Summary
Sphingolipids (SLs) play essential roles in most eukaryotes, but in the trypanosomatid protozoan Leishmania major their functions differ significantly. Previously we showed that null mutants defective in de novo sphingoid base synthesis (spt2−) lacked SLs but grew well and retained lipid rafts while replicating as promastigotes in vitro. However, they experienced catastrophic defects in membrane trafficking on entry into stationary phase, and failed to differentiate to the infective metacyclic form. Here we showed this mutant retained the ability to enter macrophages silently and inhibit activation, although as expected most parasites were destroyed. However, in mouse infections, after a delay rapidly progressive lesions appeared, and purified amastigotes were fully virulent to macrophages and mice. Mass spectrometry of spt2− amastigote lipids revealed the presence of high levels of parasite-specific inositol phosphorylceramides (IPCs) not synthesized by the mammalian hosts. Inhibitor studies showed that salvage occurs at the level of complex SLs, suggesting that parasites carry out ‘headgroup’ remodelling. Additionally, we describe a new defect of the spt2− promastigotes involving ‘empty’ acidocalcisomes (ACs), which may point to the origin of this organelle from the lysosome-related organelle/multivesicular body biogenesis pathway. However, ACs in spt2− amastigotes appeared quantitatively and morphologically normal. Thus salvage of SLs and other molecules by intracellular amastigotes play key roles in AC biogenesis and parasite survival in the host.
Introduction
The protozoan parasite Leishmania alternates between two distinct developmental stages and hosts: promastigotes that replicate in the midgut of sand flies, and amastigotes that reside within the phagolysosomes of vertebrate macrophages. Leishmania promastigotes are covered with a variety of interrelated glycosylphosphatidyl-inositol (GPI)-anchored molecules, including lipophosphoglycan (LPG), glycosylinositolphospholipids (GIPLs), proteophosphoglycan (PPG) and GPI-anchored proteins, all of which have been implicated in various steps important to the infectious cycle (Ilgoutz and McConville, 2001). In Leishmania major, previous work showed that LPG plays important roles in survival and pathogenesis in both the sand fly host and mammalian host (Spath et al., 2000; Sacks and Kamhawi, 2001; Spath et al., 2003). Studies on GIPLs and GPI-anchored proteins also suggest these molecules may be important determinants for L. major viability and virulence at key steps in the infectious cycle (Joshi et al., 2002; Zufferey et al., 2003).
In contrast, the role of sphingolipids (SLs), another abundant membrane component of trypanosomatid parasites, has not been as extensively studied. Like fungi, Leishmania synthesize inositol phosphorylceramide (IPC) instead of sphingomyelin or glycosphingolipids. IPCs account for 5–10% of total cellular lipids in Leishmania (Kaneshiro et al., 1986) and are enriched in raft-associated membrane fractions (Denny et al., 2001; Ralton et al., 2002). SLs are ubiquitous membrane components in most eukaryotic cells, and play important roles in cell signalling, vesicular trafficking and the formation of membrane rafts (Merrill Jr et al., 1993; Kolter et al., 2002; van Meer and Lisman, 2002). Moreover SL synthetic precursors and/or metabolites have been implicated in cellular signalling, apoptosis and stress responses (Spiegel and Milstien, 2002).
Recently, the functions of SLs were probed in L. major with an SL-free mutant (spt2−) created by deleting an essential subunit gene of serine palmitoyltransferase (SPT), the first enzyme in the de novo SL biosynthesis pathway responsible for sphingoid base (SB) synthesis (Zhang et al., 2003; Denny et al., 2004). Despite a complete loss of IPCs and other SLs, spt2− parasites were fully viable as log phase promastigotes, showing only modest effects in growth rate, cell shape and vesicular trafficking. Most surprising was the observation that these parasites maintained the presence of membrane microdomains commonly referred to as ‘lipid rafts’. Despite their relatively normal growth during log phase, SL-null mutants (spt2−) failed to differentiate to the infective metacyclic form on entry into stationary phase and instead died. Importantly, death did not occur through an apoptotic-like process, or changes in signal transduction affecting the expression of developmentally regulated genes, but from catastrophic defects in membrane trafficking accompanied by accumulation of multivesicular body (MVB)-like vesicles (Zhang et al., 2003). The metacyclic-stage specificity of these defects may reflect increased demands on trafficking pathways at this developmental transition, involving changes in membrane organization, as well as events associated with remodelling of the parasite shape and size (Mullin et al., 2001; Waller and McConville, 2002; Zhang et al., 2003; Denny and Smith, 2004; Denny et al., 2004).
Here we demonstrate that as expected, the viability and metacyclogenesis defects of SL-deficient spt2− parasites rendered them poorly infective to macrophages. Nonetheless, following mouse infection, after some delay spt2− parasites gave rise to lesions that progressed rapidly, and purified spt2− amastigotes showed normal virulence. Because SPT2 is downregulated in metacyclic and amastigote stages (Zhang et al., 2003; Denny et al., 2004), this raised the possibility that SLs were not required at all for the survival of amastigotes. However, we show that both wild type (WT) and spt2− amastigotes contain parasite-specific IPCs which arise by remodelling of SLs salvaged from the host, occurring at levels nearly 10-fold higher than the abundant amastigote GIPLs. We present evidence which argues that salvage is likely to be important for amastigote survival, based on the in vitro finding that the biogenesis of a key parasite organelle, acidocalcisomes (ACs) requires de novo SL synthesis in the promastigote stage.
Results
Sphingolipid-free L. major promastigotes are poorly infective but retain the ability to inhibit macrophage activation
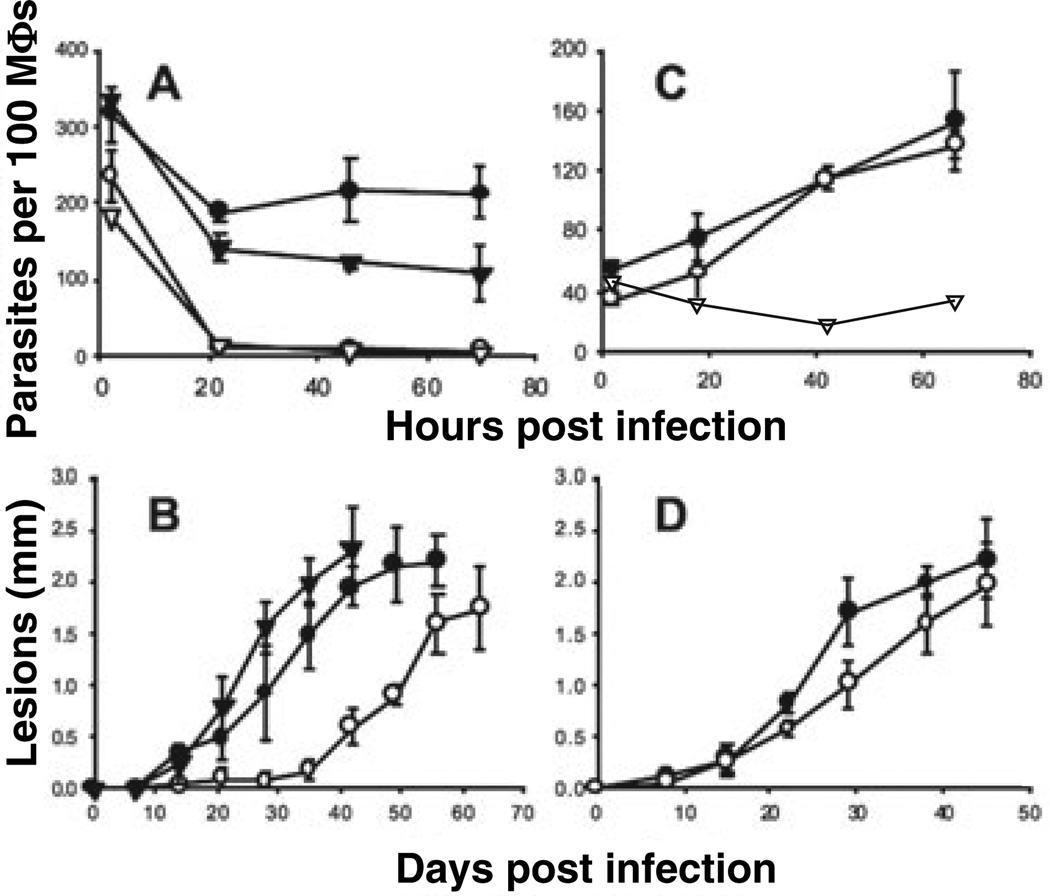
Promastigotes of spt2−L. major were shown to be unable to differentiate to viable infective metacyclic forms in vitro (Zhang et al., 2003). Correspondingly, stationary phase promastigotes of spt2− were unable to establish infections in cultured murine peritoneal macrophages (PEMs; Fig. 1A). Most spt2− parasites were rapidly killed by PEMs within 24 h after infection, whereas WT and the ‘add-back’ control spt2−/+SPT2 survived well (Fig. 1A). This arose specifically from the SL deficiency, as spt2− parasites grown with daily supplementation of SBs completely regained metacyclogenesis (Zhang et al., 2003) and infectivity (data not shown).
Fig. 1.
Virulence of spt2− stationary promastigotes and amastigotes in macrophage and mouse infections. In A and C, PEMs were infected at ratios of 10 promastigotes (A) or one amastigote (C) per macroph-age respectively. Cell survival was monitored daily by counting the number of intracellular parasites per one hundred macrophages. As a control, WT parasites were used to infect activated macrophages (stimulated with 100 ng ml−1 of LPS and 100 ng ml−1 of IFN-γ, open triangles in A and C). In B and D, BALB/c mice were infected with 106 promastigotes per mouse (B) and 2 × 104 amastigotes per mouse (D). Lesion sizes were monitored weekly. WT: filled circles, spt2−: open circles, spt2−/+SPT2: filled triangles. Error bars represent standard deviations.
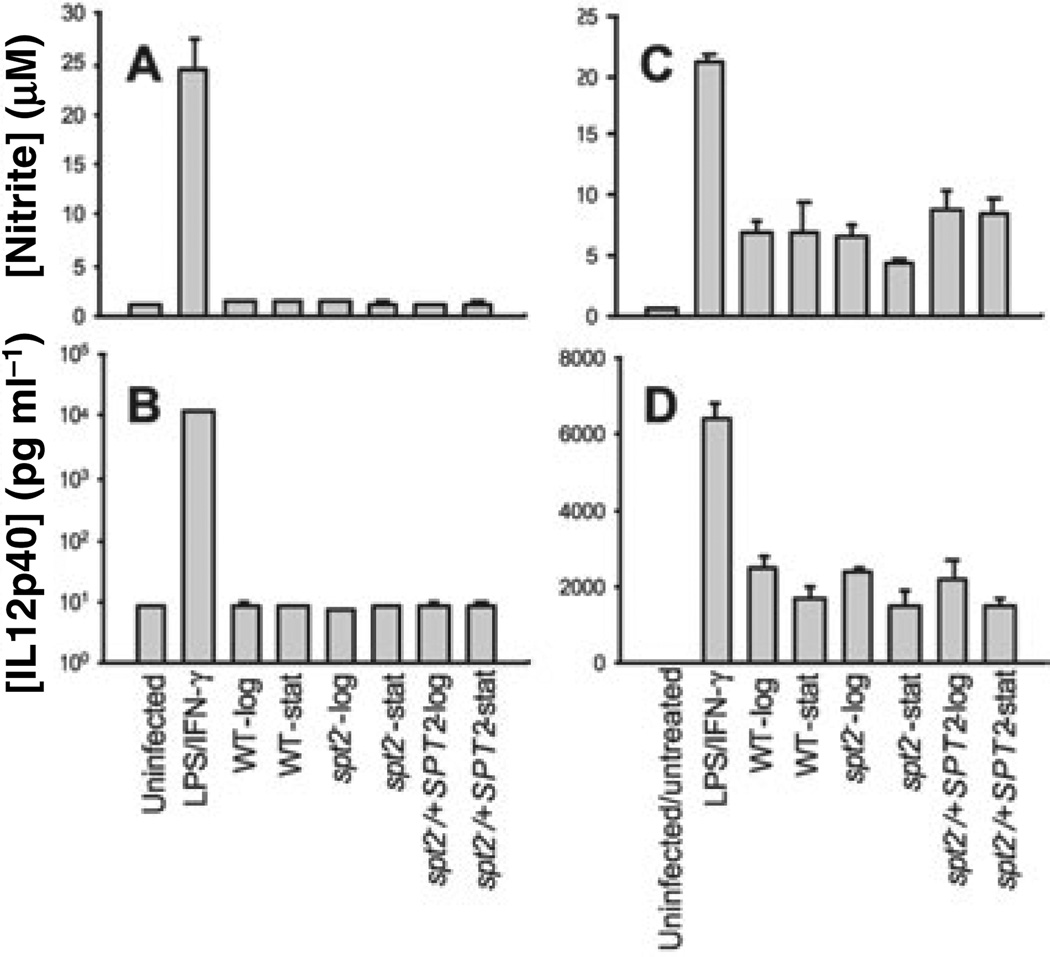
Studies by many groups have shown that WT Leishmania invasion of PEMs is relatively silent, in terms of its induction of host cell signalling pathways, and that following establishment of infection, parasites further inhibit host cell activation (Sacks and Noben-Trauth, 2002). Given the inability of spt2− promastigotes to survive in macrophages, we asked whether these parasites were altered in these properties by following NO and IL-12 production, two markers of macrophage activation especially relevant to Leishmania survival.
First, both WT and spt2− promastigotes, regardless of growth stage (log or stationary), entered macrophages efficiently without inducing the production of NO or IL12 (Fig. 2A and B). Second, after entering host cells, both WT and spt2− parasites inhibited macrophage NO or IL12 production following LPS and interferon-γ treatment(Fig. 2C and D). In both assays, uninfected macrophages produced high levels of nitrite and IL12 on activation (Fig. 2A – D). This suggested that the defect in spt2− survival and virulence did not affect the ability to inhibit macrophage activation.
Fig. 2.
spt2− parasites are able to enter macrophages silently and suppress activation following entry. Log or stationary phase promas-tigotes of WT, spt2−, or spt2−/+SPT2 were used to infect PEMs at a ratio of 20 parasites per macrophage as described in the Experimental procedures. After infection, macrophages were transferred into medium alone (A and B) or medium containing 50 ng ml−1 LPS and 50 ng ml−1 IFN-γ (C and D). After overnight incubation, concentrations of nitrite and IL12 (p40) in the supernatant were determined. In A–D, uninfected macrophages were used as negative control, while uninfected macrophages treated with 50 ng ml−1 LPS and 50 ng ml−1 IFN-γ were used as positive controls. Error bars represent standard deviations.
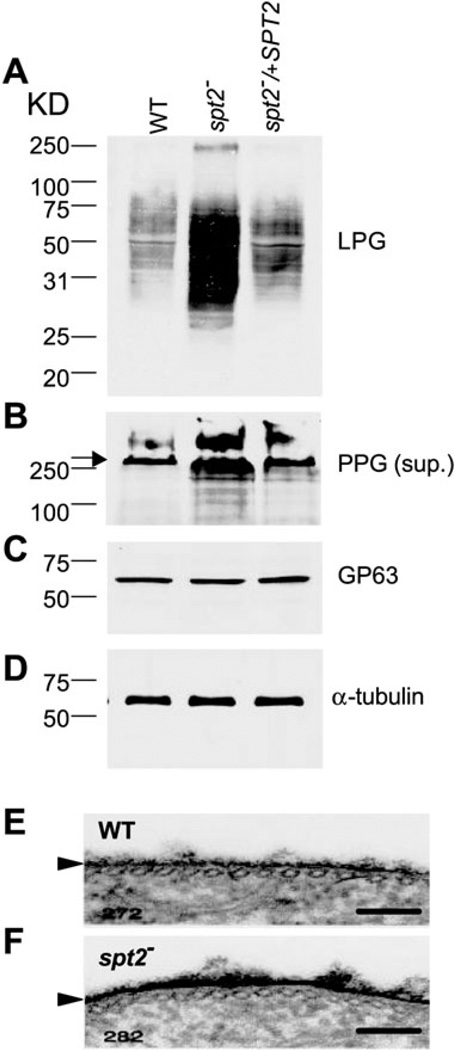
We examined spt2− promastigotes for the synthesis of key glycoconjugates thought to play a role in other aspects of parasite virulence, such as LPG, PPG and the abundant surface protease GP63. Log phase spt2− promastigotes showed elevated levels of LPG and PPG, but WT levels of GP63 (Fig. 3A – C). Correspondingly, the LPG surface coat appeared to be thickened in the spt2− parasite (Fig. 3E and F). Consistent with roles postulated for LPG in parasite virulence, the log phase spt2− parasites showed elevated survival in macrophage infections, and increased virulence in mouse infections (Fig. S1, see Supplementary material). However, in the mouse infection assay, the virulence of both WT and spt2− log phase parasites was far less than WT stationary phase parasites, as expected because they had not undergone metacyclogenesis (Fig. S1). Thus, the promastigote virulence defect of spt2− did not involve loss of other well characterized virulence markers.
Fig. 3.
Increased expression of LPG and PPG in spt2− parasites. A–D. Log phase cell extracts or culture supernatants of WT, spt2−, or spt2−/+SPT2 were resolved by SDS-PAGE and probed with mAb WIC79.3 (1:1000, to detect LPG and PPG, A and B, the arrow in B indicated the interface between stacking gel and separating gel), anti-gp63 mAb235 (1:1000, to detect gp63, C), or anti-α-tubulin Ab (1:10000, to ensure equal loading, D). In A, C and D, each lane contained material from 1 × 106 parasites. In (B), each lane contained 15 ml of log phase culture supernatant (parasite conditioned medium). E and F. Log phase parasites of WT and spt2− were fixed in the presence of 0.15% ruthenium red and subjected to transmission electron microscopy analysis as described in Experimental procedures. Arrowheads marked the cell surface glycocalyx in WT and spt2−. Bars = 100 nm.
Sphingolipid-free L. major can generate amastigotes that are fully infective
In keeping with the macrophage findings, spt2− parasites were compromised in their ability to induce progressive lesion pathology in highly susceptible BALB/c mice (Fig. 1B). However, after an initial delay of about 4 weeks relative to WT or the add-back control, the spt2− parasites gave rise to lesions that progressed thereafter at a rate similar to that of controls. Lesion sizes correlated with parasite burden, as determined by limiting dilution assays (data not shown).
This ‘delayed’ lesion phenotype of spt2− resembled that reported previously for the LPG-deficient lpg1− line of L. major; there the delay was shown to be a result of defects in the ability of the parasite to ‘establish’ macrophage infections but not to its ability to replicate as amastigotes, which normally lack LPG (Spath et al., 2000; Spath et al., 2003). To test this whether a similar explanation was applicable here, spt2− and WT amastigotes were recovered from 1 to 2 mm lesions such as shown in Fig. 1B, and used immediately. In contrast to spt2− promastigotes, spt2− amastigotes survived and replicated as well as WT amastigotes in PEMs (Fig. 1C) and gave rise to lesions at the same time and progressed as rapidly as WT in BALB/ c mice (Fig. 1D). Controls where the spt2− amastigotes were allowed to differentiate to promastigotes and then tested in PEMs or mice gave results similar to those shown in Fig. 1A and B, ruling out the possibility of compensatory alterations (data not shown). Thus SPT-medi-ated synthesis of SBs is not required for amastigote survival. In combination with the glycoconjugate studies above, this suggests that the ‘delayed lesion’ phenotype arises solely from the metacyclogenesis/viability defect in the spt2− parasite.
spt2−amastigotes contain parasite-specific IPCs
The survival of spt2− amastigotes in mouse lesion macrophages could reflect (i) the existence of an amastigote-specific SPT activity, (ii) an ability to salvage SLs from the mammalian host and/or (iii) that SBs and/or SLs are not required at all for amastigote viability and virulence. Current evidence argues against significant amastigote SPT activity, as SPT2 protein levels decline to undetectable levels in amastigotes (Zhang et al., 2003; Denny et al., 2004), and neither purified WT nor spt2− amastigotes incorporated serine into ceramide or IPC (data not shown).
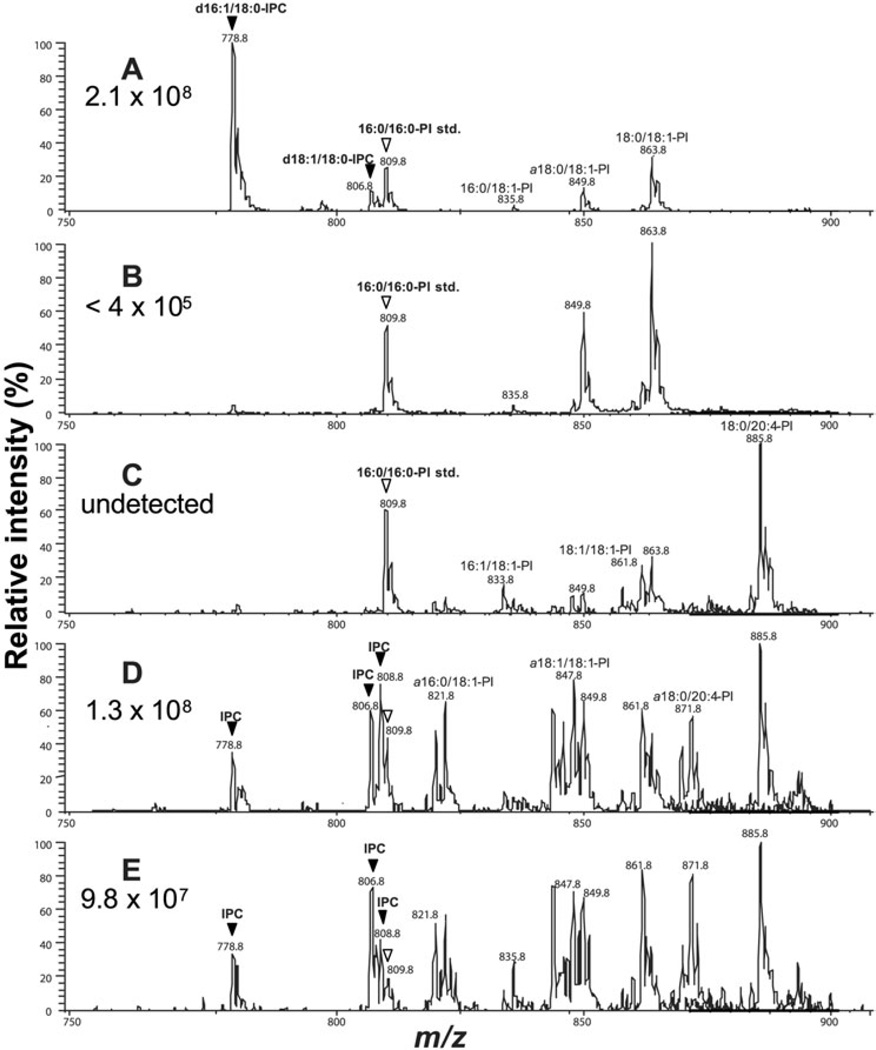
We then compared SLs present in WT or spt2− promastigotes and lesion amastigotes. To specifically detect IPC and PI molecular species, negative ion electrospray ionization/tandem mass spectrometry (ESI/MS/MS) scanning was performed to identify parents of the ion of m/z 241, which represents a characteristic fragment from the phosphoinositol moiety (Hsu and Turk, 2000). As expected from previous work (Zhang et al., 2003), L. major WT promas-tigotes synthesized high levels of IPCs, represented by ions at m/z 778.8 and 806.8 corresponding to d16:1/18:0-IPC (phosphoryl inositol N-stearoylhexadecasphing-4-enine) and d18:1/18:0-IPC (phosphoryl inositol N-stearoyl-sphingosine), whereas spt2− promastigotes lack these SLs (Fig. 4A and B). Lipids from uninfected mouse tissue showed the presence of several ionic species corresponding to known mammalian PI lipids such as 18:1/18:1-PI (1,2-dioleoyl-sn-glycero-3-phosphoinositol, represented by [M-H]− at m/z 861.8) and 18:0/20:4-PI (1-stearoyl-2-arachidonoyl-3-phosphinositol, represented by [M-H]− at m/z 885.8) but lacked IPCs as expected (Fig. 4C). In contrast, both WT and spt2− amastigote lipid preparations displayed several ions corresponding to Leishmania-specific IPCs at m/z 778.8, 806.8 and 808.8 (Fig. 4D and E), which were absent from uninfected mouse tissue (Fig. 4C). Lipids from both WT and spt2− amastigotes also contained elevated amounts of 1-alkyl-2-acyl-PIs (including but not limited to a16:0/18:1-PI at m/z 821.6, a18:1/ 18:1-PI at m/z 847.8, and a18:0/20:4-PI at m/z 871.8 in Fig. 3D and E). Because both Leishmania and mammals are known to make these lipids, and lesions contain a different distribution of host inflammatory cells than uninfected tissues, we cannot be sure of the extent to which these lipids are of host or parasite origin at present.
Fig. 4.
Amastigotes contain abundant parasite-specific IPCs. Lipids from log phase WT (A) or spt2− (B) promastigotes, from foot pads of uninfected mice (C), or amastigotes purified from mice infected with WT (D) or spt2− (E) parasites, were extracted and analysed by negative-ion ESI/MS using precursor-ion scan of m/z 241 (specific for IPCs and PIs). Before lipid extraction, a PI standard (16:0/16:0-PI at m/z 809.8) was added as an internal standard to each sample, and abundance of IPCs was estimated as described in the text; these are shown in each panel. IPCs were indicated by filled arrowheads and the PI standards were indicated by open arrowheads.
Abbreviations: d16:1/18:0-IPC, phosphoryl inositol N-stearoylhexadecasphing-4-enine; d18:1/18:0-IPC: phosphoryl inositol N-stearoylsphingosine; a16:0/18:1-PI: 1-O-hexadecyl-2-oleoyl-sn-glycero-3-phosphoinositol; 16:1/18:1-PI: 1-palmitoleoyl-2-oleoyl-sn-glycero-3-phosphoinositol; 16:0/ 18:1-PI: 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoinositol; a18:0/18:1-PI: 1-O-octadecanyl-2-octadecenoyl- sn-glycero-3-phosphoinositol (plas-manyl phosphoinositol); a18:1/18:1-PI: 1-O-octadeceyl-2-oleoyl-sn-glycero-3-phosphoinositol; 18:1/18:1-PI: 1,2-dioleoyl-sn-glycero-3-phosphoi-nositol; a18:0/20:4-PI: 1-O-octadecyl-2-arachidonoyl-3-phosphoinositol; 18:0/20:4-PI: 1-stearoyl-2-arachidonoyl-3-phosphinositol; 16:0/16:0-PI std: internal PI standard (1,2-dipalmitoyl-sn-glycero-3-phosphoinositol).
We estimated the relative abundance of PI molecular species by comparing the intensity of the ion current for a 1,2-dipalmitoylphosphoinositol internal standard (16:0/16:0-PI, [M-H]− at m/z 809.8) to the intensities of the ion currents for various IPC and PI species in negative ion ESI/MS analyses. Controls showed a linear relationship between the amount of internal standard added and its ion abundance, relative to the abundances of ions representing endogenous lipid species (Fig. S2, see Supplementary material). IPC levels were estimated by comparing their corresponding ion peaks to that of the PI standard (data not shown) and normalized to the number of cells analysed. WT Leishmania (both promastigotes and amastigotes) contained high levels of IPC (∼2.1 × 108 cell−1 and 1.3 × 108 cell−1, respectively, Fig. 4A and D), values similar to those calculated from literature estimates (Wassef et al., 1985; Kaneshiro et al., 1986). While spt2− promastigotes lacked IPCs (Fig. 4B), spt2− amastigotes contained IPCs at levels similar to WT (0.98 × 108 cell−1; Fig. 4E).
Because neither WT nor spt2− amastigotes express a de novo SB synthetic pathway, these data imply that amastigote IPCs must arise through salvage of host SLs. We tested whether this was through salvage of de novo synthesized host SB or ceramide, respectively, in L. major infections of macrophages treated with myriocin, a specific inhibitor of SPT, or fumonisin B1 (FB1), a specific inhibitor of dihydroceramide synthase (Wang et al., 1991; Mandala and Harris, 2000). In these experiments, macrophages were cultured in the presence of 10 µM myriocin or 20 µM of FB1 before, during and after infection. At these concentrations, myriocin and FB1 greatly reduce the de novo SL biosynthesis in mammalian cells, although they will not affect ceramide generation through catabolism of complex SLs (Yoo et al., 1992; Hanada et al., 2000). Neither of these compounds showed activity against Leishmania promastigote survival (at 10 and 20 µM, respectively, data not shown), nor did they show an ability to inhibit parasite replication in amastigotes (Table 1). Interestingly, FB1 showed inhibition of parasite uptake, which we attribute to non-specific effects of SL inhibition on phagocytosis arising from perturbation of host cell lipid domains (Dermine et al., 2001), although this was not explicitly confirmed. Tests using the fungal IPC synthase inhibitor aureobasidin A were inconclusive, and preliminary studies suggested that growth inhibition by this compound was not simply related to inhibition of IPC synthesis in promastigotes. Similar results have been reported for Trypanosoma cruzi, where the effect of this compound on different classes of glycoconjugates appears to be complex (Salto et al., 2003; Figueiredo et al., 2005). In total, the available data argue against salvage dependent on either de novo host SB or ceramide synthesis.
Table 1.
Effect of sphingolipid biosynthetic inhibitors on WT and spt2−Leishmania major amastigote infections of macrophages.
| WT |
spt2− |
||||
|---|---|---|---|---|---|
| Drug | Concentration | Parasites per 100 MΦ at 0 h | Fold increase in 72 h | Parasites per 100 MΦ at 0 h | Fold increase in 72 h |
| Myriocin | 0 | 246 ± 10 | 2.8 | 80 ± 8.7 | 3.5 |
| 10 µm | 283 ± 42 | 2.7 | 108 ± 18 | 3.3 | |
| Fumonisin B1 | 0 | 131 ± 17 | 3.5 | 88.9 ± 6.4 | 4.1 |
| 20 µM | 47 ± 1.5 | 7.0 | 34.7 ± 3.0 | 4.2 | |
De novo SB biosynthesis is required for the formation of normal acidocalcisomes in promastigotes
Further analysis of the spt2− mutant revealed an additional defect involving ACs, which are acidic organelles rich in pyrophosphate and polyphosphate with bound calcium and other cations (Docampo and Moreno, 2001). ACs were visualized by their characteristic morphology in transmission electron microscopy (EM), and reactivity with antisera to an AC membrane proton-pumping pyrophos-phatase (Fig. 5).
Fig. 5.
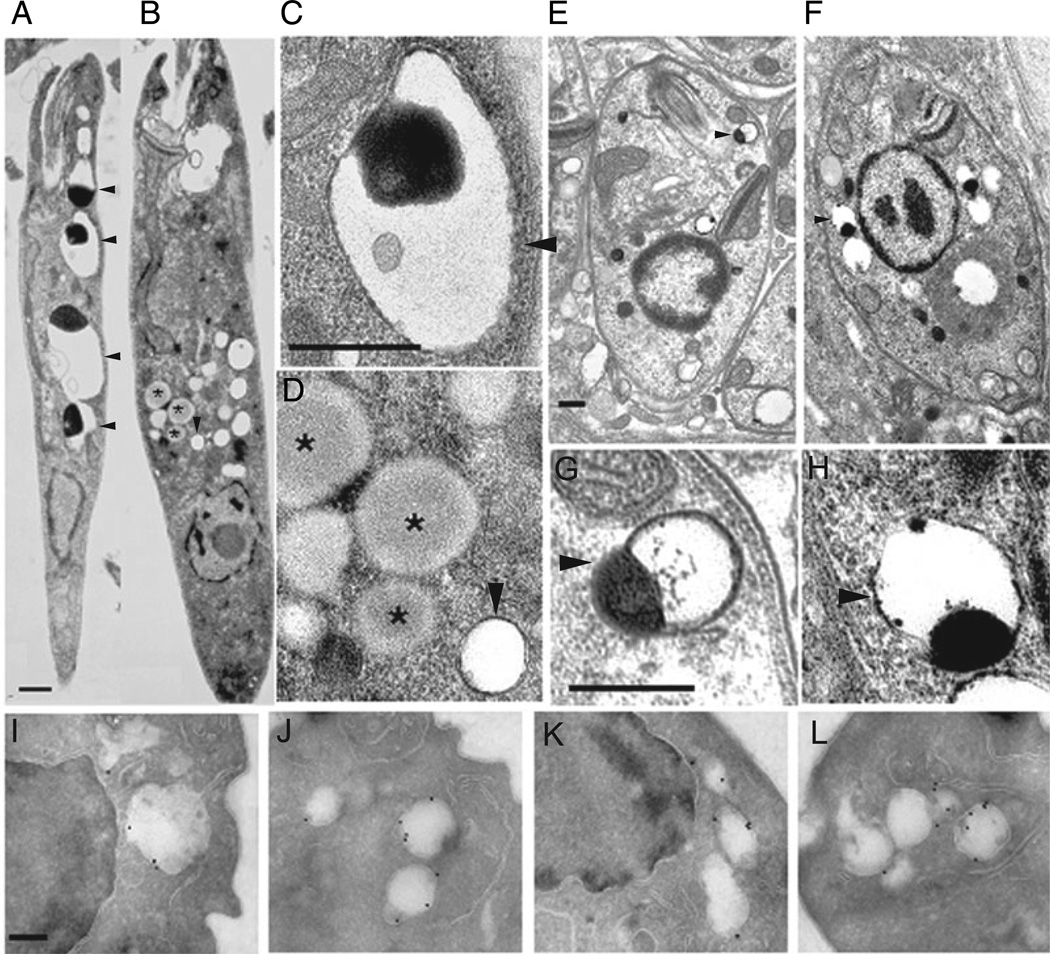
EM and immuno-EM analysis of ACs in WT and spt2−Leishmania. Stationary phase promastigotes of WT (A: WT, close-up in C) and spt2− (B, close up in D), showing normal ACs in WT and ‘empty’ ACs in spt2− promastigotes. Arrowheads indicate ACs (A–F) and asterisks indicate lipid inclusions accumulated in spt2− promastigotes (D). Amastigotes of WT (E, close-up in G) and spt2− (F, close-up in H) contain normal AC s. (I–L) Immuno-EM micrographs of promastigotes labelled with mouse anti-T. cruzi Ppase followed by anti-mouse antibody conjugated with colloidal gold (18 nm). (I) WT in log phase; (J) spt2− in log phase; (K) WT in stationary phase; (L) spt2− in stationary phase. Scale bars: 0.5 µm in A–D; 0.2 µm in E–L.
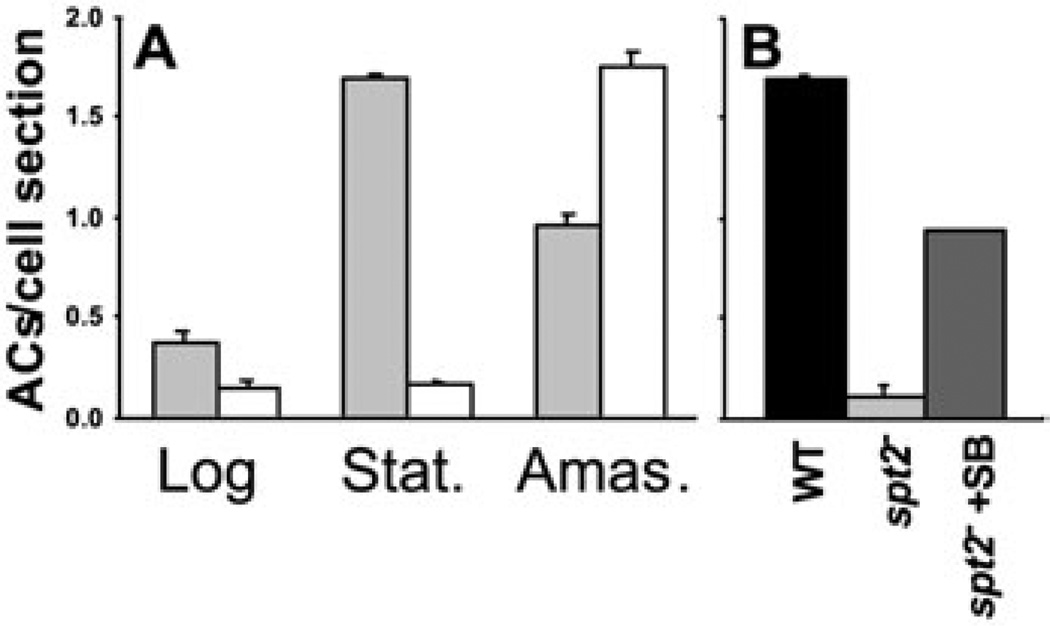
In WT parasites, AC abundance was low in log phase cells but rose greatly in stationary phase (Fig. 6). In contrast, normal ACs were uncommon in both log and stationary phase spt2− promastigotes (Figs 5B and D and 6). Stationary phase spt2− parasites instead contained a variety of vacuolated structures often bearing smaller vesicles, as previously described (Zhang et al., 2003), elevated levels of lipid bodies (visualized as moderately electron dense bodies lacking a detectable membrane bilayer; Fig. 5B and D) and many membrane-delimited vesicles that appeared to be ‘empty’ ACs (Fig. 5B and D). While AC morphology can be tricky to preserve in EM preparations, our findings were consistent in several preparations, and were confirmed by quantitative analysis of the number of apparently ‘normal’ ACs/cell section (Fig. 6A). Evidence that the seemingly ‘empty’ vesicles were indeed related to ACs was obtained by immunofluorescence and immuno-EM microscopy with an antiserum against the T. cruzi AC vacuolar membrane pyrophos-phatase (Luo et al., 2001). By immuno-EM, both WT and spt2− cells showed specific labelling of AC membranes (Fig. 5I – L). Similar results were obtained in immunofluorescence assay using the same antiserum (data not shown).
Fig. 6.
Stationary phase promastigotes but not amastigotes of spt2− contain fewer ACs than WT.
A. The average number of ‘normal’ ACs (as defined by results shown in Fig. 5) per cell section was determined by counting 100–130 EM images (as shown in Fig. 5) for promastigotes and 30–40 images for amastigotes. Grey bars, WT; open bars, spt2−.
B. Promastigotes of WT, spt2− and spt2− grown in the presence of 2 µM of 3-ketodihydrosphingosine (spt2−+SB, added daily) were cultured to late stationary phase, and the average number of ACs per cell section was determined by EM as described in (A).
Error bars represent standard deviations.
We measured total cellular levels of components typically associated with ACs in stationary phase parasites, such as calcium, zinc, short and long-chain polyphos-phates (less than 50 and 700–800 residues respectively). The most striking result was that long chain poly P levels in spt2− declined to only 2% of WT, while cellular Ca2+ and short chain poly P levels were unchanged and Zn2+ levels rose about twofold (Table 2). In combination with the EM studies above, these data suggest that the lack of long chain poly P in spt2− promastigotes leads to the absence of electron-dense matrix in their ACs, which then appeared ‘empty’.
Table 2.
Analysis of cellular materials associated with acidocalcisomes.
| Ca2+ (µg per mg protein) |
Zn2+ (µg per mg protein) |
Short chain PP (nmol per mg protein) |
Long chain PP (nmol per mg protein) |
|
|---|---|---|---|---|
| WT | 0.38 ± 0.04 | 0.39 ± 0.12 | 8.67 ± 0.74 | 25.8 ± 3 |
| Spt2− | 0.27 ± 0.10 | 0.83 ± 0.12 | 8.57 ± 2.39 | 0.58 ± 0.17 |
| Spt2−/+SPT2 | 0.34 ± 0.10 | 0.37 ± 0.09 | 6.67 ± 1.49 | 27.3 ± 6 |
Assays were performed using whole cell extracts from stationary phase promastigotes. Results shown are the average of three experiments ± standard deviation.
PP, polyphosphate.
The dependency of AC formation on SB synthesis was confirmed by growing parasites into stationary phase in the presence of exogenous SB (3-ketodihydrosphin-gosine, 2 µM provided daily), which we showed previously was able to rescue the viability and metacyclogenesis spt2− defects. This treatment was able to restore AC levels to about 60% of WT levels (Fig. 6B).
We then performed transmission EM on sections from lesions induced following infection by WT or spt2− parasites. In contrast to the results with spt2− promastigotes, lesion-derived spt2− amastigotes were morphologically normal, and specifically showed normal ACs at similar levels as WT amastigotes (Figs 5E – H and 6A). Because de novo SB synthesis is required for normal AC biogenesis in promastigotes, these data suggest that amastigote SLs may also be required for the assembly of functional ACs.
Discussion
Previous work showed that Leishmania lacking detectable SLs through genetic ablation of the de novo SB synthetic pathway were relatively normal in log phase, but showed a number of defects on entry into stationary phase, including failure to differentiate and loss of viability. Hence one would not expect such parasites to be very infective, and indeed this was seen in both macroph-age and mouse infections (Fig. 1; Zhang et al., 2003; Denny et al., 2004). Notably, stationary phase spt2− maintained the ability to inhibit macrophage pathways leading to the production of IL12 or NO (Fig. 2). These data rule out an exclusive role for parasite-derived SLs in macrophage deactivation by promastigotes, although they could function redundantly with other GPI-anchored parasite molecules such as LPG and the smaller GIPLs (McNeely et al., 1989; Proudfoot et al., 1995; Chawla and Vishwakarma, 2003).
Remarkably, following mouse infections, spt2− parasites were able to produce lesions that appeared after a short delay and progressed normally thereafter (Fig. 1B). Purified spt2− amastigotes behaved normally in both macrophage and mouse infections, arguing that de novo SB synthesis was not required for parasite virulence. Thus SBs are required for generating the infective metacyclic stage required for establishment of macrophage infections, but not thereafter as amastigotes. Similar results were presented recently for an independent spt2− null mutant generated in a different strain of L. major (Denny et al., 2004).
This raised the possibility that, like log phase promastigotes, amastigotes have no need for SBs or SLs. However, this conclusion is compromised by the fact that amastigotes from all species of Leishmania examined thus far contain host-derived SLs (McConville and Black-well, 1991; Schneider et al., 1993; Winter et al., 1994), which can reach levels comparable to the abundant GIPLs comprising much of the amastigote surface (McConville and Blackwell, 1991). While the significance of host-derived SLs was previously unknown, we obtained two lines of evidence that the host-derived SLs were likely to contribute to parasite survival.
First, our studies showed that amastigotes avidly acquire and remodel host SLs, thereby generating significant levels of IPC, an SL not synthesized by mammalian cells (Fig. 4). We estimated that both promastigotes and amastigotes contain ∼108 IPC molecules per cell, much higher than the level of GIPLs. Moreover, while spt2− pro-mastigotes lacked IPCs, spt2− amastigotes maintained IPCs at the high WT levels (∼108 cell−1, Fig. 4). These data prove that Leishmania has an efficient mechanism for acquiring host metabolites necessary for abundant IPC synthesis, and that the dominant inositol phospholipids of Leishmania are IPCs, rather than GIPLs. While modest levels of upregulation of LPG and/or GIPL biosynthesis were seen in spt2− mutants (Fig. 3; Denny et al., 2004), quantitative considerations suggest these are unlikely to be able to compensate completely for the loss of SLs in these mutants.
Potentially, amastigote IPC synthesis could arise through salvage of host SB, ceramide or glycosphingolip-ids. Tests employing specific inhibitors on amastigotes in macrophages suggest that amastigote replication does not require de novo host SB or ceramide synthesis (Table 1). However, ceramide arising through hydrolysis of complex host SLs, either within the host (Ghosh et al., 2001) or following acquisition by the parasite, could serve as a substrate for Leishmania IPC synthase. In yeast, diacylglycerol GPI-protein anchors are replaced by ceramide through the action of an as yet unidentified ‘remodelase’ (Reggiori and Conzelmann, 1998). Potentially a similar reaction occurs in Leishmania, but now focused on ‘headgroup’ exchange. It is also possible that this reaction occurs directly, without a free ceramide intermediate.
Having established that Leishmania avidly acquires and then remodels host SLs into IPCs which dominate the amastigote surface, we considered the importance of this process in amastigote survival. As in other organisms, one might gravitate towards models postulating that the lack of SB/SLs compromises one or more important constitutive functions in the amastigotes. However, spt2− pro-mastigotes do not require SBs or SLs for normal growth, or to maintain normal membrane architecture including lipid rafts (Zhang et al., 2003; Denny et al., 2004). While the spt2−L. major strain studied by Denny et al. (Denny et al., 2004) shows reductions in the trafficking of GPI anchored proteins to lipid rafts, this does not occur in the spt2− strain studied here (unpubl. data), suggesting that the effects described by these authors do not specifically arise from SL-deficiency. In total, these data raise the possibility that as in promastigotes, SLs may not play a prominent role in ‘housekeeping’ processes of growth, membrane trafficking and lipid microdomain dynamics in amastigotes, although minor roles cannot be excluded.
Alternatively, it is likely that amastigote SLs play prominent roles in pathways tied specifically to parasite virulence and survival in the host. Perhaps in a manner similar to that seen in metacyclic parasites, increased demands on SL-dependent pathways important to amastigote survival may be critical. For example, ‘snatching’ of host SLs by amastigote salvage could lead to disruption of SL-dependent signalling pathways in macrophages. In this respect it is interesting that Leishmania donovani infection leads to elevated ceramide levels in macrophages (Ghosh et al., 2001). Alternatively, IPCs arising through SL remodelling may contribute directly to amastigote survival, as seen in the fungal pathogen Cryptococcus neoformans (Luberto et al., 2001), although they do not appear essential for the inhibition of macrophage signalling by invading promastigotes (Fig. 1). Our data suggest the involvement of SB metabolism in AC biogenesis as a possibility. ACs are acidic organelles containing high levels of long chain polyphosphates and relevant enzymes and activities, occurring in a variety of organisms including trypanosomatids (Docampo and Moreno, 2001). ACs have been implicated in the ability of microbes to respond to various forms of stress, including changes in pH and osmotic pressure resembling those seen during the parasite infectious cycle (Rao and Kornberg, 1996; Kornberg et al., 1999; Ruiz et al., 2001; Kim et al., 2002). RNAi-based inactivation studies have shown that genes encoding AC-associated activities are essential in bloodstream form trypanosomes (Lemercier et al., 2004; Luo et al., 2004).
In L. major ACs occurred at low levels in replicating promastigotes, but increased in infective stationary pro-mastigotes to levels similar to that found in amastigotes (Figs 5A, E and G and 6). This progression had not been reported previously and is consistent with an important role for ACs in the infective stages. Notably, spt2− promas-tigotes were defective in AC biogenesis, in both log and stationary phase promastigotes (Figs 5 and 6A). The defect specifically affected AC contents, as spt2− promas-tigotes continued to make ACs, as judged by staining with an AC membrane protein antiserum, but these ACs were ‘empty’ as judged by EM and biochemical criteria (Fig. 5; Table 2). We showed also that the spt2− AC defect could be rescued by exogeneous supplementation with SB (Fig. 6B). Because this treatment also rescued the stationary phase metacyclogenesis defect of the spt2− parasites (Zhang et al., 2003), one must ask whether rescue of the AC defect occurs indirectly through restoration of metacyclogenesis, rather than directly through SB/SLs. We favour the ‘direct’ model presently, because healthy log phase spt2− parasites also manifest the AC defect (Fig. 6A). Thus, current data establish a requirement for de novo SB synthesis for proper AC biogenesis in pro-mastigotes, although they do not establish whether SBs themselves or a downstream metabolite are responsible.
We also noted an increase in lipid inclusions in stationary phase spt2− promastigotes, a phenomenon seen in other trypanosomatids on perturbation of lipid metabolism by treatment with inhibitors of sterol synthesis (Rodrigues et al., 2002). However, elevated numbers of lipid inclusions have also been seen in taxol-treated T . cruzi, raising the possibility that lipid body formation may be a nonspecific response rather than a specific response to disruption of lipid synthetic and trafficking pathways (Dantas et al., 2003).
In contrast to promastigotes, both WT and spt2− amastigotes contained similar levels of normal ACs (Fig. 5E – G). Given the results above with promastigotes, we propose that salvage of SBs (or a relevant metabolite) may contribute to the ‘repair’ of the AC defect in amastigotes, which in turn is important for survival in the host.
Why should SL depletion cause AC defects? This question is difficult because relatively little about AC biogenesis has been reported since their discovery. We note here that the properties of ACs resemble a class of eukaryotic organelles termed lysosome-related organelles (LROs), including melanosomes and non-classical, secretory lysosomes (Blott and Griffiths, 2002; Raposo et al., 2002; Ruiz et al., 2004). The similarities between the platelet dense granule LROs and ACs are especially striking (Ruiz et al., 2004). Notably, LROs may originate from cellular endosomal compartments with characteristics of the MVB-like compartment (Mullins and Bonifacino, 2001). This provides a potential link to our findings about SB metabolism and AC biogenesis, in that previous studies in our laboratory had suggested that stationary phase spt2− parasites exhibited defects involving the MVB pathway (Zhang et al., 2003). While our proposal was challenged recently based on the finding that spt2− parasites showed normal endocytic uptake (Denny and Smith, 2004), this criterion can be rejected because endocytosis can occur normally in trypanosomatids in the face of severe disruptions in the endosomal pathway trafficking (Hall et al., 2004).
We suggest that the deleterious effect of SB/SL deficiency on the MVB/endosomal pathway, and the potential relationship of this pathway to LRO/AC formation in parasites, provides a plausible model linking SB metabolism and AC biogenesis in Leishmania. More specifically, the ‘empty AC’ phenotype of the spt2− mutant may arise through a defect in the assembly/packaging of AC contents as it arose through the endosomal MVB/LRO pathway either directly (through incorporation AC luminal proteins or enzymes) or indirectly, through alterations in AC membrane properties affecting these processes. Given the rapidity with which our knowledge of parasite genomes, genetics and cell biology is growing, we expect tests of this model to be forthcoming in the near future. If confirmed, we suggest that the spt2− mutant will provide an attractive setting in the future for probing AC as well as MVB biogenesis, a process shared by many organisms including humans.
Our study strongly suggests that SLs are important to Leishmania infectivity throughout its life cycle, but for reasons that differ substantially from those that might be predicted from studies of yeast or humans. In the promas-tigote stage, de novo biosynthesis is the dominant pathway and plays a vital role in generating infective meta-cyclic parasites able to establish infections. For amastigotes, however, parasites mostly depend on salvage to provide SLs essential for parasite survival and generation of normal ACs. Moreover this must take place through amastigote-specific pathways, as promastigotes are unable to incorporate exogenous ceramide or complex SLs into IPCs (Zhang et al., 2003). Our findings suggest that the salvage and subsequent metabolism of host glycolipids is likely to be an important area for future study with important implications for Leishmania chemotherapy as well as virulence.
Experimental procedures
Leishmania promastigote culture
Wild type (WT) L. major LV39 clone 5 (Rho/SU/59/P), spt2− (Δspt2::HYG/Δspt2::PAC) and spt2−/+SPT2 ‘add-back’ (Δspt2::HYG/Δspt2::PAC/+pXG-SPT2) cells were grown as described (Zhang et al., 2003). Before infectivity studies, parasites were inoculated at a high multiplicity (5 × 107 promastigotes per mouse) into susceptible BALB/c mice and recovered after one month.
Mouse foot pad infection
Parasite virulence was evaluated in vivo by mouse foot pad infections (Titus et al., 1991). Parasites were grown in vitro for three passages, and log (2 × 106 cells mL−1) or stationary phase (3 days at constant maximal density) promastigotes were resuspended in DMEM media at 2 × 107 cells mL−1 and injected into the foot pads of five to six female BALB/c mice (8 week old) at 106 cells per mouse. To recover amastigotes, foot pads from infected mice were minced and homogenized at 4°C. Homogenates were then cleared (by low speed cen-trifugation) and amastigotes were collected by centrifugation at 2000 g for 10 min. These were immediately injected into mice at 2 × 104 amastigotes per mouse. Lesion sizes were measured with a vernier caliper and parasite numbers in the infected foot pads were determined by limiting dilution assays (Titus et al., 1985).
Macrophage infections and determination of NO/IL-12 production
Infection of mouse PEMs was performed using C5-deficient mouse serum opsonized promastigotes (Racoosin and Beverley, 1997; Spath et al., 2003). Amastigotes isolated from infected mouse lesions were directly applied to PEMs without opsonization (multiplicity of infection or moi varies from 1:1 to 3:1). For inhibitor studies, PEMs were cultured with drug (10 µM for myriocin and 20 µM for Fumonisin B1) for 20 h before being infected with WT or spt2− amastigotes. The same drug concentration was maintained during and after infection. At 2 or 70 h post infection, amastigote survival was determined by counting the number of parasites in 100 macrophages. For NO and IL12 production, promastigotes were allowed to infect PEMs for 4 h at moi = 20:1. Then macrophages were washed and transferred to either plain medium or medium containing 50 ng mL−1 LPS and 50 ng mL−1 IFN-γ. After overnight incubation, levels of nitrite and IL12 (p40) in the supernatant were determined using the Griess Reagent (Sigma) and the OptEIA Mouse IL-12 (p40) Set (Pharmingen) respectively.
Western blotting and immunofluorescence microscopy
Western blots and immunofluorescence microscopy were performed as described (Zhang et al., 2004). Indirect immu-nofluorescent microscopy was performed using the mouse anti-T. cruzi pyrophosphatase antiserum (1:1000), followed by goat anti-mouse IgG-FITC antibody (1:1000).
Transmission and immuno-electron microscopy
Cryo-EM was performed as previously described (Zhang et al., 2003). For ultrastructural analysis of the surface glycocalyx, parasites were fixed in freshly prepared 2% paraformaldehyde/2.5% glutaraldehyde/0.15% ruthenium red (Electron Microscopy Sciences, Ft Washington, PA) in 100 mM phosphate buffer, pH 7.2 for 1 h at room temperature. Following three washes in phosphate buffer, parasites were postfixed in 1% osmium tetroxide (Polysciences)/ 0.15% ruthenium red for 1 h. Samples were then rinsed extensively in dH2O to remove excess phosphate before en bloc staining with 1% aqueous uranyl acetate (Ted Pella, Redding, CA) for 1 h. Following several rinses in dH2O, samples were dehydrated in a graded series of ethanol and embedded in Eponate 12 resin (Ted Pella). Sections of 70–80 nm were cut, stained with uranyl acetate and lead citrate, and viewed on a JEOL 1200 EX transmission electron microscope (JEOL USA, Peabody, MA). For immunolocalization, parasites were fixed in 4% paraformaldehyde/0.2% glutaraldehyde (Polysciences, Warrington, PA) in 100 mM PIPES/0.5 mM MgCl2, pH 7.2 for 1 h at 4°C. Samples were then embedded in 10% gelatin and infiltrated overnight with 2.3M sucrose/20% polyvinyl pyrrolidone in PIPES/MgCl2 at 4°C. Samples were trimmed, frozen in liquid nitrogen, and sectioned with a Leica Ultracut UCT cryoultramicrotome (Leica Microsystems, Bannockburn, IL). Sections (70 nm) were blocked with 5% fetal bovine serum/5% normal goat serum for 30 min and subsequently incubated with mouse anti-T. cruzi pyrophosphatase antibody overnight at 4°C. Sections were then washed in block buffer and probed with 18 nm colloidal gold-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove PA) for 1 h at room temperature. Sections were washed in PIPES buffer followed by a water rinse, and stained with 0.3% uranyl acetate/2% polyvinyl alcohol. Samples were viewed with a JEOL 1200EX transmission electron microscope (JEOL USA, Peabody, MA). All labelling experiments were conducted in parallel with controls omitting the primary antibody, which were consistently negative at the concentration secondary antibodies used.
Calcium, zinc and polyphosphate assays
Promastigotes were washed three times in 116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 50 mM Hepes, pH 7.4, and treated with 70 mM perchloric acid in this buffer for 1 h on ice, followed by centrifugation (2 min, 10 000xg) and assay of the supernatant fraction by inductively coupled plasma spectroscopy in the Veterinary Diagnostic Laboratory of the University of Illinois, Urbana. Protein was measured with the Bio-Rad Coomassie blue method. Short-(less than 50 phosphate residues) and long- (∼700–800 residues) chain polyphosphate were assayed as before (Ruiz et al., 2001).
Lipid analysis by ESI/MS
Internal standard 1,2-dipalmitoyl-sn-glycero-3-phosphoinositol (16:0/16:0-PI) was added to promastigote and amastigote samples at 15 and 5 µg per 108 cells, respectively, before lipid extraction (these concentrations were determined empirically; see the Supplementary material). Total lipids were isolated as previously described (Zhang et al., 2003). Samples were infused into the ESI source of a Finnigan TSQ 7000 triple stage mass spectrometer, and the ESI/MS total negative ion current was determined (Zhang et al., 2003). Negative ion ESI/MS/MS scanning for parents of the ion of m/z 241 was performed to identify molecular species of IPCs and glycerophosphoinositol (PI) lipids (Hsu and Turk, 2000). Col-lisionally activated dissociation of precursor ions was performed at a collision energy of 38 eV with argon (2.3 mtorr) collision gas. The abundance of IPC molecular species relative to the internal standard was estimated from the ESI/MS total negative ion current tracing.
Supplementary Material
Acknowledgements
We thank Betty Lu for assistance in macrophage infections, Wandy Beatty for EM analysis, Felix A. Ruiz for help with the polyphosphate determination, Keith Gull for discussions concerning lipid inclusions, Mike Ferguson for information relevant to calculation of parasite membrane lipid composition, Norton Heise for information on IPC synthesis in T. cruzi, and Althea Capul, Deb Dobson, and Kelly Robinson for insightful discussions and comments on this manuscript. This work was funded by NIH Grant AI21903 to SMB and AI23259 to RD. Mass spectrometric analyses were performed in the Washington University Medicine Department mass spec-trometry facility, which is supported by NIH Grants P41-RR-00954, P60-DK-20579 and P30-DK-56341.
Footnotes
Supplementary material
The following material is available from http://www.blackwellpublishing.com/product/journals/suppmat/mmi/mmi4493/mmi4493sm.htm
Fig. S1. Log phase promastigotes of spt2− have higher infectivity than log phase WT.
Fig. S2. The height of PI standard peak is proportional to its concentration.
Appendix S1 Virulence of spt2− log phase promastigotes and validation of IPC quantitation by mass spectrometry using 16:0/16:0 PI as an internal standard.
References
- Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3:122–131. doi: 10.1038/nrm732. [DOI] [PubMed] [Google Scholar]
- Chawla M, Vishwakarma RA. Alkylacylglycer-olipid domain of GPI molecules of Leishmania is responsible for inhibition of PKC-mediated c-fos expression. J Lipid Res. 2003;44:594–600. doi: 10.1194/jlr.M200296-JLR200. [DOI] [PubMed] [Google Scholar]
- Dantas AP, Barbosa HS, De Castro SL. Biological and ultrastructural effects of the anti-microtubule agent taxol against Tr ypanosoma cruzi . J Submicrosc Cytol Pathol. 2003;35:287–294. [PubMed] [Google Scholar]
- Denny PW, Smith DF. Rafts and sphingolipid biosynthesis in the kinetoplastid parasitic protozoa. Mol Microbiol. 2004;53:725–733. doi: 10.1111/j.1365-2958.2004.04208.x. [DOI] [PubMed] [Google Scholar]
- Denny PW, Field MC, Smith DF. GPI-anchored proteins and glycoconjugates segregate into lipid rafts in Kinetoplastida. FEBS Lett. 2001;491:148–153. doi: 10.1016/s0014-5793(01)02172-x. [DOI] [PubMed] [Google Scholar]
- Denny PW, Goulding D, Ferguson MA, Smith DF. Sphingolipid-free Leishmania are defective in membrane trafficking, differentiation and infectivity. Mol Microbiol. 2004;52:313–327. doi: 10.1111/j.1365-2958.2003.03975.x. [DOI] [PubMed] [Google Scholar]
- Dermine JF, Duclos S, Garin J, St-Louis F, Rea S, Parton RG, Desjardins M. Flotillin-1-enriched lipid raft domains accumulate on maturing phagosomes. J Biol Chem. 2001;276:18507–18512. doi: 10.1074/jbc.M101113200. [DOI] [PubMed] [Google Scholar]
- Docampo R, Moreno SN. The acidocalcisome. Mol Biochem Parasitol. 2001;114:151–159. doi: 10.1016/s0166-6851(01)00246-8. [DOI] [PubMed] [Google Scholar]
- Figueiredo JM, Dias WB, Mendonca-Previato L, Previato JO, Heise N. Characterization of the inositol phosphorylceramide synthase activity from Trypa-nosoma cruzi . Biochem J. 2005 doi: 10.1042/BJ20041842. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Bhattacharyya S, Das S, Raha S, Maulik N, Das DK, et al. Generation of ceramide in murine macrophages infected with Leishmania donovani alters macrophage signaling events and aids intracellular parasitic survival. Mol Cell Biochem. 2001;223:47–60. doi: 10.1023/a:1017996609928. [DOI] [PubMed] [Google Scholar]
- Hall BS, Pal A, Goulding D, Field MC. Rab4 is an essential regulator of lysosomal trafficking in trypa-nosomes. J Biol Chem. 2004;279:45047–45056. doi: 10.1074/jbc.M407271200. [DOI] [PubMed] [Google Scholar]
- Hanada K, Nishijima M, Fujita T, Kobayashi S. Specificity of inhibitors of serine palmitoyltrans-ferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells. A novel evaluation system using an SPT-defective mammalian cell mutant. Biochem Pharmacol. 2000;59:1211–1216. doi: 10.1016/s0006-2952(00)00251-3. [DOI] [PubMed] [Google Scholar]
- Hsu FF, Turk J. Characterization of pho-sphatidylinositol, phosphatidylinositol-4-phosphate, and phosphatidylinositol-4,5-bisphosphate by electrospray ionization tandem mass spectrometry: a mechanistic study. J Am Soc Mass Spectrom. 2000;11:986–999. doi: 10.1016/S1044-0305(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Ilgoutz SC, McConville MJ. Function and assembly of the Leishmania surface coat. Int J Parasitol. 2001;31:899–908. doi: 10.1016/s0020-7519(01)00197-7. [DOI] [PubMed] [Google Scholar]
- Joshi PB, Kelly BL, Kamhawi S, Sacks DL, McMaster WR. Targeted gene deletion in Leish-mania major identifies leishmanolysin (GP63) as a virulence factor. Mol Biochem Parasitol. 2002;120:33–40. doi: 10.1016/s0166-6851(01)00432-7. [DOI] [PubMed] [Google Scholar]
- Kaneshiro ES, Jayasimhulu K, Lester RL. Characterization of inositol lipids from Leishmania dono-vani promastigotes: identification of an inositol sphingo-phospholipid. J Lipid Res. 1986;27:1294–1303. [PubMed] [Google Scholar]
- Kim KS, Rao NN, Fraley CD, Kornberg A. Inorganic polyphosphate is essential for long-term survival and virulence factors in Shigella and Salmonella spp . Proc Natl Acad Sci USA. 2002;99:7675–7680. doi: 10.1073/pnas.112210499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter T, Proia RL, Sandhoff K. Combinatorial ganglioside biosynthesis. J Biol Chem. 2002;277:25859–25862. doi: 10.1074/jbc.R200001200. [DOI] [PubMed] [Google Scholar]
- Kornberg A, Rao NN, Ault-Riche D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- Lemercier G, Espiau B, Ruiz FA, Vieira M, Luo S, Baltz T, et al. A pyrophosphatase regulating poly-phosphate metabolism in acidocalcisomes is essential for Trypanosoma brucei virulence in mice. J Biol Chem. 2004;279:3420–3425. doi: 10.1074/jbc.M309974200. [DOI] [PubMed] [Google Scholar]
- Luberto C, Toffaletti DL, Wills EA, Tucker SC, Casa-devall A, Perfect JR, et al. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans . Genes Dev. 2001;15: 201–212. doi: 10.1101/gad.856001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Rohloff P, Cox J, Uyemura SA, Docampo R. Trypanosoma brucei plasma membrane-type Ca(2+)-ATPase 1 (TbPMC1) and 2 (TbPMC2) genes encode functional Ca(2+)-ATPases localized to the aci-docalcisomes and plasma membrane, and essential for Ca(2+) homeostasis and growth. J Biol Chem. 2004;279:14427–14439. doi: 10.1074/jbc.M309978200. [DOI] [PubMed] [Google Scholar]
- Luo S, Vieira M, Graves J, Zhong L, Moreno SN. A plasma membrane-type Ca(2+)-ATPase co-localizes with a vacuolar H(+)-pyrophosphatase to acidocalci-somes of Toxoplasma gondii . EMBO J. 2001;20:55–64. doi: 10.1093/emboj/20.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville MJ, Blackwell JM. Developmental changes in the glycosylated phosphatidylinositols of Leish- mania donovani. Characterization of the promastigote and amastigote glycolipids. J Biol Chem. 1991;266:15170–15179. [PubMed] [Google Scholar]
- McNeely TB, Rosen G, Londner MV, Turco SJ. Inhibitory effects on protein kinase C activity by lipophosphoglycan fragments and glycosylphosphatidyli-nositol antigens of the protozoan parasite Leishmania . Bio-chem J. 1989;259:601–604. doi: 10.1042/bj2590601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala SM, Harris GH. Isolation and characterization of novel inhibitors of sphingolipid synthesis: australifungin, viridiofungins, rustmicin, and khafrefungin. Meth Enzymol. 2000;311:335–348. doi: 10.1016/s0076-6879(00)11094-8. [DOI] [PubMed] [Google Scholar]
- van Meer G, Lisman Q. Sphingolipid transport: rafts and translocators. J Biol Chem. 2002;277:25855–25858. doi: 10.1074/jbc.R200010200. [DOI] [PubMed] [Google Scholar]
- Merrill AH, Jr, Hannun YA, Bell RM. Introduction: sphingolipids and their metabolites in cell regulation. Adv Lipid Res. 1993;25:1–24. [PubMed] [Google Scholar]
- Mullin KA, Foth BJ, Ilgoutz SC, Callaghan JM, Zawadzki JL, McFadden GI, McConville MJ. Regulated degradation of an endoplasmic reticulum membrane protein in a tubular lysosome in Leishmania mexicana . Mol Biol Cell. 2001;12:2364–2377. doi: 10.1091/mbc.12.8.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins C, Bonifacino JS. The molecular machinery for lysosome biogenesis. Bioessays. 2001;23:333–343. doi: 10.1002/bies.1048. [DOI] [PubMed] [Google Scholar]
- Proudfoot L, O’Donnell CA, Liew FY. Glycoi-nositolphospholipids of Leishmania major inhibit nitric oxide synthesis and reduce leishmanicidal activity in murine macrophages. Eur J Immunol. 1995;25:745–750. doi: 10.1002/eji.1830250318. [DOI] [PubMed] [Google Scholar]
- Racoosin EL, Beverley SM. Leishmania major: promastigotes induce expression of a subset of chemokine genes in murine macrophages. Exp Parasitol. 1997;85:283–295. doi: 10.1006/expr.1996.4139. [DOI] [PubMed] [Google Scholar]
- Ralton JE, Mullin KA, McConville MJ. Intra-cellular trafficking of glycosylphosphatidylinositol (GPI) – anchored proteins and free GPIs in Leishmania mexicana . Biochem J. 2002;363:365–375. doi: 10.1042/0264-6021:3630365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NN, Kornberg A. Inorganic polyphosphate supports resistance and survival of stationary-phase Escherichia coli . J Bacteriol. 1996;178:1394–1400. doi: 10.1128/jb.178.5.1394-1400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Fevrier B, Stoorvogel W, Marks MS. Lysosome-related organelles: a view from immunity and pigmentation. Cell Struct Funct. 2002;27:443–456. doi: 10.1247/csf.27.443. [DOI] [PubMed] [Google Scholar]
- Reggiori F, Conzelmann A. Biosynthesis of inositol phosphoceramides and remodeling of glyco-sylphosphatidylinositol anchors in Saccharomyces cerevi-siae are mediated by different enzymes. J Biol Chem. 1998;273:30550–30559. doi: 10.1074/jbc.273.46.30550. [DOI] [PubMed] [Google Scholar]
- Rodrigues JC, Attias M, Rodriguez C, Urbina JA, Souza W. Ultrastructural and biochemical alterations induced by 22,26-azasterol, a delta (24(25)-sterol methyltransferase inhibitor, on promastigote and amastigote forms of Leishmania amazonensis . Antimicrob Agents Chemother. 2002;46:487–499. doi: 10.1128/AAC.46.2.487-499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- Ruiz FA, Rodrigues CO, Docampo R. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi . J Biol Chem. 2001;276:26114–26121. doi: 10.1074/jbc.M102402200. [DOI] [PubMed] [Google Scholar]
- Sacks D, Kamhawi S. Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu Rev Microbiol. 2001;55:453–483. doi: 10.1146/annurev.micro.55.1.453. [DOI] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- Salto ML, Bertello LE, Vieira M, Docampo R, Moreno SN, de Lederkremer RM. Formation and remodeling of inositolphosphoceramide during differentiation of Trypanosoma cruzi from trypomastigote to amastigote. Eukaryot Cell. 2003;2:756–768. doi: 10.1128/EC.2.4.756-768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Rosat JP, Ransijn A, Ferguson MA, McConville MJ. Characterization of glycoi-nositol phospholipids in the amastigote stage of the protozoan parasite Leishmania major . Biochem J. 1993;295:555–564. doi: 10.1042/bj2950555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath GF, Epstein L, Leader B, Singer SM, Avila HA, Turco SJ, Beverley SM. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major . Proc Natl Acad Sci USA. 2000;97:9258–9263. doi: 10.1073/pnas.160257897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath GF, Garraway LA, Turco SJ, Beverley SM. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc Natl Acad Sci USA. 2003;100:9536–9541. doi: 10.1073/pnas.1530604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine 1-phos-phate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- Titus RG, Marchand M, Boon T, Louis JA. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Titus RG, Muller I, Kimsey P, Cerny A, Behin R, Zink-ernagel RM, Louis JA. Exacerbation of experimental murine cutaneous leishmaniasis with CD4+ Leishmania major-specific T cell lines or clones which secrete interferon-gamma and mediate parasite-specific delayed-type hypersensitivity. Eur J Immunol. 1991;21:559–567. doi: 10.1002/eji.1830210305. [DOI] [PubMed] [Google Scholar]
- Waller RF, McConville MJ. Developmental changes in lysosome morphology and function Leishmania parasites. Int J Parasitol. 2002;32:1435–1445. doi: 10.1016/s0020-7519(02)00140-6. [DOI] [PubMed] [Google Scholar]
- Wang E, Norred WP, Bacon CW, Riley RT, Merrill AH., Jr Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme . J Biol Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- Wassef MK, Fioretti TB, Dwyer DM. Lipid analyses of isolated surface membranes of Leishmania donovani promastigotes. Lipids. 1985;20:108–115. doi: 10.1007/BF02534216. [DOI] [PubMed] [Google Scholar]
- Winter G, Fuchs M, McConville MJ, Stierhof YD, Overath P. Surface antigens of Leishmania mexi-cana amastigotes: characterization of glycoinositol phos-pholipids and a macrophage-derived glycosphingolipid. J Cell Sci. 1994;107:2471–2482. doi: 10.1242/jcs.107.9.2471. [DOI] [PubMed] [Google Scholar]
- Yoo HS, Norred WP, Wang E, Merrill AH, Jr, Riley RT. Fumonisin inhibition of de novo sphingolipid biosynthesis and cytotoxicity are correlated in LLC-PK1 cells. Toxicol Appl Pharmacol. 1992;114:9–15. doi: 10.1016/0041-008x(92)90090-f. [DOI] [PubMed] [Google Scholar]
- Zhang K, Barron T, Turco SJ, Beverley SM. The LPG1 gene family of Leishmania major . Mol Biochem Parasitol. 2004;136:11–23. doi: 10.1016/j.molbiopara.2004.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Showalter M, Revollo J, Hsu FF, Turk J, Beverley SM. Sphingolipids are essential for differentiation but not growth in Leishmania . EMBO J. 2003;22:6016–6026. doi: 10.1093/emboj/cdg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Allen S, Barron T, Sullivan DR, Denny PW, Almeida IC, et al. Ether phospholipids and glycosylinositolphospholipids are not required for amastig-ote virulence or for inhibition of macrophage activation byLeishmania major . J Biol Chem. 2003;278:44708–44718. doi: 10.1074/jbc.M308063200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.