Abstract
Emerging evidence suggests that the transcription factor Forkhead Box M1 (FoxM1) is associated with aggressive human carcinomas, including breast cancer. Because elevated expression of FoxM1 has been observed in human breast cancers, FoxM1 has attracted much attention in recent years as a potential target for the prevention and/or therapeutic intervention in breast cancer. However, no information is currently available regarding how downregulation of FoxM1 could be achieved for breast cancer prevention and therapy. Here, we report for the first time that 3,3′-diindolylmethane (DIM), a nontoxic dietary chemopreventive agent could effectively downregulate FoxM1 in various breast cancer cell lines. Using gene transfection, real-time reverse transcription-PCR, Western blotting, invasion and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays, we found that DIM could enhance Taxotere-induced growth inhibition of breast cancer cells, and decreased invasive capacity of breast cancer cells was observed after either treatment alone or the combination. These effects were associated with downregulation of FoxM1. We also found that knock down of FoxM1 expression by small interfering RNA (siRNA) transfection increased DIM-induced cell growth inhibition, whereas over-expression of FoxM1 by cDNA transfection attenuated DIM-induced cell growth inhibition, suggesting the mechanistic role of FoxM1. Most importantly, the combination treatment significantly inhibited tumor growth in severe combined immunodeficiency (SCID) mice, and the results were correlated with the downregulation of FoxM1 in tumor remnants. We conclude that inactivation of FoxM1 and its target genes by DIM could enhance the therapeutic efficacy of Taxotere in breast cancer, which could be a useful strategy for the prevention and/or treatment of breast cancer.
Keywords: FoxM1, DIM, Taxotere, breast cancer
Breast cancer is the second leading cause of cancer death in females, accounting for 15% of all female cancer deaths, thus early diagnosis and prevention of this disease are urgently needed to control the high incidence of breast cancer in our society. According to cancer statistics 2010 and surveillance epidemiology and end results, the 5-year survival rate of locally confined breast cancer is nearly 98% but the 5-year survival rate of metastatic breast cancer is just 23.4%, resulting in over 25,000 deaths annually.1,2 Thus, understanding the mechanisms by which breast cancer develops, and designing drug strategies by which the progression of breast cancer could be prevented is of utmost importance.
FoxM1, an oncogenic transcription factor, has been found in aggressive human breast cancer.3,4 It is believed that FoxM1 plays an important role in regulating the expression of genes involved in cell proliferation, differentiation and transformation.4–6 Recent studies have also suggested that activation of FoxM1 upregulates its downstream target genes, which contribute to aggressive tumor growth, angiogenesis and invasion, resulting in tumor progression.7 FoxM1 is overexpressed in cancer cells and acts as an important regulator of cancer cell growth and survival.4,8–10 It has been reported that FoxM1 is a key cell cycle regulator of both the transition from G1 to S phase and the progression to mitosis.11–13 Loss of FoxM1 expression generates mitotic spindle defects, delays cells in mitosis and induces mitotic catastrophes.14 FoxM1 has also been shown to regulate transcripts of cell cycle genes essential for G1-S and G2-M progression, including p27 and p21.11,12,15 Because FoxM1 plays important roles in cellular developmental pathways, including proliferation and apoptosis, alterations in FoxM1 expression are associated with tumorigenesis.16 These observations suggest that dysfunction of FoxM1 prevents differentiation, ultimately guiding undifferentiated cells toward malignant transformation. Furthermore, it has been reported that the FoxM1 expression is frequently deregulated in human malignancies with upregulated expression of FoxM1 in lung, glioblastomas, prostate, basal cell carcinomas, hepatocellular carcinoma, pancreatic cancer and primary breast cancer.4,5,9,16–18 These results suggest that FoxM1 plays an important role in the oncogenesis of several malignancies including breast malignancy.
Elevated expression of FoxM1 has been noted in a variety of aggressive human carcinomas including breast cancer, suggesting that the FoxM1 is an attractive target for the prevention and/or therapeutic intervention in breast cancer.19,20 No reports have been published regarding the role of chemopreventive agents in the regulation of FoxM1 in breast cancer. Previous studies have shown that a bioavailable, nontoxic dietary chemopreventive agent DIM can inactivate Akt/ Nuclear factor-κB (NF-κB) signaling and induce apoptosis in cancer cells.21–25 Other investigators have reported FoxM1 downregulation using drugs, namely antibiotic thiostrepton26 and epidermal growth factor receptor (EGFR) inhibitor gefitinib.27 These observations clearly suggest the role of FoxM1 in the progression of breast cancer; however, the molecular mechanism(s) by which FoxM1 contributes to the aggressiveness of breast cancer as well as the consequence of FoxM1 downregulation and the exact mechanism(s) have not been fully elucidated. To fill this void in our understanding, we investigated a novel molecular target, FoxM1, whose role in breast cancer is not understood, and whether DIM, a nontoxic dietary component, could inhibit this target in breast cancer. We also tested whether FoxM1 is necessary for cell survival by cDNA and siRNA approaches in DIM-treated breast cancer cells. In addition, we investigated whether downregulation of FoxM1 by DIM could inhibit its downstream target genes such as vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) that are known to be important for multiple cellular processes in carcinogenesis.28,29 From our study, we conclude that the effect of DIM may be mediated through the downregulation of FoxM1 signaling, resulting in the inhibition of its downstream signaling, which could lead to chemosensitization of breast cancer cells to chemotherapeutic agents such as Taxotere. We believe that targeting FoxM1 by DIM will be a novel approach for the treatment of breast cancer.
Material and Methods
Cell lines and experimental reagents
Breast cancer cell lines, MDA-MB-231, MCF-7, MDA-MB-468 and SKBR3 were obtained from ATCC (Manassas, VA). The cell lines have been tested and authenticated in core facility Applied Genomics Technology Center at Wayne State University. Primary antibodies for FoxM1, E2F1, cyclin-dependent kinase 2 (CDK2), survivin, p21 and p27 were purchased from Santa Cruz Biotechnology, and the monoclonal antibody to β-actin was from Sigma-Aldrich (St. Louis, MO). All secondary antibodies were obtained from Pierce. DIM was generously provided by Dr. Michael Zeligs (BioResponse, CO) and was dissolved in dimethyl sulfoxide (DMSO) to make a 50 mmol/L stock solution. Taxotere (Aventis Pharmaceuticals, NJ) was dissolved in DMSO to make a 4-μM stock solution.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Breast cells were seeded in 96-well plates. After 24 hr, the cells were treated with DIM (5, 10, 25, or 40 μM) followed by treatment of Taxotere (0.5, 1.0, or 1.5 nM) for 24–72 hr. The cell growth studies were performed by MTT as described earlier.21,23
Western blot analysis
Breast cancer cells were treated with DIM (25 or 40 μM) followed by treatment with and without Taxotere (1.0 nM) for 72 hr. Total proteins were fractionated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), electrotransferred, and probed with specific antibodies as described earlier. 21,23
Real-time reverse transcription-PCR (RT-PCR) analysis
The total RNA from transfected cells was isolated by TRIzol (Invitrogen) according to the manufacturer's protocols. One microgram of total RNA from each sample was subjected to first-strand cDNA synthesis using TaqMan reverse transcription reagents kit (Applied Biosystems). RT reaction conditions and primers used in this study were exactly the same as described previously.30
Plasmids and transfection
Breast cancer cells were transfected with FoxM1 siRNA (Santa Cruz) and FoxM1 cDNA (OriGene Technologies, MD), respectively, using LipofectAMINE 2000. 23,31 The transfected cells were treated with 25 or 40 μM DIM for 48 hr or 72 hr. The growth of transfected cells with and without treatments was measured by MTT assay.
VEGF and MMP-9 assay
Cells were seeded in six-well plates. After 24 hr of seeding, cells were treated with DIM (25 or 40 μM) followed by Taxotere (1.0 nM) treatment, and after another 24 hr, the culture media were collected. The medium was centrifuged at 800g for 3 min at 4°C to remove cell debris. The supernatant was used for VEGF assay immediately using commercially available ELISA kits (R&D Systems). For the MMP-9 activity assay, the cells in the plate were trypsinized, and the total number of cells was determined by cell counting. The assay was done using MMP-9 ELISA kits (R&D System) according to the manufacturer's instruction.
Invasion assay
The invasive activity breast cancer cells (5 × 104) followed by DIM (25 or 40 μM)/Taxotere (1.0 nM) treatment was tested using the BD BioCoat Tumor Invasion Assay System (BD Biosciences).
Animal studies
Female homozygous ICR SCID mice, ages 4 weeks, were used for our study. To initiate the xenografts, 5 × 106 MDA-MB-231 cells (in serum-free medium) were injected s.c. bilaterally in the flank areas of two SCID mice. Animals were developed tumors and removed after euthanizing all animals. Small pieces of tumors were then transplanted s.c. into right and left flanks of a new group of SCID mice using 12-gauge trocar. Animals were examined thrice per week for the development of palpable tumors (usually in 2 weeks). Once palpable tumors developed (0.5 cm × 0.5 cm, 63 mg), animals were randomly divided into four groups, 10 animals in each group. Group I was assigned as control and received only sesame seed oil without DIM, whereas Group II mice were given DIM by oral gavage (3.5 mg/day/animal by oral gavage). The mice from Group III received three doses of Taxotere (5 mg/kg, i.v.) every 72 hr. Group IV was administered DIM and was also treated with Taxotere. The mice in the intervention groups were given DIM by oral gavage every day for 3 weeks. Mice from all experimental groups were sacrificed 3 weeks after the start of all treatments. At that time, the tumors were harvested from each animal and processed for immunohistochemical staining and molecular analysis. The volume of the tumor in each group was determined by weekly caliper measurement according to the formula ab2/2, wherein a is length and b is cross-sectional diameter.21,22,30
Immunohistochemistry
Tissue collection, fixation and immunohistochemical staining were done according to the methods described earlier.21,22,30
Statistical analysis
The experimental results presented in the figures are representative of three or more independent observations. The data are presented as the mean values ± SE. Comparisons between groups were evaluated by Student's t test. Values of p < 0.05 were considered statistically significant.
Results
Cell growth inhibition by DIM and Taxotere treatment
We tested several doses of DIM and Taxotere at different time points, and we found that treatment of breast cancer cells with 25 μM DIM (Fig. 1a) or 1.0 nM Taxotere (Fig. 1b) for 72 h caused 30–50% growth inhibition. However, a combination of these doses of DIM and Taxotere resulted in 75–90% growth inhibition (Fig. 1c), suggesting the greater inhibitory effect of combination treatment. The highly aggressive, MDA-MB-231 breast cancer cells were treated with 40 μM DIM in combination with 1.0 nM Taxotere, which showed 78% inhibition of cell growth (Fig. 1c). These results show that the combination of DIM, along with a lower dose of Taxotere, elicited significantly greater inhibition of cancer cell growth compared with either agent alone (Figs. 1a–1c). Because inhibition of cell proliferation observed by MTT assay could be due to altered regulation of several genes such as FoxM1, we wanted to test the functional implication of such downregulation of FoxM1 by DIM and, therefore, we further investigated the expression of FoxM1 in breast cancer cells followed by DIM treatment.
Figure 1.

FoxM1 expression and its effects on cell growth after DIM and Taxotere treatment. For single-agent treatment, breast cancer cells were treated with DIM (5, 10, 25, or 40 μM), Taxotere (0.5, 1.0, or 1.5 nM) alone for 72 hr (a and b). For combination, breast cancer cells were treated with 25 or 40 (MDA-MB-231 only) μM of DIM and then exposed to 1.0 nM of Taxotere for 72 hr (c). DIM in combination with Taxotere significantly inhibited cell proliferation in all breast cancer cells (c) as measured by MTT assay. FoxM1 expression was evaluated by real-time RT-PCR and Western blotting (d). FoxM1 mRNA levels were downregulated by DIM, followed by Taxotere treatment as measured by real-time RT-PCR (e–h). Tax, Taxotere. β-actin protein was used as protein loading control for Western blot, whereas GAPDH was used as internal control for the real-time RT-PCR analysis. The data was obtained from three individual experiments. *, p < 0.05; **, p < 0.01 relatively control.
Downregulation of FoxM1 mRNA levels by DIM
The baseline expression of FoxM1 was determined in a panel of human breast cancer cell lines (data not shown) and a total of four cell lines, MDA-MB-231, MCF-7, SKBR3 and MDA-MB-468 were chosen for the current study based on their relative expression of FoxM1. First, we examined the relative mRNA levels of FoxM1 in the selected cell lines using real-time RT-PCR (Fig. 1d) and verified the results by analyzing the relative expression of FoxM1 in these cell lines by Western blot analysis (Fig. 1d). The first two cell lines (MDA-MB-231 and MDA-MB-468) showed higher expression of FoxM1, whereas the other two cell lines (MCF-7 and SKBR3) showed little or no detectable expression of FoxM1 (Fig. 1d). To determine whether FoxM1 could be an effective therapeutic target for breast cancer cells, the ability of DIM, Taxotere and combination to knock down FoxM1 mRNA was confirmed by real-time RT-PCR (Figs. 1e–1h). We observed that FoxM1 mRNA levels were significantly reduced in DIM or Taxotere treated cells for 72 hr compared with control cells, suggesting that DIM or Taxotere could inhibit cell growth partly through the downregulation of FoxM1.
Downregulation of FoxM1 expression by siRNA followed by DIM and Taxotere treatment
To study the functional relevance of DIM-mediated alteration of FoxM1 expression in breast cancer cells, the effect of FoxM1 siRNA on cell growth was examined in MDA-MB-231 and MDA-MB-468 cells, which express high levels of endogenous FoxM1. The cell viability was determined by MTT, and the effect of FoxM1 siRNA on cell growth is shown in Figure 2. We found that the downregulation of FoxM1 expression by siRNA transfection induced the cell growth inhibitory effect of DIM, Taxotere as well as the combination in both the cell lines (Figs. 2a and 2b).
Figure 2.
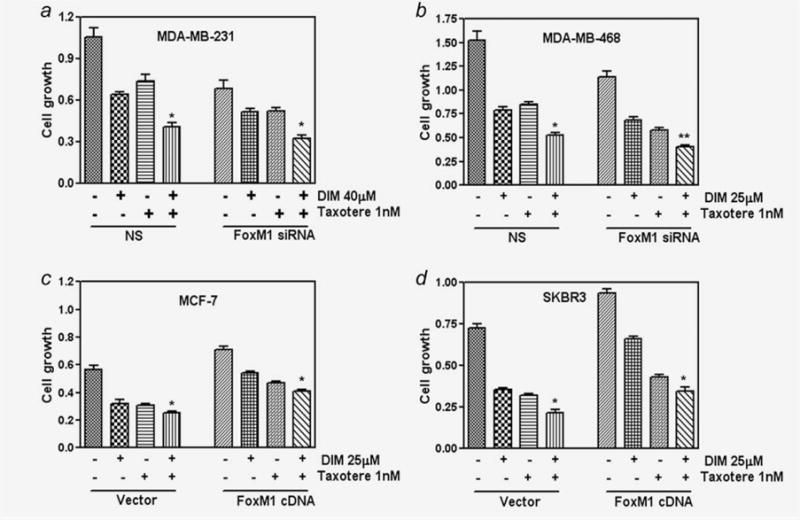
Regulation of FoxM1 expression by siRNA or cDNA followed by DIM and Taxotere treatment. Downregulation of FoxM1 expression by siRNA promotes DIM and Taxotere-induced cell growth inhibition in breast cancer cells (a and b). NS, Nonspecific. Overexpression of FoxM1 by FoxM1 cDNA transfection abrogated DIM and Taxotere-induced cell growth inhibition in breast cancer cells (c and d). The experiments were repeated thrice. *, p < 0.05; **, p < 0.01 relatively control.
Overexpression of FoxM1 by cDNA transfection reduced DIM and Taxotere-induced cell growth inhibition
To further confirm the role of FoxM1 in the growth of breast cancer cells, we transfected FoxM1 cDNA in two breast cancer cell lines (MCF-7 and SKBR3) which both express very low levels of FoxM1. We found that the overexpression of FoxM1 in these cell lines resulted in a significant increase in cell growth (Figs. 2c and 2d) compared to their parental cells. Overexpression of FoxM1 was also found to minimize DIM and Taxotere-induced cell growth inhibition. These results provide evidence for a potential role of FoxM1 during DIM enhanced Taxotere-induced cell growth inhibition in breast cancer cells.
Effect of FoxM1 on cell cycle regulatory factors
In view of the known effects of FoxM1 on the cell cycle,12,14,32 we examined the levels of a few cell cycle regulatory factors in cell lines expressing FoxM1 and studied the effects of downregulation of FoxM1. As seen in Figure 3 (upper left and right panel), the expression of CDK2 and E2F transcription factor 1 (E2F1) was found to be decreased upon FoxM1 downregulation, as determined by real time RT-PCR. Further, downregulation of FoxM1 was also found to result in an increase in the expression of CDK inhibitors such as p27 and p21 (Fig. 3, lower left and right panel), suggesting the mechanistic roles of these molecules during cell cycle arrest by DIM and in combination with Taxotere. Our data suggests that FoxM1 influences the cell cycle progression by positively regulating the factors that favor cell cycle progression and also by negatively influencing the inhibitors of cell cycle.
Figure 3.
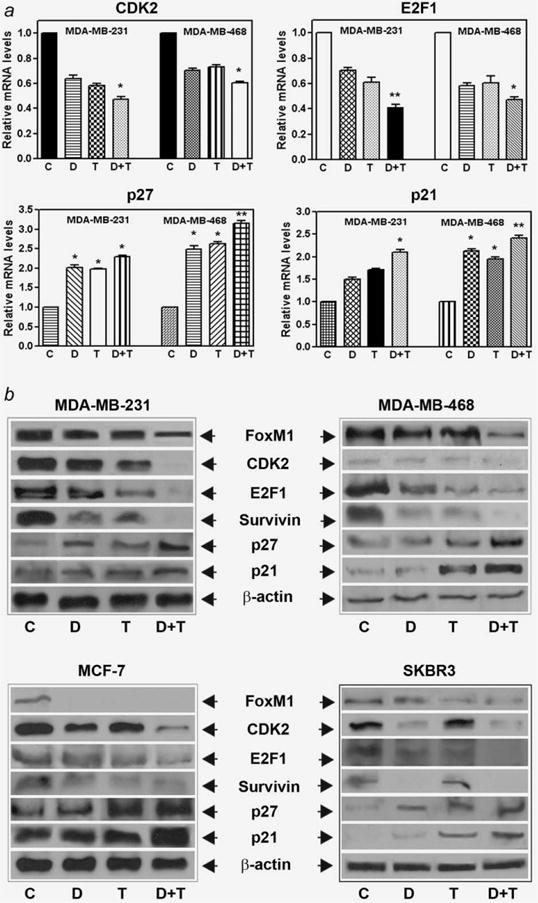
Effect of FoxM1 on cell cycle regulatory factors. The expression level of several known cell cycle regulatory factors, as measured by real-time RT-PCR (a) and Western blotting (b) in all four breast cancer cells. C, Control; D, DIM [25 or 40 (MDA-MB-231) μM]; T, Taxotere (1.0 nM); D+T, DIM + Taxotere. The experiments were repeated thrice. *, p < 0.05; **, p < 0.01 relatively control.
To further verify the regulation of gene expression at the protein level by DIM and Taxotere treatment, we conducted Western blot analysis only for selected genes that are critically important for the inhibition of cell growth and induction of apoptosis. Western blot analysis showed that the expression of FoxM1 target genes involved in cell proliferation and survival, such as CDK2, E2F1 and survivin was downregulated, and the expression of inhibitory p27 and p21 was upregulated in DIM as well as Taxotere-treated cells (Fig. 3b). These results suggest that DIM regulates the transcription of genes involved in apoptosis and cell cycle arrest, angiogenesis and tumor cell invasion.
Downregulation of FoxM1 decreased MMP-9 and VEGF expression and their activities
FoxM1 is known to regulate the expression of MMP-9, which plays a role in tumor cell invasion and metastatic processes of breast cancer cells.29,33 Interestingly, MMP-9 expression is elevated in the liver of FoxM1B transgenic mice.10 Therefore, we investigated whether the downregulation of FoxM1 by DIM could lead to a decrease in MMP-9 activity. We observed a marked decrease in the activity of MMP-9 in all breast cancer cells treated with DIM, followed by Taxotere treatment (Fig. 4, upper panel). VEGF is another molecule that is involved in tumor cell invasion and metastasis.28 It has been reported that FoxM1 regulates VEGF signaling in various cell types.28,34 To further explore whether DIM treatment reduced VEGF activity, we examined the activity of VEGF secreted in the culture medium. We found that downregulation of FoxM1 by DIM, followed by Taxotere treatment, could lead to a decrease in the levels of VEGF secreted in the culture medium (Fig. 4, lower panel). We also investigated the activity of MMP-9 and VEGF in FoxM1 siRNA and FoxM1cDNA transfected breast cancer cells followed by DIM and Taxotere treatment, and we observed significant downregulation by siRNA followed by DIM plus Taxotere treatment, whereas cDNA transfection attenuated in the expression of MMP-9 and VEGF (data not shown). These results clearly suggest that tumor progression could be attenuated by the downregulation of FoxM1.
Figure 4.
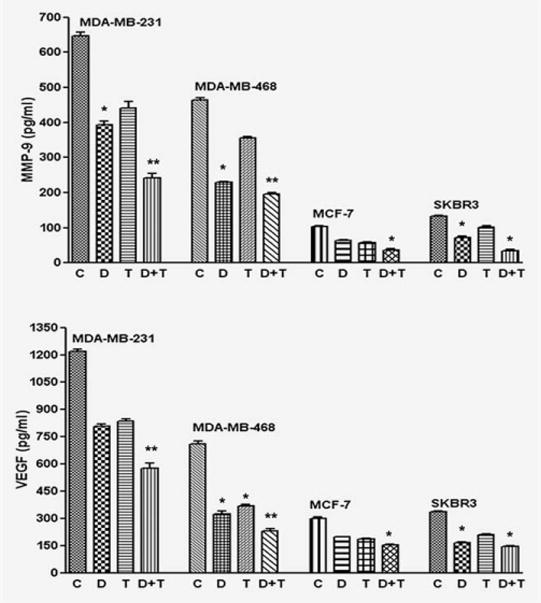
Downregulation of FoxM1 decreased MMP-9 and VEGF activities. Effects of FoxM1 downregulation on the activities of MMP-9 and VEGF, as measured by respective ELISA analysis conducted using conditioned media. C, Control; D, DIM [25 or 40 (MDA-MB-231) μM]; T, Taxotere (1.0 nM); D+T, DIM + Taxotere. All the analyses were done at least thrice. *, p < 0.05; **, p < 0.01 relatively control.
Downregulation of FoxM1 decreased breast cancer cell invasion
Because silencing of FoxM1 inhibited the expression and activity of several factors that are critically involved in the processes of tumor cell, invasion and metastasis,5,35 we tested the effect of FoxM1 downregulation on cancer cell invasion (Fig. 5). We found that downregulation of FoxM1 decreased MDA-MB-231 and MDA-MB-468 breast cancer cell invasion (Fig. 5). Moreover, as illustrated in Figure 5, DIM- or Taxotere-treated cells showed a low level of penetration through the Matrigel-coated membrane (invasion) compared with the control cells. These results verify the functional implication of downregulation of factors such as VEGF resulting from FoxM1 silencing.
Figure 5.
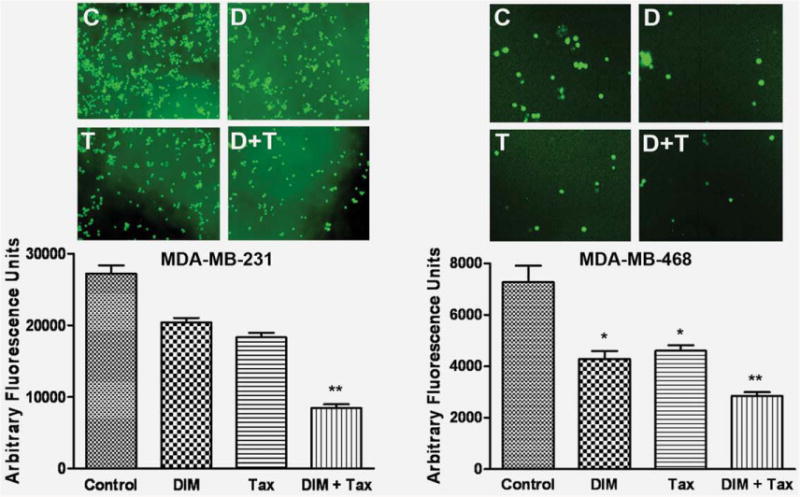
DIM decreased breast cancer cell invasion. Invasion assay showing that DIM-treated breast cancer cells resulted in low penetration through the Matrigel-coated membrane, compared with control cells. MDA-MB-231 and MDA-MB-468 cell were with 25 and 40 μM of DIM, respectively. The bar graphs show the fluorescence values from the invasive cells. The value indicated the comparative amount of invaded cells. C, Control; D, DIM 25 or 40 μM; T, Taxotere (1.0 nM); D+T, DIM + Taxotere; Tax, Taxotere; DIM+Tax, DIM + Taxotere. The experiments were repeated thrice. *, p < 0.05; **, p < 0.01 relatively control. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DIM enhances in vivo therapeutic efficacy of Taxotere
We found that DIM and Taxotere significantly inhibited MDA-MB-231 tumor growth 40% and 65%, respectively (Fig. 6a). DIM potentiated tumor growth inhibition induced by Taxotere (80%), demonstrating an enhanced inhibitory effect of DIM/Taxotere combination treatment on the in vivo model of breast cancer (Fig. 6a). The expression of FoxM1 was significantly decreased in MDA-MB-231 tumors in SCID mice receiving DIM, compared with the control (Fig. 6b). Importantly, the combination of DIM and Taxotere showed a greater degree of downregulation of FoxM1 (Fig. 6b). To explore the molecular mechanism by which DIM potentiated the anti-tumor and anti-metastatic activity of Taxotere, we further analyzed the FoxM1 target gene altered by DIM or Taxotere treatment. Our results clearly show that FoxM1 and its effectors genes, such as E2F1, CDK2, p27 and survivin, were significantly downregulated in specimens obtained from the combination group (Fig. 6c). These in vivo results confirmed our in vitro findings, suggesting that the inactivation of FoxM1 is at least one of the molecular events by which the drug combination potentiates antitumor activity in our experimental model.
Figure 6.
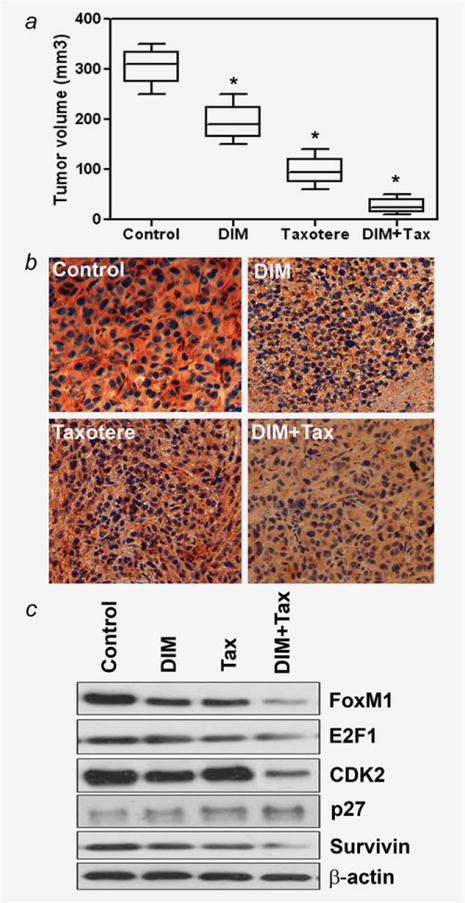
Inhibition of tumor growth in vivo by DIM alone or in combination with Taxotere. DIM or Taxotere alone significantly inhibited MDA-MB-231 tumor growth (40% and 65%, respectively). Treatment of animals with DIM in combination with Taxotere caused 80% reduction in tumor volume compared with control group (a). DIM+Tax, DIM + Taxotere. *, p < 0.05 relatively control. Immunohistochemical staining for FoxM1 in DIM plus Taxotere-treated and -untreated animal tumors done on randomly selected tumor tissues (b). Tumor cells in untreated control group show intensive staining of FoxM1 (b). DIM+Tax, DIM + Taxotere. In contrast, tumor cells in DIM plus Taxotere-treated group show weaker staining of FoxM1 (b). The total proteins were extracted from randomly selected frozen tumor tissues obtained from each treatment group of animals and subjected to Western blot analysis (c). Results showed that the combination of DIM and Taxotere was effective in downregulating FoxM1, E2F1, CDK2, p27 and survivin in treated animals relative to the control tumors (c). Tax, Taxotere; DIM+Tax, DIM + Taxotere. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
Overexpression of FoxM1, an oncogenic transcription factor, has been found in a variety of aggressive human cancers, including breast cancer.4,36 Several studies have suggested that the activation of FoxM1 upregulates its downstream target genes, resulting in the activation of migration, invasion and the angiogenic processes.28,29,33,34 Therefore, FoxM1 appears to be an attractive target for therapy, and it has rightly been pointed out that by inhibiting this single transcription factor it should be possible to target multiple facets of tumorigenesis.37 Further, a large-scale analysis of microarray results revealed that FoxM1 is one of the most common genes over expressed in a majority of solid tumors.38 In breast cancer, one of the earliest clues for the involvement of FoxM1 was provided by a bioinformatic approach.38 Another study revealed that FoxM1 is significantly downregulated by docetaxel, either alone or in combination with other drugs, in prostate cancer cells.39 A recent review article reported that FoxM1 could be inhibited by Siomycin A.19 Because FoxM1 gene is upregulated in breast cancer,3,4 the mechanism(s) of action of FoxM1 as well as the consequence of FoxM1 downregulation by DIM and the exact mechanism(s) have not been fully elucidated. More importantly, there is no strategy by which FoxM1-driven cellular signaling could be inhibited by DIM which may result in a better treatment outcome. Therefore, to identify the molecular basis for the effects of DIM in breast cancer, we investigated the role of FoxM1 in cell proliferation of metastatic and invasive breast cancer cell lines. We found that downregulation of FoxM1 by DIM resulted in a significant decrease in the aggressiveness of breast cancer cells. These results provide clear evidence in support of the role of FoxM1 as an oncogene in breast cancer cells.
Because downregulation of FoxM1 reduced cell growth, we assumed inhibition of cell growth could be due to the induction of cell cycle arrest. The importance of FoxM1 with respect to cell cycle is well recognized.36,40 Recent studies also indicate that the overexpression of FoxM1 in tumors is associated with poor prognosis and increased tumor recurrence because it may overcome the G1-S checkpoint imposing progression of cells through cell cycle conferring proliferative advantage.11–14 Accordingly, we observed that DIM was effective in downregulating FoxM1 in breast cancer cells as well as inducing G1-S cell cycle arrest. Our results also showed that mRNA expression of E2F1 and CDK2 was downregulated in DIM-treated breast cancer cells, whereas p21 and p27 were upregulated, suggesting that DIM inhibited the growth of breast cancer cells through the arrest of cell cycle and inhibition of proliferation. Similar effects on the cell cycle have been observed in DIM-treated prostate cancer cells.41 It has been shown that cyclins binds to CDK to control the cell cycle process.42 The CDK inhibitors, including p21 and p27, have been shown to arrest the cell cycle and inhibit the growth of cancer cells.11,43,44 Our observations confirm these earlier published results supporting the role of FoxM1 in breast cancer progression. Based on our findings, one can argue that DIM in combination with Taxotere treatment may be effective in blocking cell cycle progression in the G1-S phase resulting from DIM-induced inhibition of FoxM1 that leads the cells to undergo apoptosis.45,46 This highlights yet another important molecular mechanism of action of DIM in enhancing the therapeutic efficacy of cytotoxic agents because G1-S cell cycle arrest has emerged as an attractive therapeutic target for cancer therapy.
FoxM1 is known to regulate the expression of the MMP-2 and MMP-9, both of which play a role in tumor cell invasion.29,33 FoxM1 has been reported to promote both invasion and angiogenesis in certain tumor models and inhibition of FoxM1 has been shown to reduce tumor cell proliferation and angiogenesis in hepatocellular carcinomas.5,35 It is known that MMPs are critically involved in the processes of tumor cell invasion and metastasis and that MMPs are directly associated with angiogenesis and metastatic processes.47 MMP-9 has been implicated in metastasis because of its role in the degradation of basement membrane collagen.48 MMP-9 expression was also elevated in the liver of FoxM1B transgenic mice.10 We hypothesized that downregulation of FoxM1 by any agent(s) could potentiate anti-tumor and anti-angiogenic activities, partly through the downregulation of the expression of MMPs in breast cancer cells. In this study, we found that the downregulation of FoxM1 led to a reduced expression of MMP-9. VEGF is another molecule that is involved in tumor cell invasion and metastasis.28 Many studies have documented that VEGF is a critical mediator of angiogenesis and regulates most of the steps in the angiogenic cascade including proliferation, migration and tube formation of endothelial cells.49 Recent studies have shown that VEGF promotes migration and invasion of breast cancer cells.50 It has been reported that FoxM1 regulates VEGF signaling in various cell types.34 These findings confirm our current findings, showing reduced levels and activity of VEGF secreted in the culture medium. To fully understand the consequence of such downregulation in the expression and the activity of VEGF and MMPs, we performed invasion assays, where downregulation of FoxM1 led to a significant reduction in the invasive potential of MDA-MB-231 and MDA-MB-468 cells.
To test whether DIM has any anti-tumor effects in vivo, and whether FoxM1 is inactivated and correlated with down-regulation of it target genes, we conducted an animal experiment using a xenograft model of experimental breast cancer. We found that treatment of MDA-MB-231 breast cancer cells with DIM sensitizes these cells to Taxotere, which could be mechanistically related to downregulation of FoxM1 in tumor tissues. These results further support the conclusion that downregulation of FoxM1 promotes Taxotere-induced inhibition of breast cancer cells growth in vivo. The expression of FoxM1, an important molecule of tumor cell survival and metastasis, in our immunohistochemical analysis, was significantly decreased in MDA-MB-231 tumors in mice receiving the DIM and Taxotere compared with control. In general, our in vitro and in vivo results showed that the use of a dietary agent such as DIM alone or in combination with other chemotherapeutic agents could be useful for the treatment of breast cancer. Importantly, our observations from preclinical animal model and in vitro studies revealed the downregulation of FoxM1 in sensitization experiments, which may be effective in contributing to the potentiation of Taxotere cytotoxicity by DIM. These observations on alterations in the expression status of FoxM1 by DIM are of considerable importance, which strongly suggest that DIM sensitizes the breast cancer cells to the cytotoxic effect of chemotherapeutic drugs.
In conclusion, our present findings suggest that DIM may function as an inhibitor of FoxM1, ultimately causing cell growth inhibition and induction of apoptosis, which may lead to chemosensitization of breast cancer to Taxotere. The downregulation of FoxM1 by DIM and Taxotere reduced the levels of E2F1 and CDK2 and increased p21 and p27 expression. We believe that downregulation of FoxM1 by DIM, followed by Taxotere treatment could lead to a decrease in the levels of VEGF and MMP-9, which is likely to result in the inhibition of invasion, angiogenesis and metastasis of breast cancer. Taken together, the results from our in vitro as well as in vivo experiments clearly suggest that the combination of DIM and Taxotere could be a potential regimen for the treatment of patients diagnosed with breast cancer. Further, in-depth testing of combinations of various drugs with DIM are needed in support of designing mechanism-based anticancer therapies, particularly for metastatic breast cancer.
Acknowledgments
Department of Defense; Grant numbers: W81XWH-04-1-0689, W81XWH-05-1-0505
This work was partially funded by Department of Defense to KM. W. R.
References
- 1.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldon W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975-2007. USA: National Cancer Institute; 2010. [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Bektas N, Haaf A, Veeck J, Wild PJ, Luscher-Firzlaff J, Hartmann A, Knuchel R, Dahl E. Tight correlation between expression of the Forkhead transcription factor FOXM1 and HER2 in human breast cancer. BMC Cancer. 2008;8:1–9. doi: 10.1186/1471-2407-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madureira PA, Varshochi R, Constantinidou D, Francis RE, Coombes RC, Yao KM, Lam EW. The Forkhead box M1 protein regulates the transcription of the estrogen receptor alpha in breast cancer cells. J Biol Chem. 2006;281:25167–76. doi: 10.1074/jbc.M603906200. [DOI] [PubMed] [Google Scholar]
- 5.Kalin TV, Wang IC, Ackerson TJ, Major ML, Detrisac CJ, Kalinichenko VV, Lyubimov A, Costa RH. Increased levels of the FoxM1 transcription factor accelerate development and progression of prostate carcinomas in both TRAMP and LADY transgenic mice. Cancer Res. 2006;66:1712–20. doi: 10.1158/0008-5472.CAN-05-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida Y, Wang IC, Yoder HM, Davidson NO, Costa RH. The forkhead box M1 transcription factor contributes to the development and growth of mouse colorectal cancer. Gastroenterology. 2007;132:1420–31. doi: 10.1053/j.gastro.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Penzo M, Massa PE, Olivotto E, Bianchi F, Borzi RM, Hanidu A, Li X, Li J, Marcu KB. Sustained NF-kappaB activation produces a short-term cell proliferation block in conjunction with repressing effectors of cell cycle progression controlled by E2F or FoxM1. J Cell Physiol. 2009;218:215–27. doi: 10.1002/jcp.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandran UR, Ma C, Dhir R, Bisceglia M, Lyons-Weiler M, Liang W, Michalopoulos G, Becich M, Monzon FA. Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer. 2007;7:1–21. doi: 10.1186/1471-2407-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim IM, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV, Major ML, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006;66:2153–61. doi: 10.1158/0008-5472.CAN-05-3003. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Bhattacharyya D, Dennewitz MB, Kalinichenko VV, Zhou Y, Lepe R, Costa RH. Rapid hepatocyte nuclear translocation of the Forkhead Box M1B (FoxM1B) transcription factor caused a transient increase in size of regenerating transgenic hepatocytes. Gene Expr. 2003;11:149–62. doi: 10.3727/000000003108749044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Hung NJ, Costa RH. Earlier expression of the transcription factor HFH-11B diminishes induction of p21(CIP1/ WAF1) levels and accelerates mouse hepatocyte entry into S-phase following carbon tetrachloride liver injury. Hepatology. 2001;33:1404–14. doi: 10.1053/jhep.2001.24666. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Kiyokawa H, Dennewitz MB, Costa RH. The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci USA. 2002;99:16881–6. doi: 10.1073/pnas.252570299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang IC, Chen YJ, Hughes DE, Ackerson T, Major ML, Kalinichenko VV, Costa RH, Raychaudhuri P, Tyner AL, Lau LF. FoxM1 regulates transcription of JNK1 to promote the G1/S transition and tumor cell invasiveness. J Biol Chem. 2008;283:20770–8. doi: 10.1074/jbc.M709892200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wonsey DR, Follettie MT. Loss of the forkhead transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe. Cancer Res. 2005;65:5181–9. doi: 10.1158/0008-5472.CAN-04-4059. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Krupczak-Hollis K, Tan Y, Dennewitz MB, Adami GR, Costa RH. Increased hepatic Forkhead Box M1B (FoxM1B) levels in old-aged mice stimulated liver regeneration through diminished p27Kip1 protein levels and increased Cdc25B expression. J Biol Chem. 2002;277:44310–16. doi: 10.1074/jbc.M207510200. [DOI] [PubMed] [Google Scholar]
- 16.Liu M, Dai B, Kang SH, Ban K, Huang FJ, Lang FF, Aldape KD, Xie TX, Pelloski CE, Xie K, Sawaya R, Huang S. FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer Res. 2006;66:3593–602. doi: 10.1158/0008-5472.CAN-05-2912. [DOI] [PubMed] [Google Scholar]
- 17.Kalinichenko VV, Major ML, Wang X, Petrovic V, Kuechle J, Yoder HM, Dennewitz MB, Shin B, Datta A, Raychaudhuri P, Costa RH. Foxm1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19ARF tumor suppressor. Genes Dev. 2004;18:830–50. doi: 10.1101/gad.1200704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Res. 2002;62:4773–80. [PubMed] [Google Scholar]
- 19.Adami GR, Ye H. Future roles for FoxM1 inhibitors in cancer treatments. Future Oncol. 2007;3:1–3. doi: 10.2217/14796694.3.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad A, Wang Z, Kong D, Ali S, Li Y, Banerjee S, Ali R, Sarkar FH. FoxM1 down-regulation leads to inhibition of proliferation, migration and invasion of breast cancer cells through the modulation of extra-cellular matrix degrading factors. Breast Cancer Res Treat. 2010;122:337–46. doi: 10.1007/s10549-009-0572-1. [DOI] [PubMed] [Google Scholar]
- 21.Rahman KM, Ali S, Aboukameel A, Sarkar SH, Wang Z, Philip PA, Sakr WA, Raz A. Inactivation of NF-kB by 3,3′-diindolylmethane (DIM) contributes to increased apoptosis induced by chemotherapeutic agent in breast cancer cells. Mol Cancer Ther. 2007;6:1–9. doi: 10.1158/1535-7163.MCT-07-0336. [DOI] [PubMed] [Google Scholar]
- 22.Rahman KM, Banerjee S, Ali S, Ahmad A, Wang Z, Kong D, Sakr WA. 3,3′-Diindolylmethane enhances taxotere-induced apoptosis in hormone-refractory prostate cancer cells through survivin down-regulation. Cancer Res. 2009;69:4468–75. doi: 10.1158/0008-5472.CAN-08-4423. [DOI] [PubMed] [Google Scholar]
- 23.Rahman KM, Sarkar FH. Inhibition of nuclear translocation of nuclear factor-{kappa}B contributes to 3,3′-diindolylmethane-induced apoptosis in breast cancer cells. Cancer Res. 2005;65:364–71. [PubMed] [Google Scholar]
- 24.Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. 3,3′-Diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27:717–28. doi: 10.1093/carcin/bgi270. [DOI] [PubMed] [Google Scholar]
- 25.Kassie F, Anderson LB, Scherber R, Yu N, Lahti D, Upadhyaya P, Hecht SS. Indole-3-carbinol inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo(a)pyrene-induced lung tumorigenesis in A/J mice and modulates carcinogen-induced alterations in protein levels. Cancer Res. 2007;67:6502–11. doi: 10.1158/0008-5472.CAN-06-4438. [DOI] [PubMed] [Google Scholar]
- 26.Kwok JM, Myatt SS, Marson CM, Coombes RC, Constantinidou D, Lam EW. Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression. Mol Cancer Ther. 2008;7:2022–32. doi: 10.1158/1535-7163.MCT-08-0188. [DOI] [PubMed] [Google Scholar]
- 27.McGovern UB, Francis RE, Peck B, Guest SK, Wang J, Myatt SS, Krol J, Kwok JM, Polychronis A, Coombes RC, Lam EW. Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in breast cancer. Mol Cancer Ther. 2009;8:582–91. doi: 10.1158/1535-7163.MCT-08-0805. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N. History of discovery: vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29:789–91. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- 29.Wan R, Mo Y, Zhang X, Chien S, Tollerud DJ, Zhang Q. Matrix metalloproteinase-2 and –9 are induced differently by metal nanoparticles in human monocytes: the role of oxidative stress and protein tyrosine kinase activation. Toxicol Appl Pharmacol. 2008;233:276–85. doi: 10.1016/j.taap.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman KM, Sarkar FH, Banerjee S, Wang Z, Liao DJ, Hong X, Sarkar NH. Therapeutic intervention of experimental breast cancer bone metastasis by indole-3-carbinol in SCID-human mouse model. Mol Cancer Ther. 2006;5:2747–56. doi: 10.1158/1535-7163.MCT-06-0221. [DOI] [PubMed] [Google Scholar]
- 31.Rahman KM, Li Y, Sarkar FH. Inactivation of akt and NF-kappaB play important roles during indole-3-carbinol-induced apoptosis in breast cancer cells. Nutr Cancer. 2004;48:84–94. doi: 10.1207/s15327914nc4801_12. [DOI] [PubMed] [Google Scholar]
- 32.Ye H, Holterman AX, Yoo KW, Franks RR, Costa RH. Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Mol Cell Biol. 1999;19:8570–80. doi: 10.1128/mcb.19.12.8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai B, Kang SH, Gong W, Liu M, Aldape KD, Sawaya R, Huang S. Aberrant FoxM1B expression increases matrix metalloproteinase-2 transcription and enhances the invasion of glioma cells. Oncogene. 2007;26:6212–19. doi: 10.1038/sj.onc.1210443. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Zhang N, Dai B, Liu M, Sawaya R, Xie K, Huang S. FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells. Cancer Res. 2008;68:8733–42. doi: 10.1158/0008-5472.CAN-08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gusarova GA, Wang IC, Major ML, Kalinichenko VV, Ackerson T, Petrovic V, Costa RH. A cell-penetrating ARF peptide inhibitor of FoxM1 in mouse hepatocellular carcinoma treatment. J Clin Invest. 2007;117:99–111. doi: 10.1172/JCI27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7:126–36. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- 37.Radhakrishnan SK, Gartel AL. FOXM1: the Achilles' heel of cancer? Nat Rev Cancer. 2008;8:c1. doi: 10.1038/nrc2223-c1. author reply c2. [DOI] [PubMed] [Google Scholar]
- 38.Pilarsky C, Wenzig M, Specht T, Saeger HD, Grutzmann R. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6:744–50. doi: 10.1593/neo.04277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Hong X, Hussain M, Sarkar SH, Li R, Sarkar FH. Gene expression profiling revealed novel molecular targets of docetaxel and estramustine combination treatment in prostate cancer cells. Mol Cancer Ther. 2005;4:389–98. doi: 10.1158/1535-7163.MCT-04-0244. [DOI] [PubMed] [Google Scholar]
- 40.Costa RH. FoxM1 dances with mitosis. Nat Cell Biol. 2005;7:108–10. doi: 10.1038/ncb0205-108. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Li X, Sarkar FH. Gene expression profiles of I3C- and DIM-treated PC3 human prostate cancer cells determined by cDNA microarray analysis. J Nutr. 2003;133:1011–19. doi: 10.1093/jn/133.4.1011. [DOI] [PubMed] [Google Scholar]
- 42.Obaya AJ, Sedivy JM. Regulation of cyclin-Cdk activity in mammalian cells. Cell Mol Life Sci. 2002;59:126–42. doi: 10.1007/s00018-002-8410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawamata N, Morosetti R, Miller CW, Park D, Spirin KS, Nakamaki T, Takeuchi S, Hatta Y, Simpson J, Wilcyznski S, Lee YY, Bartram CR. Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res. 1995;55:2266–9. [PubMed] [Google Scholar]
- 44.Leung KC, Hsin MK, Chan JS, Yip JH, Li M, Leung BC, Mok TS, Warner TD, Underwood MJ, Chen GG. Inhibition of thromboxane synthase induces lung cancer cell death via increasing the nuclear p27. Exp Cell Res. 2009;315:2974–81. doi: 10.1016/j.yexcr.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Hong C, Kim HA, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane (DIM) induces a G(1) cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21(WAF1/CIP1) expression. Carcinogenesis. 2002;23:1297–305. doi: 10.1093/carcin/23.8.1297. [DOI] [PubMed] [Google Scholar]
- 46.Song J, Salek-Ardakani S, So T, Croft M. The kinases aurora B and mTOR regulate the G1-S cell cycle progression of T lymphocytes. Nat Immunol. 2007;8:64–73. doi: 10.1038/ni1413. [DOI] [PubMed] [Google Scholar]
- 47.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 48.Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000;36:1621–30. doi: 10.1016/s0959-8049(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 49.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–27. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 50.Hatake K, Tokudome N, Ito Y. Next generation molecular targeted agents for breast cancer: focus on EGFR and VEGFR pathways. Breast Cancer. 2007;14:132–49. doi: 10.2325/jbcs.977. [DOI] [PubMed] [Google Scholar]


