Abstract
Purpose.
We examined the treatment period necessary to restore retinal and visual stability following trauma to the optic nerve.
Methods.
Cats received unilateral optic nerve crush and no treatment (NT), treatment of the injured eye with brain-derived neurotrophic factor (BDNF), or treatment of the injured eye combined with treatment of visual cortex for 2 or 4 weeks. After 1-, 2-, 4-, or 6-week survival periods, pattern electroretinograms (PERGs) were obtained and retinal ganglion cell (RGC) survival determined.
Results.
In the peripheral retina, RGC survival for NT, eye only, and eye + cortex animals was 55%, 78%, and 92%, respectively, at 1 week, and 31%, 60%, and 93%, respectively, at 2 weeks. PERGs showed a similar pattern of improvement. After 4 weeks, RGC survival was 7%, 29%, and 53% in each group, with PERGs in the dual-treated animals similar to the 1- to 2-week animals. For area centralis (AC), the NT, eye only, and eye + cortex animals showed 47%, 78%, and 82% survival, respectively, at 2 weeks, and 13%, 54%, and 81% survival, respectively, at 4 weeks. Removing the pumps at 2 weeks resulted in ganglion cell survival levels of 76% and 74% in the AC at 4 and 6 weeks postcrush, respectively. The PERGs from 2-week treated, but 4- and 6-week survival animals were comparable to those of the 2-week animals.
Conclusions.
Treating the entire central visual pathway is important following optic nerve trauma. Long-term preservation of central vision may be achieved with as little as 2 weeks of treatment using this approach.
Keywords: neurotrophins, retina, optic neuropathy
These studies emphasize the importance of treating the entire central visual pathway following trauma to the optic nerve. They also indicate that, by doing so, one might achieve long-term preservation of central vision with minimal intervention.
Introduction
The axons of retinal ganglion cells (RGCs) form the optic nerve by which visual information is conveyed to higher centers of the brain. However, because of its relatively long path (approximately 50–60 mm), the optic nerve is highly susceptible to injury from accidents and disease (e.g., glaucoma). Even minor injury can initiate progressive axonal damage that ultimately leads to axotomy, retinal ganglion cell degeneration, and blindness. Fortunately, since most cases of optic nerve trauma involve partial and delayed, rather than complete and immediate, axotomy, the potential for therapeutic intervention often exists.
Over the past several years, a number of studies have demonstrated that direct application of various neurotrophic materials to the eye following optic nerve injury can slow the rate of retinal ganglion cell degeneration.1–10 Much of this research, including our own, has focused on the use of brain-derived neurotrophic factor (BDNF), an especially potent neuroprotectant in the mammalian retina, and central visual pathway in general.1–6,8–22 This work has demonstrated that BDNF not only promotes ganglion cell survival following optic nerve injury, but that it also has an important role in preserving the structural integrity and visual responsiveness of these neurons.
A central challenge to the development of a trophic factor-based strategy for treatment of optic neuropathy is the apparent inability of the drug to provide long-term neuroprotection when applied directly to the eye—typically <10 days. To address this issue, more recent work in rodents has focused on the use of viral vectors and mesenchymal stem cells to deliver a more sustained supply of various trophic factors, including BDNF, to the eye.11,13,17–19,21,23–33 While these studies have shown considerable promise, they are not without concerns.34,35 In our pursuit of a strategy for long-term recovery following trauma to the optic nerve, we have focused on the close relation between retinal ganglion cells and their target neurons in the visual thalamus. Neuron–target dependence is a well-known phenomenon that affects the developing and mature nervous system.36
Based on the hypothesis that target health has a critical role in the recovery and long-term survival of presynaptic neurons following nerve trauma, we demonstrated recently that treating the retina and its primary target following optic nerve injury provides a more significant and sustained level of neuroprotection relative to treating the eye alone.15 The studies presented here extend these initial findings, suggesting that by treating the eye and visual thalamus, it is possible to enhance retinal ganglion cell survival and preserve central visual function for at least 6 weeks, the longest period studied, following as little as 2 weeks of treatment. This reduces the potential for adverse side effects, a warranted concern associated with long-term applications of trophic factors to the central nervous system.
Methods
The 19 adult cats studied were maintained in a free-run environment with food and water provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee at Michigan State University, and all adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
The basic surgical and treatment procedures have been described in detail previously, and, thus, only procedures specific to the present studies are presented here.15 In general, all animals were anesthetized, intubated, and received a mild crush of the left optic nerve via the frontal sinus using sterile surgical procedures. In all animals the nerve crush was performed by 15-minute application of a vascular clamp, 2 to 3 mm posterior to the globe, that exerted a force of approximately 15 g. In addition, the route via the frontal sinus allowed only one angle of approach for placement of the clamp onto the nerve. Combined, these helped to standardize the crush across different cats. Vital signs were monitored continuously, postoperative pain medication was provided, and they were monitored every 2 hours during recovery, then daily.
All cats used in the present study received either a 4- or 6-week survival period relative to the time of the nerve crush—for continuity and comparison with our previous work, in some cases, as noted, data obtained from the 1- and 2-week studies also are provided. Of the 15 cats receiving a 4-week survival period, 1 received no treatment (4-week/NT), 4 received treatment of the eye alone (4-week/eye), 7 received a single treatment of the eye plus 4 weeks of treatment to visual cortex (4-week/4-week), and 3 received treatment of the eye, but only 2 weeks of treatment to the visual cortex (2-week/4-week). The remaining 4 animals received the eye treatment along with 2 weeks of treatment to the visual cortex, followed by an additional 4-week survival period (2-week/6-week, Table 1). As in our previous study, the eye treatments involved intravitreal injection of BDNF, while treatment of the visual cortex was bilateral via osmotic minipumps.15 The cortical infusions of BDNF were made at approximately the representation of central vision (P4.0, L2.0).37 The BDNF was delivered at a rate of 0.25 μL/h for either 2 or 4 weeks (84 μL/168 μL), at which time the pumps were removed and the brain cannulae tubing heat sealed if a longer survival period was used.
Table 1.
Summary of Animals and Treatment Versus Survival Condition
|
Survival |
NT |
Eye |
DT |
2 wk/4 wk DT |
2 wk/6 wk DT |
| 1 wk | 6 | 6 | 6 | NM | NM |
| 2 wk | 6 | 7 | 6 | NM | NM |
| 4 wk | 1 | 4 | 7 | 3 | NM |
| 6 wk | NM | NM | NM | NM | 4 |
The 1- and 2-week data are from the study of Weber et al.15 NM, not measured; DT, dual treatment; 2 wk/4 wk, 2-week treatment/4-week postcrush survival; 2 wk/6 wk, 2-week treatment/6-week postcrush survival.
Following the survival period, all cats were prepared for noninvasive electrophysiologic analysis, as described previously.15 Pattern electroretinograms (PERGs) were acquired using an Espion E2 electrophysiologic system and pattern stimulus generator (NEC AccuSync 120; Diagnosys LLC, Lowell, MA). Stimuli were luminance modulations of either a uniform field or contrast-reversed grating patterns. Square-wave luminance modulations were used in spatial and temporal domains. Stimuli spatial frequencies (SF; 0.063–2 cycles per degree [cpd]) were presented at 2 Hz. At the viewing distance, the stimulus field subtended 44.6° horizontally and 33.9° vertically. The minimum and maximum luminances were 2 and 96 cd/m2 (95% contrast). Responses were averaged over 3 trials and 50 presentations/SF.
Following the recording session, and without recovery from anesthesia, the animals received an overdose of pentobarbital sodium, and were perfused transcardially with physiologic saline and mixed aldehyde fixatives (1.5% paraformaldehyde/2.0% glutaraldehyde in 0.1 M phosphate buffer at pH 7.4). The brains were placed into the same fixative for future processing, while the eyes were removed, bisected at the ora serrata, and postfixed for 1 to 2 days. The retinae then were dissected, whole-mounted, dehydrated in graded alcohols, defatted, stained with cresyl violet, and coverslipped.
Retinal ganglion cell counts were obtained using the computer-based imaging system described previously.15 Two regions were selected for quantitative analysis. One region occupied approximately 1.7 × 106 um2, and was located 3.5 mm above and 1.5 mm temporal to the area centralis (AC), and was chosen because of the relatively constant size and density of ganglion cells in this peripheral location of the cat retina, and for comparison with our previous studies.12,14,15 The second region comprised 1.7 × 105 um2, and was centered on the AC, the cat equivalent of the fovea in primates. Neurons were classified as ganglion cells based on the criteria of Stone.38,39 In particular, they needed to display a distinct nucleus and nucleolus, and have a continuous ring of cytoplasm surrounding the nucleus. In addition, for analysis, we set a minimum size limit of 12 μm diameter. While this might have resulted in the exclusion of some small ganglion cells, it eliminated any significant inclusion of displaced amacrine cells, the majority of which have somata less than 10 μm in diameter.40–45
Images were obtained systematically using a high resolution color video camera (Microfire; Optronics, Inc., Goleta, CA) and either ×20 (peripheral) or ×40 (central) objective. Double counting was avoided by using the previous image as a reference. Cell size and number were measured directly from the digital images using Image Pro Plus image analysis software (Media Cybernetics, Bethesda, MD).
All data are presented as mean ± SE. The retinal cell counts and mean soma sizes were normalized by taking the square root of each measurement, determining the difference between the experimental and normal fellow eye of the same animal, and comparing these differences for all of the animals across the different experimental conditions and survival times using a 1-way ANOVA followed by the Bonferroni test for multiple comparisons (Prism 5.0; GraphPad, Inc., San Diego, CA). Mean PERG response amplitudes, obtained from the Espion system (NEC AccuSync 120; Diagnosys LLC), were compared using a 2-tailed Student's t-test (SigmaPlot; SPSS, Inc., Chicago, IL). Cell size distributions, normalized for differences in the numbers of eyes examined under each condition, were compared using a Kruskal-Wallis nonparametric test with a Dunns post hoc analysis. In all cases, P = 0.05 was used as the level of significance.
Results
Qualitative Observations
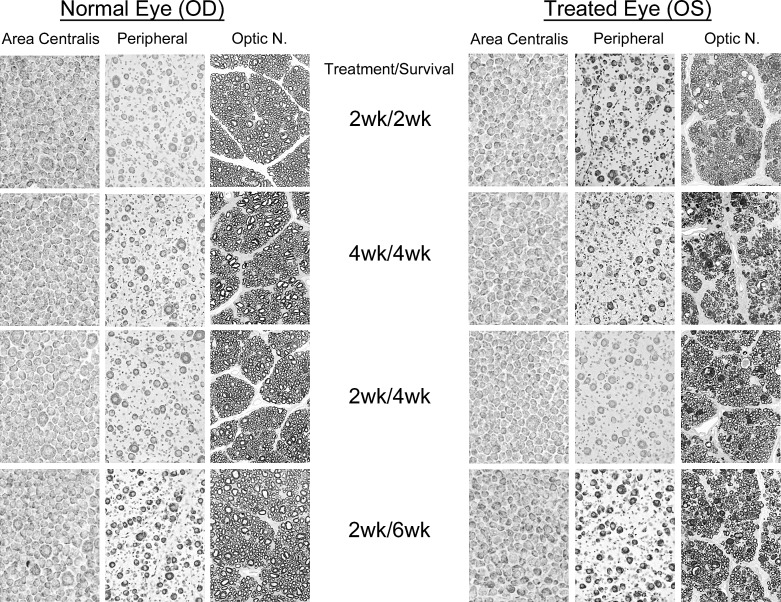
Comparisons of the normal and affected retinae and optic nerves for the different treatment and survival paradigms are shown in Figure 1. Qualitatively, there was little difference in the cellular appearance of the AC across conditions; however, one noticeable effect of the nerve injury was the loss or reduction of the few large ganglion cells seen typically in this region. While their loss was consistent, the extent of their retention with treatment was highly variable, and seen primarily with combined eye and brain treatment versus treatment of the eye alone. In peripheral retina, ganglion cells from all of the treated animals had relatively normal appearances, characterized by well-defined membranes, oval-shaped somata with centrally located nuclei, and an abundance of Nissl substance, with the exception being the 4-week treated and 4-week survival animals. Similarly, despite the scattered presence of numerous degenerating profiles, the optic nerves from these animals also contained a significant number of axons that were well-myelinated and arranged into discrete bundles.
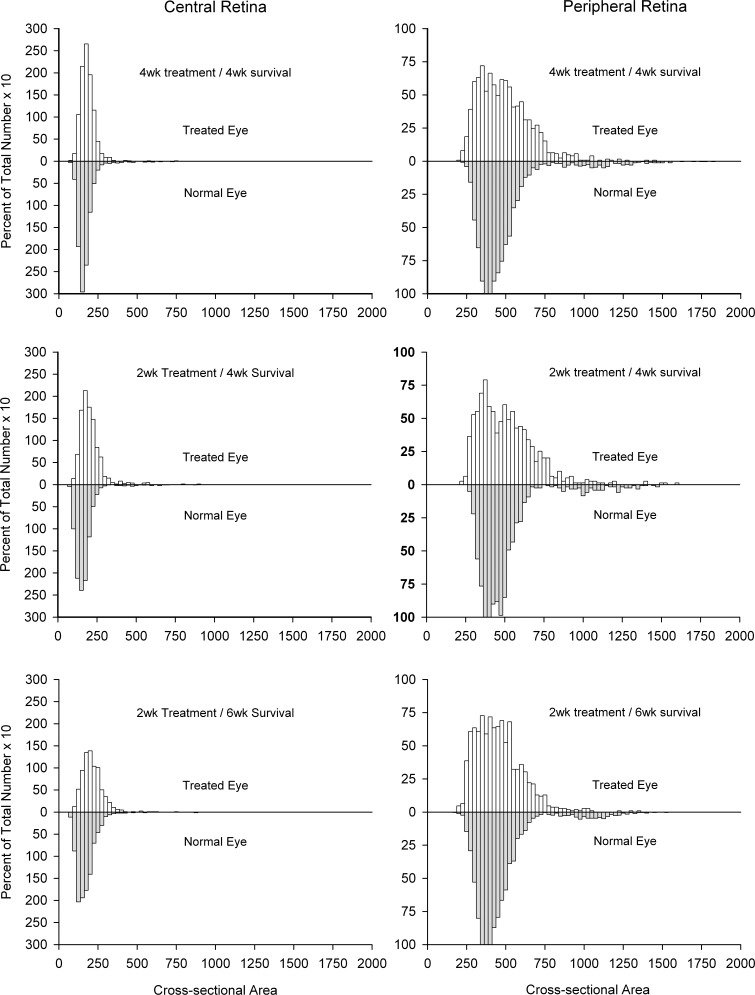
Figure 1.
Morphologic comparisons of the retinae and optic nerves from normal eyes, and eyes that received a mild, unilateral, nerve crush combined with intravitreal injection of BDNF at the time of the nerve injury, and either 2 or 4 weeks of BDNF infusion into visual cortex bilaterally. Postcrush survival periods were 2, 4, or 6 weeks in duration. Ganglion cell images represent matched regions of the AC and peripheral retina. The 2-week treatment/survival data are from the study of Weber et al.15
Quantitative Observations: Cell Survival and Visual Responses
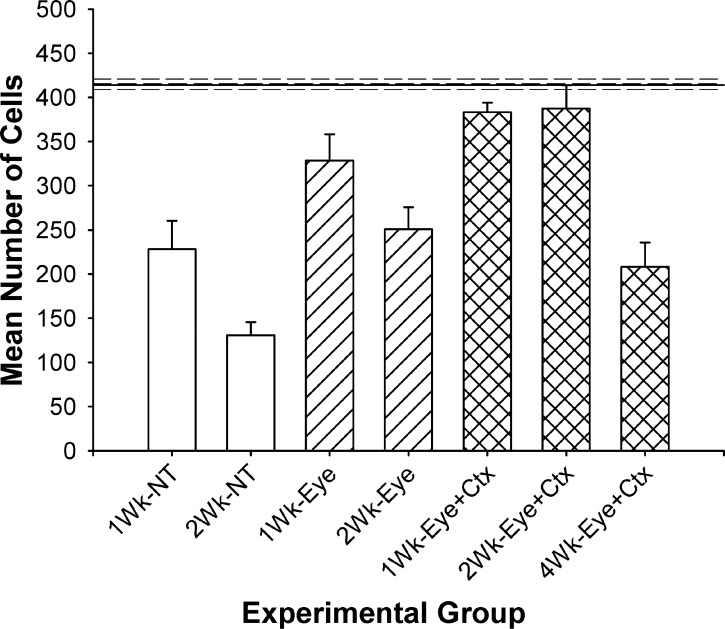
In our previous study, the goal was to determine whether treatment of the eye and its primary target, the dorsal lateral geniculate nucleus of the thalamus (dLGN), results in greater preservation of retinal ganglion cells following optic nerve injury compared to treatment of the eye alone. The data from these 2-week studies, shown to the left in Figure 2, indicated a significant increase in ganglion cell survival in peripheral retina at 1 week (92% vs. 78%) and 2 weeks (93% vs. 60%) post-injury/treatment by applying this dual-treatment (DT) strategy. Extending this approach to 4 weeks, however, did not produce the same result (Fig. 2), but rather showed a decrease in peripheral ganglion cell survival to approximately 50% of normal, a level comparable to animals receiving either no treatment and 1-week survival or treatment of the eye alone and 2-week survival.
Figure 2.
Comparison of mean ganglion cell survival in the peripheral retina under each experimental condition. Solid line represents the mean number of cells measured in matched regions of the normal fellow eye. Values are mean ± SEM. NT and eye only values versus N, P < 0.001 to 0.05; NT versus eye, P < 0.05; eye versus DT, 1-week, NS; 2-week, P < 0.05; DT versus N, P > 0.05; DT versus 4-week, P < 0.05. The 1- and 2-week data are from the study of Weber et al.15
Despite this decrease, the PERG responses for the 4-week dual-treated animals were significantly higher than those of animals receiving no treatment. Also, although not significantly different from the responses of the 2-week dual-treated animals, they were consistently better across all spatial frequencies tested (Fig. 3). Because of the strong PERG responses, we then performed a second analysis in each of four retinal quadrants immediately adjacent to the AC.
Figure 3.
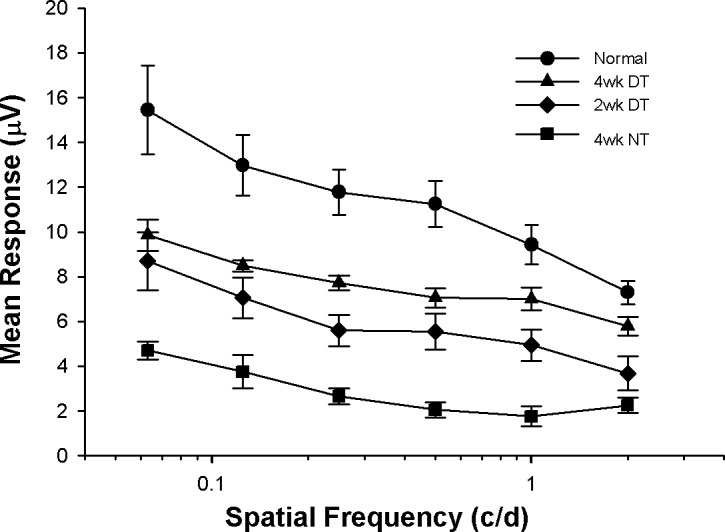
Pattern ERG responses to gratings of different spatial frequency for each experimental condition. Although not normal, the responses measured in the treated animals are significantly better than those measured in animals not receiving treatment. Note also that the 4-week DT responses are consistently stronger than the 2-week DT responses. Values are mean ± SEM. N versus DT, P < 0.05; 2-week versus 4-week, P > 0.05; DT versus NT, P < 0.05.
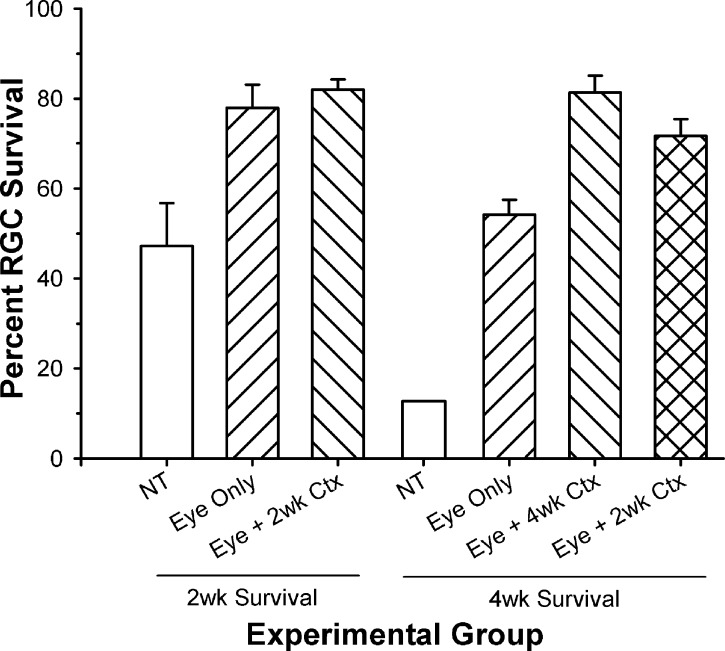
Figure 4 compares ganglion cell survival in the AC for animals receiving optic nerve injury and the different treatment strategies used, followed by either a 2- or 4-week survival period. In the 2-week animal cell counts, obtained by reanalyzing the retinae from our previous study,15 ganglion cell survival in the AC was only 47% of normal without treatment, but significantly greater following treatment of the eye alone (78%) and treatment of the eye plus visual cortex (82%). For the 4-week survival animals, those that received the nerve injury and NT showed a significant loss (13% survival) in ganglion cell numbers in the central retina. Treatment of the eye alone resulted in a significant increase in cell survival (54%), but this still was significantly less than that seen following treatment of the eye and cortex (81%).
Figure 4.
Comparison of the percent ganglion cell survival in the AC for animals receiving different treatment strategies, and either 2- or 4-week survival periods. At 2 weeks after injury/treatment, there was a significant increase in ganglion cell survival following treatment of the eye alone and the eye + cortex (P < 0.05) compared to NT. Following a 4-week survival period, all treatment conditions enhanced ganglion cell survival significantly relative to NT (P < 0.05), with the DT animals showing a significant increase over those receiving treatment of the eye alone (P < 0.05). Although those receiving only 2 weeks of treatment, followed by an additional 2-week survival period without treatment, showed a slight reduction in ganglion cell survival in the AC, they were not different from those animals receiving 4 weeks of treatment (P > 0.05). The 2-week survival data are from the study of Weber et al.15
Based on the similar levels of neuroprotection in the AC for the 2-week DT and 4-week DT animals (82% vs. 81%), we then asked whether 2 weeks of cortical treatment might be sufficient to stabilize ganglion cell survival in the central retina. For cats that received a single injection to the eye and continuous application of BDNF to the visual cortex for 2 weeks, but then were allowed to survive an additional 2 weeks without further treatment, the number of surviving central ganglion cells was only slightly less than that seen with 4 weeks of treatment (76% vs. 81%), but remained significantly higher than that seen with treatment of the eye alone (54%; Fig. 4, right bar). Comparisons of the PERG responses from DT animals receiving 2 weeks of treatment and either 2- or 4-week survival periods are shown in Figure 5. The 2-week treatment/4-week survival animals (2-week/4-week DT) had visual responses that were between those of the 2-week treatment/survival animals (2-week/2-week DT) and normal, and were not significantly different from either.
Figure 5.
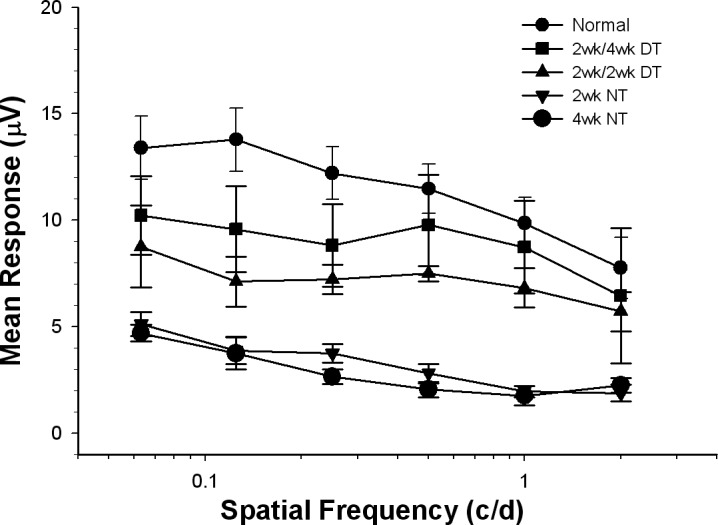
PERG responses to gratings of different spatial frequency for 2- and 4-week experimental conditions. Normal and treated animal responses were significantly stronger than those measured in the eyes of the NT animals. The DT animal responses were not different from each other; however, the responses from the 2-week treatment/4-week survival animals were consistently stronger than those measured in the 2-week DT animals, and not different from normal at the higher spatial frequencies. Values are mean ± SEM. N versus NT, P < 0.001; DT versus NT, P < 0.05.
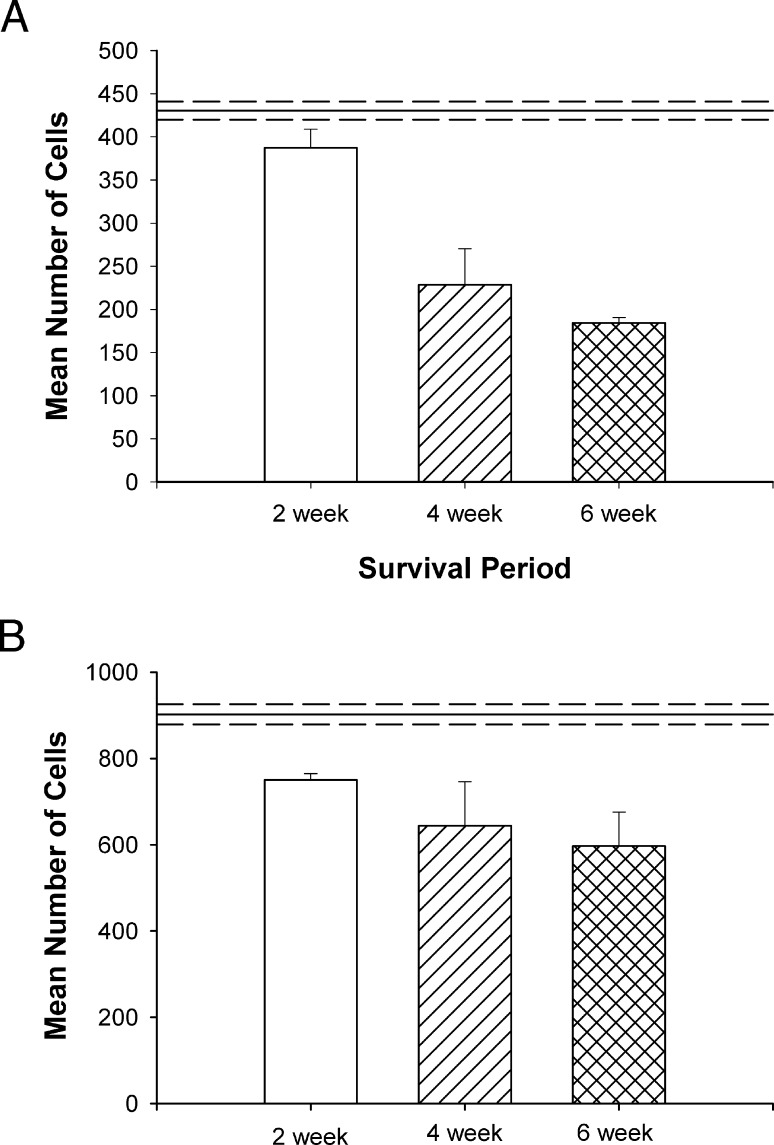
Due to the strong visual responses in these animals, a final set of 4 animals received an optic nerve injury and treatment of the eye, combined with 2 weeks of BDNF treatment to visual cortex, and a 6 week postinjury survival period. Comparisons of cell survival in the peripheral and central retinae of these animals are presented in Figures 6A and 6B, respectively, and their comparison with all of the other animals is presented in Table 2.
Figure 6.
Comparison of the mean number of surviving ganglion cells measured in peripheral (A) and central (B) retina following 2 weeks of treatment followed by either a 2-, 4-, or 6-week survival period. In the peripheral retina there was a significant decrease in ganglion cell survival between 2 and 4 weeks (P < 0.01). Although there was an additional 9% loss between 4 and 6 weeks, this additional change was not significant. In the central retina there was only a 13% to 14% decrease in ganglion cell survival at 4 to 6 weeks, even though treatment had been stopped at 2 weeks. This decrease was not significant for either time period (P > 0.05).
Table 2.
Summary of Ganglion Cell Survival: Peripheral Versus Central Retina
|
1 wk |
2 wk |
4 wk |
2 wk/4 wk |
2 wk/6 wk |
|
| NT | 55 | 31 vs. 47* | 7 vs. 13* | NM | NM |
| Eye only | 78 | 60 vs. 78* | 29 vs. 54* | NM | NM |
| Eye + brain | 92 | 93 vs. 82* | 53 vs. 81* | 50 vs. 76* | 44 vs. 74* |
Values are shown as percent survival. The 1- and 2-week data are derived from animals used in the study of Weber et al.15
Central retina data.
For the dual-treated animals, in peripheral retina, there was a 40% difference in ganglion cell survival between the 2- and 4-week survival periods (93% vs. 53%), a 43% difference between the 2-week treatment and survival animals versus those that received 2 weeks of treatment and an additional 2-week survival with no further treatment (93% vs. 50%). Extending the survival period with no treatment for an additional 2 weeks resulted in an additional 6% decrease in ganglion cell survival (50% vs. 44%). In the central retina, there was only a 1% difference in ganglion cell survival between the 2- and 4-week DT animals (82% vs. 81%), a 6% difference measured following an additional 2-week survival without further treatment (82% vs. 76%), and only a 2% additional decrease by extending the nontreatment period an additional 2 weeks (76% vs. 74%). In agreement with this sustained level of centrally-located ganglion cells, the PERG responses for the 6-week animals were not significantly different from those of the 2-or 4-week survival animals (Fig. 7).
Figure 7.
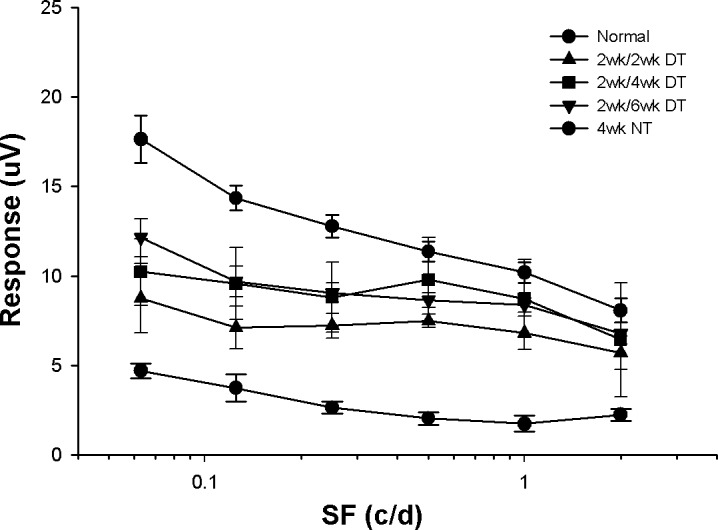
PERG responses for each of the different experimental conditions tested. All of the treated animals displayed responses that were significantly stronger than those measured in animals without treatment (P < 0.05). There was no significant difference among the treated animals, nor between the responses of the longer survival animals and normals at the higher spatial frequencies tested (P > 0.05).
Cell Size
Figure 8 shows the cell size distributions for the normal and treated eyes of ganglion cells measured in the central and peripheral retina. Although very similar in shape, there was a small, but significant, shift toward larger soma sizes in the treated animals. This also was seen when comparing the mean soma sizes within each group against their normal companion eyes (central, 188.3 ± 9.2 vs. 158.9 ± 2.3 μm2; peripheral, 473.9 ± 15.9 vs. 466.6 ± 21.9 μm2). Examining the proportions of small, medium, and large ganglion cells in central and peripheral retina did not indicate any differences across the different treatment conditions for either region—in the central retina, on average, 6.6% of the cells had somata less that 125 μm2 in size, 86.4% were in the 125 to 300 μm2 range, and 7% had somata greater than 300 μm2, while in the peripheral retina 2% had somata < 250 μm2, 74.6% were in the 250 to 600 μm2 range, and 23.4% had somata greater than 600 μm2 in area.
Figure 8.
Size distributions of ganglion cells in the central and peripheral retina of experimental (top) and normal (bottom) eyes as a percentage of the total number of ganglion cells in the sample region. Although not significant, there was a slight shift toward larger cell size in the treated versus normal animals.
However, as noted, for both regions there was a significant shift toward larger sized cell somata in the experimental eyes versus normal eyes, regardless of the treatment—in the central retina the proportion of small ganglion cells decreased from 24.2% in the normal to 6.6% in the treated eyes, while the proportion of medium-sized ganglion cells increased from 71.8% to 86.4%. In the peripheral retina, the proportion of medium-sized cells decreased from 88.4% in the normal eyes to 74.6% in the experimental eyes, while the proportion of cells with somata greater than 600 μm2 increased from 9.8% to 23.2%. Despite these changes, when the mean soma sizes for affected versus normal eyes were compared within each individual animal, there were no significant differences for either central or peripheral retina (P range = 0.15–0.94).
Discussion
The goal of this study was to extend our current understanding of the use of BDNF as a retinal neuroprotectant, as well as to emphasize further the importance of treating the eye and entire central visual pathway following injury to the optic nerve. In our previous work, we demonstrated that treatment of the eye and central visual pathway following optic nerve trauma is more beneficial than treating the eye alone. The focus here was to gain additional insight with respect to the relation between duration of treatment, and ganglion cell survival and function. This is an important consideration in most treatment paradigms, where cost, patient compliance, and possible contraindications are concerns.
The data suggest that, following optic nerve injury, direct treatment of the eye with BDNF, combined with as little as 2 weeks of treatment provided to the rest of the central visual pathway, might be sufficient to preserve critical central vision, and prevent the progressive retinal degeneration seen commonly following such trauma. As expected, animals that received no treatment showed the greatest level of retinal degeneration and loss of function, followed by those that received a single intraocular injection of BDNF at the time of the injury. Cats that received the eye injection combined with chronic delivery of the drug to the visual cortex showed the highest levels of ganglion cell survival; however, by 4 weeks there was a clear distinction between central and peripheral retina—between 2 and 4 weeks after injury, ganglion cell loss was significant in the peripheral, but not central, retina. Furthermore, stopping treatment after 2 weeks resulted in an additional 49% decrease (93%–44%) in ganglion cell survival in the peripheral retina at 6 weeks after injury, but only an 8% additional decrease (82%–74%) in ganglion cell survival in the central retina (Table 2).
There are several possible explanations for this central-peripheral difference. First, because ganglion cells of all types display increased soma and dendritic field sizes with increased retinal eccentricity,42–46 it might be the case that the larger-sized cells in the peripheral retina, regardless of cell type, require a higher level of neurotrophic support relative to their smaller counterparts residing in the central retina. This also might explain the conspicuous loss of central large cells; however, the loss is not exclusive to these neurons. Second, the difference might reflect variations in the sensitivity of different classes of ganglion cells to exogenous application of BDNF—medium-sized beta cells dominate the AC of the cat,38,39,45 and previous studies by us and others have indicated that, although these neurons are readily susceptible to nerve trauma, they also are highly responsive to the administration of BDNF.12,14,47–50 While this might suggest differences with respect to receptor numbers for large alpha versus smaller beta cells, previous work comparing mRNA levels of BDNF and trkB receptors in spinal motor neurons does not support a correlation between cell size/function and trophic factor/receptor content.51 Unfortunately, at present, there is little or no information concerning the density and/or distribution of neurotrophic receptors on specific classes of ganglion cells, or on those of the same or different class located in different regions of the retina. Finally, it is possible that the enhanced survival of central versus peripheral ganglion cells simply reflects the fact that, in all cases, the cannulae used to infuse BDNF into visual cortex were directed at the cortical representation of the AC. Thus, there was an inherent treatment bias in favor of those neuronal components associated with central versus peripheral vision. Since the visual field representation in the cat cortex is relatively large and well-defined,37 it would be of interest to test whether targeting treatment toward a different cortical area might result in a predictable shift in the region of enhanced retinal ganglion cell survival, with a concomitant reduction in ganglion cell survival in the central retina.
The goal of any neuroprotection strategy is to provide long-term stability to the injured system. In the case of damage to the optic nerve, be it via physical trauma or disease, the primary focus has been on the preservation of the retinal ganglion cells whose axons comprise the optic nerve. As noted in the Introduction, initial studies in this area focused on the direct injection of different trophic materials to the eye. These studies were beneficial in defining the potential of the different compounds, and their effective duration. Of the different compounds, ciliary neurotrophic factor (CNTF) and BDNF have received the most attention. Unfortunately, despite their strong ability to prevent ganglion cell degeneration short-term, their effectiveness long-term is limited when provided as a single injection, or even as multiple injections. This is due partly to the limited supply and presence of the protein, but also to downregulation of the receptors they use, and desensitization of the signal transduction pathways they drive.12,13,52–58 In the case of BDNF, to our knowledge DiPolo et al. were the first to show that, relative to direct injection of the protein into the eye, one could enhance ganglion cell survival in the rat following axotomy by intraocular injection of a viral vector containing the BDNF gene, thus providing a slower and more prolonged delivery of the drug.11 Nevertheless, the effect still was short-lived, with ganglion cell survival falling from a maximum of 65% at 10 days after axotomy/injection to approximately 10% at 4 weeks. To address the possibility that this reduction might be the result of drug-induced downregulation of the BDNF TrkB receptor, Cheng et al. then combined direct application of BDNF to the eye with transfection of the retina using an adeno-associated viral (AAV) vector containing the TrkB gene.13 While this resulted in a 76% survival rate at 2 weeks after axotomy, survival again declined to 17% of normal at 4 weeks. In 2003, Martin et al.19 applied a modified AAV.BDNF vector to the eyes of rats with chronic elevation of intraocular pressure and experimental glaucoma, and reported a ganglion cell survival rate, based on axon counting, of 68% at 4 weeks after induction of the disease. A slightly lower result (61% survival) was achieved by Pease et al.27 using a comparable CNTF-based AAV vector (see also CNTF-based studies by others23–25,28,34). More recently, Ren et al.21 examined ganglion cell survival, and visual function in rats undergoing acute elevation of intraocular pressure, and ischemic insult to the eye and optic nerve followed by combined treatment of the eye with a direct injection of BDNF and an AAV.BDNF vector. They reported ganglion cell density levels at 9 weeks after injury/treatment that were 84% of normal, as well as significantly enhanced visual evoked and spatial contrast responses as far out as 70 weeks. While these studies support the potential use of viral vectors as a means of providing prolonged drug delivery to the retina, and, thus, extended neuroprotection via a single injection, it is important to keep several factors in mind. First, the studies involve very different mechanisms of injury to the optic nerve, and thus the success of each must be evaluated on that basis. Second, the studies involve the treatment of eyes that are much smaller than human eyes, and, thus, the ability to provide widespread neuroprotection to the retina via intravitreal injection is much greater—our own experience with the use of AAV and Ad vectors in cat eyes, which are comparable in size to that of humans, is that there is little diffusion of the viral vector from the site of injection via intravitreal application. Similar results have been described by colleagues in the dog, even following injection at the retinal surface. This problem can be remedied, to some extent, by preinjection vitrectomy, but this adds an additional layer of potential complicating factors. Furthermore, the location of the injection can result in initiation of an immune response that reduces the effectiveness of additional treatments using the same vector.59,60 To overcome these issues, considerable emphasis has been placed on enhancing the transfection range, efficiency, and safety of AAV vectors via the development of different serotypes and/or modification of the viral capsid.61–64
A second approach that recently has been adopted as a means of providing prolonged delivery of neuroprotection to the retina, while avoiding the safety issues associated with the use of viral vectors, is intravitreal transplantation of bone marrow-derived mesenchymal stem cells.18,29–33,35 These cells, which have been shown to secrete a number of different trophic factors naturally, can be obtained readily and used for autologous transplantation. In addition, they can be induced to upregulate the production of various trophic factors via manipulation of the culture media,30 or by transfection using different viral vectors.18,31
While long-term delivery of different trophic factors generally is considered necessary for long-term neuroprotection and maintenance of function, too much of a good thing can be problematic. Rodger et al.34 examined the morphology of single ganglion cells in the rat 5 to 8 months after optic nerve transection, transplantation of a peripheral nerve stalk to enhance axon regeneration, and intravitreal injection of AAV vectors capable of transfecting these neurons with either the CNTF or BDNF gene. Compared to eyes receiving saline injections, or injection with vectors expressing either GAP-43 or the GFP gene alone, eyes receiving trophic factor expressing vectors showed a significant increase (>65% vs. <15%) in the proportion of ganglion cells with abnormal somal and dendritic morphologies, with some of the effects being trophic factor– or cell class–specific. In addition, long-term gene expression also resulted in abnormal stratification of ganglion cell dendrites into inappropriate functional laminae of the inner plexiform layer. Equally interesting, the effects were seen not only in transfected ganglion cells, but nontransfected neighboring cells as well. Ongoing studies suggest that ganglion cell changes also occur in normal eyes receiving vector-based trophic factor treatment (Harvey A, personal communication, April 2013). Since ganglion cells receive all of their input via their dendrites, these induced changes in dendritic morphology suggest a potential concern with respect to possible alterations in the visual response properties of the surviving cells. We have not seen similar changes, most likely due to the much shorter exposure to the drug.14
In the present study, we continue to focus on a nonviral vector approach to long-term neuroprotection, emphasizing instead the well-known importance of the neuron–target relation in the developing and mature nervous system.56,65–70 In addition, our goal is preservation of existing neurons and function following nerve trauma, and not the much more difficult task of regeneration. Under normal conditions, we envision that healthy ganglion cells provide a basal level of electrical activity to their target neurons, which in turn stimulates them to express trophic materials that then are taken up by the ganglion cell axon terminals and transported back to the retina. Trauma to the optic nerve affects not only the retrograde transport of trophic materials, but also the level of target activation.71–76 The resulting decrease in target neuronal activity then leads to a general reduction in target trophic levels, which has a negative impact not only on the injured neurons, but their healthy neighbors as well; thus, contributing to the progressive degeneration commonly seen even after removal of the primary insult. Although the dLGN, and not the visual cortex, is the primary target of retinal ganglion cell axons, the ease of accessing the visual cortex versus the dLGN and the massive reciprocal connection between the two make it possible to impose a major influence on the dLGN via treatment of visual cortex.77–80 With respect to BDNF, it is likely that cortical application of the drug acts on the dLGN on several levels. First, since it has been shown that BDNF can enhance electrical activity in neurons,81 it is highly likely that its application to cortex results in electrical stimulation of the corticogeniculate pathway, and, thus, increased electrical excitation of neurons within the dLGN. Second, it is well-known that BDNF can be transported anterogradely and retrogradely,53,73,76,82–86 the result being increased levels of dLGN BDNF and activation of BDNF-related intracellular signaling pathways in dLGN neurons. Two well-studied pathways are the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase-protein kinase B (PI3K-Akt) pathways.13,87,88 The collective result of these different events would be a sustained level of trophic material and cellular activity within the dLGN; thus, helping to preserve ganglion cells whose axons were not affected directly by the trauma, but which potentially could be affected adversely by secondary processes. The question, then, is how to achieve the same central neuroprotective effect, but without direct injection of trophic material into either the dLGN or visual cortex. One possible approach might be through the use of transcranial magnetic stimulation (TMS). A number of studies have demonstrated the ability to excite and inhibit visual cortex using TMS.89–91
While the data presented here support our longstanding notion that treatment beyond the eye is a critical component in the preservation of retinal ganglion cells and vision following trauma to the optic nerve, the equally important finding was the sustained neuronal survival and visual responses measured in animals that received only 2 weeks of treatment, but were allowed an additional 2- to 4-week survival period without any further treatment. This, combined with the fact that animals receiving longer survival periods in general showed consistently better PERG responses at all spatial frequencies tested (Figs. 3, 5, 7), suggests that under these experimental conditions and treatment paradigm, 2 weeks might represent the minimum period needed for surviving ganglion cells to recover fully and remain stable following trauma to the optic nerve. Nevertheless, while observation of the animals in their free-run environment did not indicate any obvious vision-related impairment, additional studies that include a more complete regimen of behavioral testing are needed to determine the full degree of functional recovery, and the extent to which long-term stability in visual function has been achieved. If these support the current findings, then many of the concerns associated with long-term trophic factor exposure might be alleviated by combining a single treatment of the eye with short-term treatment of the central visual pathway in general.
Acknowledgments
Supported by National Institutes of Health (NIH)/National Eye Institute (NEI) Grant EY11159 (AJW).
Disclosure: A.J. Weber, None; C.D. Harman, None
References
- 1. Aguayo AJ, Clarke DB, Jelsma TN, Kittlerova P, Friedman HC, Bray GM. Effects of neurotrophins on the survival and regrowth of injured retinal neurons. Ciba Found Symp. 1996; 196: 135– 144 [DOI] [PubMed] [Google Scholar]
- 2. Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994; 91: 1632– 1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993; 602: 304– 317 [DOI] [PubMed] [Google Scholar]
- 4. Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996; 37: 489– 500 [PubMed] [Google Scholar]
- 5. Sawai H, Clarke DB, Kittlerova P, Bray GM, Aguayo AJ. Brain-derived neurotrophic factor and neurotrophin-4/5 stimulate growth of axonal branches from regenerating retinal ganglion cells. J Neurosci. 1996; 16: 3887– 3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parrilla-Reverter G, Agudo M, Sobrado-Calvo P, Salinas-Navarro M, Villegas-Perez MP, Vidal-Sanz M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Exp Eye Res. 2009; 89: 32– 41 [DOI] [PubMed] [Google Scholar]
- 7. Koeberle P, Ball A. Neurturin enhances the survival of axotomized retinal ganglion cells in vivo: combine effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. Neuroscience. 2002; 110: 555– 567 [DOI] [PubMed] [Google Scholar]
- 8. Ikeda K, Tanihara H, Tatsuno T, Noguchi H, Nakayama C. Brain-derived neurotrophic factor shows a protective effect and improves recovery of the ERG b-wave response in light damage. J Neurochem. 2003; 87: 290– 296 [DOI] [PubMed] [Google Scholar]
- 9. Galindo-Romero C, Valiente-Soriano F, Jimenez-Lopez M, et al. Effect of brain-derived neurotrophic factor on mouse axotomized retinal ganglion cells and phagocytic microglia. Invest Ophthalmol Vis Sci. 2013; 54: 974– 985 [DOI] [PubMed] [Google Scholar]
- 10. Sanchez-Migallon M, Nadal-Nicolas F, Jimenez-Lopez M, Sobrado-Calvo P, Vidal-Sanz M, Agudo-Barriuso M. Brain derived neurotrophic factor maintains Brn3a expression in axotomized rat retinal ganglion cells. Exp Eye Res. 2011; 92: 260– 267 [DOI] [PubMed] [Google Scholar]
- 11. Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Müller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998; 95: 3978– 3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen H, Weber AJ. BDNF enhances retinal ganglion cell survival in cats with optic nerve damage. Invest Ophthalmol Vis Sci. 2001; 42: 966– 974 [PubMed] [Google Scholar]
- 13. Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002; 22: 3977– 3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weber A, Harman C. BDNF preserves the dendritic morphology of alpha and beta ganglion cells in the cat retina after optic nerve injury. Invest Ophthalmol Vis Sci. 2008; 49: 2456– 2463 [DOI] [PubMed] [Google Scholar]
- 15. Weber AJ, Viswanathan S, Ramanathan C, Harman CD. Combined application of BDNF to the eye and brain enhances ganglion cell survival and function in the cat after optic nerve injury. Invest Ophthalmol Vis Sci. 2010; 51: 327– 334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weibel D, Kreutzberg GW, Schwab ME. Brain-derived neurotrophic factor (BDNF) prevents lesion-induced axonal die-back in young rat optic nerve. Brain Res. 1995; 679: 249– 254 [DOI] [PubMed] [Google Scholar]
- 17. Harper M, Adamson L, Blits B, Bunge M, Grozdanic S, Sakaguchi D. Brain-derived neurotrophic factor released from engineered mesenchymal stem cells attenuates glutamate- and hydrogen peroxide-mediated death of staurosporine-differentiated RGC-5 cells. Exp Eye Res. 2009; 89: 538– 548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harper M, Grozdanic S, Blits B, et al. Transplantation of BDNF-secreting mesenchymal stem cells provides neuroprotection in chronically hypertensive rat eyes. Invest Ophthalmol Vis Sci. 2011; 52: 4506– 4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin KR, Quigley HA, Zack DJ, et al. Gene therapy with brain-derived neurotrophic factor as a protection: retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003; 44: 4357– 4365 [DOI] [PubMed] [Google Scholar]
- 20. Ko M, Hu D, Ritch R, Scharma S, Chen C. Patterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of rats. Neurosci Lett. 2001; 305: 139– 142 [DOI] [PubMed] [Google Scholar]
- 21. Ren R, Li Y, Liu Z, Liu K, He S. Long-term rescue of rat retinal ganglion cells and visual function by AAV-mediated BDNF expression after acute elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2012; 53: 1003– 1011 [DOI] [PubMed] [Google Scholar]
- 22. Cuenca N, Pinilla I, Fernández-Sánchez L, et al. Changes in the inner and outer retinal layers after acute increase of the intraocular pressure in adult albino Swiss mice. Exp Eye Res. 91: 273– 285 [DOI] [PubMed] [Google Scholar]
- 23. Harvey AR, Hu Y, Leaver SG, et al. Gene therapy and transplantation in CNS repair: the visual system. Prog Retin Eye Res. 2006; 25: 449– 489 [DOI] [PubMed] [Google Scholar]
- 24. Hellstrom M, Harvey AR. Retinal ganglion cell gene therapy and visual system repair. Curr Gene Ther. 2011; 11: 116– 131 [DOI] [PubMed] [Google Scholar]
- 25. Hellstrom M, Pollett MA, Harvey AR. Post-injury delivery of rAAV2-CNTF combined with short-term pharmacotherapy is neuroprotective and promotes extensive axonal regeneration after optic nerve trauma. J Neurotrauma. 2011; 28: 2475– 2483 [DOI] [PubMed] [Google Scholar]
- 26. Cui Q, Yip H, Zhao R, So KF, Harvey A. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci. 2003; 22: 49– 61 [DOI] [PubMed] [Google Scholar]
- 27. Pease M, Zack D, Berlinicke C, et al. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci. 2009; 50: 2194– 2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leaver S, Cui Q, Plant G, et al. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006; 13: 1328– 1341 [DOI] [PubMed] [Google Scholar]
- 29. Sasaki M, Radtke C, Tan A, et al. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J Neurosci. 2009; 29: 14932– 14941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levkovitch-Verbin H, Sadan O, Vander S, et al. Intravitreal injections of neurotrophic factors secreting mesenchymal stem cells are neuroprotective in rat eyes following optic nerve transection. Invest Ophthalmol Vis Sci. 2010; 51: 6394– 6400 [DOI] [PubMed] [Google Scholar]
- 31. Park H, Kim J, Kim H, Park C. Stem cell-based delivery of brain-derived neurotrophic factor gene in the rat retina. Brain Res. 2012; 1469: 10– 23 [DOI] [PubMed] [Google Scholar]
- 32. Johnson T, Bull ND, Hunt DP, Marina N, Tomarev S, Martin K. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010; 51: 2051– 2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson T, Bull N, Martin K. Stem cell therapy for glaucoma: possibilities and practicalities. Exp Rev Ophthalmol. 2011; 6: 165– 174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodger J, Drummond E, Hellstroem M, Robertson D, Harvey A. Long-term gene therapy causes transgene-specific changes in the morphology of regenerating retinal ganglion cells. PLoS One. 2012; 7: e31061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson TV, Bull ND, Martin KR. Neurotrophic factor delivery as a protective treatment for glaucoma. Exp Eye Res. 2011; 93: 196– 203 [DOI] [PubMed] [Google Scholar]
- 36. Purves D. The trophic theory of neural connections. Trends Neurosci. 1986; 9: 486– 489 [Google Scholar]
- 37. Tusa R, Palmer L, Rosenquist A. The retinotopic organization in area 17 (striate cortex) in the cat. J Comp Neurol. 1978; 177: 213– 235 [DOI] [PubMed] [Google Scholar]
- 38. Stone J. A quantitative analysis of the distribution of ganglion cells in the cat's retina. J Comp Neurol. 1965; 124: 337– 352 [DOI] [PubMed] [Google Scholar]
- 39. Stone J. The number and distribution of ganglion cells in the cat's retina. J Comp Neurol. 1978; 180: 753– 771 [DOI] [PubMed] [Google Scholar]
- 40. Wassle H, Chun M, Muller F. Amacrine cells in the ganglion cell layer of the cat retina. J Comp Neurol. 1987; 265: 391– 408 [DOI] [PubMed] [Google Scholar]
- 41. Wong RO, Hughes A. The morphology, number, and distribution of a large population of confirmed displaced amacrine cells in the adult cat retina. J Comp Neurol. 1987; 255: 159– 177 [DOI] [PubMed] [Google Scholar]
- 42. Berson DM, Pu M, Famiglietti EV. The zeta cell: a new ganglion cell type in cat retina. J Comp Neurol. 1998; 399: 269– 288 [DOI] [PubMed] [Google Scholar]
- 43. Berson DM, Isayama T, Pu M. The Eta ganglion cell type of cat retina. J Comp Neurol. 1999; 408: 204– 219 [PubMed] [Google Scholar]
- 44. Isayama T, Berson DM, Pu M. Theta ganglion cell type of cat retina. J Comp Neurol. 2000; 417: 32– 48 [DOI] [PubMed] [Google Scholar]
- 45. Stein JJ, Johnson SA, Berson DA. Distribution and coverage of beta cells in the cat retina. J Comp Neurol. 1996; 372: 597– 617 [DOI] [PubMed] [Google Scholar]
- 46. Boycott BB, Wassle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974; 240: 397– 419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watanabe M, Sawai H, Fukuda Y. Survival of axotomized retinal ganglion cells in adult mammals. Clin Neurosci. 1997; 4: 233– 239 [PubMed] [Google Scholar]
- 48. Watanabe M, Inukai N, Fukuda Y. Survival of retinal ganglion cells after transection of the optic nerve in adult cats: a quantitative study within two weeks. Vis Neurosci. 2001; 18: 137– 145 [DOI] [PubMed] [Google Scholar]
- 49. Watanabe M, Tokita Y, Kato M, Fukuda Y. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003; 116: 733– 742 [DOI] [PubMed] [Google Scholar]
- 50. Watanabe M, Fukuda Y. Survival and axonal regeneration of retinal ganglion cells in adult cats. Prog Ret Eye Res. 2002; 21: 529– 553 [DOI] [PubMed] [Google Scholar]
- 51. Copray S, Kernell D. Neurotrophins and trk-receptors in adult rat spinal motoneurons: differences related to cell size but not to ‘slow/fast' specialization. Neurosci Lett. 2000; 289: 217– 220 [DOI] [PubMed] [Google Scholar]
- 52. Frank L, Ventimiglia R, Anderson K, Lindsay R, Rudge J. BDNF down-regulates neurotrophin responsiveness, TrkB protein and TrkB mRNA levels in cultured rat hippocampal neurons. Eur J Neurosci. 1996; 8: 1220– 1230 [DOI] [PubMed] [Google Scholar]
- 53. Frank L, Wiegand S, Siuciak J, Lindsay R, Rudge J. Effects of BDNF infusion on the regulation of TrkB protein and message in adult rat brain. Exp Neurol. 1997; 145: 62– 70 [DOI] [PubMed] [Google Scholar]
- 54. Chen H, Weber A. Brain-derived neurotrophic factor reduces TrkB protein and mRNA in the normal retina and following optic nerve crush in adult rats. Brain Res. 2004; 1011: 99– 106 [DOI] [PubMed] [Google Scholar]
- 55. Sommerfeld MT, Schweigreiter R, Barde YA, Hoppe E. Down-regulation of the neurotrophin receptor TrkB following ligand binding. Evidence for an involvement of the proteasome and differential regulation of TrkA and TrkB. J Biol Chem. 2000; 275: 8982– 8990 [DOI] [PubMed] [Google Scholar]
- 56. Spalding KL, Cui Q, Harvey AR. Retinal ganglion cell neurotrophin receptor levels and trophic requirements following target ablation in the neonatal rat. Neuroscience. 2005; 131: 387– 395 [DOI] [PubMed] [Google Scholar]
- 57. DiStefano P, Boulton T, Stark J, et al. Ciliary neurotrophic factor induces down-regulation of its receptor and desensitization of signal transduction pathways in vivo: non-equivalence with pharmacological activity. J Biol Chem. 1996; 271: 22839– 22846 [DOI] [PubMed] [Google Scholar]
- 58. Miotke J, MacLennan A, Meyer R. Immunochemical localization of CNTFR-alpha in adult mouse retina and optic nerve following intraorbital nerve crush: evidence for the axonal loss of a trophic factor receptor after injury. J Comp Neurol. 2007; 500: 384– 400 [DOI] [PubMed] [Google Scholar]
- 59. Li Q, Miller R, Han P, et al. Intraocular route of AAV2 vector administration define humoral immune response and therapeutic potential. Mol Vis. 2008; 14: 1760– 1769 [PMC free article] [PubMed] [Google Scholar]
- 60. Millar J, Pang IH, Wang WH, Wang Y, Clark A. Effect of immunomodulation with anti-CD40L antibody on adenoviral-mediated transgene expression in mouse anterior segment. Mol Vis. 2008; 14: 10– 19 [PMC free article] [PubMed] [Google Scholar]
- 61. Vandenberghe L, Wilson J, Gao G. Tailoring the AAV vector capsid for gene therapy. Gene Ther. 2009; 16: 311– 219 [DOI] [PubMed] [Google Scholar]
- 62. Boyle S, Boyle S, Lewin A, Hauswirth W. A comprehensive review of retinal gene therapy. Mol Ther. 2013; 21: 509– 519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gabriel N, Hareendran S, Sen D, et al. Bioengineering of AAV2 capsid at specific serine, threonine, or lysine residues improves its transduction efficiency in vitro and in vivo. Hum Gene Ther Meth. 2013; 24: 80– 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aslanidi G, Rivers A, Ortiz L, et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: the final threshold? PLoS One. 2013; 8: e59142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kalil RE. Removal of visual cortex in the cat: effects on the morphological development of the retino-geniculo-cortical pathway. In: Stone J, Dreher B, Rapaport DH. eds Development of Visual Pathways in Mammals. New York, NY: Alan R; Liss; 1984: 257– 274 [Google Scholar]
- 66. Harvey AR, Robertson D. Time-course and extent of retinal ganglion cell death following ablation of the superior colliculus in neonatal rats. J Comp Neurol. 1992; 325: 83– 94 [DOI] [PubMed] [Google Scholar]
- 67. Spalding KL, Rush RA, Harvey AR. Target-derived and locally derived neurotrophins support retinal ganglion cell survival in the neonatal rat retina. J Neurobiol. 2004; 60: 319– 327 [DOI] [PubMed] [Google Scholar]
- 68. Cui Q, Harvey AR. At least two mechanisms are involved in the death of retinal ganglion cells following target ablation in neonatal rats. J Neurosci. 1995; 15: 8143– 8155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pearson HE, Labar DR, Payne BR, Cornwell P, Aggarwal N. Transneuronal retrograde degeneration in the cat retina following neonatal ablation of visual cortex. Brain Res. 1981; 212: 470– 475 [DOI] [PubMed] [Google Scholar]
- 70. Pearson HE, Stoffler DJ. Retinal ganglion cell degeneration following loss of postsynaptic target neurons in the dorsal lateral geniculate nucleus of the adult cat. Exp Neurol. 1992; 116: 163– 171 [DOI] [PubMed] [Google Scholar]
- 71. Holländer H, Sbisti S, Maffei L, Hebel R. Electroretinographic responses and retrograde changes of retinal morphology after intracranial optic nerve section. A quantitative analysis in the cat. Exp Brain Res. 1984; 55: 483– 493 [DOI] [PubMed] [Google Scholar]
- 72. Iwabe S, Moreno-Mendoz NA, Trigo-Tavera F, Crowder C, Garcia-Sanchex GA. Retrograde axonal transport obstruction of brain-derived neurotrophic factor (BDNF) and its TrkB receptor in the retina and optic nerve of American cocker spaniels dogs with spontaneous glaucoma. Vet Ophthalmol. 2007; 10: 12– 19 [DOI] [PubMed] [Google Scholar]
- 73. Pease ME, McKinnon SJ, Quigley HA, Kerrigan-Baumrind LA, Zack DJ. Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000; 41: 764– 774 [PubMed] [Google Scholar]
- 74. Weber AJ, Harman CD. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Invest Ophthalmol Vis Sci. 2005; 46: 3197– 3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Smith EI, Chino Y, Harwerth R, Ridder WI, Crawford M, DeSantis L. Retinal inputs to the monkey lateral geniculate nucleus in experimental glaucoma. Clin Vis Sci. 1993; 8: 113– 139 [Google Scholar]
- 76. Quigley HA, McKinnon SJ, Zack DJ, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci. 2000; 41: 3460– 3466 [PubMed] [Google Scholar]
- 77. Gilbert CD, Kelly JP. The projection of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975; 163: 81– 106 [DOI] [PubMed] [Google Scholar]
- 78. Kawamura S, Sprague JM, Niimi K. Corticofugal projections from the visual cortices to the thalamus, pretectum, and superior colliculus in the cat. J Comp Neurol. 1974; 158: 339– 362 [DOI] [PubMed] [Google Scholar]
- 79. Updyke BV. Topographic organization of the projection from cortical area 17, 18, and 19 onto the thalamus, pretectum and superior colliculus in the cat. J Comp Neurol. 1977; 173: 81– 122 [DOI] [PubMed] [Google Scholar]
- 80. Weber AJ, Kalil RE. Development of corticogeniculate synapses in the cat. J Comp Neurol. 1987; 264: 171– 192 [DOI] [PubMed] [Google Scholar]
- 81. Kafitz KW, Rose CR, Thoenen H, Konnerth A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature. 1999; 401: 918– 921 [DOI] [PubMed] [Google Scholar]
- 82. Altar C, Cai N, Bliven T, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997; 389: 856– 860 [DOI] [PubMed] [Google Scholar]
- 83. Fawcett JP, Bamji SX, Causing CG, et al. Functional evidence that BDNF is an anterograde neuronal trophic factor in the CNS. J Neurosci. 1998; 18: 2808– 2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Caleo M, Menna E, Chierzi S, Cenni M, Maffei L. Brain-derived neurotrophic factor is an anterograde survival factor in the rat visual system. Curr Biol. 2000; 10: 1155– 1161 [DOI] [PubMed] [Google Scholar]
- 85. Caleo M, Medini P, von Bartheld C, Maffei L. Provision of brain-derived neurotrophic factor via anterograde transport from the eye preserves the physiological responses of axotomized geniculate neurons. J Neurosci. 2003; 23: 287– 296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. DiStefano P, Friedman B, Radziejewski C, et al. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992; 8: 983– 993 [DOI] [PubMed] [Google Scholar]
- 87. Klöcker N, Kermer P, Weishaupt JH, Labes M, Ankerhold R, Bahr M. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3′-kinase/protein kinase B signaling. J Neurosci. 2000; 20: 6962– 6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nakazawa T, Tamai M, Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest Ophthalmol Vis Sci. 2002; 43: 3319– 3326 [PubMed] [Google Scholar]
- 89. Merabet L, Theoret H, Pascual-Leone A. Transcranial magnetic stimulation as an investigative tool in the study of visual function. Optom Vis Sci. 2003; 80: 356– 368 [DOI] [PubMed] [Google Scholar]
- 90. Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000; 406: 147– 150 [DOI] [PubMed] [Google Scholar]
- 91. Salminen-Vaparanta N, Noreika V, Revonsuo A, Koivisto M, Vanni S. Is selective primary visual cortex stimulation achievable with TMS? Hum Brain Mapp. 2012; 33: 652– 665 [DOI] [PMC free article] [PubMed] [Google Scholar]