of cells from the blood stream or from the underlying mesenchymal populations. In calvarial cultures of bones bordering the mouse sagittal suture, implantation experiments indicated that mesenchymal cells proliferating at the osteogenic front are the precursors of osteoblasts for the expanding bone, although a small minority may come from the suture mesenchyme (Lana-Elola et al., 2007). Since a small amount of suture mesenchyme becomes incorporated into the ossifying bone, a small number of circulating osteoblast progenitors might also contribute to the ossifying calvarial bones.
Osteoclasts found during initial osteogenesis are likely to arise from blood-borne monocytes (Caplan et al., 1983). Osteoclast precursors, which have a hematopoietic origin, are known to circulate in the blood supply with monocytes (Fujikawa et al., 1996), providing the primary pool of osteoclasts for bone resorption associated with bone remodeling (Eriksen et al., 2007). Osteoclasts are critical during early bone remodeling, as they provide the mechanism for removal of bone that is a fundamental part of changes in bone shape necessary for normal growth. For instance, osteoclast resorption within calvarial bones is a necessary step in altering cranial vault curvature and in general bone remodeling across the craniofacial complex (Enlow, 2000). If chondroclasts and osteoclasts differentiate from the same precursor population (Hall, 2005), circulating monocyte precursors also provide the source for chondroclasts that precede endothelial cells during angiogenesis into the cartilage models of endochondral bones (Lewinson and Silbermann, 1992; Streeten and Brandi, 1990).
Regulatory Interactions between Endothelial and Osteogenic Cells
Growing vessel networks not only provide a source of circulating factors and cells to previously avascular sites, but their migrating endothelial cells are an active part of the regulatory network underlying bone formation and remodeling that includes osteoblasts, osteoclasts, macrophages, and stromal cells. Endothelial cells, with known proximity to differentiating osteoblasts in newly ossifying bone, are known to respond to factors produced by osteoblastic lineage cells (see Regulation of Intramembranous Angiogenesis) and produce factors that can regulate the differentiation, metabolism, and survival of osteoblastic lineage cells (Collin-Osdoby, 1994; Brandi and Collin-Osdoby, 2006).
Many of the potential regulatory interactions between factors produced by endothelial cells and bone cells remain untested, although the addition of endothelial cells into mesenchymal stem cells enhances tissue-engineered bone formation, presumably because of factors expressed by the endothelial cells (Grellier et al., 2009; Usami et al., 2009). There is evidence that endothelial expression of endothelin-1 may influence osteoprogenitor cell proliferation and differentiation (Von Schroeder et al., 2003). Hypoxia, VEGF, and oscillatory shear stress are known to upregulate expression of BMP-2 in vascular endothelial cells (Bouletreau et al., 2002; Sorescu et al., 2003), which can induce osteoblast differentiation (Yamaguchi et al., 2000), as well as upregulate VEGF expression and angiogenesis during endochondral ossification (Towler, 2008). In fact, vascular smooth muscle and endothelial cells have been identified as the primary source of BMP-2 during distraction osteogenesisis (Matsubara et al., 2012), suggesting that vascular expression of this or other factors might provide a significant regulatory basis for normal osteoblast activity. Further tests of the effects of these and other factors produced by endothelial cells on osteoblast differentiation and activity during osteogenesis are necessary to verify their importance and specify their role in ossifying intramembranous and endochondral bones.
Endothelial cells may also regulate osteoclasts during early osteogenesis. Active time and location dependent regulation of osteoclast movement through the endothelial cell layer is likely necessary for the delivery of osteoclasts to appropriate locations (Parfitt, 2000; Brandi and Collin-Osdoby, 2006). Endothelial cells are known to produce factors that can regulate cells of the osteoclast lineage, including macrophage-colony stimulating factor and a host of other molecules (Brandi and Collin-Osdoby, 2006). Regardless of the exact ways in which endothelial cells participate in the intercellular regulatory network of osteogenesis, the close proximity they have to osteoblasts and osteoclasts virtually guarantees that they interact with these cells, beyond allowing for the delivery of factors from the blood supply.
Perspectives
Although the development of endochondral long bones has been better studied, craniofacial endochondral elements may be a more appropriate reference for a model of intramembranous osteogenesis. The few comparisons of cranial and postcranial endochondral ossification suggest a largely conserved pattern of gene expression (Eames and Helms, 2004) that is temporally delayed in cranial base precursors compared to postcranial bones, leading to delayed ossification (Balczerski et al., 2012). Although any differences between craniofacial and postcranial endochondral bones may be relatively minor, it is possible that some of these differences stem from cellular origin and location within the craniofacial context, which are shared between intramembranous and endochondral bones of the skull. Further studies on the regulation of early craniofacial osteogenesis, including the role of angiogenesis, might do well to focus on both intramembranous and endochondral bones in order to determine whether intramembranous osteogenesis is more similar to craniofacial endochondral osteogenesis than to postcranial endochondral osteogenesis.
A Model of Angiogenesis during Intramembranous Osteogenesis
Angiogenesis into the mid-diaphysis and epiphysis of long bones and into the distraction site during distraction osteogenesis is necessary for the initiation of mineralization at these sites. This relationship and the noted association between growing capillaries, osteoblasts, and mineralization within the chick frontal bone (Thompson et al., 1989) strongly suggests that angiogenesis is a necessary prerequisite for ossification during intramembranous osteogenesis. Based on data presented in preceding sections, we introduce a model of the association between angiogenesis and intramembranous bone osteogenesis (Fig 5). Because most information on preosteogenic mesenchymal expansion and intramembranous osteogenesis come from studies of vault bones, it is possible that this model does not represent osteogenesis of intramembranous facial bones. Consequently, the expectations of the model should be tested for both vault and non-vault bones in order to determine its validity and generalizability.
Figure 5.
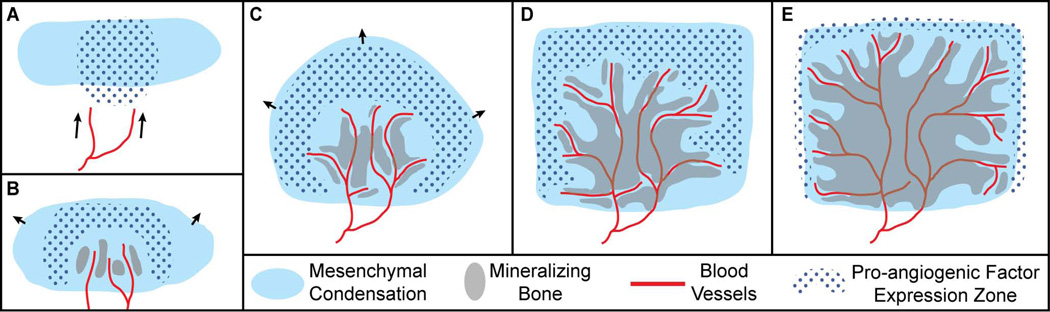
Hypothetical schematic of the association between mesenchymal precursors, pro-angiogenic factor (e.g. VEGF, HIF) expression, invading blood vessels, and bone formation during the initial period of intramembranous ossification. A) Blood vessels (red line) are drawn (arrows) to the border of the avascular mesenchymal condensation (light blue solid) by pro-angiogenic factors (blue dots). B) As mesenchymal cells migrate outwards (arrows), blood vessels invade the condensation near the center of ossification at or around the time of initial ossification (grey solid), which occurs in proximity to invading vasculature. C,D) Migration of cells derived from the original condensation continues outward (arrows) until they receive some signal to stop, often at sutures that form between the advancing mesenchymal fronts of two bones. Vessels continue to extend outward through the mesenchymal condensation towards regions of pro-angiogenic factor expression. New bone mineralization occurs proximate to sprouting vessels, as previous sites of bone formation begin to merge and mature. E) After the end of rapid mesenchymal cell expansion from the condensation, vessels and associated regions of ossification approach the edge, forming an ossification front at the suture margin that will allow for continued calvarial growth.
Just before the initial ossification of a given avascular preosteogenic mesenchymal condensation, we expect capillaries to enter a surrounding layer of avascular loose mesenchyme and come into proximity with the condensation (Fig 5A). Then, these capillaries will enter the preosteogenic condensation near the site of and around the time of its initial ossification (Fig 5B). This movement of vasculature into proximity with and the vascular invasion of the condensation are likely based on a reduction in the expression of currently unidentified anti-angiogenic factors and an increase in the expression of pro-angiogenic factors (i.e., VEGF, HIF). While we expect vasculature to originally approach only the initial sites of ossification, as occurs during endochondral ossification, no feature analogous to a bone collar has yet been identified for intramembranous bones.
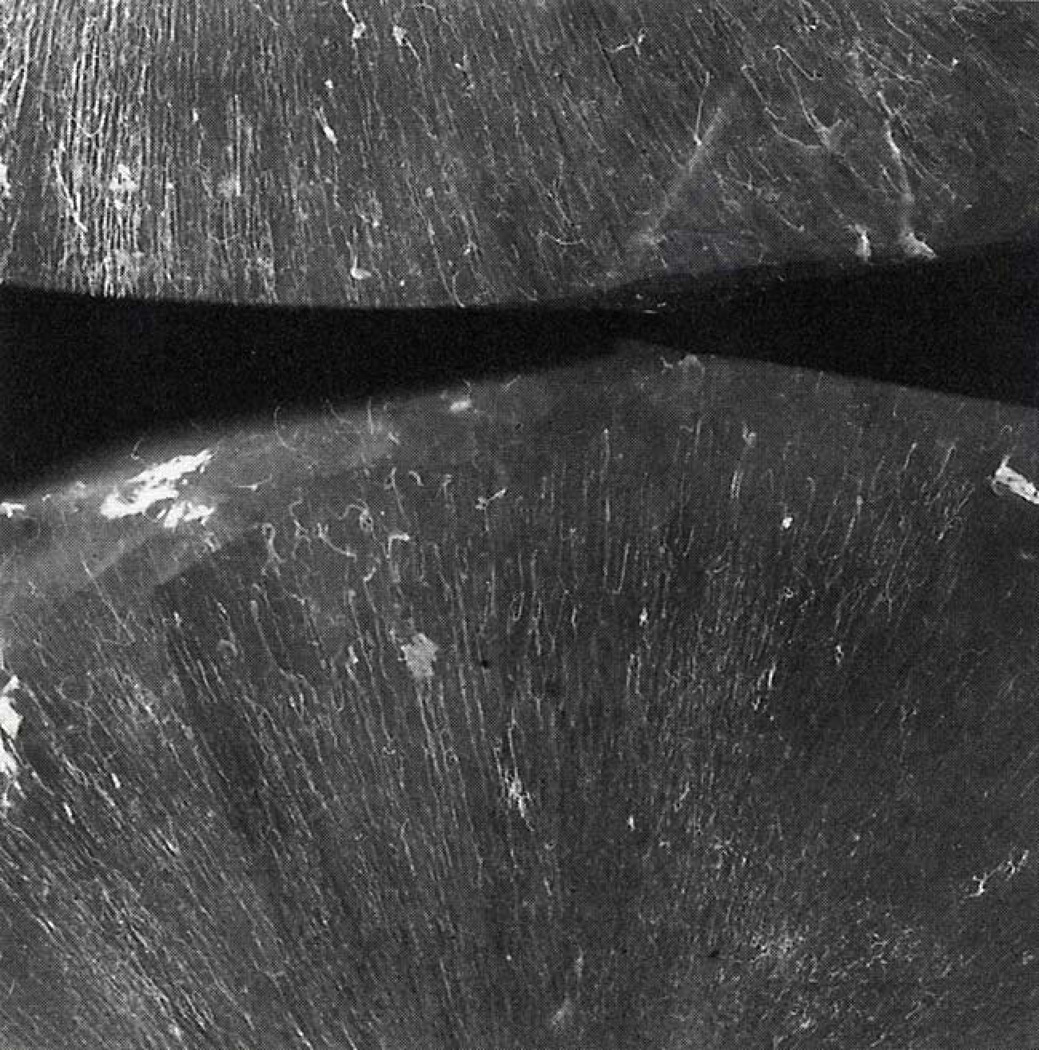
As ossification occurs at the site of initial blood vessel invasion, capillaries continue growing outward from the vessels already within the ossifying condensation, rather than from vessels outside of it, in order to vascularize the quickly expanding population of mesenchymal cells (Fig 5C). Radiographic images of Thorotrast filled calvarial capillaries in fetal human parietals show vessels radiating outward like spokes from a center of ossification towards the bone edges (Fig 6) (Brookes and Revell, 1998), supporting the idea that angiogenesis within developing intramembranous bones stem from central points of vascular invasion. We anticipate that the pro-angiogenic factors first expressed by mesenchymal cells near the initial site of ossification will next be expressed by mesenchymal cells that make up the expanding bone precursor. Evidence of the close association between preosteoblasts and sprouting capillaries during endochondral osteogenesis (Maes et al., 2010) suggests that growing vessels and migrating preosteoblastic cells of the expanding mesenchymal condensation might keep pace with each other, maintaining an association during the earliest stages of intramembranous osteogenesis.
Figure 6.
Radiographic image of the two parietal bones of a human fetal skull after vascular perfusion with radioactive Thorotrast (white). The vasculature within the developing parietal bones can be seen radiating outward from their centers. Image reproduced with kind permission of Springer Science+Business Media (p65, Brookes and Revell, 1998).
We expect mineralization of an intramembranous bone to first occur in proximity to capillaries growing through the expanding condensation, because they may be directly associated with migrating preosteoblasts and are a source of factors necessary for ossification. We then expect ossification to continue at sites progressively farther from these capillaries, serving to fill in unossified portions of the bone; as the process of bone remodeling and maturation begins (Fig 5D). Once the mesenchymal condensation has stopped its period of rapid expansion, growing capillaries and the front of mineralizing bone will approach the presumptive sutures (Fig 5E). This will initiate the better described process of bone growth at the suture margin, during which angiogenesis is also likely to play an important role. Finally, as ossification occurs at the suture margins, earlier ossified portions of bone and the vascular network within it will continue to mature and continued angiogenesis may play a continual role in this process.
Studies of the three-dimensional association between blood vessels, the initially avascular mesenchymal condensation, and osteoblasts across the earliest period of osteogenesis will be necessary to verify the associations between all three tissues defined within this model (Fig 5), particularly for the morphologically complex intramembranous bones of the facial skeleton. In addition, the results of such studies can be used to test the assumption that invading capillaries are a necessary prerequisite for intramembranous osteogenesis.
Though variation in arterial and venous structure is marked, the region across which vessels grow and the timing of vessel growth is likely stable, based on typical timing and location of pro-angiogenic factor expression. If angiogenesis is a necessary prerequisite for intramembranous osteogenesis, a change in the regions of vessel growth within a preosteogenic mesenchymal condensation should contribute to a change in the shape of the associated ossifying bone, while a change in the timing or rate of angiogenesis should modify the speed of mineralization and bone maturation, but may also contribute to shape change. The severity of any resulting change in bone phenotype is likely to be associated with the timing and severity of angiogenesis dysregulation, but also on whether vessel proximity is sufficient to trigger local ossification rather than just being necessary for it. To answer this question, studies of gene expression and experimental manipulation of possible regulatory interactions are necessary to determine the signals that upregulate both angiogenesis into and osteoblast differentiation within a given portion of the mesenchyme.
Knowledge of the expression patterns of pro-angiogenic factors within mesenchymal cells and cells of the osteoblast lineage is necessary to determine the possible sources of the signal that draw capillaries toward and into the initial mesenchymal condensation as well as throughout the expanding mesenchymal bone precursor. Comparisons of the co-expression of pro-angiogenic factors and factors associated with osteoblast precursor proliferation and differentiation are necessary to test whether the regulatory basis for patterns of angiogenesis and osteogenesis are similar and/or coupled. Although inhibition of angiogenesis into the perichondrium can prevent endochondral ossification, inhibition of perichondrial osteoblastogenesis is also associated with a severe reduction in vascular invasion (Komori et al., 1997; Otto et al., 1997; Colnot et al., 2005). This suggests that signals for the upregulation of angiogenesis, which is necessary for ossification through the activity of mature osteoblasts, may be associated with preosteoblast differentiation. Consequently, angiogenesis may not be sufficient to trigger ossification within mesenchymal condensations.
Specific Hypotheses
Based on research reviewed in this paper and on our proposed model of angiogenesis and intramembranous osteogenesis, we suggest specific hypotheses to be tested. Rather than being groundbreaking in any sense, these hypotheses represent the explicit and implicit assumptions of research into fundamental properties of intramembranous bone osteogenesis. We expect these hypotheses to be supported by future research, but any aspects that are not supported will provide important windows into differences in the association between angiogenesis and osteogenesis in intramembranous and endochondral bone formation.
Hypotheses:
Avascularity is necessary for normal preosteogenic condensation growth that precedes intramembranous ossification.
Pro-angiogenic signals that promote angiogenesis towards, into, and through preosteogenic condensations come from regions of hypoxia within these condensations.
The pattern of intramembranous osteogenesis described in our model (Fig 5) is accurate for both vault bones and morphologically complex bones of the face. This includes a rapid expansion of mesenchymal cells from the initial condensation into a shape roughly resembling the adult bone at the same time that ossification is occurring.
Proximity to capillaries is necessary, but not sufficient, for local ossification within a mesenchymal condensation. Instead, regulatory signals from within a mesenchymal condensation promote both angiogenesis into the condensation and differentiation of osteoblasts from within it.
Variation in the regulation of angiogenesis can have a significant influence on osteogenesis and produce evolutionary and medically relevant variation in craniofacial skeletal phenotypes.
While future studies of the association between blood vessels, mesenchymal condensations, and mineralizing bone are likely to reveal covariation between the development of blood vessel networks and craniofacial skeletal phenotypes, it is also critical to study the regulatory interactions between factors carried in the blood or produced by endothelial cells and cells of the osteoblast lineage. Only with a combination of phenotypic, developmental, and regulatory data can the causal basis of the association between blood vessels and mineralizing bone be illuminated. Given the likely role of angiogenesis during intramembranous osteogenesis, alterations in angiogenesis regulation are likely to contribute to craniofacial skeletal variation fundamental to evolutionary change or clinically relevant phenotypes. Modifications to angiogenesis regulation may be associated with changes in rate of intramembranous ossification and bone maturation, in the shape of craniofacial bones, the relationship of cranial elements to one another, and in the production of craniofacial dysmorphology. To determine the potential impact of causal links between angiogenesis and osteogenesis in these contexts, the basic developmental associations between the two tissues must be illuminated for intramembranous bones. While information gained from the study of endochondral osteogenesis and distraction osteogenesis can provide a useful beginning for understanding intramembranous osteogenesis, verification of these processes is necessary to further elucidate the contribution of intramembranous ossification in development, disease and evolution.
Salient Features of the Manuscript.
Angiogenesis is critical to intramembranous osteogenesis, but the nature of this association is not well studied.
Proximity to vasculature is likely necessary for intramembranous osteogenesis, because vessels provide access to circulating factors, may be associated with migrating bone cell precursors, and vascular endothelial cells likely play a role in regulating osteoblast activity.
Dysregulation of angiogenesis may serve as a basis of craniofacial skeletal variation.
Future research on the spatio-temporal association and regulatory interactions of mesenchymal, vascular, and bone cells is required to illuminate the development of the craniofacial complex.
Acknowledgements
Thanks to Kazuhiko Kawasaki for his many helpful suggestions and comments on this paper. Thanks also to Kenneth Weiss, Tim Ryan, and Patrick Drew for their comments on a previous version of this manuscript. This work was funded in part by grants from the National Science Foundation (BCS-1061554) to CJP and National Institute of Dental and Craniofacial Research and the American Recovery and Reinvestment Act (R01DE018500; 3R01DE018500-02S1; R01DE022988) to JTR.
Contributor Information
Christopher J. Percival, Department of Anthropology, Penn State University
Joan T. Richtsmeier, Department of Anthropology, Penn State University
References
- Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007;134:3133–3144. doi: 10.1242/dev.002709. [DOI] [PubMed] [Google Scholar]
- Al-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. Journal of Dental Research. 2008;87:107–118. doi: 10.1177/154405910808700215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amizuka N, Hasegawa T, Oda K, Luiz de Freitas P, Hoshi K, Li M, Ozawa H. Histology of epiphyseal cartilage calcification and endochondral ossification. Front Biosci. 2012;4:2085–2100. doi: 10.2741/e526. [DOI] [PubMed] [Google Scholar]
- Aronson J, Good B, Stewart C, Harrison B, Harp J. Preliminary studies of mineralization during distraction osteogenesis. Clinical Orthopaedics and Related Research. 1990;250:43–49. [PubMed] [Google Scholar]
- Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clinical Orthopaedics and Related Research. 1994;301:124–131. [PubMed] [Google Scholar]
- Balczerski B, Zakaria S, Tucker A, Borycki A, Koyama E, Pacifici M, Francis-West P. Distinct spatiotemporal roles of hedgehog signalling during chick and mouse cranial base and axial skeleton development. Developmental Biology. 2012 doi: 10.1016/j.ydbio.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom W, Fawcett DW. A Textbook of Histology. New York, NY: Chapman & Hall; 1994. [Google Scholar]
- Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, Greenwald JA, Longaker MT. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plastic and Reconstructive Surgery. 2002;109:2384–2397. doi: 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- Boyde A, Shapiro IM. Morphological observations concerning the pattern of mineralization of the normal and the rachitic chick growth cartilage. Anatomy and Embryology. 1987;175:457–466. doi: 10.1007/BF00309681. [DOI] [PubMed] [Google Scholar]
- Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. Journal of Bone and Mineral Research. 2006;21:183–192. doi: 10.1359/JBMR.050917. [DOI] [PubMed] [Google Scholar]
- Brookes M, Revell WJ. Blood Supply of Bone. London: Springer; 1998. [Google Scholar]
- Caplan AI, Syftestad G, Osdoby P. The development of embryonic bone and cartilage in tissue culture. Clinical Orthopaedics and Related Research. 1983;174:243–263. [PubMed] [Google Scholar]
- Choi IH, Ahn JH, Chung CY, Cho TJ. Vascular proliferation and blood supply during distraction osteogenesis: a scanning electron microscopic observation. Journal of Orthopaedic Research. 2000;18:698–705. doi: 10.1002/jor.1100180504. [DOI] [PubMed] [Google Scholar]
- Choi IH, Chung CY, Cho TJ, Yoo WJ. Angiogenesis and mineralization during distraction osteogenesis. Journal of Korean Medical Science. 2002;17:435–447. doi: 10.3346/jkms.2002.17.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun Y, Kim M, Park J. Oxygen-dependent and -independent regulation of HIF-1alpha. Journal of Korean Medical Science. 2002;17:581–588. doi: 10.3346/jkms.2002.17.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin-Osdoby P. Role of vascular endothelial cells in bone biology. Journal of Cellular Biochemistry. 1994;55:304–309. doi: 10.1002/jcb.240550306. [DOI] [PubMed] [Google Scholar]
- Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. Journal of Bone and Mineral Research. 2009;24:274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot C, De la Fuente L, Huang S, Hu D, Lu C, St-Jacques B, Helms JA. Indian hedgehog synchronizes skeletal angiogenesis and perichondrial maturation with cartilage development. Development. 2005;132:1057–1067. doi: 10.1242/dev.01649. [DOI] [PubMed] [Google Scholar]
- Colnot C, Lu C, Hu D, Helms JA. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Developmental Biology. 2004;269:55–69. doi: 10.1016/j.ydbio.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Colnot C, Zhang X, Tate MLK. Current insights on the regenerative potential of the periosteum: Molecular, cellular, and endogenous engineering approaches. Journal of Orthopaedic Research. 2012;30:1869–1878. doi: 10.1002/jor.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Rabie A. VEGF: an essential mediator of both angiogenesis and endochondral ossification. Journal of Dental Research. 2007;86:937–950. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- Delloye C, Delefortrie G, Coutelier L, Vincent A. Bone regenerate formation in cortical bone during distraction lengthening: an experimental study. Clinical Orthopaedics and Related Research. 1990;250:34–42. [PubMed] [Google Scholar]
- Depew MJ, Tucker AS, Sharpe PT. Craniofacial development. In: Rossant J, Tam PPL, editors. Mouse development, patterning, morphogenesis, and organogenesis. San Diego: Academic Press; 2002. pp. 421–498. [Google Scholar]
- Dietrich EM, Antoniades K. Bone-vasculature interactions in the mandible: Is bone an angiogenic tissue? Medical Hypotheses. 2012;79(5):582–584. doi: 10.1016/j.mehy.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Drushel RF, Pechak DG, Caplan AI. The anatomy, ultrastructure and fluid dynamics of the developing vasculature of the embryonic chick wing bud. Cell Differentiation. 1985;16:13–28. doi: 10.1016/0045-6039(85)90603-7. [DOI] [PubMed] [Google Scholar]
- Eames BF, Helms JA. Conserved molecular program regulating cranial and appendicular skeletogenesis. Developmental Dynamics. 2004;231:4–13. doi: 10.1002/dvdy.20134. [DOI] [PubMed] [Google Scholar]
- Eames BF, De La Fuente L, Helms JA. Molecular ontogeny of the skeleton. Birth Defects Research Part A: Clinical and Molecular Teratology. 2003;69:93–101. doi: 10.1002/bdrc.10016. [DOI] [PubMed] [Google Scholar]
- Eghbali-Fatourechi GZ, Mödder UIL, Charatcharoenwitthaya N, Sanyal A, Undale AH, Clowes JA, Tarara JE, Khosla S. Characterization of circulating osteoblast lineage cells in humans. Bone. 2007;40:1370–1377. doi: 10.1016/j.bone.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enlow D. Normal craniofacial growth. In: Cohen MM Jr, MacLean RE, editors. Craniosynostosis: Diagnosis, Evaluation, and Management. Second. New York: Oxford University Press; 2000. pp. 35–47. [Google Scholar]
- Eriksen EF, Eghbali-Fatourechi GZ, Khosla S. Remodeling and vascular spaces in bone. J Bone Miner Res. 2007;22:1–6. doi: 10.1359/jbmr.060910. [DOI] [PubMed] [Google Scholar]
- Eshkar-Oren I, Viukov SV, Salameh S, Krief S, Akiyama H, Gerber HP, Ferrara N, Zelzer E. The forming limb skeleton serves as a signaling center for limb vasculature patterning via regulation of Vegf. Development. 2009;136:1263–1272. doi: 10.1242/dev.034199. [DOI] [PubMed] [Google Scholar]
- Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, Song HJM, Carano RA, Filvaroff EH, Bednarski MD, Giaccia AJ, et al. Angiogenesis is required for successful bone induction during distraction osteogenesis. Journal of Bone and Mineral Research. 2005;20:1114–1124. doi: 10.1359/JBMR.050301. [DOI] [PubMed] [Google Scholar]
- Feinberg RN, Latker CH, Beebe DC. Localized vascular regression during limb morphogenesis in the chicken embryo. I. Spatial and temporal changes in the vascular pattern. The Anatomical Record. 1986;214:405–409. doi: 10.1002/ar.1092140411. [DOI] [PubMed] [Google Scholar]
- Fujikawa Y, Quinn J, Sabokbar A, McGee J, Athanasou N. The human osteoclast precursor circulates in the monocyte fraction. Endocrinology. 1996;137:4058–4060. doi: 10.1210/endo.137.9.8756585. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nature Medicine. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Grellier M, Bordenave L, Amedee J. Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends in Biotechnology. 2009;27:562–571. doi: 10.1016/j.tibtech.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Hall BK. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. Academic Press; 2005. [Google Scholar]
- Hansen ES. Microvascularization, osteogenesis, and myelopoiesis in normal and pathological conditions. In: Schoutens A, Arlet J, Gardeniers JWM, Hughes SPF, editors. Bone Circulation and Vascularization in Normal and Pathological Conditions. NATO ASI Series A: Life Sciences. New York: Plenum Press; 1993. pp. 29–42. [Google Scholar]
- Heaney RP. Principles of Bone Biology. 3rd ed. Vol. 2. San Diego: Academic Press; 2008. Calcium; pp. 1697–1710. [Google Scholar]
- Hunter WL, Arsenault AL, Hodsman AB. Rearrangement of the metaphyseal vasculature of the rat growth plate in rickets and rachitic reversal: a model of vascular arrest and angiogenesis renewed. The Anatomical Record. 1991;229:453–461. doi: 10.1002/ar.1092290404. [DOI] [PubMed] [Google Scholar]
- Iseki S, Wilkie A, Heath J, Ishimaru T, Eto K, Morriss-Kay G. Fgfr2 and osteopontin domains in the developing skull vault are mutually exclusive and can be altered by locally applied FGF2. Development. 1997;124:3375–3384. doi: 10.1242/dev.124.17.3375. [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Developmental Biology. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Jourdeuil K, Franz-Odendaal TA. Vasculogenesis and the induction of skeletogenic condensations in the avian eye. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2012;295:691–698. doi: 10.1002/ar.22429. [DOI] [PubMed] [Google Scholar]
- Kanczler J, Oreffo R. Osteogenesis and angiogenesis: the potential for engineering bone. European Cells and Materials. 2008;15:100–114. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- Karaplis AC. Embryonic development of bone and regulation of intramembranous and endochondral bone formation. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of Bone Biology. Vol. 1. 3rd ed. Academic Press; 2008. pp. 53–84. [Google Scholar]
- Kawaguchi H, Nakamura K, Tabata Y, Ikada Y, Aoyama I, Anzai J, Nakamura T, Hiyama Y, Tamura M. Acceleration of fracture healing in nonhuman primates by fibroblast growth factor-2. Journal of Clinical Endocrinology & Metabolism. 2001;86:875–880. doi: 10.1210/jcem.86.2.7199. [DOI] [PubMed] [Google Scholar]
- Kish PE, Bohnsack BL, Gallina D, Kasprick DS, Kahana A. The eye as an organizer of craniofacial development. genesis. 2011;49:222–230. doi: 10.1002/dvg.20716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson R, Gao Y-H, Inada M, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Kuettner KE, Pauli BU, Hall BK. Cartilage. Vol. 1. New York, NY: Academic Press; 1983. Vascularity of cartilage; pp. 281–312. [Google Scholar]
- Lana-Elola E, Rice R, Grigoriadis AE, Rice DPC. Cell fate specification during calvarial bone and suture development. Developmental Biology. 2007;311:335–346. doi: 10.1016/j.ydbio.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Langenfield EM, Langenfield J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Molecular Cancer Research. 2004;2:141–149. [PubMed] [Google Scholar]
- Levin M, Ewald AJ, McMahon M, Werb Z, Mostov K. A model of intussusceptive angiogenesis. In: Chadwick DJ, Goode J, editors. Vascular development. Chichster, UK: John Wiley and Sons; 2007. pp. 37–45. [DOI] [PubMed] [Google Scholar]
- Lewinson D, Silbermann M. Chondroclasts and endothelial cells collaborate in the process of cartilage resorption. The Anatomical Record. 1992;233:504–514. doi: 10.1002/ar.1092330403. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tang Z, Kundu RK, Wu L, Luo W, Zhu D, Sangiorgi F, Snead ML, Maxson RE., Jr Msx2 gene dosage influences the number of proliferative osteogenic cells in growth centers of the developing murine skull: a possible mechanism for MSX2-mediated craniosynostosis in humans. Developmental Biology. 1999;205:206–274. doi: 10.1006/dbio.1998.9114. [DOI] [PubMed] [Google Scholar]
- Liu Z, Lavine KJ, Hung IH, Ornitz DM. FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Developmental Biology. 2007;302:80–91. doi: 10.1016/j.ydbio.2006.08.071. [DOI] [PubMed] [Google Scholar]
- Mackie E, Ahmed Y, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. The International Journal of Biochemistry & Cell Biology. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Developmental Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks SC, Odgren PR. Structure and development of the skeleton. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. San Diego: Academic Press; 2002. pp. 3–16. [Google Scholar]
- Matsubara H, Hogan DE, Morgan EF, Mortolock DP, Einhorn TA, Gerstenfeld LC. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone. 2012;51:168–180. doi: 10.1016/j.bone.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBratney-Owen B, Iseki S, Bamforth SD, Olsen BR, Morriss-Kay GM. Development and tissue origins of the mammalian cranial base. Developmental Biology. 2008;322:121–132. doi: 10.1016/j.ydbio.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PDF. Bones: a study of the development and structure of the vertebrate skeleton. Cambridge, UK: Cambridge University Press; 1985. [Google Scholar]
- Nakamura H, Yukita A, Ninomiya T, Hosoya A, Hiraga T, Ozawa H. Localization of Thy-1-positive cells in the perichondrium during endochondral ossification. Journal of Histochemistry & Cytochemistry. 2010;58:455–462. doi: 10.1369/jhc.2010.955393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. Journal of Anatomy. 2005;207:575–601. doi: 10.1111/j.1469-7580.2005.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperman LA, Rawlins JT. The extracellular matrix environment in suture morphogenesis and growth. Cells Tissues Organs. 2005;181:127–135. doi: 10.1159/000091374. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Parfitt A. The mechanism of coupling: a role for the vasculature. Bone. 2000;26:319–323. doi: 10.1016/S8756-3282(00)80937-0. [DOI] [PubMed] [Google Scholar]
- Parfitt A. The bone remodeling compartment: a circulatory function for bone lining cells. Journal of Bone and Mineral Research. 2001;16:1583–1585. doi: 10.1359/jbmr.2001.16.9.1583. [DOI] [PubMed] [Google Scholar]
- Patel J, Landers K, Mortimer R, Richard K. Regulation of hypoxia inducible factors (HIF) in hypoxia and normoxia during placental development. Placenta. 2010;31:951–957. doi: 10.1016/j.placenta.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Percival CJ. The Influence of Angiogenesis on Craniofacial Development and Evolution. (Doctoral dissertation) University Park, PA: Penn State University; 2013. [Google Scholar]
- Pignolo RJ, Kassem M. Circulating osteogenic cells: implications for injury, repair, and regeneration. Journal of Bone and Mineral Research. 2011;26:1685–1693. doi: 10.1002/jbmr.370. [DOI] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nature medicine. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Giaccia AJ, Schipani E. A central role for hypoxic signaling in cartilage, bone, and hematopoiesis. Current Osteoporosis Reports. 2011;9:46–52. doi: 10.1007/s11914-011-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Aberg T, Chan Y, Tang Z, Kettunen PJ, Pakarinen L, Maxson R, Thesleff I. Integration of FGF and TWIST in calvarial bone and suture development. Development. 2000;127:1845–1855. doi: 10.1242/dev.127.9.1845. [DOI] [PubMed] [Google Scholar]
- Rice R, Rice DPC, Olsen BR, Thesleff I. Progression of calvarial bone development requires Foxc1 regulation of Msx2 and Alx4. Developmental Biology. 2003;262:75–87. doi: 10.1016/s0012-1606(03)00355-5. [DOI] [PubMed] [Google Scholar]
- Saadeh PB, Mehrara BJ, Steinbrech DS, Spector JA, Greenwald JA, Chin GS, Ueno H, Gittes GK, Longaker MT. Mechanisms of fibroblast growth factor-2 modulation of vascular endothelial growth factor expression by osteoblastic cells. Endocrinology. 2000;141:2075–2083. doi: 10.1210/endo.141.6.7502. [DOI] [PubMed] [Google Scholar]
- Von Schroeder H, Veillette C, Payandeh J, Qureshi A, Heersche J. Endothelin-1 promotes osteoprogenitor proliferation and differentiation in fetal rat calvarial cell cultures. Bone. 2003;33:673–684. doi: 10.1016/s8756-3282(03)00215-1. [DOI] [PubMed] [Google Scholar]
- Shapiro IM, Golub EE, Chance B, Piddington C, Oshima O, Tuncay OC, Haselgrove JC. Linkage between energy status of perivascular cells and mineralization of the chick growth cartilage. Developmental Biology. 1988;129:372–379. doi: 10.1016/0012-1606(88)90384-3. [DOI] [PubMed] [Google Scholar]
- Sims NA, Clément-Lacroix P, Da Ponte F, Bouali Y, Binart N, Moriggl R, Goffin V, Coschigano K, Gaillard-Kelly M, Kopchick J, et al. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. Journal of Clinical Investigation. 2000;106:1095–1103. doi: 10.1172/JCI10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. Journal of Biological Chemistry. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- De Spiegelaere W, Cornillie P, Casteleyn C, Burvenich C, Van Den Broeck W. Detection of hypoxia inducible factors and angiogenic growth factors during foetal endochondral and intramembranous ossification. Anatomia, Histologia, Embryologia. 2010;39:376–384. doi: 10.1111/j.1439-0264.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- Streeten EA, Brandi ML. Biology of bone endothelial cells. Bone and Mineral. 1990;10:85–94. doi: 10.1016/0169-6009(90)90084-s. [DOI] [PubMed] [Google Scholar]
- Takai S, Tokuda H, Hanai Y, Harada A, Yasuda E, Matsushima-Nishiwaki R, Kato H, Ogura S, Ohta T, Kozawa O. Negative Regulation by p70 S6 Kinase of FGF-2-Stimulated VEGF Release Through Stress-Activated Protein Kinase/c-Jun N-Terminal Kinase in Osteoblasts. Journal of Bone and Mineral Research. 2007;22:337–346. doi: 10.1359/jbmr.061209. [DOI] [PubMed] [Google Scholar]
- Takimoto A, Nishizaki Y, Hiraki Y, Shukunami C. Differential actions of VEGF-A isoforms on perichondrial angiogenesis during endochondral bone formation. Developmental Biology. 2009;332:196–211. doi: 10.1016/j.ydbio.2009.05.552. [DOI] [PubMed] [Google Scholar]
- Thiriet M. Biology and Mechanics of Blood Flows. Springer; 2008. [Google Scholar]
- Thoma R. Untersuchungen über das schädelwachstum und seine störungen. 2. Das fetale wachstum. Virchows Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin. 1913;212:1. [Google Scholar]
- Thompson TJ, Owens PD, Wilson DJ. Intramembranous osteogenesis and angiogenesis in the chick embryo. Journal of Anatomy. 1989;166:55–65. [PMC free article] [PubMed] [Google Scholar]
- Ting M-C, Wu NL, Roybal PG, Sun J, Liu L, Yen Y, Maxson RE. EphA4 as an effector of Twist1 in the guidance of osteogenic precursor cells during calvarial bone growth and in craniosynostosis. Development. 2009;136:855–864. doi: 10.1242/dev.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson RE, McKenzie JA, Schmieder AH, Wohl GR, Lanza GM, Silva MJ. Angiogenesis is required for stress fracture healing in rats. Bone. 2013;52:212–219. doi: 10.1016/j.bone.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler DA. Vascular endothelial growth factor and osteogenic-angiogenic coupling. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of bone biology. 3rd ed. Vol. 2. Academic Press; 2008. pp. 1133–1144. [Google Scholar]
- Trueta J. The role of the vessels in osteogenesis. Journal of Bone and Joint Surgery-British Volume. 1963;45:402–418. [PubMed] [Google Scholar]
- Usami K, Mizuno H, Okada K, Narita Y, Aoki M, Kondo T, Mizuno D, Mase J, Nishiguchi H, Kagami H, et al. Composite implantation of mesenchymal stem cells with endothelial progenitor cells enhances tissue-engineered bone formation. Journal of Biomedical Materials Research Part A. 2009;90:730–741. doi: 10.1002/jbm.a.32142. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere M, Guldberg RE, Gerstenfeld LC, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. Journal of Clinical Investigation. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl GR, Towler DA, Silva MJ. Stress fracture healing: fatigue loading of the rat ulna induces upregulation in expression of osteogenic and angiogenic genes that mimic the intramembranous portion of fracture repair. Bone. 2009;44:320–330. doi: 10.1016/j.bone.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocrine Reviews. 2000;21:393–411. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
- Yin M, Pacifici M. Vascular regression is required for mesenchymal condensation and chondrogenesis in the developing limb. Developmental Dynamics. 2001;222:522–533. doi: 10.1002/dvdy.1212. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. Cell lineage in mammalian craniofacial mesenchyme. Mechanisms of Development. 2008;125:797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Yu JC, Lucas JH, Fryberg K, Borke JL. Extrinsic tension results in FGF-2 release, membrane permeability change, and intracellular Ca++ increase in immature cranial sutures. Journal of Craniofacial Surgery. 2001;12:391–398. doi: 10.1097/00001665-200107000-00018. [DOI] [PubMed] [Google Scholar]
- Zelzer E, McLean W, Ng YS, Fukai N, Reginato AM, Lovejoy S, D’Amore PA, Olsen BR. Skeletal defects in VEGF120/120 mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129:1893–1904. doi: 10.1242/dev.129.8.1893. [DOI] [PubMed] [Google Scholar]
- Zelzer E, Olsen BR. Multiple roles of vascular endothelial growth factor (VEGF) in skeletal development, growth, and repair. Current Topics in Developmental Biology. 2004;65:169–187. doi: 10.1016/S0070-2153(04)65006-X. [DOI] [PubMed] [Google Scholar]
- Zernik J, Twarog K, Upholt WB. Regulation of alkaline phosphatase and alpha2 (I) procollagen synthesis during early intramembranous bone formation in the rat mandible. Differentiation. 1990;44:207–215. doi: 10.1111/j.1432-0436.1990.tb00619.x. [DOI] [PubMed] [Google Scholar]