Abstract
Purpose.
We have previously shown that TGF-β3 (T3) stimulates extracellular matrix (ECM) assembly while maintaining antifibrotic characteristics in a model using human corneal fibroblasts (HCFs). This model, however, requires non-physiological levels of serum. In the current study, we tested whether T3 could stimulate human corneal keratocytes (HCKs) in vitro to assemble a functional ECM, while maintaining their characteristics.
Methods.
Human corneal keratocytes and HCFs were isolated and cultured using 1% or 10% serum, respectively ±T3. The constructs were processed for indirect immunofluorescence (IF), transmission electron microscopy (TEM), and qRT-PCR, analyzing for keratocyte marker, keratocan, and ECM components, collagen (col) types I, III, and V.
Results.
Quantitative reverse transcriptase PCR data showed that keratocan, col I, and V were all upregulated in HCKs compared with HCFs, whereas col III was expressed at low levels in HCKs. Transforming growth factor beta 3 stimulation further enhanced the level of change. Without T3, HCK constructs were very thin, approximately 5 μm; however, as with HCFs, upon stimulation with T3, HCK constructs increased in thickness by approximately 5-fold. Cell counts and ECM production revealed that HCKs assembled more ECM per unit area compared with HCFs, and IF revealed downregulation of fibrotic markers, col III, and thrombospondin-1, with T3 stimulation. Transmission electron microscopy data revealed aligned ECM with long fibrils for all conditions except HCK Controls. Human corneal keratocytes+T3 also showed denser collagen fibrils with more consistent fibril diameter.
Conclusions.
Overall, the data suggests that it is possible to stimulate matrix secretion and assembly by HCKs in vitro by using a single growth factor, T3.
Keywords: human corneal keratocytes, TGF-β3, extracellular matrix
In the current study, we stimulated human corneal keratocytes (HCKs) in order to test whether HCKs can assemble a functional extracellular matrix, while maintaining their characteristics. Transforming growth factor-beta 3 stimulation enhanced matrix secretion and assembly by HCKs in vitro without losing keratocyte markers and phenotype.
Introduction
The embryonic development of the cornea has been studied extensively in a variety of species, with the chick model by Hay being the most detailed.1 There are three main corneal layers (epithelium, stroma, and endothelium), with the corneal stroma representing approximately 85% to 90% of the total corneal thickness. The corneal stroma consists of highly regular collagenous lamellae and extracellular matrix (ECM) components that are secreted by the human corneal keratocytes (HCK),2,3 and consists mainly of type I, V, and VI collagens.2,3 The HCKs are a population of quiescent, mesenchymal-derived cells and are found between the collagen lamellae of the mature corneal stroma. Human corneal keratocytes exhibit slow turnover and occupy only 10% of the stromal size. Despite their quiescence, HCKs play a key role in corneal transparency and integrity.
Upon injury, some HCKs undergo apoptosis immediately, while others are transformed into human corneal fibroblasts (HCFs) and myofibroblasts.2,4–6 In most cases, any damage or interruption in the precisely orchestrated process of healing, results in corneal scarring and leads to the activation of HCKs.2,6,7 These activated HCKs are often referred to as “active keratocytes” or HCFs, which secrete a disorganized ECM that ultimately affects transparency and corneal integrity.6,8 Therefore, when creating corneal tissue for engineering studies, it might be better to use a more keratocyte-like cell.
In humans, there are three TGF-β isoforms, TGF-β1, -β2 and -β3 (T1, T2, and T3, respectively). Despite the fact that they share 70% to 80% amino acid sequence homology9 and bind to the same receptors, their functions vary considerably. All three isoforms affect a wide variety of biological processes, such as cell proliferation, differentiation, and migration,10–12 which are all critical events following injury. However, T1 and T2 are known for their profibrotic role, whereas T3 is better known for its antifibrotic activity.13–16 In fact, our previous studies15 have shown that T3 can stimulate HCFs to secrete larger amounts of ECM, while maintaining nonfibrotic characteristics.
In the current study, we investigated whether HCKs can be stimulated to secrete and assemble an organized ECM, similar to that in a mature cornea, while maintaining their keratocyte characteristics. We also investigated the role of T3 on HCKs, and whether T3 helped increase ECM secretion while maintaining keratocyte characteristics in vitro. Our data shows that when HCKs were stimulated with T3, the HCKs maintained the vital keratocyte marker, keratocan, as well as their morphology. In addition, HCKs secreted copious amounts of ECM, while maintaining high alignment and organization and minimizing fibrotic marker expression. This is vital for corneal tissue engineering since it can lead to new alternatives and therapeutic solutions for sight-threatening cases.
Materials and Methods
Primary Culture of Human Corneal Fibroblasts
Human corneas were obtained from the National Disease Research Interchange (NDRI; Philadelphia, PA). All research adhered to the tenets of the Declaration of Helsinki. Cells were isolated as described.17 Briefly, corneal epithelium and endothelium were scrapped off and removed from the stroma using a razor blade. The stromal tissue was cut into small pieces (∼2 × 2 mm), put into 6-well plates (4 or 5 pieces per well) and allowed to adhere to the bottom of the wells. Explants were split into two groups: (1) explants cultured with Eagle's Minimum Essential Medium (EMEM: American Type Culture Collection [ATCC]; Manassas, VA) containing 10% fetal bovine serum (FBS; ATCC), and (2) explants cultured with EMEM and 1% FBS. For the purpose of this study, cells exposed to 10% FBS will be referred to as HCFs, indicating their differentiation from a normal keratocyte phenotype to a fibroblastic phenotype, as previously demonstrated by Beales et al.,4 and cells cultured in 1% FBS will be referred to as HCKs, denoting the maintenance, at least partially, of a normal keratocyte phenotype.4 All cultures were allowed 1 to 2 weeks of cultivation, at which point cells were passaged into a 100-mm cell culture plate. The cells were allowed to grow to 100% confluence before being used in the culture system.
Assembly of Extracellular Matrix
Both cell types (HCFs and HCKs) were plated on transwell 6-well plates containing polycarbonate membrane inserts with 0.4-μm pores (Costar, Charlotte, NC) at a density of 106 cells/mL. Cells were cultured in EMEM with 1% or 10% FBS and 0.5 mM 2-O-α-D-glucopyranosyl-L-ascorbic acid (VitC; Wako Chemicals USA, Inc., Richmond, VA). The cultures were allowed to grow for 4 weeks ±0.1 ng/mL T3. This concentration of T3 was chosen after comparing both cell types, HCFs15,18 and HCKs, with a concentration series ranging from 0.1 to 10 ng/ml. When HCKs were stimulated with concentrations higher than 0.1 ng/mL, their self-assembled ECM contracted. Therefore, 0.1 ng/mL T3 concentration was chosen as the optimum for both cell types. Cultures without T3 served as controls. The morphology of the cultures was examined using brightfield and transmission electron microscopy (TEM). In addition, indirect immunofluorescence (IF) was used to identify specific markers of stromal components, such as collagens type I (col I), type III (col III), and type V (col V), as well as thrombospondin-1 (TSP-1).
Identical procedures were followed when cells were cultured on 6-well plates and processed for qRT-PCR in order to investigate the expression of keratocyte marker, keratocan, and ECM components, col I, III, and V. In addition to the controls without T3, a no VitC control was included to assess for any significant effects on the expression of these probes with or without VitC.
Transmission Electron Microscopy
The constructs were collected after 4 weeks in culture, fixed in Karnovsky's fixative (2% paraformaldehyde, 2.5% gluteraldehyde in cacodylate buffer, pH 7.4) and processed for TEM using standard procedures, as described previously.19 Briefly, constructs were rinsed in PBS, processed through post-fixation in 2% osmium tetroxide, en bloc stained in 0.5% uranyl oxide, dehydrated with alcohol to propylene oxide, and embedded (Embed 812; Electron Microscopy Sciences, Hatfield, PA). A diamond knife on an ultramicrotome (LKB, Bromma, Sweden) was used to cut thin sections transverse to the plane of the construct. The sections were viewed and photographed with an electron microscope (Tecnai G2 Spirit; FEI Company, Hillsboro, OR).
Indirect Immunofluorescence
The constructs were collected, fixed in 4% paraformaldehyde, and stained for IF, as previously described.6 In brief, constructs were incubated overnight at 4°C with primary antibodies against col III (Southern Biotech, Birmingham, AL), col I (Abcam, Cambridge, MA), col V (Novus Biologicals, Littleton, CO), and TSP-1 (NeoMarkers, Fremont, CA) in 1% BSA+0.1% Triton-X. The next day, constructs were washed and incubated overnight at 4°C with the corresponding secondary antibody, donkey anti-goat (col III), anti-rabbit (col I), and anti-rabbit (col V and TSP-1) in 1% BSA+0.1% Triton-X. All constructs were counterstained with iodide (TO-PRO-3; Life Technologies, Grand Island, NY), a marker of all cell nuclei. Constructs were washed, mounted (Vectashield; Vector Laboratories, Burlingame, CA), and observed, and photographed with a confocal microscope (TCS-SP2; Leica Microsystems, Bannockburn, IL). Negative controls, where the primary antibody was omitted, were run with all experiments.
The construct thicknesses were also measured, as described previously,15,18 using the confocal microscope's z-scans. Measurements started from the top of the construct (first cell visible) and ended at the bottom of the construct (last cell visible). Thicknesses were analyzed (5–7 samples per condition) for significant difference (P < 0.05).
qRT-PCR
Total RNA was extracted from the cells (GeneJet RNA Purification Kit, K0731; ThermoScientific, Waltham, MA). Genomic DNA was removed by incubation with RNase-free DNase I (M0303S; New England BioLabs, Ipswich, MA) in the presence of RNase inhibitor. The RNA was annealed with oligo dt and random hexamer primers and first strand synthesis carried out with MuLV reverse transcriptase. Negative controls were performed without reverse transcriptase. Quantitative reverse transcriptase PCR was done on Vii7A (Life Technologies) using ABI TaqMan gene expression assays: col1A1: Hs00164004_m1, col3A1: Hs00943809_m1, col5A1: Hs00609088_m1, and keratocan: Hs00559942_m1, and the eukaryotic 18S rRNA endogenous control, 4308329. Results were calculated using the ΔΔCt method using 18S rRNA as the endogenous control.
Collagen Fibril Measurements
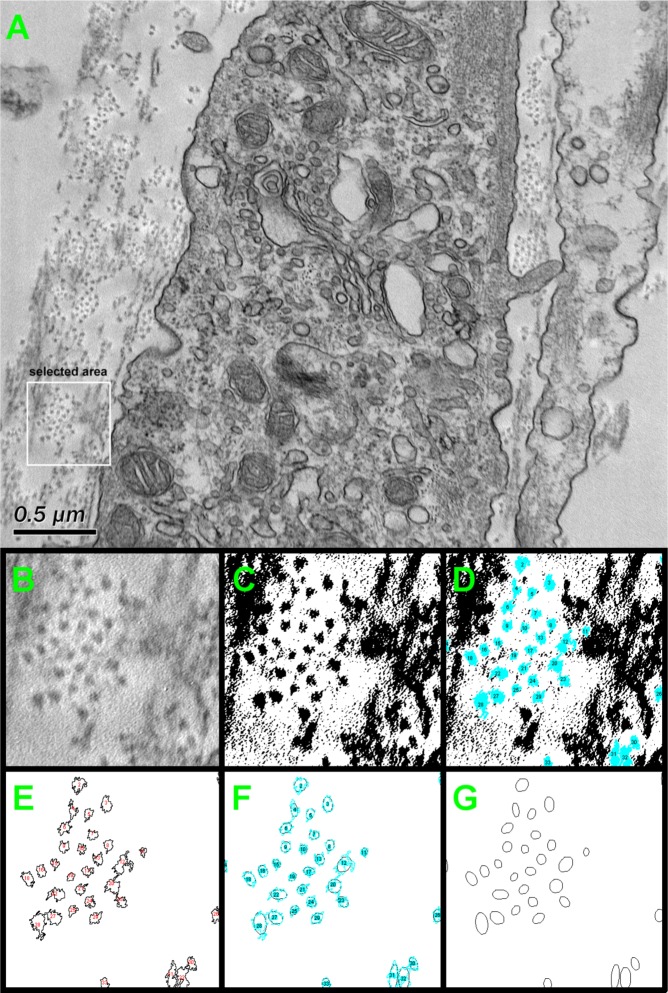
Fibril densities for each condition were measured and compared (ImageJ v.1.44p; National Institutes of Health, Bethesda, MD). At least five randomly chosen electron micrographs were used for each condition and six areas per micrograph were analyzed, an example of which is seen in Figure 1. Figure 1A shows a selected TEM image, as a representative example, with a selected area of 0.5 μm × 0.5 μm (white box). The selected area was cropped (Fig. 1B), processed and transformed to a binary image (Fig. 1C). The collagen fibrils were identified, detected by edge detection method and highlighted in blue, as shown in Figure 1D. The outlines of each detected fibril was then extracted (Fig. 1E) and an ellipse was fitted (Fig. 1F). Collagen fibril density was then calculated by counting the collagen fibrils within the selected area (Fig. 1G). The scale bar for the TEM was used to calibrate the measurements. Results were plotted and analyzed for significance (P < 0.05).
Figure 1.
Schematic representation of the method used to quantify the fibril density from TEM images with ImageJ software. (A) The original TEM image with random 0.5 μm × 0.5 μm area selected (white box); (B) selected area magnified; (C) magnified image converted to a binary black/white image; (D) collagen fibrils identified by edge detection method (blue spots); (E) outlines of each detected area; (F) fitted ellipses to detected area; and (G) final highlighted ellipses showing the collagen fibrils. The fibril density was then calculated by counting the fibrils within the selected area.
Cell Numbers and ECM Production
As described previously,15,18 we quantified the total cell number per construct per condition, as well as, cell per unit volume (Image Pro Plus, v.7; Media Cybernetics, Bethesda, MD). Briefly, the number of cells in each section (plane-of-focus) of a confocal z-series was counted and the number of cells per construct was quantitated. A minimum of three confocal z-series was used for each condition, and their average was plotted and analyzed.
Statistical Analysis
All experiments were repeated at least three times and data was analyzed for significant variations (P < 0.05) using the Student's t-test and Dunnett's multiple comparison test (GraphPad Prism v.5.0b; GraphPad, La Jolla, CA).
Results
Cell Phenotype and qRT-PCR
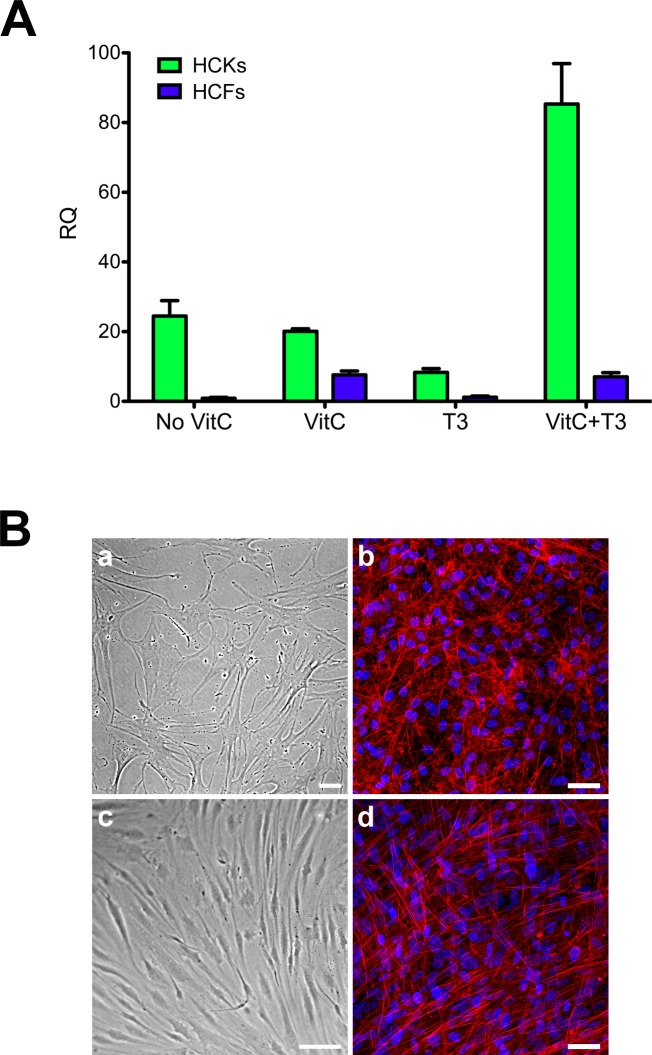
We performed qRT-PCR to investigate keratocan expression by both HCKs and HCFs. We tested four conditions: No VitC, VitC, T3, and VitC+T3. Figure 2A clearly shows that HCKs expressed significantly higher amounts of keratocan under all conditions compared with HCFs (P < 0.01), ranging from 12-fold upregulation when both VitC and T3 were present to 27-fold with No VitC. In terms of morphology, HCKs maintained their dendritic morphology (Figs. 2Ba, 2Bb) and were distinctively different from HCFs, which had a more fibroblastic, elongated morphology (Figs. 2Bc, 2Bd). The morphologic characteristics were maintained both with cells in culture (Figs. 2Ba, 2Bc) and cells on polycarbonate membranes (Figs. 2Bb, 2Bd).
Figure 2.
(A) Quantitative reverse transcriptase PCR analysis of both HCKs and HCFs for keratocan expression. Four conditions were tested: No VitC, VitC, T3, and VitC+T3. Under all conditions HCKs expressed significantly higher levels of Keratocan when compared with HCFs (P < 0.01). (B) Representative images showing the morphology for both HCKs (a, b) and HCFs (c, d) in two-dimensional (2D) (a, c) and 3D (b, d) cultures. HCKs maintained their dendritic morphology (a, b), whereas HCFs showed a more fibroblastic, elongated morphology (c, d). Red = Phalloidin, Blue = TOPRO-3. Scale bars: 50 μm.
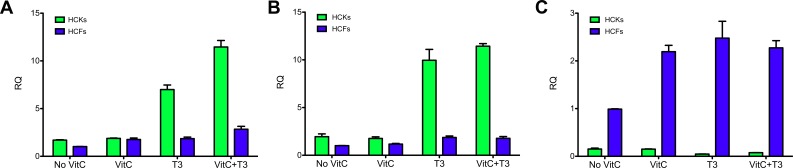
We further characterized HCK and HCF differences by investigating several essential probes for corneal stromal cells. We performed qRT-PCR for col I, III, and V. Collagen I and V are routinely found in healthy adult corneal stroma, whereas col III is a sign of myofibroblast differentiation and a wounded/scarred cornea. For HCKs, our data showed significant upregulation of col I and V (∼4–6-fold, P < 0.01) when T3 was present independent of VitC stimulation, as shown in Figures 3A and 3B, respectively. Human corneal fibroblasts also showed upregulation of both col I and V, but on a much smaller scale (∼2-fold, P < 0.05). Collagen III (Fig. 3C), on the other hand, was significantly upregulated (P < 0.01) in HCFs under all conditions when compared with HCKs, ranging from approximately 6-fold (P < 0.001) with No VitC to approximately 53-fold with T3 stimulation (P < 0.01).
Figure 3.
Quantitative reverse transcriptase PCR analysis of both HCKs and HCFs for (A) Col I, (B) Col V, and (C) Col III expression. Four conditions were tested: No VitC, VitC, T3, and VitC+T3. HCKs showed significant upregulation of Col I and V (∼4–6-fold, P < 0.01) when T3 was present independent of VitC stimulation [A] and [B], respectively). On a smaller scale HCFs also showed upregulation of both Col I and V (∼2-fold, P < 0.05). HCKs expressed Col I and V at higher levels compared with HCFs under all conditions. Col III (C), on the other hand, was significantly upregulated in HCFs under all conditions when compared with HCKs (∼6–53-fold, P < 0.01).
Construct Characterization
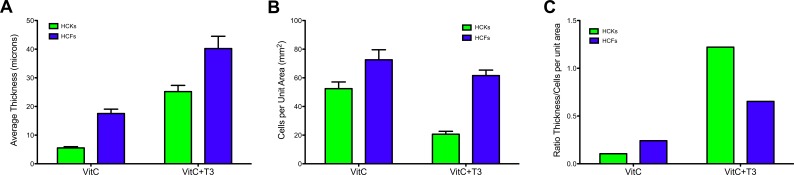
Keratocyte differentiation is characterized by the secretion of matrix components unique to the corneal stroma and essential for corneal transparency.20 Therefore, we investigated the amount of ECM secreted for each condition in order to characterize the HCKs' ability to secret ECM in vitro. As seen in Figure 4A, the thickness of the construct increased with T3 stimulation, independent of cell type (HCK 3-fold, HCF 1.6-fold, P < 0.001). Human corneal fibroblasts with VitC+T3 had the thickest construct, which is in agreement with our previous studies.15 In contrast, HCK constructs with VitC only were extremely thin (∼5 μm); however, when T3 was added, the cells secreted and assembled ECM components, in fact, these constructs reached a thickness of approximately 20 μm, which was significantly higher than VitC only (P < 0.001).
Figure 4.
Graph of (A) the average construct thickness, (B) total number of cells per unit area (millimeter squared), and (C) the ratio of constructs' thickness over cell per unit area for both HCKs and HCFs stimulated with VitC ± T3. (A) Both HCK and HCF constructs' thicknesses were significantly upregulated (3- and 1.6-fold, respectively; P < 0.001) when stimulated with T3. HCFs total thickness was higher (2–4-fold) under all conditions when compared with HCKs. (B) HCFs had a higher number of cells per unit area ± T3 stimulation when compared with HCKs. A significant decrease in number of cells per unit area following T3 stimulation (P < 0.001) is shown for HCKs compared with VitC only. (C) The ratio of construct thickness/cells per unit area shows the difference in ECM secretion between HCKs and HCFs ± T3. The amount of ECM per cell was dramatically increased in HCKs upon T3 stimulation (∼11-fold) compared with that of the HCFs (∼3-fold), suggesting a greater ability of the HCKs to increase ECM production than HCFs.
In order to determine if this increase in thickness was a result of an increase in the number of cells or an increase in the amount of matrix produced per cell, we examined the number of cells per unit area of ECM. Results showed a significant decrease in the number of cells per unit area following T3 stimulation of HCK compared with HCK with VitC only (2.5-fold; P < 0.001) (Fig. 4B) and HCFs with VitC+T3 (∼3-fold; P < 0.001). Results indicate that the amount of matrix per cell was dramatically increased in HCKs upon T3 stimulation (Fig. 4C; ∼11-fold). Therefore, Figure 4C shows the ratio of construct thickness/cells per unit area, clearly showing the difference in ECM secretion between HCKs and HCFs ± T3. Overall, our data suggests that HCK have a much greater ability to upregulate matrix production than HCF (3-fold). In fact, preliminary data (not shown here) shows that HCKs in longer cultures (8 weeks) continue to secrete ECM, to a far greater extent than HCFs.
TEM
Transmission electron microscopy examination revealed cell–matrix interactions, as well as matrix organization and alignment as seen previously.15,17,18 Cells appeared elongated and were surrounded by ECM (data not shown).
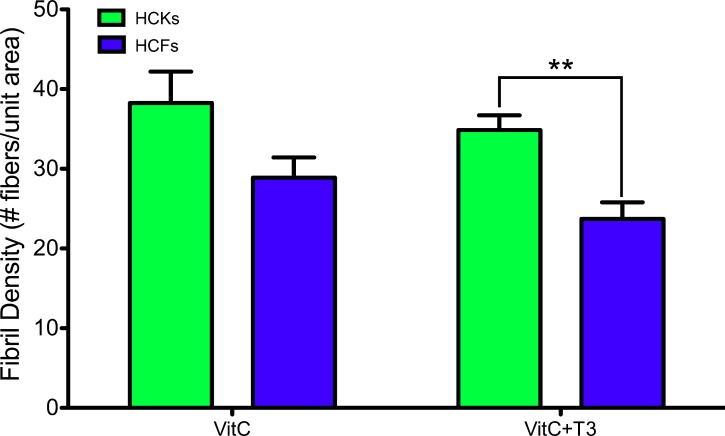
Collagen fibril organization was identified in all conditions and the density of the fibrils was quantified. Figure 5 shows the average fibril densities obtained from representative TEM images. Interestingly, HCKs' ECM had denser fibrillar organization than HCFs ± T3 stimulation, and with T3, the fibril density of the HCKs was significantly greater than HCFs (P < 0.01).
Figure 5.
Graph demonstrating the fibril densities obtained from the TEM images for HCK and HCF constructs' ±T3. HCKs showed higher density of fibrils per unit area when compared with HCFs, both ±T3, with T3 stimulation of the HCKs causing the density to become significantly greater than with the HCFs (**P < 0.01).
Immunofluorescence Microscopy
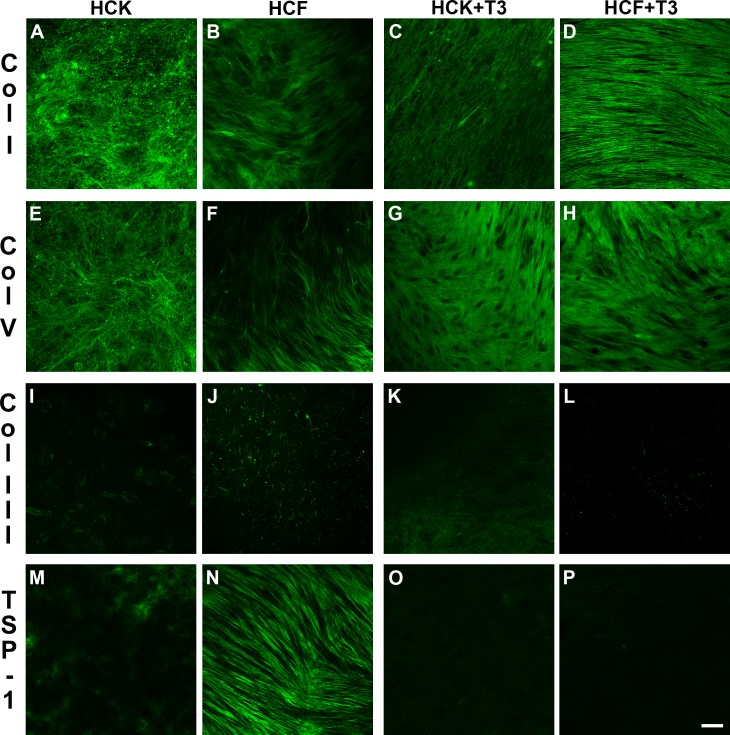
In this study, we investigated the expression of the three major collagen types (col I, III, and V), as well as a multifunctional matrix protein, TSP-1. Irrespective of the cell type (HCK or HCF), col I (Figs. 6A, 6B) and V (Figs. 6E, 6F) were present throughout the constructs with VitC only. Upon T3 stimulation, col I (Figs. 6C, 6D) and V (Figs. 6G, 6H) remained present. Interestingly, both collagen types seemed to be more aligned upon T3 stimulation (Figs. 6C, 6D, 6G, 6H) when compared with VitC only (Figs. 6A, 6B, 6E, 6F). On the other hand, col III, which has been linked to irregular or fibrotic corneas, was found to be at low levels, if any, in both the HCKs and HCFs ± T3 (Figs. 6I–L). The HCF data agrees with what was previously published.15
Figure 6.
Confocal images of the immunolocalization of Col I (A–D), Col V (E–H), Col III (I–L), and TSP-1 (M–P) in HCK and HCF constructs ±T3 stimulation. Both Col I (A–D) and V (E–H) were present throughout the constructs under all conditions. Upon T3 stimulation, the amounts of Col I (C, D) and V (G, H) remained unchanged; however, the collagen seemed to be more aligned when compared with No T3 [A, B] and [E, F]. Little, if any, Col III expression was apparent in, both the HCKs and HCFs ± T3 stimulation (I–L). Also, little, if any, TSP-1 expression was present in HCKs ± T3 stimulation (M, O); however, high levels were apparent with HCFs without T3 stimulation (N). Upon T3 stimulation, the expression of TSP-1 decreased (P).
A similar effect was seen for TSP-1 (Figs. 6M–P). Thrombospondin-1 does not appear to be expressed in the unwounded stroma,21 but several reports have indicated that it is present after wounding.22–24 In our in vitro model, HCFs without T3 stimulation showed high levels of TSP-1 expression (Fig. 6N), whereas upon T3 stimulation, the expression of TSP-1 was minimum (Fig. 6P). Human corneal keratocytes, on the other hand, showed little, if any, TSP-1 expression ± T3 stimulation (Figs. 6M, 6O).
Overall, our data shows that T3 increases the amount of matrix deposited by HCKs, while maintaining all the vital ECM components present in a mature in vivo cornea.
Discussion
Corneal stroma is a major contributor to the cornea's overall integrity and clarity. The ECM of the stroma is highly organized and consists of a dense network of collagen in which the stromal cells, termed keratocytes, reside. Keratocytes in vivo generally remain quiescent until an injury and/or trauma stimulate them to differentiate into fibroblasts and myofibroblasts.25–27 Myofibroblasts are generally responsible for wound contraction, as well as ECM deposition and organization during repair.28 Unfortunately, once the corneal integrity has been disrupted, the newly secreted ECM is highly disorganized and opaque, which ultimately results in partial or full loss of vision.29 For a number of years, in vitro studies have concentrated on both characterizing these cells, as well as investigating ways to alter the end result of corneal injuries.
It is widely accepted that once primary keratocytes are exposed to conventional 10% FBS in vitro, they become fibroblasts and/or myofibroblasts. Previous studies, however, have shown the ability to expand keratocytes in vitro using serum free media.30–32 While this is true, these studies used a growth factor mix in their media to maintain the keratocyte phenotype, such as albumin, insulin, and VitC. This suggests that the regular medium, MEM/Dulbecco's modified Eagle's medium (DMEM) without serum, is not enough for the expansion of keratocytes in vitro. However, in this study, we have reduced the serum concentration from 10% to 1% and have shown that 1% FBS is enough to maintain the keratocyte phenotype. In order to verify this, we examined the cells for keratocan expression. Keratocan is a widely accepted marker for the native keratocyte phenotype,30–33 and has been reported to decrease upon fibroblast/myofibroblast differentiation29,34 in a manner reminiscent of in vivo wound healing.34 Furthermore, we tested the effect of TGF-β3 on the keratocyte phenotype since we previously identified this growth factor as a key player in corneal wound healing and nonfibrotic responses.15 In this study, levels of keratocan were enhanced by the presence of both VitC and TGF-β3 in the media. This suggests that VitC and/or TGF-β3 may be necessary for corneal stromal ECM restoration in vivo.
Extracellular matrix restoration in vivo is critical for corneal tissue engineering applications. One of the challenges is the ECM assembly by keratocytes, which are the resident cells in human corneas. As shown by previous studies, the keratocytes' ability to secrete ECM is limited in vitro; however, Funderburgh and coworkers have shown secretion of ECM by a population of stromal keratocytes within spheroidal aggregates.35
In our current study, we attempted to stimulate keratocytes to secrete and deposit their own organized ECM in a three-dimensional (3D) culture model. In vivo, the stroma makes up approximately 90% of the corneal thickness and is composed of ECM and cells. The ECM is made of exquisitely aligned and organized collagen, such as types I and V, with type I collagen being the most abundant.36,37 These collagens, along with various proteoglycans, ensure the integrity and tensile strength of the stroma. Both col I and V were present in our cultures in both HCF and HCK constructs, indicating their ability to self-assemble an ECM with components similar to that found in the human stroma. Interestingly, type III collagen, which is associated with corneal fibrosis, was not expressed by HCKs and was minimized following T3 stimulation in HCFs. This is in agreement with our previous observations.15 Together our data indicates that it is possible for HCKs to secrete and assemble significant amounts of ECM without losing their characteristics and without promoting fibrotic responses.
Furthermore, we examined the expression of TSP-1. In the adult cornea, TSP-1 has been reported to localize in the endothelium, Descemet's membrane24,38,39 and immediately subjacent to the corneal epithelial basal cells.39 In addition, several reports have indicated that TSP-1 is present after wounding.21,22,24 In our study, TSP-1 was highly expressed in the HCF constructs with VitC only, whereas the HCK constructs showed minimal expression. Interestingly, T3 was found to minimize the TSP-1 expression in the HCF constructs, but had no effect on the HCK constructs, thereby mimicking an unwounded in vivo situation. This observation strengthens our recent report that TSP-1 localization in the stromal ECM is involved in the transformation of keratocytes into myofibroblasts.21
Overall, the data shown here is vital for corneal tissue engineering since the removal of FBS can lead to a new therapeutic solution for sight-threatening defects. Even though we have not completely removed serum from our cultures, we are moving in the right direction. We have shown that it is possible for HCKs to secrete and assemble significant amounts of ECM without losing their characteristics. Previous studies with 0% serum have failed to secrete great amounts of ECM30–32; however, our novel system maintained the stromal cells with a keratocyte-like phenotype, which were able to secrete a well-organized ECM with components similar to that found in a healthy human stroma. We aim to further our investigations and optimize our system in order to remove FBS completely from our system. We have previously shown the effects of varying the concentration of T3 with HCFs.15 Interestingly, when the T3 concentration was increased with HCKs, we found that all the constructs contracted, making it impossible to analyze (data not shown). The concentrations used were 0.25, 0.5, and 1 ng/mL, and further studies are currently ongoing in order to fully characterize the effect of T3 at higher concentrations with HCKs. It is our hypothesis that an optimum T3 concentration might be the answer to completely removing the serum from our cultures.
Acknowledgments
Supported by National Institutes of Health/National Eye Institute Grants EY03790 (Core-JDZ), EY005665 (JDZ), EY020886 (JDZ, DK), and EY06000 (VT-R).
Disclosure: D. Karamichos, None; C.B. Rich, None; R. Zareian, None; A.E.K. Hutcheon, None; J.W. Ruberti, None; V. Trinkaus-Randall, None; J.D. Zieske, None
References
- 1. Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1980; 63: 263– 322 [DOI] [PubMed] [Google Scholar]
- 2. Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999; 18: 529– 551 [DOI] [PubMed] [Google Scholar]
- 3. Linsenmayer TF, Fitch JM, Schmid TM, et al. Monoclonal antibodies against chicken type V collagen: production, specificity, and use for immunocytochemical localization in embryonic cornea and other organs. J Cell Biol. 1983; 96: 124– 132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beales MP, Funderburgh JL, Jester JV, Hassell JR. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999; 40: 1658– 1663 [PubMed] [Google Scholar]
- 5. Wilson SE, He YG, Weng J, et al. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996; 62: 325– 327 [DOI] [PubMed] [Google Scholar]
- 6. Zieske JD, Guimaraes SR, Hutcheon AE. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res. 2001; 72: 33– 39 [DOI] [PubMed] [Google Scholar]
- 7. Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003; 278: 45629– 45637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp Eye Res. 2007; 85: 305– 311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998; 67: 753– 791 [DOI] [PubMed] [Google Scholar]
- 10. Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000; 1: 169– 178 [DOI] [PubMed] [Google Scholar]
- 11. Roberts AB. The ever-increasing complexity of TGF-beta signaling. Cytokine Growth Factor Rev. 2002; 13: 3– 5 [DOI] [PubMed] [Google Scholar]
- 12. Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002; 307: 1– 14 [DOI] [PubMed] [Google Scholar]
- 13. Brunet CL, Sharpe PM, Ferguson MW. Inhibition of TGF-beta 3 (but not TGF-beta 1 or TGF-beta 2) activity prevents normal mouse embryonic palate fusion. Int J Dev Biol. 1995; 39: 345– 355 [PubMed] [Google Scholar]
- 14. Carrington LM, Albon J, Anderson I, Kamma C, Boulton M. Differential regulation of key stages in early corneal wound healing by TGF-beta isoforms and their inhibitors. Invest Ophthalmol Vis Sci. 2006; 47: 1886– 1894 [DOI] [PubMed] [Google Scholar]
- 15. Karamichos D, Hutcheon AE, Zieske JD. Transforming growth factor-beta3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J Tissue Eng Regen Med. 2011; 5: e228– e238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995; 108: 985– 1002 [DOI] [PubMed] [Google Scholar]
- 17. Guo X, Hutcheon AE, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2007; 48: 4050– 4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karamichos D, Guo XQ, Hutcheon AE, Zieske JD. Human corneal fibrosis: an in vitro model. Invest Ophthalmol Vis Sci. 2010; 51: 1382– 1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gipson IK, Grill SM, Spurr SJ, Brennan SJ. Hemidesmosome formation in vitro. J Cell Biol. 1983; 97: 849– 857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Funderburgh JL. Corneal proteoglycans. In: Iozzo RV. ed Proteoglycans: Structure, Biology, and Molecular Interactions. New York, NY: Marcel Dekker; 2000: 237– 273 [Google Scholar]
- 21. Matsuba M, Hutcheon AE, Zieske JD. Localization of thrombospondin-1 and myofibroblasts during corneal wound repair. Exp Eye Res. 2011; 93: 534– 540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao Z, Wu HK, Bruce A, Wollenberg K, Panjwani N. Detection of differentially expressed genes in healing mouse corneas, using cDNA microarrays. Invest Ophthalmol Vis Sci. 2002; 43: 2897– 2904 [PubMed] [Google Scholar]
- 23. Hiscott P, Armstrong D, Batterbury M, Kaye S. Repair in avascular tissues: fibrosis in the transparent structures of the eye and thrombospondin 1. Histol Histopathol. 1999; 14: 1309– 1320 [DOI] [PubMed] [Google Scholar]
- 24. Uno K, Hayashi H, Kuroki M, et al. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem Biophys Res Commun. 2004; 315: 928– 934 [DOI] [PubMed] [Google Scholar]
- 25. Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999; 18: 311– 356 [DOI] [PubMed] [Google Scholar]
- 26. Moller-Pedersen T, Vogel M, Li HF, Petroll WM, Cavanagh HD, Jester JV. Quantification of stromal thinning, epithelial thickness, and corneal haze after photorefractive keratectomy using in vivo confocal microscopy. Ophthalmology. 1997; 104: 360– 368 [DOI] [PubMed] [Google Scholar]
- 27. Petroll WM, New K, Sachdev M, Cavanagh HD, Jester JV. Radial keratotomy. III. Relationship between wound gape and corneal curvature in primate eyes. Invest Ophthalmol Vis Sci. 1992; 33: 3283– 3291 [PubMed] [Google Scholar]
- 28. West-Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006; 38: 1625– 1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berryhill BL, Kader R, Kane B, Birk DE, Feng J, Hassell JR. Partial restoration of the keratocyte phenotype to bovine keratocytes made fibroblastic by serum. Invest Ophthalmol Vis Sci. 2002; 43: 3416– 3421 [PubMed] [Google Scholar]
- 30. Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta-induced keratocyte-myofibroblast transdifferentiation. J Biol Chem. 2001; 276: 44173– 44178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994; 35: 730– 743 [PubMed] [Google Scholar]
- 32. Lakshman N, Petroll WM. Growth factor regulation of corneal keratocyte mechanical phenotypes in 3-D collagen matrices. Invest Ophthalmol Vis Sci. 2012; 53: 1077– 1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garagorri N, Fermanian S, Thibault R, et al. Keratocyte behavior in three-dimensional photopolymerizable poly(ethylene glycol) hydrogels. Acta Biomater. 2008; 4: 1139– 1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hahnel C, Somodi S, Weiss DG, Guthoff RF. The keratocyte network of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2000; 19: 185– 193 [DOI] [PubMed] [Google Scholar]
- 35. Funderburgh ML, Mann MM, Funderburgh JL. Keratocyte phenotype is enhanced in the absence of attachment to the substratum. Mol Vis. 2008; 14: 308– 317 [PMC free article] [PubMed] [Google Scholar]
- 36. Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001; 32: 223– 237 [DOI] [PubMed] [Google Scholar]
- 37. Linsenmayer TF, Fitch JM, Gordon MK, et al. Development and roles of collagenous matrices in the embryonic avian cornea. Prog Retin Eye Res. 1998; 17: 231– 265 [DOI] [PubMed] [Google Scholar]
- 38. Hiscott P, Seitz B, Schlotzer-Schrehardt U, Naumann GO. Immunolocalisation of thrombospondin 1 in human, bovine and rabbit cornea. Cell Tissue Res. 1997; 289: 307– 310 [DOI] [PubMed] [Google Scholar]
- 39. Sekiyama E, Nakamura T, Cooper LJ, et al. Unique distribution of thrombospondin-1 in human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2006; 47: 1352– 1358 [DOI] [PubMed] [Google Scholar]