Abstract
Purpose.
To describe Heidelberg Retina Tomograph (HRT) measures, their principal components, and their associations in a British population.
Methods.
The European Prospective Investigation of Cancer (EPIC)-Norfolk Eye Study is nested within a multicenter cohort study. Measurements were taken with the HRT-2 and the software subsequently updated to yield HRT-3 parameters. Principal components analysis (PCA) was used to identify distinct components of the HRT variables. Generalized estimating equation models were used to examine associations of these components with age, sex, height, body mass index (BMI), blood pressure, social class, education, alcohol intake, smoking status, axial length, IOP, and lens status.
Results.
Complete data were available from 10,859 eyes of 6430 participants with a mean age of 68 years. Principal components analysis identified three components with an eigenvalue greater than 1, explaining 79.9% of the variance of all the HRT measures. These were named cup, retinal nerve fiber layer (RNFL), and rim based on the factor loadings they were most correlated with. Older age was significantly associated with a greater cup (P = 0.003), smaller RNFL (P < 0.001), and smaller rim (P < 0.001). Female sex (P = 0.001), higher education (P < 0.001), and shorter axial length (P < 0.001) were associated with a greater RNFL. Lower BMI and higher IOP were associated with a greater cup (both, P < 0.001) and a smaller rim (BMI, P = 0.001; IOP, P < 0.001).
Conclusions.
Heidelberg Retina Tomograph measures in this cohort were largely explained by three principal components related to optic disc cup, RNFL, and rim. Associations with cup and rim were distinct to associations with RNFL, suggesting different underlying determinants.
Keywords: diagnostic techniques, epidemiology, axial length, glaucoma, body mass index, optic disk
Analysis of Heidelberg Retina Tomograph measurements from a large population-based sample identified three distinct components (optic disc “cup”, “rim” and retinal nerve fiber layer [“RNFL”]). Associations with “cup” and “rim” were distinct to associations with “RNFL”, suggesting different underlying determinants.
Introduction
Structural assessment of the optic disc is an important component of the workup of patients with suspected or established glaucoma.1,2 Optic disc photography is a common, low-cost method of assessment, but may suffer from high intra- and interobserver variability for the diagnosis of glaucoma.3 Newer imaging devices provide quantitative structural measures that may be useful for the diagnosis of glaucoma and detection of disease progression.4,5 The Heidelberg Retina Tomograph (HRT) is one such device which creates reproducible 3-dimensional (3D) images of the optic nerve head using scanning laser ophthalmoscopy.6,7
While some risk factors for POAG are well established (such as age, race, family history, and raised IOP),8 the evidence for others such as sex,9 and anthropometric measures10 is inconsistent. This may be a result of small effects not being detected due to misclassification of glaucoma as a dichotomous outcome. Certainly, the reported prevalence of glaucoma varies greatly depending on the definition used.11 Objective quantitative measures related to glaucoma, such as HRT-derived parameters, may provide greater power for detecting weak associations. This “endophenotype” approach has been fruitful in genetic association studies for glaucoma.12 Understanding what influences optic nerve head structural measures at a population level may provide insight into the aetiology of glaucoma.
The aims of this study were to:
Describe HRT-derived optic nerve head measures in a predominantly White British population;
Examine the correlation structure of the glaucoma-related HRT measures to determine their principal components; and
Describe systemic and ocular associations with these HRT component measures.
Methods
Participants
The European Prospective Investigation into Cancer (EPIC) study is a pan-European prospective population-based cohort study.13 EPIC-Norfolk , one of the United Kingdom (UK) arms of EPIC, recruited and examined 25,639 residents aged 40 to 79 years between 1993 and 1997 for the baseline examination.14 Recruitment was via general practices in the city of Norwich and the surrounding small towns and rural areas, and methods have been described in detail previously.14 Since virtually all residents in the UK are registered with a general practitioner through the National Health Service, general practice lists serve as population registers. Ophthalmic assessment formed part of the third health examination and this is termed the EPIC-Norfolk Eye Study.15 In total, 8623 participants were seen for the ophthalmic examination, between 2004 and 2011. The EPIC-Norfolk Eye Study was carried out following the principles of the Declaration of Helsinki and the Research Governance Framework for Health and Social Care. The study was approved by the Norfolk Local Research ethics committee (05/Q0101/191) and the East Norfolk & Waveney National Health Science Research Governance Committee (2005EC07L). All participants gave written, informed consent.
Measurements
Scanning laser ophthalmoscopy of each eye was carried out using the HRT 2 (Heidelberg Engineering, Heidelberg, Germany) following entering the participant's keratometry and refraction (Auto-Refractor 500; Humphrey Instruments, San Leandro, California). If image quality was poor (topography SD > 40 μm) a repeat scan was undertaken. Contours around the disc margins were manually drawn and subsequently checked by an ophthalmologist (and redrawn if necessary). The HRT software was subsequently updated to Glaucoma Module Premium Edition (software version 3.1; Heidelberg Engineering) and data exported following this. This derived data that is equivalent to HRT-3–derived parameters.
Axial length was measured using a Zeiss IOLMaster Optical Biometer (Carl Zeiss Meditech Ltd., Welwyn Garden City, UK). Five measurements were taken per eye and a mean was calculated. Intraocular pressure was measured using a noncontact appliance, the Ocular Response Analyzer (ORA; Reichert, Corp., Buffalo, NY). Three readings were taken per eye and the single best value of the Goldmann-correlated value used (based on the best quality pressure waveform as assessed by the ORA software). All ocular examinations were carried out without pupil dilation.
Height and weight were measured at the third health examination, with participants wearing light clothing and no shoes. Height was measured to 0.1 cm using a stadiometer, and weight was measured to the nearest 0.1 kg using digital scales (Tanita UK Ltd., Middlesex, UK). Body mass index (BMI) was calculated as weight/height.2 Self-reported alcohol intake and smoking status were also ascertained at the third health examination. Alcohol intake was calculated as units consumed per week based on a questionnaire asking how much of beer/cider/lager (half pints), wine (glasses), sherry/fortified wine (glasses), or spirits (single measures) were drunk for each day in the last week. Blood pressure was also measured in the third health examination using an objective measurement device (Accutorr Plus; Datascope Patient Monitoring, Mindray UK, Ltd., Huntington, UK). Social class and educational level were ascertained at the first health examination. Social class was recorded according to the Registrar-General's occupation-based classification system and was based on the participant's last occupation if they were retired. Educational level was recorded and classified into four groups according to the highest qualification achieved.
Statistical Analysis
Description of HRT Parameters and Associations With Disc Area.
For t-test comparisons of HRT parameters between men and women, we considered the mean value of both eyes for each participant. For all subsequent analyses, we considered data from both eyes of each participant and used generalized estimating equation models to account for the correlation between eyes. Univariable linear regression models were used to examine associations of optic disc area (outcome variable) with age, sex, height, BMI, systolic and diastolic blood pressure, social class, educational level, alcohol intake, smoking status, axial length, IOP, and lens status (explanatory variables). All parameters significant at the P less than 0.1 level were then included together in one multivariable linear regression model to examine independent associations with disc area.
Principal Components Analysis.
Correlations between HRT variables were examined by the construction of a correlation matrix. Principal components analysis (PCA) was used to identify distinct components of all HRT variables, except for disc area. We did not include disc area in the PCA as our aim was to identify components of HRT variables that change with progressive glaucomatous damage and that describe the spectrum of health of an optic disc. However, when examining associations with the HRT components in subsequent analyses, we adjusted for disc area given the correlation with several HRT parameters (see below). Components with an eigenvalue greater than 1 after varimax rotation of the factor loadings were retained. An eigenvalue quantifies the amount of variance explained by a component, and a threshold of greater than 1 is commonly used to select principal components as this equates to each component explaining at least as much information as one original variable. The components were named according to the HRT variables they were most strongly correlated with (there were 3 components, which were named cup, retinal nerve fiber layer [RNFL] and rim – see Results section).
Associations With Components.
Linear regression models were used to examine crude associations between the three components (outcome variables) and age, sex, height, BMI, systolic and diastolic blood pressure, social class, educational level, alcohol intake, smoking status, axial length, IOP, and lens status (explanatory variables). All models were adjusted for disc area given that rim area16–23 and cup-to-disc ratio23–26 are consistently positively associated with optic disc area. All parameters significant at the P less than 0.1 level for any of the components were then included together in three multivariable linear regression models, one for each component. Again, these models were all adjusted for disc area. While the HRT algorithm makes use of keratometry and refraction information, this may not account for ocular magnification completely. Axial length has been shown to be more closely related to magnification than keratometry.27 Magnification correction for axial length is linear. We also repeated analyses further adjusted for the square of axial length for area parameters, such as disc area. To further examine the association between sex and RNFL, we repeated the analysis stratified by tertiles of age. Since the PCA-derived components had no specific units or scale that were of clinical significance, three further multivariable linear regression models were constructed replacing the PCA component outcome variable with a HRT parameter that was familiar clinically and well correlated with the component (linear cup-to-disc ratio, mean RNFL thickness, and rim area). These three regression analyses were then repeated in participants not reporting a history of glaucoma medication use or a glaucoma procedure. Stata version 12.1 (StataCorp LP, College Station, TX) was used for all analyses.
Results
In total, 15,694 eyes of 8064 participants underwent examination with the HRT, of which 11,946 eyes of 7009 participants had good quality scans (topography SD ≤ 40). Of these, there were complete data for all covariables from 10,859 eyes of 6430 participants and the main analyses refer to these eyes. Compared with excluded participants (those attending the EPIC-Norfolk Eye Study but without a good quality HRT image or complete data for covariables, n = 2193), included participants were of similar weight, BMI, social class, and educational level, but were significantly younger (P < 0.001) and more were female (P = 0.007). Included participants had a mean age of 68 years (range 48–90) and 56% were women.
Mean values of global HRT parameters for the cohort, stratified by sex, are presented in Table 1. These are crude values and not adjusted for height. There were intersex differences for all parameters except cup shape measure. On average, men had larger disc area and cup-related measures. Women had larger rim-related measures (except rim area) and RNFL-related measures.
Table 1.
HRT Variables by Sex
|
Men n
= 2825 |
Women n
= 3605 |
P
Value |
|
| Disc area, mm2 | 1.897 (0.418) | 1.832 (0.400) | <0.001 |
| Cup area, mm2 | 0.478 (0.349) | 0.433 (0.328) | <0.001 |
| Rim area, mm2 | 1.420 (0.326) | 1.399 (0.324) | 0.013 |
| Cup-to-disc area ratio | 0.238 (0.144) | 0.224 (0.141) | <0.001 |
| Rim-to-disc area ratio | 0.762 (0.144) | 0.776 (0.141) | <0.001 |
| Cup volume, mm3 | 0.102 (0.113) | 0.087 (0.100) | <0.001 |
| Rim volume, mm3 | 0.345 (0.129) | 0.354 (0.132) | 0.007 |
| Mean cup depth, mm | 0.191 (0.089) | 0.183 (0.086) | 0.001 |
| Maximum cup depth, mm | 0.516 (0.209) | 0.497 (0.204) | <0.001 |
| Height variation contour, mm | 0.357 (0.098) | 0.364 (0.091) | 0.003 |
| Cup shape measure | −0.175 (0.062) | −0.175 (0.063) | 1.00 |
| Mean RNFL thickness, mm | 0.215 (0.063) | 0.225 (0.065) | <0.001 |
| RNFL cross-sectional area, mm2 | 1.040 (0.305) | 1.069 (0.315) | <0.001 |
| Horizontal cup-to-disc ratio | 0.448 (0.208) | 0.425 (0.204) | <0.001 |
| Vertical cup-to-disc ratio | 0.358 (0.226) | 0.329 (0.228) | <0.001 |
| CLM temporal–superior, mm | 0.169 (0.067) | 0.178 (0.068) | <0.001 |
| CLM temporal–inferior, mm | 0.141 (0.072) | 0.153 (0.072) | <0.001 |
| Linear cup-to-disc ratio | 0.453 (0.173) | 0.437 (0.173) | <0.001 |
Numbers presented are means (SD). P values were derived from t-tests comparing means in men and women. CLM, contour line modulation.
Univariable associations with optic disc area are presented in Supplementary Table SA. In the multivariable analysis (Table 2), only height and axial length were significantly associated with disc area (0.027 mm2 per 10 cm taller height, P = 0.001; and 0.038 mm2 per millimeter longer axial length, P < 0.001). Age, sex, and IOP were only significant at the 1% level in the univariable models.
Table 2.
Adjusted Associations With Optic Disc Area
|
β |
95% CI |
P
Value |
|
| Age, per decade | −0.011 | (−0.025, 0.003) | 0.14 |
| Sex | |||
| Male | Ref | ||
| Female | −0.007 | (−0.037, 0.022) | 0.62 |
| Height, per 10 cm | 0.027 | (0.011, 0.043) | 0.001 |
| DBP, per 10 mm Hg | 0.009 | (−0.002, 0.020) | 0.10 |
| Education level | |||
| Degree | Ref | ||
| A level | 0.014 | (−0.013, 0.042) | 0.31 |
| O level | −0.014 | (−0.050, 0.023) | 0.47 |
| Less than O level | 0.012 | (−0.020, 0.043) | 0.46 |
| Alcohol intake | |||
| No intake | Ref | ||
| > 0 < 7 units/wk | −0.010 | (−0.038, 0.017) | 0.46 |
| ≥ 7 < 14 units/wk | 0.022 | (−0.007, 0.051) | 0.14 |
| ≥ 14 < 21 units/wk | 0.019 | (−0.016, 0.055) | 0.29 |
| ≥ 21 units/wk | −0.010 | (−0.041, 0.021) | 0.53 |
| Axial length, mm | 0.038 | (0.030, 0.047) | <0.001 |
| IOP, mm Hg | 0.002 | (0.000, 0.004) | 0.039 |
| Lens status | |||
| Phakic | Ref | ||
| Pseudophakic | −0.016 | (−0.050, 0.018) | 0.35 |
Results are from one multiple linear regression model with all variables presented in the model. P values <0.01 are in bold. O and A levels are standard educational examinations taken at 16 and 18 years of age in the UK. DBP, diastolic blood pressure. Ref, reference level.
Many HRT variables were strongly correlated with other HRT variables (Supplementary Table SB, Supplementary Fig. SA). Exploratory PCA identified three principal components with an eigenvalue greater than 1, explaining 79.9% of the variance of the data. Examination of the factor loadings of these three components (Table 3) found the first component to be correlated most with cup (and cup-disc ratio) related measures; the second component with disc margin RNFL related measures; and the third component with rim related measures. The components were therefore named cup, RNFL, and rim. Summary statistics and the correlation structure for the three components are summarized in Supplementary Tables SC and SD. A larger (more positive) cup or rim component measure represents a structurally larger cup or rim. A larger (more positive) RNFL component represents a thicker RNFL or more RNFL modulation at the disc margin.
Table 3.
Results from Principal Components Analysis
|
Component 1 Cup |
Component 2 RNFL |
Component 3 Rim |
|
| Cup area, mm2 | 0.364 | −0.078 | 0.178 |
| Rim area, mm2 | 0.012 | −0.060 | 0.777 |
| Cup-to-disc area ratio | 0.336 | −0.045 | −0.039 |
| Rim-to-disc area ratio | −0.336 | 0.045 | 0.039 |
| Cup volume, mm3 | 0.338 | −0.034 | 0.129 |
| Rim volume, mm3 | −0.051 | 0.221 | 0.506 |
| Mean cup depth, mm | 0.327 | 0.164 | −0.041 |
| Maximum cup depth, mm | 0.283 | 0.210 | −0.108 |
| Height variation contour, mm | 0.007 | 0.382 | −0.049 |
| Cup shape measure | 0.192 | −0.063 | 0.126 |
| Mean RNFL thickness, mm | −0.055 | 0.482 | −0.098 |
| RNFL cross-sectional area, mm2 | 0.050 | 0.442 | 0.176 |
| Horizontal cup-to-disc ratio | 0.304 | 0.003 | −0.076 |
| Vertical cup-to-disc ratio | 0.316 | −0.024 | −0.026 |
| CLM temporal–superior, mm | 0.001 | 0.402 | −0.046 |
| CLM temporal–inferior, mm | −0.027 | 0.355 | −0.023 |
| Linear cup-to-disc ratio | 0.321 | −0.006 | −0.090 |
Following rotation, three components with an eigenvalue >1 explained 79.9% of the variance. The factor loadings are presented below, and based on these, the components were named cup, RNFL, and rim. Factor loading magnitudes >0.25 are in bold.
Crude associations with the three components (adjusted for disc area only) are presented in Supplementary Table SE. Table 4 presents results from multivariable models for the three components. Similar associations were found for the cup and rim components, which were both different to associations found with the RNFL component. Older age was significantly associated with all three components. Women had a significantly larger RNFL component, but there were no significant sex differences for cup or rim. Similarly, higher educational level and shorter axial length were associated with a larger RNFL component, but there were no significant associations with cup or rim. Conversely, higher BMI was associated with a smaller cup component and a larger rim component, but there was no significant association with RNFL. Higher IOP was associated with a larger cup and smaller rim, but there was no significant association with RNFL. There appeared to be more significant associations between height and lens status with RNFL than cup and rim components. The multivariable associations presented in Table 4 did not change significantly after further adjustment for the square of axial length. A comparison of the association between female sex and RNFL across tertiles of age is shown in the Figure. The association was strongest in the youngest tertile (+0.308 [95% confidence interval (CI) 0.093, 0.524], P = 0.005), weaker in the middle tertile (+0.272 [0.060, 0.484], P = 0.012), and no longer significant in the oldest tertile (+0.118 [−0.108, 0.343], P = 0.31).
Table 4.
Multivariable Associations With Component Scores
|
Cup Component |
RNFL Component |
Rim Component |
|||||||
|
β |
95% CI |
P
Value |
β |
95% CI |
P
Value |
β |
95% CI |
P
Value |
|
| Age, per decade | 0.132 | (0.046, 0.218) | 0.003 | −0.409 | (−0.472, −0.345) | <0.001 | −0.076 | (−0.112, −0.039) | <0.001 |
| Sex | |||||||||
| Male | Ref | Ref | Ref | ||||||
| Female | −0.010 | (−0.183, 0.163) | 0.91 | 0.220 | (0.094, 0.346) | 0.001 | 0.021 | (−0.05, 0.094) | 0.58 |
| Height, per 10 cm | 0.124 | (0.027, 0.221) | 0.012 | 0.096 | (0.026, 0.167) | 0.008 | −0.050 | (−0.09, −0.009) | 0.016 |
| BMI, per 5 kg/m2 | −0.133 | (−0.202, −0.064) | <0.001 | 0.008 | (−0.043, 0.058) | 0.76 | 0.051 | (0.021, 0.080) | 0.001 |
| SBP, per 10 mm Hg | −0.023 | (−0.060, 0.013) | 0.21 | 0.010 | (−0.017, 0.036) | 0.49 | 0.009 | (−0.007, 0.024) | 0.26 |
| Education level | |||||||||
| Degree | Ref | Ref | Ref | ||||||
| A level | 0.144 | (−0.022, 0.309) | 0.09 | −0.259 | (−0.379, −0.138) | <0.001 | −0.093 | (−0.163, −0.023) | 0.009 |
| O Level | −0.030 | (−0.247, 0.188) | 0.79 | −0.055 | (−0.213, 0.103) | 0.50 | 0.024 | (−0.068, 0.116) | 0.61 |
| Less than O level | 0.147 | (−0.039, 0.333) | 0.12 | −0.293 | (−0.429, −0.157) | <0.001 | −0.071 | (−0.150, 0.007) | 0.08 |
| Axial length, mm | 0.011 | (−0.040, 0.063) | 0.66 | −0.221 | (−0.260, −0.182) | <0.001 | −0.014 | (−0.036, 0.009) | 0.23 |
| IOP, mm Hg | 0.048 | (0.035, 0.061) | <0.001 | −0.002 | (−0.012, 0.009) | 0.73 | −0.020 | (−0.025, −0.014) | <0.001 |
| Lens status | |||||||||
| Phakic | Ref | Ref | Ref | ||||||
| Pseudophakic | 0.210 | (0.008, 0.412) | 0.041 | −0.313 | (−0.474, −0.151) | <0.001 | −0.106 | (−0.194, −0.017) | 0.019 |
Results are from three multiple linear regression models with the component score as dependent variable, and all explanatory variables presented included together in each model. P values <0.01 are in bold. SBP, systolic blood pressure.
Figure.
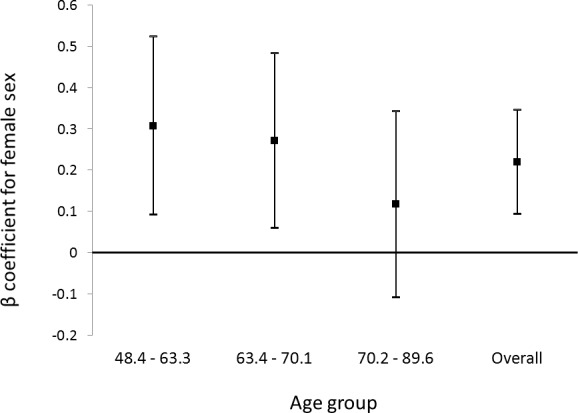
Beta coefficients with 95% CIs for the association of female sex with the RNFL component, stratified by tertiles of age, and for the whole cohort. The coefficients were derived from multivariable linear regression models adjusted for age, height, BMI, systolic blood pressure, education level, axial length, IOP, and lens status.
Given the arbitrary scale of the PCA-derived components, a clinically familiar representative measure was chosen for each component based on the factor loadings (Table 3) and frequency of use in clinical practice. Linear cup-to-disc ratio was chosen for cup, mean RNFL thickness for RNFL, and rim area for rim. Table 5 presents results from the multivariable analyses with the representative variables as the outcome variables. Similar patterns of association were found as with the principal components. Following exclusion of 203 participants with a history of glaucoma medication use (n = 174) or a glaucoma procedure (n = 47), repeated regression analyses revealed similar associations as for the full cohort (Supplementary Table SF). There were small reductions in magnitude of the coefficients for age, and pseudophakic lens status.
Table 5.
Multivariable Associations With HRT Variables Representative of Each Principle Component
|
Linear Cup-to-Disc Ratio |
Mean RNFL Thickness, mm |
Rim Area, mm2 |
|||||||
|
β |
95% CI |
P
Value |
β |
95% CI |
P
Value |
β |
95% CI |
P
Value |
|
| Age, per decade | 0.010 | (0.004, 0.015) | 0.001 | −0.015 | (−0.017, −0.012) | <0.001 | −0.022 | (−0.031, −0.013) | <0.001 |
| Sex | |||||||||
| Male | Ref | Ref | Ref | ||||||
| Female | 0.004 | (−0.008, 0.015) | 0.52 | 0.006 | (0.002, 0.011) | 0.003 | 0.000 | (−0.019, 0.018) | 0.97 |
| Height, per 10 cm | 0.009 | (0.002, 0.015) | 0.008 | 0.003 | (0.000, 0.005) | 0.036 | −0.010 | (−0.020, 0.000) | 0.06 |
| BMI, per 5 kg/m2 | −0.008 | (−0.013, −0.004) | <0.001 | 0.001 | (−0.001, 0.002) | 0.37 | 0.013 | (0.006, 0.020) | <0.001 |
| SBP, per 10 mm Hg | −0.001 | (−0.003, 0.001) | 0.44 | 0.000 | (−0.001, 0.001) | 0.58 | 0.003 | (−0.001, 0.007) | 0.11 |
| Education level | |||||||||
| Degree | Ref | Ref | Ref | ||||||
| A level | 0.010 | (−0.001, 0.021) | 0.06 | −0.008 | (−0.012, −0.004) | <0.001 | −0.016 | (−0.034, 0.001) | 0.07 |
| O Level | −0.003 | (−0.017, 0.012) | 0.71 | −0.001 | (−0.007, 0.004) | 0.60 | 0.004 | (−0.019, 0.027) | 0.72 |
| Less than O level | 0.009 | (−0.003, 0.021) | 0.15 | −0.009 | (−0.014, −0.005) | <0.001 | −0.014 | (−0.034, 0.005) | 0.15 |
| Axial length, mm | 0.002 | (−0.001, 0.006) | 0.18 | −0.006 | (−0.008, −0.005) | <0.001 | −0.005 | (−0.010, 0.001) | 0.08 |
| IOP, mm Hg | 0.003 | (0.002, 0.004) | <0.001 | 0.000 | (−0.001, 0.000) | 0.15 | −0.005 | (−0.006, −0.003) | <0.001 |
| Lens status | |||||||||
| Phakic | Ref | Ref | Ref | ||||||
| Pseudophakic | 0.01s7 | (0.003, 0.030) | 0.016 | −0.011 | (−0.017, −0.006) | <0.001 | −0.030 | (−0.052, −0.008) | 0.007 |
Results are from three multivariable linear regression models with the HRT variable as dependent variable, and all explanatory variables presented included together in each model. P values <0.01 are in bold.
Discussion
The HRT generates many correlated variables (Supplementary Table SB) and there is no consensus on which variables are most important clinically or for research when examining associations with optic disc structure. Principal components analysis is a useful technique in such situations as it can reduce the data into fewer components. The process attempts to derive uncorrelated components, and therefore helps to identify distinct attributes of the data. We found 80% of the variance of the global HRT parameters to be explained by three components, which we named cup, RNFL, and rim according to the raw parameters with which they were most correlated. We therefore suggest, when assessing patients clinically or analyzing data in a research setting, examining at least one parameter from each category may be helpful. Linear cup-to-disc ratio, mean RNFL thickness and rim area yielded similar associations as the three component scores and these may be suitable variables (Table 5).
Interestingly, we found the RNFL component to have different systemic and ocular associations from the cup or rim components. Higher IOP and lower BMI were associated with larger cup and smaller rim components, but not associated with RNFL. Female sex, higher educational level, and shorter axial length were associated with a larger RNFL component, but not associated with cup or rim components. This contrasting epidemiology suggests there may be distinct determinants of optic disc anatomy and peripapillary RNFL anatomy, and is in keeping with current evidence regarding the association between IOP and optic nerve head structure. Raised IOP has been previously associated with optic disc parameters in population-based studies.28–30 However, while hospital-based studies have reported thinner RNFL in ocular hypertensive patients compared with controls31 and faster rates of RNFL thinning amongst glaucoma patients with higher IOP,32 to the best of our knowledge, a significant association between IOP and RNFL measures has never been reported at a population level. A recent report from the Beijing Eye Study 2011 found no significant association between IOP and SD-OCT measured RNFL.33 Furthermore, data from the Singapore Chinese Eye Study found IOP to be associated with SD-OCT measured rim area and vertical cup-to-disc ratio, but not average RNFL thickness.34 Based on these findings, and the results of the present study, it may be hypothesized that there are different underlying mechanisms for optic disc cupping compared with RNFL thinning. For example, while RNFL thinning may mostly reflect retinal ganglion cell (RGC) loss, optic disc cupping may reflect both RGC loss and mechanical posterior deformation of the lamina cribrosa. In other words, IOP-induced stress and strain at the optic nerve head may induce some disc cupping independently of RGC loss, with minimal effect on RNFL thickness. Supporting this hypothesis is evidence of reduced disc cupping35 and reversal of lamina cribrosa posterior displacement36 following trabeculectomy in glaucoma patients.
We found higher BMI to be associated with a smaller optic disc cup and larger rim (both in a direction consistent with a reduced prevalence of POAG). A positive association between BMI and rim area has previously been reported,37,38 as well as a protective effect of BMI on POAG.10,39 Xu and colleagues postulated that the association between higher BMI and less optic disc cupping may be mediated by a higher cerebrospinal fluid pressure providing more lamina cribrosa support in opposition to IOP.38 If BMI is mediating its effect via mechanical forces at the lamina cribrosa, the findings of an association of BMI with cup and rim, but not RNFL, would be in keeping with our hypothesis that mechanical changes at the optic nerve head may result in cupping that is partly independent of RGC loss, and with minimal change to RNFL thickness.
We also found associations between HRT parameters and age, sex and, axial length. We found older age to be independently associated with a larger cup, smaller rim, and thinner RNFL. While these associations were mildly reduced following exclusion of participants with a history of glaucoma therapy, it would appear that a change in these parameters occurs with age in healthy individuals, albeit at a likely slower rate. While there are several studies reporting a decline in RNFL thickness with age,40–43 there is a surprising paucity of evidence for a decline in optic disc rim with age.34,44,45 More studies have not reported a significant decline of rim area with age.22,23,38,46 We found women to have a significantly larger RNFL component, consistent with a lower prevalence of POAG. The evidence for a sex predilection in POAG is inconsistent,9 though our results may support the finding of a Bayesian meta-analysis, which suggested an increased risk in men.47 Furthermore, our finding of a stronger sex effect in younger participants is consistent with results reported from the Melbourne Visual Impairment project, which showed higher rates of incident open-angle glaucoma in men for all age groups except the oldest (80+).48 This may be related to a protective effect of endogenous oestrogens in premenopausal women, consistent with the finding that earlier menopause may be a risk factor for glaucoma.49,50 We found a longer axial length to be associated with a smaller RNFL component, but found no association with cup or rim. This lack of association with disc parameters is in contrast to several studies that have reported significant associations between rim parameters and axial length29,46 or refraction.30,51–53 However, there was no adjustment for disc area in any of these studies. In our population, the crude positive associations between axial length and rim or cup (data not shown) were explained by confounding by disc area. In other words, a longer axial length was not associated with a larger rim or cup over and above that expected with a larger disc area.
Heidelberg Retina Tomograph variables derived using software version 3 or later (as in the current study) have been shown to differ significantly from those derived using earlier software versions. Heidelberg Retina Tomograph-2–derived disc area, rim area, cup area, cup-disc area ratio, rim-disc area ratio, and rim volume were found to be systematically larger than HRT-3–derived equivalent variables in participants of the Singapore Malay Eye Study.54 We found mean optic disc area to be 1.90 mm2 and 1.83 mm2 in men and women respectively; this is of similar magnitude to other published HRT-3–derived optic disc area measures, which have ranged from 1.77 mm2 to 2.07 mm.2,53–55 There is some evidence suggesting that larger optic disc area is a risk factor for POAG56,57 and this has prompted work examining the determinants of disc area. Genetic polymorphisms in the ATOH7 gene have been found to be associated with disc area (considered an endophenotype for POAG)58–60 and this gene has subsequently been found to be associated with POAG.61 We found significantly larger optic discs in participants who were taller and had longer axial length. Height was also significantly associated with disc area in the Rotterdam study,51 although no association was found in other studies.37,62–64 A significant, positive association between axial length and disc area was found in Chinese Singaporean adults,29 and consistent with this, larger disc area has been found in participants who were more myopic in several studies.51–53,63,65 While there is consistent evidence for myopia and longer axial length as risk factors for POAG,66 height has not been reported as a risk factor for POAG. We did not find age to be independently associated with optic disc area; this is in agreement with the majority of the literature16,17,21,44,64,67,68 and suggests that optic disc size does not alter significantly with time. We found women to have smaller discs, but this was explained largely by height (i.e., sex was not independently associated with disc size). Significantly smaller discs in women has been reported in the Baltimore Eye Survey68 and the Tajimi Study,30 but these were crude associations without adjustment for height. Several studies using confocal scanning laser ophthalmoscopy have found no statistically significant sex difference for disc size.16,63,67,69–72
Strengths of the present study include the population-based design and large sample size. Furthermore, detailed ophthalmic examination has been undertaken allowing adjustment for important confounders in analyses. There are limitations of the present study design. The EPIC-Norfolk Eye Study cohort is healthy and selected. It is likely that potential EPIC participants with significant visual impairment were not examined due to difficulty with required questionnaires or travel to the research clinic. Nevertheless, this would only result in a reduced study power to detect associations unless the direction of association was opposite in those not included, which is unlikely. Similarly, the exclusion of eyes without good quality HRT scans or complete data would most likely result in reduced power to detect associations, as it is unlikely that the associations examined would be in the opposite direction in the excluded eyes. The present study is cross-sectional, and therefore any causal inference is limited. The associations we found with age may be due to true longitudinal changes, or may be a cohort effect and we cannot be certain which of these is important from a cross-sectional study. A limitation of HRT is that many parameters rely on a reference plane, which is based on the position of a user-defined contour line. Therefore, there is a degree of subjectivity in the parameters given contour line placement can vary even amongst experienced users.73 Furthermore, physiological age-related decline in RNFL thickness will affect the position of the HRT reference plane, with a small posterior translation with increasing age. For any given optic nerve head, a more posterior reference plane is associated with a larger rim (and smaller cup) area estimate. Thus, the effect of a posterior translation of the reference plane with greater age is that the decline with age in rim (and increase in cup) observed in this study could be an underestimate. Another limitation of the HRT is that RNFL parameters are calculated relative to the reference plane at the disc margin, and may therefore be considered a surrogate measure rather than a true anatomical measure of the peripapillary RNFL. It should also be noted that while many of the associations we found were highly statistically significant, the coefficients were relatively small and this therefore limits any predictive ability of the regression models.
In summary, we found that HRT parameters in a population setting had three main components related to the cup, RNFL, and rim. We suggest examining at least one parameter related to each of these three components when assessing patients. The different components appear to have different associations within the present study cohort, potentially suggesting different contributory mechanisms for optic disc cupping and RNFL thinning.
Supplementary Material
Acknowledgments
Supported by grants from the Medical Research Council (G1000143) and Cancer Research UK (C864/A14136) (EPIC-Norfolk infrastructure and core functions); Research into Ageing (262) the Richard Desmond Charitable Trust (via Fight for Sight) and the Department for Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital, and the University College London Institute of Ophthalmology for a specialist Biomedical Research Centre for Ophthalmology (PJF).
Disclosure: A.P. Khawaja, None; M.P.Y. Chan, None; D.C. Broadway, None; D.F. Garway-Heath, None; R. Luben, None; J.L.Y. Yip, None; S. Hayat, None; K.-T. Khaw, None; P.J. Foster, None
References
- 1. Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002; 86: 238– 242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quigley HA. Glaucoma. Lancet. 2011; 377: 1367– 1377 [DOI] [PubMed] [Google Scholar]
- 3. Gaasterland DE, Blackwell B, Dally LG, Caprioli J, Katz LJ, Ederer F. The Advanced Glaucoma Intervention Study (AGIS): 10. Variability among academic glaucoma subspecialists in assessing optic disc notching. Trans Am Ophthalmol Soc. 2001; 99: 177– 184, discussion 184–185 [PMC free article] [PubMed] [Google Scholar]
- 4. Sharma P, Sample PA, Zangwill LM, Schuman JS. Diagnostic tools for glaucoma detection and management. Surv Ophthalmol. 2008; 53 (suppl 16S): S17– S32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chauhan BC, Nicolela MT, Artes PH. Incidence and rates of visual field progression after longitudinally measured optic disc change in glaucoma. Ophthalmology. 2009; 116: 2110– 2118 [DOI] [PubMed] [Google Scholar]
- 6. Lusky M, Bosem ME, Weinreb RN. Reproducibility of optic nerve head topography measurements in eyes with undilated pupils. J Glaucoma. 1993; 2: 104– 109 [PubMed] [Google Scholar]
- 7. Mikelberg FS, Wijsman K, Schulzer M. Reproducibility of topographic parameters obtained with the Heidelberg retina tomograph. J Glaucoma. 1993; 2: 101– 103 [PubMed] [Google Scholar]
- 8. Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008; 53 (suppl 1): S3– S10 [DOI] [PubMed] [Google Scholar]
- 9. Vajaranant TS, Nayak S, Wilensky JT, Joslin CE. Gender and glaucoma: what we know and what we need to know. Curr Opin Ophthalmol. 2010; 21: 91– 99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pasquale LR, Willett WC, Rosner BA, Kang JH. Anthropometric measures and their relation to incident primary open-angle glaucoma. Ophthalmology. 2010; 117: 1521– 1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wolfs RC, Borger PH, Ramrattan RS, et al. Changing views on open-angle glaucoma: definitions and prevalences–The Rotterdam Study. Invest Ophthalmol Vis Sci. 2000; 41: 3309– 3321 [PubMed] [Google Scholar]
- 12. Burdon KP. Genome-wide association studies in the hunt for genes causing primary open-angle glaucoma: a review. Clin Experiment Ophthalmol. 2012; 40: 358– 363 [DOI] [PubMed] [Google Scholar]
- 13. Riboli E, Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997; 26 (suppl 1): S6– S14 [DOI] [PubMed] [Google Scholar]
- 14. Day N, Oakes S, Luben R, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer. 1999; 80 (suppl 1): 95– 103 [PubMed] [Google Scholar]
- 15. Khawaja AP, Chan MPY, Hayat S, et al. The EPIC-Norfolk Eye Study: rationale, methods and a cross-sectional analysis of visual impairment in a population-based cohort. BMJ open. 2013; 3: 1– 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bowd C, Zangwill LM, Blumenthal EZ, et al. Imaging of the optic disc and retinal nerve fiber layer: the effects of age, optic disc area, refractive error, and gender. J Opt Soc Am A Opt Image Sci Vis. 2002; 19: 197– 207 [DOI] [PubMed] [Google Scholar]
- 17. Britton RJ, Drance SM, Schulzer M, Douglas GR, Mawson DK. The area of the neuroretinal rim of the optic nerve in normal eyes. Am J Ophthalmol. 1987; 103: 497– 504 [DOI] [PubMed] [Google Scholar]
- 18. Budde WM, Jonas JB, Martus P, Gründler AE. Influence of optic disc size on neuroretinal rim shape in healthy eyes. J Glaucoma. 2000; 9: 357– 362 [DOI] [PubMed] [Google Scholar]
- 19. Garway-Heath DF, Hitchings RA. Quantitative evaluation of the optic nerve head in early glaucoma. Br J Ophthalmol. 1998; 82: 352– 361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jonas JB, Budde WM, Lang P. Neuroretinal rim width ratios in morphological glaucoma diagnosis. Br J Ophthalmol. 1998; 82: 1366– 1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kee C, Koo H, Ji Y, Kim S. Effect of optic disc size or age on evaluation of optic disc variables. Br J Ophthalmol. 1997; 81: 1046– 1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988; 29: 1151– 1158 [PubMed] [Google Scholar]
- 23. Arvind H, George R, Raju P, et al. Neural rim characteristics of healthy South Indians: the Chennai Glaucoma Study. Invest Ophthalmol Vis Sci. 2008; 49: 3457– 3464 [DOI] [PubMed] [Google Scholar]
- 24. Crowston JG, Hopley CR, Healey PR, Lee A, Mitchell P. The effect of optic disc diameter on vertical cup to disc ratio percentiles in a population based cohort: the Blue Mountains Eye Study. Br J Ophthalmol. 2004; 88: 766– 770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garway-Heath DF, Ruben ST, Viswanathan A, Hitchings RA. Vertical cup/disc ratio in relation to optic disc size: its value in the assessment of the glaucoma suspect. Br J Ophthalmol. 1998; 82: 1118– 1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jonas JB, Bergua A, Schmitz-Valckenberg P, Papastathopoulos KI, Budde WM. Ranking of optic disc variables for detection of glaucomatous optic nerve damage. Invest Ophthalmol Vis Sci. 2000; 41: 1764– 1773 [PubMed] [Google Scholar]
- 27. Garway-Heath DF, Rudnicka AR, Lowe T, Foster PJ, Fitzke FW, Hitchings RA. Measurement of optic disc size: equivalence of methods to correct for ocular magnification. Br J Ophthalmol. 1998; 82: 643– 649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klein BEK, Klein R, Lee KE, Hoyer CJ. Does the intraocular pressure effect on optic disc cupping differ by age? Trans Am Ophthalmol Soc. 2006; 104: 143– 148 [PMC free article] [PubMed] [Google Scholar]
- 29. Bourne RRA, Foster PJ, Bunce C, et al. The morphology of the optic nerve head in the Singaporean Chinese population (the Tanjong Pagar study): part 2–biometric and systemic associations. Br J Ophthalmol. 2008; 92: 310– 314 [DOI] [PubMed] [Google Scholar]
- 30. Abe H, Shirakashi M, Tsutsumi T, et al. Laser scanning tomography of optic discs of the normal Japanese population in a population-based setting. Ophthalmology. 2009; 116: 223– 230 [DOI] [PubMed] [Google Scholar]
- 31. Bowd C, Weinreb RN, Williams JM, Zangwill LM. The retinal nerve fiber layer thickness in ocular hypertensive, normal, and glaucomatous eyes with optical coherence tomography. Arch Ophthalmol. 2000; 118: 22– 26 [DOI] [PubMed] [Google Scholar]
- 32. Medeiros FA, Alencar LM, Zangwill LM, Sample PA, Weinreb RN. The relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009; 116: 1125– 33.e1 –e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Pan Z, Zhao L, You Q, Xu L, Jonas J. Retinal nerve fiber layer thickness. The Beijing Eye Study 2011. PLoS One. 2013; 8: 2– 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheung CY, Chen D, Wong TY, et al. Determinants of quantitative optic nerve measurements using spectral domain optical coherence tomography in a population-based sample of non-glaucomatous subjects. Invest Ophthalmol Vis Sci. 2011; 52: 9629– 9635 [DOI] [PubMed] [Google Scholar]
- 35. Kotecha A, Siriwardena D, Fitzke FW, Hitchings RA, Khaw PT. Optic disc changes following trabeculectomy: longitudinal and localisation of change. Br J Ophthalmol. 2001; 85: 956– 961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee EJ, Kim T-W, Weinreb RN. Reversal of lamina cribrosa displacement and thickness after trabeculectomy in glaucoma. Ophthalmology. 2012; 119: 1359– 1366 [DOI] [PubMed] [Google Scholar]
- 37. Zheng Y, Cheung CYL, Wong TY, Mitchell P, Aung T. Influence of height, weight, and body mass index on optic disc parameters. Invest Ophthalmol Vis Sci. 2010; 51: 2998– 3002 [DOI] [PubMed] [Google Scholar]
- 38. Xu L, Wang YX, Wang S, Jonas JB. Neuroretinal rim area and body mass index. PLoS One. 2012; 7: e30104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramdas WD, Wolfs RCW, Hofman A, de Jong PTVM, Vingerling JR, Jansonius NM. Lifestyle and risk of developing open-angle glaucoma: the Rotterdam study. Arch Ophthalmol. 2011; 129: 767– 772 [DOI] [PubMed] [Google Scholar]
- 40. Alasil T, Wang K, Keane PA, et al. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma. 2012; 22: 1– 10 [DOI] [PubMed] [Google Scholar]
- 41. Parikh RS, Parikh SR, Sekhar GC, Prabakaran S, Babu JG, Thomas R. Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology. 2007; 114: 921– 926 [DOI] [PubMed] [Google Scholar]
- 42. Varma R, Bazzaz S, Lai M. Optical tomography-measured retinal nerve fiber layer thickness in normal latinos. Invest Ophthalmol Vis Sci. 2003; 44: 3369– 3373 [DOI] [PubMed] [Google Scholar]
- 43. Sung KR, Wollstein G, Bilonick RA, et al. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. Ophthalmology. 2009; 116: 1119– 1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Garway-Heath DF, Wollstein G, Hitchings RA. Aging changes of the optic nerve head in relation to open angle glaucoma. Br J Ophthalmol. 1997; 81: 840– 845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. See JLS, Nicolela MT, Chauhan BC. Rates of neuroretinal rim and peripapillary atrophy area change: a comparative study of glaucoma patients and normal controls. Ophthalmology. 2009; 116: 840– 847 [DOI] [PubMed] [Google Scholar]
- 46. Rao HL, Kumar AU, Babu JG, Kumar A, Senthil S, Garudadri CS. Predictors of normal optic nerve head, retinal nerve fiber layer, and macular parameters measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 1103– 1110 [DOI] [PubMed] [Google Scholar]
- 47. Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006; 47: 4254– 4261 [DOI] [PubMed] [Google Scholar]
- 48. Mukesh BN, McCarty CA, Rait JL, Taylor HR. Five-year incidence of open-angle glaucoma: the visual impairment project. Ophthalmology. 2002; 109: 1047– 1051 [DOI] [PubMed] [Google Scholar]
- 49. Pasquale LR, Rosner BA, Hankinson SE, Kang JH. Attributes of female reproductive aging and their relation to primary open-angle glaucoma: a prospective study. J Glaucoma. 2007; 16: 598– 605 [DOI] [PubMed] [Google Scholar]
- 50. Hulsman CA, Westendorp IC, Ramrattan RS, et al. Is open-angle glaucoma associated with early menopause? The Rotterdam Study. Am J Epidemiol. 2001; 154: 138– 144 [DOI] [PubMed] [Google Scholar]
- 51. Ramrattan RS, Wolfs RC, Jonas JB, Hofman A, de Jong PT. Determinants of optic disc characteristics in a general population: the Rotterdam Study. Ophthalmology. 1999; 106: 1588– 1596 [DOI] [PubMed] [Google Scholar]
- 52. Wu R-Y, Wong T-Y, Zheng Y-F, et al. Influence of refractive error on optic disc topographic parameters: the Singapore Malay Eye Study. Am J Ophthalmol. 2011; 152: 81– 86 [DOI] [PubMed] [Google Scholar]
- 53. Ramdas WD, Wolfs RCW, Hofman A, de Jong PTVM, Vingerling JR, Jansonius NM. Heidelberg Retina Tomograph (HRT3) in population-based epidemiology: normative values and criteria for glaucomatous optic neuropathy. Ophthalmic Epidemiol. 2011; 18: 198– 210 [DOI] [PubMed] [Google Scholar]
- 54. Koh V, Loon SC, Wong W-L, Wong TY, Aung T. Comparing stereometric parameters between Heidelberg Retinal Tomography 2 and 3 in Asian eyes: the Singapore Malay Eye Study. J Glaucoma. 2012; 21: 102– 106 [DOI] [PubMed] [Google Scholar]
- 55. Foo L-L, Perera SA, Cheung CY, et al. Comparison of scanning laser ophthalmoscopy and high-definition optical coherence tomography measurements of optic disc parameters. Br J Ophthalmol. 2012; 96: 576– 580 [DOI] [PubMed] [Google Scholar]
- 56. Healey PR, Mitchell P. Optic disk size in open-angle glaucoma: the Blue Mountains Eye Study. Am J Ophthalmol. 1999; 128: 515– 517 [DOI] [PubMed] [Google Scholar]
- 57. Wang L, Damji KF, Munger R, et al. Increased disk size in glaucomatous eyes vs normal eyes in the Reykjavik Eye Study. Am J Ophthalmol. 2003; 135: 226– 228 [DOI] [PubMed] [Google Scholar]
- 58. Ramdas WD, van Koolwijk LME, Ikram MK, et al. A genome-wide association study of optic disc parameters. PLoS Genet. 2010; 6: e1000978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Khor CC, Ramdas WD, Vithana EN, et al. Genome-wide association studies in Asians confirm the involvement of ATOH7 and TGFBR3, and further identify CARD10 as a novel locus influencing optic disc area. Hum Mol Genet. 2011; 20: 1864– 1872 [DOI] [PubMed] [Google Scholar]
- 60. Macgregor S, Hewitt AW, Hysi PG, et al. Genome-wide association identifies ATOH7 as a major gene determining human optic disc size. Hum Mol Genet. 2010; 19: 2716– 2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ramdas WD, van Koolwijk LME, Lemij HG, et al. Common genetic variants associated with open-angle glaucoma. Hum Mol Genet. 2011; 20: 2464– 2471 [DOI] [PubMed] [Google Scholar]
- 62. Jonas JB, Gründler AE, Papastathopoulos KI. Optic disc dimensions, body length, and body weight. Br J Ophthalmol. 1998; 82: 197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nangia V, Matin A, Bhojwani K, Kulkarni M, Yadav M, Jonas JB. Optic disc size in a population-based study in central India: the Central India Eye and Medical Study (CIEMS). Acta Ophthalmol. 2008; 86: 103– 104 [DOI] [PubMed] [Google Scholar]
- 64. Arvind H, George R, Raju P, et al. Optic disc dimensions and cup-disc ratios among healthy South Indians: the Chennai Glaucoma Study. Ophthalmic Epidemiol. 2011; 18: 189– 197 [DOI] [PubMed] [Google Scholar]
- 65. Wang Y, Xu L, Zhang L, Yang H, Ma Y, Jonas JB. Optic disc size in a population based study in northern China: the Beijing Eye Study. Br J Ophthalmol. 2006; 90: 353– 356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Marcus MW, de Vries MM, Montolio FGJ, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011; 118: 1989– 1994 [DOI] [PubMed] [Google Scholar]
- 67. Agarwal HC, Gulati V, Sihota R. The normal optic nerve head on Heidelberg Retina Tomograph II. Indian J Ophthalmol (Poona City). 2003; 51: 25– 33 [PubMed] [Google Scholar]
- 68. Varma R, Tielsch JM, Quigley HA, et al. Race-, age-, gender-, and refractive error-related differences in the normal optic disc. Arch Ophthalmol. 1994; 112: 1068– 1076 [DOI] [PubMed] [Google Scholar]
- 69. Durukan AH, Yucel I, Akar Y, Bayraktar MZ. Assessment of optic nerve head topographic parameters with a confocal scanning laser ophthalmoscope. Clin Experiment Ophthalmol. 2004; 32: 259– 264 [DOI] [PubMed] [Google Scholar]
- 70. Kashiwagi K, Tamura M, Abe K, Kogure S, Tsukahara S. The influence of age, gender, refractive error, and optic disc size on the optic disc configuration in Japanese normal eyes. Acta Ophthalmol Scand. 2000; 78: 200– 203 [DOI] [PubMed] [Google Scholar]
- 71. Zangwill LM, Weinreb RN, Berry CC, et al. The confocal scanning laser ophthalmoscopy ancillary study to the ocular hypertension treatment study: study design and baseline factors. Am J Ophthalmol. 2004; 137: 219– 227 [DOI] [PubMed] [Google Scholar]
- 72. Sawada Y, Ishikawa M, Sato N, Yoshitomi T. Optic nerve head morphology assessed by laser scanning tomography in normal Japanese subjects. J Glaucoma. 2011; 20: 445– 451 [DOI] [PubMed] [Google Scholar]
- 73. Garway-Heath DF, Poinoosawmy D, Wollstein G, et al. Inter- and intraobserver variation in the analysis of optic disc images: comparison of the Heidelberg retina tomograph and computer assisted planimetry. Br J Ophthalmol. 1999; 83: 664– 669 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


