Abstract
Dopamine (DA), the most abundant catecholamine in the basal ganglia, participates in the regulation of motor functions and of cognitive processes such as learning and memory. Abnormalities in dopaminergic systems are thought to be the bases for some neuropsychiatric disorders including addiction, Parkinson’s disease, and Schizophrenia. DA exerts its arrays of functions via stimulation of D1-like (D1 and D5) and D2-like (D2, D3, and D4) DA receptors which are located in various regions of the brain. The DA D1 and D2 receptors are very abundant in the basal ganglia where they exert their functions within separate neuronal cell types. The present paper focuses on a review of the effects of stimulation of DA D1 receptors on diverse signal transduction pathways and gene expression patterns in the brain. We also discuss the possible involvement of the DA D1 receptors in DA-mediated toxic effects observed both in vitro and in vivo. Future studies using more selective agonist and antagonist agents and the use of genetically modified animals should help to further clarify the role of these receptors in the normal physiology and in pathological events that involve DA.
Keywords: Amphetamines, AP-1, apoptosis, basal ganglia, cocaine, DA receptors, Egr, signal transduction
INTRODUCTION
Dopamine (DA) is a catecholamine (CA) neurotransmitter that regulates functional network activities in various regions of the brain [1]. DA neurons are characterized by their anatomical and functional diversity, being located in the ventral midbrain, the diencephalon, and the olfactory bulb [2,3]. Dopaminergic neurons send projections to the cingulate gyrus, frontal cortex, nucleus accumbens, and the striatum [4,5] and are involved in a number of neurological and psychiatric disorders including addiction, Parkinson’s Disease (PD), and Schizophrenia [6–9]. For example, the recognition that death of neurons in the substantia nigra is responsible for the majority of the signs and symptoms of PD has been the main driving force for the development of therapeutic agents [8,10]. In the case of schizophrenia, however, the development of the DA hypothesis was driven by the fact that the majority of antipsychotic drugs are antagonists at DA receptors [11].
DA neurotransmission in the brain is dependent on the stimulation of two classes of G-protein-coupled DA receptors, the D1- and D2-like classes, which were initially distinguished on the basis of their opposite influence on adenylyl cyclase [12–14]. The DA D1-like receptor family includes D1 and D5 receptors whereas the D2 receptor class includes D2, D3, D4 subtypes. The molecular structures of these classes of DA receptors also show interesting differences, with the D1-like receptors having short third intracellular loops and long carboxyl terminal tails but the D2-like receptors having long third intracellular loops and short carboxyl terminal tails [15–17].
DOPAMINE D1 RECEPTORS, LOCALIZATION AND SIGNAL TRANSDUCTION MECHANISMS
The two members of D1-like DA receptors, D1 and D5 subtypes, are genetically distinct [16,18–20]. They share about 80% sequence homology within the highly conserved seven trans-membrane spanning domains but only 50% homology at the levels of amino acid content. They are also differentially distributed in the brain [21,22]. The present review will focus on the molecular neuropharmacology of DA D1 receptors which play major roles in dopaminergic signaling in several brain regions, participate in the control of gene expression, and appear to be important triggers of neurodegenerative effects caused by increased DA concentration in the striatum.
LOCALIZATION OF DA D1 RECEPTORS IN THE BRAIN
DA D1 receptors play important roles in learning and memory, locomotor activity, reward mechanisms, and have been implicated in the signs and symptoms of some neuropsychiatric disorders [23,24]. DA D1 receptors are widely expressed in the brain, with the highest levels being found in the caudate-putamen, the nucleus accumbens, the substantia nigra pars reticulata, and the olfactory bulb [25–27]. These binding data are consistent with the high levels of DA D1 receptor mRNA detected in neurons of the caudate-putamen and in the nucleus accumbens in human and rodent brains [28,29]. Moderate binding densities are found in the cerebral aqueduct, the third and fourth ventricles, entopeduncular nucleus, and the nucleus interstitialis stria terminalis [25]. Lower densities of D1 receptors are found in other brain areas including the dorsolateral prefrontal cortex, the cingulate cortex, the hippocampus, and the habenular [25,27]. In the striatum, D1 receptors are co-localized with DARPP-32 in medium-sized spiny neurons [30]. D1 receptors were also co-localized with DARPP-32 in fibers of the entopeduncular nucleus and the pars reticulata of the substantia nigra [30]. DA D1 receptors are highly concentrated in dendritic spines including spine heads and the postsynaptic density of neurons [31] where they can interact with other receptors and influence signaling mechanisms involved in the function of spines [32].
Lesion studies have been used extensively to examine the effects of various toxins on the expression of DA D1 receptors. It was initially reported that there were no changes in DA D1 receptors after 6-hydroxydopamine (6-OHDA)-induced lesions of the nigrostriatal DA pathway [33]. Subsequently, Berger et al. [34] found that intrastriatal injections of 6-OHDA, which caused greater than 97% and 88% respective losses of mazindol-labeled DA uptake sites in the striatum and substantia nigra pars compacta (SNpc), respectively, caused about 15% loss of D1 receptors in the striatum and 10% loss in the SNpr in animals euthanized 2 weeks after the 6-OHDA lesions. Animals that underwent similar surgical procedures but that were killed after 8 weeks showed no changes in striatal D1 receptors [35]. In contrast, excitotoxin-induced lesions of neuronal cell bodies in the striatum caused significant decreases in D1-like receptors in the striatum and nucleus accumbens, indicating that these receptors are located on intrinsic postsynaptic neurons [33]. These experimental results are consistent with data showing that DA D1 receptors show decreased expression in the brains of patients who suffer from Huntington’s disease [36,37], a neurodegenerative disorder characterized by substantial loss of medium spiny neurons in the striatum [38].
SIGNAL TRANSDUCTION MECHANISMS
Kebabian and collaborators were the first investigators to show that low DA concentrations can activate an adenylate cyclase in rat striatal homogenates, effects that were blocked by the DA antagonists, haloperidol or chlorpromazine [12,39]. D1 receptors can induce cAMP formation in brain striatal tissues [40,41], in cells transfected with the D1 receptor [19,42], and in malignant neuroblastoma cells [43]. DA can also stimulate adenylate cyclase in tissues obtained from the median eminence, olfactory tubercle, and the nucleus accumbens [40,44,45], all regions that contain moderate to high concentrations of DA D1 receptors [25]. Zhang et al. (2007) [46] recently reported that the synaptic scaffolding protein, postsynaptic density-95 (PSD-95) interacts with D1 receptors and regulates its functions. They reported, in addition, that D1-PSD-95 interaction is mediated by the carboxyl-terminal tail of D1 and the NH2 terminus of PSD-95 and that co-expression of PSD-95 with D1 in mammalian cells caused inhibition of D1-mediated cAMP accumulation. Electrothermic- or 6-OHDA-induced lesions that cause striatal dopamine depletion are associated with potentiation of the DA-mediated cAMP response in the striatum [47,48]. Although it was initially thought that D1-mediated induction of adenylyl cyclase occurred via stimulation of the G-protein, Gs, there is now evidence that the activation occurs through coupling with Golf in the striatum [49,50]. These observations are consistent with previous studies that there are very low levels of Gs but high levels of Golf in the striatum [49,51,52]. It is to be noted that Go and Gs are 88% homologous in amino acid composition and both stimulate adenylyl cyclase [53].
DA D1 receptors have also been linked to other second messenger systems [54–58]. These include receptor-mediated activation of phospholipase C (PLC) to generate inositol 1,4,5-trisphosphate [IP3] which participates in phosphoinositide turnover and calcium-regulated signaling pathways in the brain [59]. IP3 receptors are located mainly in the endoplasmic reticular (ER) membrane where IP3 can mobilize Ca2+ from intracellular stores [59]. Several recent studies have documented that D1 receptors are indeed coupled to Gq/PLC and have a significant role in phosphatidylinositol (PI)/phosphatidylinositol 4,5-biphosphate (PIP2) hydrolysis in the cortex, hippocampus, amygdala, and cerebellum [58,60,61]. DA D1 receptors are coupled differentially to multiple Galpha protein subunits in these brain regions. Specifically, DA-induced stimulation of PIP2 hydrolysis was blocked by antisera against Gq in hippocampal and in striatal membranes [61]. Dopamine also increased the binding of [35S]GTPgammaS or [alpha-32P]GTP to Gq in these brain regions, with the greatest effects being observed in the hippocampus [61]. In contrast, DA-induced binding of [35S]GTP gammaS to Gs was observed in the frontal cortex and striatum, but not in the other brain regions. These effects of DA were blocked by a DA D1 antagonist, SCH23390, further indicating a coupling of D1 receptors to both Gs and Gq proteins [61]. Interestingly, Tang and Bezprozvanny [62] reported that dopamine DA can cause D1-mediated PLC-dependent robust calcium transients in about 40% of striatal medium spiny neurons. Experiments with DARPP32 knockout mice also documented a role for DARPP32 in these DA-mediated calcium oscillations. Taken together, these observations suggest the existence of interactions of the PI and cAMP transduction mechanisms within DA D1-expressing striatal medium spiny neurons (MSN). Interestingly, Rashid et al. [63] have also provided evidence for the existence of brain heteromeric D1-D2 DA receptor signaling mechanisms that require the coincident binding of both receptors for activation and intracellular calcium release. Activation of this D1-D2 complex is associated with increased levels of calcium/calmodulin-dependent kinase IIalpha in the nucleus accumbens [63]. More studies on the PI-linked DA D1 receptors were conducted by Ma et al. [64] who used whole cell patch-clamp technique to investigate the effects of the DA D1 receptor agonist, SKF83959, on high-voltage activated (HVA) calcium currents in primary cultured striatal neurons. They found that stimulation by SKF83959 caused inhibition of HVA calcium currents in a dose-dependent manner in substance-P (SP)-immunoreactive striatal neurons. Application of D1, but not of D2, antagonists prevented the SKF83959-induced inhibition which was regulated by PLC-mediated activation of intracellular calcium stores and of calcineurin. Activation of D1 receptors in isolated nerve terminals from rat striatum by SKF81297, another selective D1-like receptor agonist, also caused increases in calcium levels in striatal synaptosomes [65]. The D1-induced changes in calcium levels appear to occur via sodium channel-mediated membrane depolarization followed by opening of voltage-gated calcium channels. These SKF-mediated effects were inhibited by the D1 receptor antagonist, SCH23390 [65]. More recently, it was reported that phosphorylation of tau at serines 199–202 and 214 in rat striatal sections occurred through activation of calcium-dependent intracellular mechanisms, subsequent to D1 receptor-induced cAMP-dependent protein kinase A (PKA), increased calcium levels and cdk5 activation [66], a process which suggests that stimulation of DA receptors might influence short and long-term changes in brain architecture [67]. Thus, when taken together, these observations indicate that DA D1 receptors are coupled to a diversity of transduction mechanisms which include SCH23390-sensitive Golf/AC/PKA, Gq/PLC, and voltage–gated calcium channels. These signaling pathways might act singly, in parallel, or in concert to modulate DA-mediated regulation of synaptic functions, neuronal plasticity, and of functional neuroanatomy of brain regions containing D1-positive neurons.
DA D1 RECEPTORS AND TRANSCRIPTIONAL REGULATION
Stimulation of striatal DA receptors has a significant role in the control of gene expression in the brain [68,69]. Table 1 provides a functional classification of genes whose expression has been reported to be influenced by administration of drugs that stimulate DA D1 receptors [68,69]. These include immediate early genes (IEGs), transcription factors, and ER stress-related genes, among others. Observations on the role of DA in the control of gene expression were initially made with the use of indirect DA agonists including cocaine and the amphetamines [70–75]. Several groups of investigators have studied the effects of direct and indirect DA agonists on the expression of members of AP-1, Egr and Nr4a families of transcription factors and have reported that these changes in gene expression are mediated, for the most part, via stimulation of DA D1 receptors [68–76]. In what follows, we discuss some of the genes shown in Table 2 which represents a partial list of genes whose expression is affected by the administration of either direct or indirect DA D1 agonists [68,69].
Table 1.
Functional classification of genes affected by stimulation of DA D1 receptor.
| Apoptosis |
| Cell-Adhesion and Mobility |
| Cell Cycle |
| Cell Differentiation or Development |
| Cell Migration |
| Cellular Transport |
| ER Stress |
| Hormone/Immune Response |
| Metabolism |
| Mitochondria |
| Signaling Transduction |
| Transcription Factors |
| Ubiquitin |
| Zinc Finger |
Table 2. Partial list of genes whose mRNA levels are affected in a DA D1 receptor-dependent fashion.
| Gene Symbol | Gene Name | Transcription factor Binding Sites | |||
|---|---|---|---|---|---|
| Abra | Actin-binding Rho activating protein | ||||
| Arc | Activity regulated cytoskeletal-associated protein | Creb | |||
| Baz1a | Bromodomain adjacent to zinc finger domain, 1A | ||||
| Btg2 | B-cell translocation gene 2, anti-proliferative | Egr | |||
| Cebpb | CCAAT/enhancer binding protein (C/EBP), beta | AP-1 | Egr | ||
| Cited2 | Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2 | Creb | Egr | ||
| Cnot6l | CCR4-NOT transcription complex, subunit 6-like | ||||
| Egr1 | Early growth response 1 | Creb | AP-1 | Nfat | Egr |
| Egr2 | Early growth response 2 | Creb | AP-1 | Nfat | Egr |
| Egr4 | Early growth response 4 | Egr | |||
| Fos | FBJ murine osteosarcoma viral oncogene homolog | Creb | AP-1 | Nfat | Egr |
| Fosl1 | fos-like antigen 1 | Creb | AP-1 | Egr | |
| Hes1 | Hairy and enhancer of split 1 (Drosophila) | Creb | Egr | ||
| Icer | Inducible cAMP early repressor | Creb | AP-1 | Nfat | |
| Icer | Inducible cAMP early repressor | Creb | AP-1 | Nfat | |
| Jun | Jun oncogene | Creb | Egr | ||
| JunB | Jun-B oncogene | Creb | Egr | ||
| Klf5 | Kruppel-like factor 5 | Creb | Egr | ||
| Klf6 | Kruppel-like factor 6 | ||||
| Maff | v-maf musculoaponeurotic fibrosarcoma oncogene family, protein F (avian) | ||||
| Mafk | v-maf musculoaponeurotic fibrosarcoma oncogene family, protein K (avian) | ||||
| Myc | myelocytomatosis viral oncogene homolog (avian) | Creb | Nfat | Egr | |
| Nab2 | Ngfi-A binding protein 2 | Egr | |||
| Nfil3 | Nuclear factor, interleukin 3 regulated | AP-1 | Nfat | ||
| Nr4a2 | Nuclear receptor subfamily 4, group A, member 2 | Creb | |||
| Nr4a3 | Nuclear receptor subfamily 4, group A, member 3 | ||||
| Per1 | period homolog 1 (Drosophila) | ||||
| Plagl1 | pleiomorphic adenoma gene-like 1 | AP-1 | |||
| Rel | Reticuloendotheliosis oncogene | ||||
| Rem2 | rad and gem related GTP binding protein 2 | ||||
| Uba2 | Ubiquitin-like 1 (sentrin) activating enzyme E1B | ||||
| Zfp189 | zinc finger protein 189 | ||||
| Zfp287 | zinc finger protein 287 | ||||
| Zfp36l1 | zinc finger protein 36, C3H type-like 1 | ||||
TRANSCRIPTION FACTORS
The effects of DA agents on the expression of transcription factors have been investigated extensively [69,77,78]. In the case of direct agonists, Simpson and Morris [79] used primary cultures of embryonic striatal neurons and reported marked SCH23390-sensitive, SKF38393-stimulated increases in the expression of c-fos and zif268 mRNA levels. Berke et al. [69] took a comprehensive approach using differential display and the 6-OHDA rat model of Parkinson’s disease. They list a set of 32 genes whose expression was rapidly induced by stimulation of striatal DA D1 receptors by the DA D1 agonist, SKF38393. The mRNAs that were affected belonged to several families of transcription factors that include c-fos, fosB, fra2, c-jun, junB, junD, egr2, egr3, CHOP, arc, nur77, zif268, and krox20. The timing of changes in gene expression varied with a large number of genes (e.g. c-jun) peaking at around 2 hours and returning to baseline expression within 4 hours whereas others (CHOP) took a longer time to normalize [69]. Svenningsson et al. [80] used DARPP32 knockout mice to assess the effects of the DA D1 agonist, SKF82958, on c-fos and NGFI-A expression. They found that injection of SKF82958 caused greater up-regulation of the expression of these immediate early genes in wild-type mice in comparison to the DARPP32 knockout mice. These results were found not to be related to changes in the expression of DA D1 receptors because there were no differences in D1 receptor expression between the two groups of mice.
Several studies have investigated the effects of the indirect DA agonists, cocaine and the amphetamines, on gene expression. Cocaine is an addictive stimulant that is widely abused [81]. Cocaine causes its biochemical effects by blocking DA re-uptake in reward related brain regions [82] and by causing dose-dependent increases in extracellular DA concentration [83]. The addictive effects of the drug are thought to be secondary to molecular adaptations secondary to changes in gene expression in various areas of the brain [84]. These ideas are consistent with the fact that cocaine can cause changes in the expression of several IEGs via activation of striatal D1 receptors [73,74,76,85,86]. A single cocaine injection caused time-dependent increases in ERK phosphorylation, c-Fos and FosB protein expression in the rat striatum [87]. Cocaine-induced increases in the expression of c-fos and fosB proteins are inhibited by pretreatment with the DA-D1 receptor antagonist, SCH23390. Genetically modified mice in which c-fos was mutated in D1 receptor-bearing neurons were used to examine the role of c-fos in mediating cocaine-induced persistent neurobiological changes [87]. These animals exhibited abnormalities in the manifestation of cocaine-mediated behavioral sensitization. Because these animals showed alterations in the composition of AP-1 transcription complexes and in the expression of some neurotransmitter receptors that are induced by chronic cocaine administration, the authors concluded that cocaine-induced c-fos expression in D1 receptor-containing neurons might be very important to the molecular and structural neuroadaptations induced by the drug [87] The role of DA D1 receptors in cocaine-induced changes in c-fos expression is supported by reports that direct DA agonists also cause SCH23390-sensitive increases in c-fos expression [88]. Bertran-Gonzalez et al. [89] used transgenic mice, in which enhanced green fluorescent protein (EGFP) expression was driven by a D1 receptor promoter (drd1a-EGFP) or a D2 receptor promoter (drd2-EGFP); the acute administration of cocaine-induced expression of c-fos and Zif268 occurred mostly in D1 receptor-expressing neurons. Repeated cocaine administration caused inhibition of cocaine-induced signaling responses. Other investigators have also shown that acute injections of cocaine can cause significant increases in the expression of phospho- cAMP response element binding protein (p-CREB) and p-Elk-1 in the mouse striatum [90]. These cocaine-induced changes are blocked by the DA D1 receptor antagonist, SCH23390 but not by the D2 antagonist, raclopride [90]. Cocaine also increases Nab2 expression [91].
The amphetamines are used clinically in the treatment of neuropsychiatric disorders including attention deficit and hyperactivity disorder (ADHD) [92]. Their abuse is very prevalent and is associated with an array of complications [93,94]. The amphetamines have also been shown to cause significant DA D1-mediated changes in gene expression. For example, Graybiel et al. [73] showed that single doses of amphetamine induced rapid expression of c-fos in the striatum, induction which was almost completely blocked by the DA D1 antagonist, SCH23390. Subsequent studies from the same laboratory also reported that amphetamine caused SCH23390-sensitive upregulation of Egr1 (Zif268, NGFI-A) expression in the rat brain [75]. These amphetamine-induced changes are dependent on D1 receptor-mediated cAMP response element binding protein (CREB) phosphorylation [74]. In addition to the effects of cocaine and amphetamine, methamphetamine (METH) has also been shown to cause substantial changes in gene expression in the rodent brain [68,77]. Wang et al. (1995) [95] used in situ hybridization and reported that a single dose of METH (15 mg/kg) increased c-fos expression in the rat striatum at 1 hour after METH with reversal to normal at 3 hours post-drug. Cadet, et al. [77] used microarray and RT-PCR analyses and found significant METH-induced increases in c-fos, fosB, Fra2 c-Jun, JunB, JunD in the frontal cortex of mouse. Jayanthi, et al. [96] also reported that a single injection of METH (40 mg/kg) caused significant increases in c-Jun mRNA which peaked at 2 hours and returned to normal between 16 and 24 hours after the drug injection. The single METH injection was also associated with increases in the expression of c-Jun protein which peaked at 4 hours and lasted for 7 days after the METH injection. In addition, there were increases in phosphorylated c-Jun which lasted for 2 days after the drug exposure. In order to test the role of D1 receptors in METH-induced changes in gene expression, we compared striatal transcriptional profiles in four groups of rats treated with saline (C), SCH23390 alone (S), METH alone (M) and METH plus SCH23390 (M + S) using Illumina rat arrays which contain 22,227 genes [68]. There were only 85 genes whose expression was influenced by the injection of SCH23990 alone (see Table 3 for a partial list). METH alone caused increases in 189 and 133 genes at the 2- and 4-hr time points, respectively [68]. METH-induced SCH23390-responsive genes include genes that participate in signal transduction, transcriptional regulation, metabolic pathways, apoptotic events, and endoplasmic reticulum stress among others [68] (see Table 1 for classification of genes). Quantitative polymerase chain reaction (PCR) was used to validate some of the array data and test the effects of SCH23390 on the METH-induced changes on the expression of a number of these transcription factors (see Figs 1–4).
Table 3.
Partial list of SCH233900-regulated genes in the rat striatum*.
| Gene Symbol | Gene Name |
|---|---|
| Bucs1 p | butyryl Coenzyme A synthetase 1 (_p) |
| Ccrk | cell cycle related kinase |
| Dok4 p | docking protein 4 (_p) |
| Edn1 | endothelin 1 |
| Efnb1 | ephrin B1 |
| Errfi1 | ERBB receptor feedback inhibitor 1 |
| Fgfrl1 | fibroblast growth factor receptor-like 1 |
| Gpd1 | glycerol-3-phosphate dehydrogenase 1 (soluble) |
| Hmga2 | high mobility group box 2 |
| Hmgb2 | high mobility group box 2 |
| Kif22 | kinesin family member 22 |
| Ms4a4a p | membrane-spanning 4-domains, subfamily A, member 4 (_p) |
| Mylk p | myosin, light polypeptide kinase (_p) |
| Nes | Nestin |
| Nfatc3 p | nuclear factor of activated T-cells, cytoplasmic, calcineurin-dependent 3 (_p) |
| Npy1r | neuropeptide Y receptor Y1 |
| Pdk4 | pyruvate dehydrogenase kinase, isoenzyme 4 |
| Phlda1 | pleckstrin homology-like domain, family A, member 1 |
| Phlpb | phospholipase B |
| Prps1l1 p | phosphoribosyl pyrophosphate synthetase 1-like 1 (_p) |
| Rap1gds1 p | RAP1, GTP-GDP dissociation stimulator 1 (_p) |
| Rps9 | ribosomal protein S9 |
| Sesn1 p | sestrin 1 (_p) |
| Sfrp2 | secreted frizzled-related protein 2 |
| Sgk | serum/glucocorticoid regulated kinase |
| Slc16a11 p | solute carrier family 16 (monocarboxylic acid transporters), member 11 (_p) |
| Slc17a6 | solute carrier family 17 (sodium-dependent inorganic phosphate cotransporter), member 6 |
| Slc19a3 p | solute carrier family 19, member 3 (_p) |
| Sntg2 p | syntrophin, gamma 2 (_p) |
| St3gal1 | ST3 beta-galactoside alpha-2,3-sialyltransferase 1 |
| Tff3 | trefoil factor 3 |
| Tmem109 | transmembrane protein 109 |
| Tmtc3 p | transmembrane and tetratricopeptide repeat containing 3 (_p) |
| Tor1b | torsin family 1, member B |
| Ubl4a p | ubiquitin-like 4a (_p) |
| Vamp1 | vesicle-associated membrane protein 1 |
The genes in Bold print were upregulated while the other genes were downregulated.
Fig. 1. Quantitative PCR analyses of SCH23390-mediated inhibition of METH-induced expression of AP1 transcription factors.
The levels of mRNA were measured by real-time quantitative PCR; mRNA levels were normalized to 18s RNA. Data represent means ± SEM of fold changes relative to the controls (n = 6 rats per group). Statistical significance was determined by ANOVA followed by protected least-squares difference (PLSD). $ = p <0.05, M vs. C; # = p < 0.05, M vs. M + S. Abbreviations are C, control; S, SCH23390; M, methamphetamine, and M + S, methamphetamine plus SCH23390.
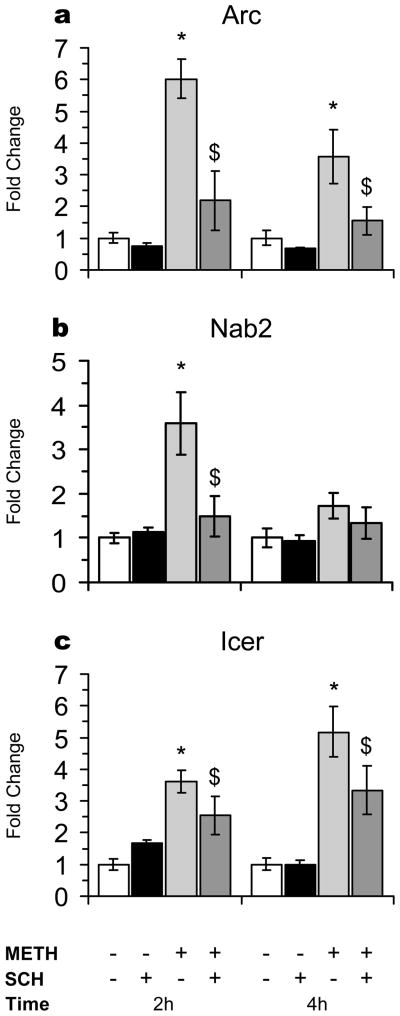
Fig. 4. Quantitative PCR analyses of the effects of METH and SCH23390 on the expression of Arc, Icer, and Nab2.
The levels of mRNA were measured by real-time quantitative PCR; mRNA levels were normalized to 18s RNA. Data represent means ± SEM of fold changes relative to the controls (n = 6 rats per group). Statistical significance was determined by ANOVA followed by protected least-squares difference (PLSD). $ = p <0.05, M vs. C; # = p < 0.05, M vs. M + S. Abbreviations are C, control; S, SCH23390; M, methamphetamine, and M + S, methamphetamine plus SCH23390.
The effects of METH on four members of AP-1 transcription factors [97] are shown in Fig. 1. Mammalian AP-1 proteins are composed of homodimers and heterodimers of basic region leucine sipper (bZIP) proteins that include c-Fos, FosB, Fra-1, and Fra2, c-Jun, JunB, and JunD [98]. Most of the studies on DA-induced regulation of gene expression have focused on c-Fos expression. Here, we show that a single METH injection can also cause substantial DA D1-dependent increases in Fra1 expression at both 2 and 4 hours after METH. METH also caused significant D1-receptor-dependent increases in Fra2 expression at the two time points. METH-induced increases in c-Jun expression were totally blocked by pretreatment with the D1 receptor antagonist, SCH23390, with JunB expression showing similar SCH23390-sensitive METH-induced changes.
Figure 2 shows the results of the METH injection on the expression of the Egr family of IEGs [99]. These include Egr1 (Krox-1, NGF1A, Zif268), Egr2 (Krox20, NGF1B), Egr3 (Pilot), and Egr4 (NGF1C). Egr1 and Egr2 mRNA levels showed significant METH-induced increases that were blocked by SCH23390. Egr3 expression was induced at the 4-hr but not at the 2-hr time point. The changes in Egr3 were also blocked by a D1 antagonist. In contrast, there were no significant METH-induced changes in Egr4 expression at the times in which measurements were taken. Our findings on METH-induced expression of the Egr members of transcription factors expand those of previous investigations that had discussed only Egr1 by providing information on the influence of METH on all four Egr transcription factors. Indeed similar to our observations, Wang and McGinty [100] had reported that pretreatment with SCH23390 was able to block METH-induced changes in Zif268 (Egr1).
Fig. 2. Quantitative PCR analyses of the effects of METH and SCH23390 on the expression of Egr family of transcription factors.
The levels of mRNA were measured by real-time quantitative PCR; mRNA levels were normalized to 18s RNA. Data represent means ± SEM of fold changes relative to the controls (n = 6 rats per group). Statistical significance was determined by ANOVA followed by protected least-squares difference (PLSD). $ = p <0.05, M vs. C; # = p < 0.05, M vs. M + S. Abbreviations are C, control; S, SCH23390; M, methamphetamine, and M + S, methamphetamine plus SCH23390.
Figure 3 shows that the METH injection used in our study also caused substantial increases in the expression of the members of the nuclear receptor Nr4a superfamily which includes Nr4a1/nur77/NGFI-B, Nr4a2/Nurr1 and Nr4a3/NOR-1 [101]. Nr4a1/Nur77 expression was induced by METH in a SCH23390-sensitive fashion. There were also D1-dependent METH-induced increases in Nr4a2/Nurr1. Nr4a3/Nor1 expression was also significantly increased by METH but, unexpectedly, SCH23390 potentiated these METH-induced changes. These results suggest that Nr4a3/Nor1 might play roles that are distinct from those of the other two Nr4a family members.
Fig. 3. Quantitative PCR analyses of the effects of METH and SCH23390 on the expression of Nr4a family of transcription factors.
The levels of mRNA were measured by real-time quantitative PCR; mRNA levels were normalized to 18s RNA. Data represent means ± SEM of fold changes relative to the controls (n = 6 rats per group). Statistical significance was determined by ANOVA followed by protected least-squares difference (PLSD). $ = p <0.05, M vs. C; # = p < 0.05, M vs. M + S. Abbreviations are C, control; S, SCH23390; M, methamphetamine, and M + S, methamphetamine plus SCH23390.
Figure 4 shows the effects of METH-induced changes on Arc, ICER/CREM, and Nab2 expression in the rat striatum. METH caused increases in Arc expression in a SCH23390-sensitive fashion. There were also significant METH-mediated increases in Nab2 expression which were blocked by SCH23390. Moreover, ICER was induced by METH. Pretreatment of the animals with SCH23390 partially blocked the the effects of METH on ICER expression. These results are consistent with the recent report that amphetamine induced ICER expression in the rat nucleus accumbens [102] and with the fact that toxic doses of METH also caused increases in cAMP responsive element modulator (CREM) expression at 3 hours after injection of METH [103]. ICER is a member of a group of proteins produced from the CREM/ICER gene by use of an internal promoter within an intron of the CREM gene [104]. ICER functions as a repressor of transcription of several CREB target genes [104,105]. The observations suggest that METH- and amphetamine (AMPH)-induced ICER expression might serve a negative-feedback role against CREB-mediated transcription. In other words, because ICER itself is a target gene for CREB-mediated transcription, which also occurs via stimulation of DA D1 receptors, its induction by METH and AMPH might serve to suppress more delayed drug-induced gene transcription. The METH-induced change in Nab2 is interesting in this respect because Nab2 is an Egr1 co-repressor [106] which can also be induced by Egr1 [107].
Members of the nuclear factor of activated T-cells (NFAT) family of transcription factors are important transcription factors that participate in a number of functions in the mammalian brain [108]. Consistent with these observations, METH has been shown to cause substantial D1-mediated changes in the expression of members of this family of transcription factors [109]. Specifically, METH administration caused shuttling of NFATc3 and NFATc4 from the cytoplasm into the nucleus. These changes were dependent on METH-induced activation of calcineurin and were blocked by the SCH23390 [109]. These results are consistent with the findings of Groth et al. [110] who reported that stimulation of D1 receptors can stimulate NFAT-dependent transcription in striatal cell cultures.
NEUROPEPTIDES
In addition to high levels of monoamines, the basal ganglia contain various neuropeptides that participate in the modulation of the pharmacology and physiology of motoric, reward-related and cognitive behaviors [111,112]. These include dynorphin, enkephalin, neuropetide Y, neurotensin, somatostatin, and substance P (SP) that have also been shown to be affected, to different degrees, by various dopaminergic agents [73,112,113]. Subacute treatment of rats with the D1 agonist, SKF38393, caused decreases in SP levels in the substantia nigra [114]. Intermittent administration of SKF38393 induced increases in dynorphin levels [115]. The D1 receptor agonist, SKF82958, also caused SCH23390-sensitive increases in the expression of striatal preprodynorphin (PPD) and SP [116]. Repeated administration of SCH23390 to rats caused increases in the SP immunoreactivity in the striatum [117]. Using organotypic striatal slices, Campbell and Walker [118] reported that SKF38393 increased preprotachykinin (PPT) mRNA expression that were blocked by SCH23390. SKF82958 also increased PPD and SP mRNA levels in the mouse striatum in a dose-dependent manner [119]. Campbell et al. [120] examined the effects of SKF82958 in rats and also reported drug-induced increases in striatal PPT mRNA in the rat brain. Low but not high doses of METH also caused increases in the extracellular concentration of SP in the substantia nigra, increases that were also blocked by D1 receptor antagonism [121]. Mice with targeted disruption of DARPP-32 show normal basal expression of D1 and D2 receptors and exhibit normal expression levels of SP and prodynorphin [80]. However, SKF82958 produced greater up-regulation of these neuropeptides in wild-type mice than in mice lacking DARPP-32, indicating the importance of DARPP-32 in DA receptor-mediated regulation of neuropetide gene expression in the basal ganglia [80].
Neurotensin (NT) is a neuropeptide that is very sensitive to perturbations in DA-mediated signaling and is thought to be involved in pathophysiological states in the CNS [122]. NT has been implicated in the pathogenesis of neuropsychiatric disorders including schizophrenia [123]. Single or multiple high doses of METH induced significant increase in the content of neurotensin-like immunoreactivity (NTLI) in the nucleus accumbens that could be prevented by SCH23390 [124]. SKF82958 increased striatal NT mRNA levels that were enhanced by prior 6-OHDA-induced depletion of striatal DA [125]. More recently, St-Hilaire et al. [126] reported that destruction of the nigrostriatal DA system by 6-OHDA results in significant increases in NT mRNA expression in striatal enkephalin (ENK)-positive cells. Repeated injections of L-DOPA caused further increases in NT mRNA levels which were blocked by SCH23390.
Neuropeptide Y (NPY) is a neuropeptide that has neuroprotective properties in several models of neurodegeneration including METH-induced neurotoxicity [127]. Intermittent injections of SKF38393 reduced NPY expression in the rodent striatum [115]. METH administration decreased NPY levels in specific regions of the nucleus accumbens and the caudate in a SCH-23390-sensitive fashion [128]. Injections of multiple high doses of METH, however, were shown to cause marked increases in striatal preproNPY (ppNPY) mRNA expression [129]. The increases in the number of ppNPY mRNA-expressing neurons were blocked by SCH22390 [129].
TROPHIC FACTORS
The use of psychostimulants is associated with structural changes that include modification of dendrites and dendritic spines in the rat brain [130]. These structural changes are probably related to changes in the expression of several genes including Arc and other transcription factors that might arise in a D1 receptor-dependent fashion [131,132]. Nevertheless, the recent reports that stimulation of D1 receptors can also affect the expression of some trophic factors suggest that D1 receptors can have effects beyond those traditionally associated with stimulation of these receptors. For example, the D1 receptor-selective agonist, SKF38393, was shown to increase brain-derived neurotrophic factor (BDNF) mRNA and protein levels in striatal cultures, effects that were inhibited by SCH23390 [133]. A subsequent study also reported that the SKF38393 also caused increases in BDNF protein in striatal and hippocampal tissue slices [134]. Another trophic factor of interest is fibroblast growth factor-2 (FGF-2) which is synthesized in and secreted by glial cells in the adult brain [135]. Activation of D1 receptors stimulates FGF-2 biosynthesis and secretion in astrocytes, in a PI-dependent fashion [136]. Moreover, blockade of intracellular calcium oscillation by IP3 inhibitors inhibited SKF83959-induced increases in FGF-2 expression [136]. When taken together with previous observations, these data indicate a potentially significant role of D1-induced PI turnover in the regulation of gene expression and synaptic plasticity induced by drugs, such as the psychostimulants, that cause marked increases in DA levels in various brain regions [83,137,138]. These ideas remain to be investigated fully.
DOPAMINE, DA D1 RECEPTORS, AND NEURODEGENERATION
Dopamine is a neurotransmitter that has recently associated with the regulation of neuronal apoptosis. For example, intrastriatal DA injections can cause DNA damage and apoptosis in the rat brain [139,140]. Striatal cells exposed to DA are also killed in vitro [141–144]. Various DA concentrations have also been shown to be toxic to several cell types including cortical cells, mesencephalic cells, striatal cells, and some established cell lines [145–150]. The manner by which DA exerts its toxic effects have been attributed, mainly, to the production of reactive quinones and other reactive species that are generated by oxidative DA metabolism [151–153]. More recently, however, substantial evidence has been put forward to suggest that stimulation of D1 receptors might also participate in DA-induced degeneration [154]. For example, chronic treatment of human SK-N-MC neuroblastoma cells with DA induces death of these cells [155]. Treatment with either the antioxidant, sodium metabisulfite, or the D1 antagonist, SCH 23390, was able to partially block the toxic effect of DA. When used together, these two agents completely blocked DA-induced cell death. In accord with a D1 receptor mode of neurotoxicity, Importantly, activation of D1 receptors with the D1 agonist SKF38393 also killed neuroblastoma and striatal cells [155]. Follow-up studies by these investigators have also revealed that DA can cause activation of p-ERK1/2, p-JNK and p-p38 MAPK [156]. In contrast, SKF38393 caused SCH23390-sensitive p-ERK1/2, but not p-JNK or p-p38 MAPK activation. Interestingly, D1-induced ERK activation was reported to cause oxidative stress and cell death. Co-immunoprecipitation studies revealed that p-ERK formed stable heterotrimeric complexes with D1 receptors and beta-arrestin2 [156]. These reports are supported by studies with METH,- Authors – what are you referring to here? METH? -which also caused SCH23390-inhibitable cell death in the rodent brain.
METH that causes its biochemical effects by causing large increases in DA release in the brain [137], can also cause degeneration of neurons of several brain regions [157,158]. These toxic effects occur via the activation of apoptotic pathways including increased production of prodeath members of the Bcl-2 family of proteins [78], induction of ER stress [68,159] and up-regulation of the calcineurin/NFAT/Fas ligand death pathway [109]. Recent studies from various laboratories have indicated that METH-induced cell death is dependent on stimulation of D1 receptors because pretreatment with the D1 antagonist, SCH23390, blocks METH-induced neurodegeneration [68,94,109] (see Krasnova and Cadet [94] for a recent review). METH-induced toxicity occurs via activation of SCH23390-sensitive calcineurin-dependent gene expression [109]. The phosphatase, calcineurin is a well known mediator of several cellular responses due to calcium overload and oxidative stress [160]. When activated by calcium, calcineurin, dephosphorylates NFAT, which translocates to the nucleus and activates genes that contribute to growth [160] or apoptosis [161], depending on the trigger. In the case of METH, we have shown that METH administration activates SCH23390-inhibitable calcineurin/NFAT-mediated increases in FasL expression and neuronal apoptosis in the rat brain [109], thus supporting the idea that DA can cause neurodegeneration by activating DA D1 receptors [155].
FUTURE PROSPECTS
In summary, the catecholamine neurotransmitter, DA, exerts significant influence on gene expression via stimulation of the DA D1 receptors located in various brain regions, but principally in the mammalian striatum. The DA D1-mediated changes have been shown to occur in genes that are involved in many functions in the brain. These include several IEGs, neuropeptides, and trophic factors. Recent use of microarray analysis has expanded the list of genes that might be involved to include several unexpected genes including genes that are involved in ER stress and in the induction of neuronal death. DA, itself, has also been shown to cause cell death through enhancement of oxidative stress and by stimulation of D1-mediated toxic events. The prevailing data suggest that METH, which causes release of large amounts of DA in the brain, produce its neurotoxic effects via events that include DA D1-medaated ER stress. The use of pharmacological agonists and antagonists in conjunction with mutant mice with targeted gene deletion of D1 receptors have helped to advance scientific understanding in this field [162]. Nevertheless, much remains to be done in order to completely elucidate the various functional roles of the D1 receptor subtype in the brain. For example, tt will be important to determine to what extent stimulation of the cAMP/PKA or the PI/PKC limb of D1-meidated signal transduction cascade might be more or less involved in D1 receptor-mediated changes in specific genes or in specific functional or behavioral changes in animal model systems. Such understanding will also improve our ability to control the pathophysiological conditions underlying these neural systems.
ABBREVIATIONS
- AP-1
activating protein 1
- cAMP
cyclic adenosine monophosphate
- CREB
cAMP response element binding protein
- CREM
cAMP responsive element modulator
- DA
Dopamine
- DARPP-32
dopamine and cAMP regulated phosphoprotein 32 kDa
- EGFP
enhanced green fluorescent protein
- Egr
early growth response
- ER
endoplasmic reticulum
- FGF-2
fibroblast growth factor-2
- GTP
guanine triphosphate
- IEGs
immediate early genes
- METH
methamphetamine
- MSN
medium spiny neurons
- NFAT
nuclear factor of activated T-cells
- NPY
Neuropeptide Y
- NT
Neurotensin
- 6-OHDA
6-hydroxydopamine
- PI
phosphatidylinositol
- PKA
protein kinase A
- PLC
phospholipase C
- PSD-95
postsynaptic density-95
- SP
substance-P
References
- 1.Harley CW. Norepinephrine and dopamine as learning signals. Neural Plast. 2004;11:191–204. doi: 10.1155/NP.2004.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fallon JH. Topographic organization of ascending dopaminergic projections. Ann N Y Acad Sci. 1988;537:1–9. doi: 10.1111/j.1749-6632.1988.tb42093.x. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez-Castellanos J, Graybiel AM. Subdivisions of the dopamine-containing A8-A9-A10 complex identified by their differential mesostriatal innervation of striosomes and extrastriosomal matrix. Neuroscience. 1987;23:223–242. doi: 10.1016/0306-4522(87)90285-5. [DOI] [PubMed] [Google Scholar]
- 4.Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Chinta SJ, Andersen JK. Dopaminergic neurons. Int J Biochem Cell Biol. 2005;37:942–946. doi: 10.1016/j.biocel.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Cadet JL. A unifying theory of movement and madness: involvement of free radicals in disorders of the isodendritic core of the brainstem. Med Hypotheses. 1988;27:59–63. doi: 10.1016/0306-9877(88)90085-0. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry. 2006;39(Suppl 1):S10–14. doi: 10.1055/s-2006-931483. [DOI] [PubMed] [Google Scholar]
- 8.Hornykiewicz O. Basic research on dopamine in Parkinson’s disease and the discovery of the nigrostriatal dopamine pathway: the view of an eyewitness. Neurodegener Dis. 2008;5:114–117. doi: 10.1159/000113678. [DOI] [PubMed] [Google Scholar]
- 9.Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahn S. The history of dopamine and levodopa in the treatment of Parkinson’s disease. Mov Disord. 2008;23(Suppl 3):S497–508. doi: 10.1002/mds.22028. [DOI] [PubMed] [Google Scholar]
- 11.Peroutka SJ, Synder SH. Relationship of neuroleptic drug effects at brain dopamine, serotonin, alpha-adrenergic, and histamine receptors to clinical potency. Am J Psychiatry. 1980;137:1518–1522. doi: 10.1176/ajp.137.12.1518. [DOI] [PubMed] [Google Scholar]
- 12.Kebabian JW, Petzold GL, Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the “dopamine receptor”. Proc Natl Acad Sci U S A. 1972;69:2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onali P, Olianas MC, Gessa GL. Selective blockade of dopamine D-1 receptors by SCH 23390 discloses striatal dopamine D-2 receptors mediating the inhibition of adenylate cyclase in rats. Eur J Pharmacol. 1984;99:127–128. doi: 10.1016/0014-2999(84)90445-x. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DM, Bier-Laning CM, Halford MK, Ahlijanian MK, Zahniser NR. Dopamine, acting through D-2 receptors, inhibits rat striatal adenylate cyclase by a GTP-dependent process. Mol Pharmacol. 1986;29:113–119. [PubMed] [Google Scholar]
- 15.Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S. Dopamine receptors--physiological understanding to therapeutic intervention potential. Pharmacol Ther. 1999;84:133–156. doi: 10.1016/s0163-7258(99)00029-7. [DOI] [PubMed] [Google Scholar]
- 16.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 17.Niznik HB, Van Tol HH. Dopamine receptor genes: new tools for molecular psychiatry. J Psychiatry Neurosci. 1992;17:158–180. [PMC free article] [PubMed] [Google Scholar]
- 18.Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;33:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- 19.Monsma FJ, Jr, Mahan LC, McVittie LD, Gerfen CR, Sibley DR. Molecular cloning and expression of a D1 dopamine receptor linked to adenylyl cyclase activation. Proc Natl Acad Sci U S A. 1990;87:6723–6727. doi: 10.1073/pnas.87.17.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunahara RK, Guan HC, O’Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, Niznik HB. Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature. 1991;350:614–619. doi: 10.1038/350614a0. [DOI] [PubMed] [Google Scholar]
- 21.Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–7836. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi WS, Machida CA, Ronnekleiv OK. Distribution of dopamine D1, D2, and D5 receptor mRNAs in the monkey brain: ribonuclease protection assay analysis. Brain Res Mol Brain Res. 1995;31:86–94. doi: 10.1016/0169-328x(95)00038-t. [DOI] [PubMed] [Google Scholar]
- 23.Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 24.Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 25.Savasta M, Dubois A, Scatton B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res. 1986;375:291–301. doi: 10.1016/0006-8993(86)90749-3. [DOI] [PubMed] [Google Scholar]
- 26.Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wamsley JK, Gehlert DR, Filloux FM, Dawson TM. Comparison of the distribution of D-1 and D-2 dopamine receptors in the rat brain. J Chem Neuroanat. 1989;2:119–137. [PubMed] [Google Scholar]
- 28.Fremeau RT, Jr, Duncan GE, Fornaretto MG, Dearry A, Gingrich JA, Breese GR, Caron MG. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive affective and neuroendocrine aspects of dopaminergic neurotransmission. Proc Natl Acad Sci U S A. 1991;88:3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mengod G, Villaro MT, Landwehrmeyer GB, Martinez-Mir MI, Niznik HB, Sunahara RK, Seeman P, O’Dowd BF, Probst A, Palacios JM. Visualization of dopamine D1, D2 and D3 receptor mRNAs in human and rat brain. Neurochem Int. 1992;20(Suppl):33S–43S. doi: 10.1016/0197-0186(92)90208-9. [DOI] [PubMed] [Google Scholar]
- 30.Langley KC, Bergson C, Greengard P, Ouimet CC. Co-localization of the D1 dopamine receptor in a subset of DARPP-32-containing neurons in rat caudate-putamen. Neuroscience. 1997;78:977–983. doi: 10.1016/s0306-4522(96)00583-0. [DOI] [PubMed] [Google Scholar]
- 31.Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 33.Altar CA, Marien MR. Picomolar affinity of 125I-SCH 23982 for D1 receptors in brain demonstrated with digital subtraction autoradiography. J Neurosci. 1987;7:213–222. doi: 10.1523/JNEUROSCI.07-01-00213.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger K, Przedborski S, Cadet JL. Retrograde degeneration of nigrostriatal neurons induced by intrastriatal 6-hydroxydopamine injection in rats. Brain Res Bull. 1991;26:301–307. doi: 10.1016/0361-9230(91)90242-c. [DOI] [PubMed] [Google Scholar]
- 35.Cadet JL, Last R, Kostic V, Przedborski S, Jackson-Lewis V. Long-term behavioral and biochemical effects of 6-hydroxydopamine injections in rat caudate-putamen. Brain Res Bull. 1991;26:707–713. doi: 10.1016/0361-9230(91)90164-f. [DOI] [PubMed] [Google Scholar]
- 36.Augood SJ, Faull RL, Emson PC. Dopamine D1 and D2 receptor gene expression in the striatum in Huntington’s disease. Ann Neurol. 1997;42:215–221. doi: 10.1002/ana.410420213. [DOI] [PubMed] [Google Scholar]
- 37.Weeks RA, Piccini P, Harding AE, Brooks DJ. Striatal D1 and D2 dopamine receptor loss in asymptomatic mutation carriers of Huntington’s disease. Ann Neurol. 1996;40:49–54. doi: 10.1002/ana.410400110. [DOI] [PubMed] [Google Scholar]
- 38.Deng YP, Albin RL, Penney JB, Young AB, Anderson KD, Reiner A. Differential loss of striatal projection systems in Huntington’s disease: a quantitative immunohistochemical study. J Chem Neuroanat. 2004;27:143–164. doi: 10.1016/j.jchemneu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Kebabian JW, Greengard P. Dopamine-sensitive adenyl cyclase: possible role in synaptic transmission. Science. 1971;174:1346–1349. doi: 10.1126/science.174.4016.1346. [DOI] [PubMed] [Google Scholar]
- 40.Clement-Cormier YC, Kebabian JW, Petzold GL, Greengard P. Dopamine-sensitive adenylate cyclase in mammalian brain: a possible site of action of antipsychotic drugs. Proc Natl Acad Sci U S A. 1974;71:1113–1117. doi: 10.1073/pnas.71.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izenwasser S, Katz JL. Differential efficacies of dopamine D1 receptor agonists for stimulating adenylyl cyclase in squirrel monkey and rat. Eur J Pharmacol. 1993;246:39–44. doi: 10.1016/0922-4106(93)90007-v. [DOI] [PubMed] [Google Scholar]
- 42.Dearry A, Gingrich JA, Falardeau P, Fremeau RT, Jr, Bates MD, Caron MG. Molecular cloning and expression of the gene for a human D1 dopamine receptor. Nature. 1990;347:72–76. doi: 10.1038/347072a0. [DOI] [PubMed] [Google Scholar]
- 43.Prasad KN, Gilmer KN. Demonstration of dopamine-sensitive adenylate cyclase in malignant neuroblastoma cells and change in sensitivity of adenylate cyclase to catecholamines in “differentiated” cells. Proc Natl Acad Sci U S A. 1974;71:2525–2529. doi: 10.1073/pnas.71.6.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clement-Cormier YC, Robison GA. Adenylate cyclase from various dopaminergic areas of the brain and the action of antipsychotic drugs. Biochem Pharmacol. 1977;26:1719–1722. doi: 10.1016/0006-2952(77)90151-4. [DOI] [PubMed] [Google Scholar]
- 45.Coronas V, Krantic S, Jourdan F, Moyse E. Dopamine receptor coupling to adenylyl cyclase in rat olfactory pathway: a combined pharmacological-radioautographic approach. Neuroscience. 1999;90:69–78. doi: 10.1016/s0306-4522(98)00460-6. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Vinuela A, Neely MH, Hallett PJ, Grant SG, Miller GM, Isacson O, Caron MG, Yao WD. Inhibition of the dopamine D1 receptor signaling by PSD-95. J Biol Chem. 2007;282:15778–15789. doi: 10.1074/jbc.M611485200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra RK, Gardner EL, Katzman R, Makman MH. Enhancement of dopamine-stimulated adenylate cyclase activity in rat caudate after lesions in substantia nigra: evidence for denervation supersensitivity. Proc Natl Acad Sci U S A. 1974;71:3883–3887. doi: 10.1073/pnas.71.10.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krueger BK, Forn J, Walters JR, Roth RH, Greengard P. Stimulation by dopamine of adenosine cyclic 3′,5′-monophosphate formation in rat caudate nucleus: effect of lesions of the nigro-neostriatal pathway. Mol Pharmacol. 1976;12:639–648. [PubMed] [Google Scholar]
- 49.Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin JP, Glowinski J, Girault JA. G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci. 1993;13:2237–2248. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuang X, Belluscio L, Hen R. G(olf)alpha mediates dopamine D1 receptor signaling. J Neurosci. 2000;20:RC91. doi: 10.1523/JNEUROSCI.20-16-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herve D, Rogard M, Levi-Strauss M. Molecular analysis of the multiple Golf alpha subunit mRNAs in the rat brain. Brain Res Mol Brain Res. 1995;32:125–134. doi: 10.1016/0169-328x(95)00070-9. [DOI] [PubMed] [Google Scholar]
- 52.Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 53.Jones DT, Reed RR. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989;244:790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- 54.Kuroiwa M, Bateup HS, Shuto T, Higashi H, Tanaka M, Nishi A. Regulation of DARPP-32 phosphorylation by three distinct dopamine D1-like receptor signaling pathways in the neostriatum. J Neurochem. 2008;107:1014–1026. doi: 10.1111/j.1471-4159.2008.05702.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, O’Dowd BF, George SR. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- 56.Mahan LC, Burch RM, Monsma FJ, Jr, Sibley DR. Expression of striatal D1 dopamine receptors coupled to inositol phosphate production and Ca2+ mobilization in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990;87:2196–2200. doi: 10.1073/pnas.87.6.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- 58.Wang HY, Undie AS, Friedman E. Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine-mediated inositol phosphate formation. Mol Pharmacol. 1995;48:988–994. [PubMed] [Google Scholar]
- 59.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 60.Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85:378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- 61.Jin LQ, Wang HY, Friedman E. Stimulated D(1) dopamine receptors couple to multiple Galpha proteins in different brain regions. J Neurochem. 2001;78:981–990. doi: 10.1046/j.1471-4159.2001.00470.x. [DOI] [PubMed] [Google Scholar]
- 62.Tang TS, Bezprozvanny I. Dopamine receptor-mediated Ca(2+) signaling in striatal medium spiny neurons. J Biol Chem. 2004;279:42082–42094. doi: 10.1074/jbc.M407389200. [DOI] [PubMed] [Google Scholar]
- 63.Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma LQ, Liu C, Wang F, Xie N, Gu J, Fu H, Wang JH, Cai F, Liu J, Chen JG. Activation of phosphatidylinositol-linked novel D1 dopamine receptors inhibits high-voltage-activated Ca2+ currents in primary cultured striatal neurons. J Neurophysiol. 2009;101:2230–2238. doi: 10.1152/jn.90345.2008. [DOI] [PubMed] [Google Scholar]
- 65.Wu J, Dougherty JJ, Nichols RA. Dopamine receptor regulation of Ca2+ levels in individual isolated nerve terminals from rat striatum: comparison of presynaptic D1-like and D2-like receptors. J Neurochem. 2006;98:481–494. doi: 10.1111/j.1471-4159.2006.03901.x. [DOI] [PubMed] [Google Scholar]
- 66.Lebel M, Patenaude C, Allyson J, Massicotte G, Cyr M. Dopamine D1 receptor activation induces tau phosphorylation via cdk5 and GSK3 signaling pathways. Neuropharmacology. 2009;57:392–402. doi: 10.1016/j.neuropharm.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 67.Jessberger S, Gage FH, Eisch AJ, Lagace DC. Making a neuron: Cdk5 in embryonic and adult neurogenesis. Trends Neurosci. 2009;32:575–582. doi: 10.1016/j.tins.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jayanthi S, McCoy MT, Beauvais G, Ladenheim B, Gilmore K, Wood W, 3rd, Becker K, Cadet JL. Methamphetamine induces dopamine D1 receptor-dependent endoplasmic reticulum stress-related molecular events in the rat striatum. PLoS ONE. 2009;4:e6092. doi: 10.1371/journal.pone.0006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR. A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci. 1998;18:5301–5310. doi: 10.1523/JNEUROSCI.18-14-05301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berretta S, Robertson HA, Graybiel AM. Dopamine and glutamate agonists stimulate neuron-specific expression of Fos-like protein in the striatum. J Neurophysiol. 1992;68:767–777. doi: 10.1152/jn.1992.68.3.767. [DOI] [PubMed] [Google Scholar]
- 71.Bhat RV, Baraban JM. Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exp Ther. 1993;267:496–505. [PubMed] [Google Scholar]
- 72.Cole AJ, Bhat RV, Patt C, Worley PF, Baraban JM. D1 dopamine receptor activation of multiple transcription factor genes in rat striatum. J Neurochem. 1992;58:1420–1426. doi: 10.1111/j.1471-4159.1992.tb11358.x. [DOI] [PubMed] [Google Scholar]
- 73.Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 74.Konradi C, Cole RL, Heckers S, Hyman SE. Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci. 1994;14:5623–5634. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moratalla R, Robertson HA, Graybiel AM. Dynamic regulation of NGFI-A (zif268, egr1) gene expression in the striatum. J Neurosci. 1992;12:2609–2622. doi: 10.1523/JNEUROSCI.12-07-02609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang D, Zhang L, Lou DW, Nakabeppu Y, Zhang J, Xu M. The dopamine D1 receptor is a critical mediator for cocaine-induced gene expression. J Neurochem. 2002;82:1453–1464. doi: 10.1046/j.1471-4159.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- 77.Cadet JL, Jayanthi S, McCoy MT, Vawter M, Ladenheim B. Temporal profiling of methamphetamine-induced changes in gene expression in the mouse brain: Evidence from cDNA array. Synapse. 2001;41:40–48. doi: 10.1002/syn.1058. [DOI] [PubMed] [Google Scholar]
- 78.Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL. Methamphetamine causes differential regulation of pro-death and anti- death Bcl-2 genes in the mouse neocortex. FASEB J. 2001;15:1745–1752. doi: 10.1096/fj.01-0025com. [DOI] [PubMed] [Google Scholar]
- 79.Simpson CS, Morris BJ. Induction of c-fos and zif/268 gene expression in rat striatal neurons, following stimulation of D1-like dopamine receptors, involves protein kinase A and protein kinase C. Neuroscience. 1995;68:97–106. doi: 10.1016/0306-4522(95)00122-y. [DOI] [PubMed] [Google Scholar]
- 80.Svenningsson P, Fienberg AA, Allen PB, Moine CL, Lindskog M, Fisone G, Greengard P, Fredholm BB. Dopamine D(1) receptor-induced gene transcription is modulated by DARPP-32. J Neurochem. 2000;75:248–257. doi: 10.1046/j.1471-4159.2000.0750248.x. [DOI] [PubMed] [Google Scholar]
- 81.Nnadi CU, Mimiko OA, McCurtis HL, Cadet JL. Neuropsychiatric effects of cocaine use disorders. J Natl Med Assoc. 2005;97:1504–1515. [PMC free article] [PubMed] [Google Scholar]
- 82.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 83.Hurd YL, Ungerstedt U. Cocaine: an in vivo microdialysis evaluation of its acute action on dopamine transmission in rat striatum. Synapse. 1989;3:48–54. doi: 10.1002/syn.890030107. [DOI] [PubMed] [Google Scholar]
- 84.Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect. 2005;3:4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cenci MA, Campbell K, Wictorin K, Bjorklund A. Striatal c-fos Induction by Cocaine or Apomorphine Occurs Preferentially in Output Neurons Projecting to the Substantia Nigra in the Rat. Eur J Neurosci. 1992;4:376–380. doi: 10.1111/j.1460-9568.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D, Katz JL, Xu M. c-Fos facilitates the acquisition and extinction of cocaine-induced persistent changes. J Neurosci. 2006;26:13287–13296. doi: 10.1523/JNEUROSCI.3795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wirtshafter D, Osborn CV. The atypical dopamine D1 receptor agonist SKF 83959 induces striatal Fos expression in rats. Eur J Pharmacol. 2005;528:88–94. doi: 10.1016/j.ejphar.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guan X, Tao J, Li S. Dopamine D1 receptor, but not dopamine D2 receptor, is a critical regulator for acute cocaine-enhanced gene expression. Neurol Res. 2009;31:17–22. doi: 10.1179/174313208X332986. [DOI] [PubMed] [Google Scholar]
- 91.Jouvert P, Dietrich JB, Aunis D, Zwiller J. Differential rat brain expression of EGR proteins and of the transcriptional corepressor NAB in response to acute or chronic cocaine administration. Neuromolecular Med. 2002;1:137–151. doi: 10.1385/NMM:1:2:137. [DOI] [PubMed] [Google Scholar]
- 92.Dopheide JA, Pliszka SR. Attention-deficit-hyperactivity disorder: an update. Pharmacotherapy. 2009;29:656–679. doi: 10.1592/phco.29.6.656. [DOI] [PubMed] [Google Scholar]
- 93.Gold MS, Kobeissy FH, Wang KK, Merlo LJ, Bruijnzeel AW, Krasnova IN, Cadet JL. Methamphetamine- and trauma-induced brain injuries: comparative cellular and molecular neurobiological substrates. Biol Psychiatry. 2009;66:118–127. doi: 10.1016/j.biopsych.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang JQ, Smith AJ, McGinty JF. A single injection of amphetamine or methamphetamine induces dynamic alterations in c-fos, zif/268 and preprodynorphin messenger RNA expression in rat forebrain. Neuroscience. 1995;68:83–95. doi: 10.1016/0306-4522(95)00100-w. [DOI] [PubMed] [Google Scholar]
- 96.Jayanthi S, McCoy MT, Ladenheim B, Cadet JL. Methamphetamine causes coordinate regulation of SRC, cas, crk, and the jun N-terminal kinase-jun pathway. Mol Pharmacol. 2002;61:1124–1131. doi: 10.1124/mol.61.5.1124. [DOI] [PubMed] [Google Scholar]
- 97.Herdegen T, Waetzig V. AP-1 proteins in the adult brain: facts and fiction about effectors of neuroprotection and neurodegeneration. Oncogene. 2001;20:2424–2437. doi: 10.1038/sj.onc.1204387. [DOI] [PubMed] [Google Scholar]
- 98.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 99.Beckmann AM, Wilce PA. Egr transcription factors in the nervous system. Neurochem Int. 1997;31:477–510. doi: 10.1016/s0197-0186(96)00136-2. discussion 517–476. [DOI] [PubMed] [Google Scholar]
- 100.Wang JQ, McGinty JF. Differential effects of D1 and D2 dopamine receptor antagonists on acute amphetamine- or methamphetamine-induced up-regulation of zif/268 mRNA expression in rat forebrain. J Neurochem. 1995;65:2706–2715. doi: 10.1046/j.1471-4159.1995.65062706.x. [DOI] [PubMed] [Google Scholar]
- 101.Li QX, Ke N, Sundaram R, Wong-Staal F. NR4A1, 2, 3--an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histol Histopathol. 2006;21:533–540. doi: 10.14670/HH-21.533. [DOI] [PubMed] [Google Scholar]
- 102.Green TA, Alibhai IN, Hommel JD, DiLeone RJ, Kumar A, Theobald DE, Neve RL, Nestler EJ. Induction of inducible cAMP early repressor expression in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. J Neurosci. 2006;26:8235–8242. doi: 10.1523/JNEUROSCI.0880-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thomas DM, Francescutti-Verbeem DM, Liu X, Kuhn DM. Identification of differentially regulated transcripts in mouse striatum following methamphetamine treatment--an oligonucleotide microarray approach. J Neurochem. 2004;88:380–393. doi: 10.1046/j.1471-4159.2003.02182.x. [DOI] [PubMed] [Google Scholar]
- 104.Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- 105.Tinti C, Conti B, Cubells JF, Kim KS, Baker H, Joh TH. Inducible cAMP early repressor can modulate tyrosine hydroxylase gene expression after stimulation of cAMP synthesis. J Biol Chem. 1996;271:25375–25381. doi: 10.1074/jbc.271.41.25375. [DOI] [PubMed] [Google Scholar]
- 106.Svaren J, Sevetson BR, Apel ED, Zimonjic DB, Popescu NC, Milbrandt J. NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol Cell Biol. 1996;16:3545–3553. doi: 10.1128/mcb.16.7.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kumbrink J, Gerlinger M, Johnson JP. Egr-1 induces the expression of its corepressor nab2 by activation of the nab2 promoter thereby establishing a negative feedback loop. J Biol Chem. 2005;280:42785–42793. doi: 10.1074/jbc.M511079200. [DOI] [PubMed] [Google Scholar]
- 108.Nguyen T, Di Giovanni S. NFAT signaling in neural development and axon growth. Int J Dev Neurosci. 2008;26:141–145. doi: 10.1016/j.ijdevneu.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Groth RD, Weick JP, Bradley KC, Luoma JI, Aravamudan B, Klug JR, Thomas MJ, Mermelstein PG. D1 dopamine receptor activation of NFAT-mediated striatal gene expression. Eur J Neurosci. 2008;27:31–42. doi: 10.1111/j.1460-9568.2007.05980.x. [DOI] [PubMed] [Google Scholar]
- 111.Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 112.Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res Brain Res Rev. 1994;19:1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 113.Augood SJ, Westmore K, Faull RL, Emson PC. Neuroleptics and striatal neuropeptide gene expression. Prog Brain Res. 1993;99:181–199. doi: 10.1016/s0079-6123(08)61346-1. [DOI] [PubMed] [Google Scholar]
- 114.Sonsalla PK, Gibb JW, Hanson GR. Opposite responses in the striato-nigral substance P system to D1 and D2 receptor activation. Eur J Pharmacol. 1984;105:185–187. doi: 10.1016/0014-2999(84)90666-6. [DOI] [PubMed] [Google Scholar]
- 115.Engber TM, Boldry RC, Kuo S, Chase TN. Dopaminergic modulation of striatal neuropeptides: differential effects of D1 and D2 receptor stimulation on somatostatin, neuropeptide Y, neurotensin, dynorphin and enkephalin. Brain Res. 1992;581:261–268. doi: 10.1016/0006-8993(92)90716-m. [DOI] [PubMed] [Google Scholar]
- 116.Wang JQ, McGinty JF. The full D1 dopamine receptor agonist SKF-82958 induces neuropeptide mRNA in the normosensitive striatum of rats: regulation of D1/D2 interactions by muscarinic receptors. J Pharmacol Exp Ther. 1997;281:972–982. [PubMed] [Google Scholar]
- 117.Oblin A, Zivkovic B, Bartholini G. Selective antagonists of dopamine receptor subtypes differentially affect substance P levels in the striatum and substantia nigra. Brain Res. 1987;421:387–390. doi: 10.1016/0006-8993(87)91314-x. [DOI] [PubMed] [Google Scholar]
- 118.Campbell BM, Walker PD. Striatal preprotachykinin mRNA levels are regulated by stimulatory agents and dopamine D1 receptor manipulation in rodent organotypic slice cultures. Brain Res. 2001;888:26–33. doi: 10.1016/s0006-8993(00)02997-8. [DOI] [PubMed] [Google Scholar]
- 119.Mao L, Conquet F, Wang JQ. Impaired preprodynorphin, but not preproenkephalin, mRNA induction in the striatum of mGluR1 mutant mice in response to acute administration of the full dopamine D(1) agonist SKF-82958. Synapse. 2002;44:86–93. doi: 10.1002/syn.10061. [DOI] [PubMed] [Google Scholar]
- 120.Campbell BM, Kreipke CW, Walker PD. Failure of MK-801 to suppress D1 receptor-mediated induction of locomotor activity and striatal preprotachykinin mRNA expression in the dopamine-depleted rat. Neuroscience. 2006;137:505–517. doi: 10.1016/j.neuroscience.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 121.Hanson GR, Bush L, Keefe KA, Alburges ME. Distinct responses of basal ganglia substance P systems to low and high doses of methamphetamine. J Neurochem. 2002;82:1171–1178. doi: 10.1046/j.1471-4159.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 122.St-Gelais F, Jomphe C, Trudeau LE. The role of neurotensin in central nervous system pathophysiology: what is the evidence? J Psychiatry Neurosci. 2006;31:229–245. [PMC free article] [PubMed] [Google Scholar]
- 123.Caceda R, Kinkead B, Nemeroff C. Peptides: Neurotensin, role in psychiatric and neurological diseases. 2006;27:2385–2404. doi: 10.1016/j.peptides.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 124.Merchant KM, Letter AA, Gibb JW, Hanson GR. Changes in the limbic neurotensin systems induced by dopaminergic drugs. Eur J Pharmacol. 1988;153:1–9. doi: 10.1016/0014-2999(88)90581-x. [DOI] [PubMed] [Google Scholar]
- 125.Hanson GR, Keefe KA. Dopamine D-1 regulation of caudate neurotensin mRNA in the presence or absence of the nigrostriatal dopamine pathway. Brain Res Mol Brain Res. 1999;66:111–121. doi: 10.1016/s0169-328x(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 126.St-Hilaire M, Landry E, Levesque D, Rouillard C. Denervation and repeated L-DOPA induce complex regulatory changes in neurochemical phenotypes of striatal neurons: implication of a dopamine D1-dependent mechanism. Neurobiol Dis. 2005;20:450–460. doi: 10.1016/j.nbd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 127.Thiriet N, Deng X, Solinas M, Ladenheim B, Curtis W, Goldberg SR, Palmiter RD, Cadet JL. Neuropeptide Y protects against methamphetamine-induced neuronal apoptosis in the mouse striatum. J Neurosci. 2005;25:5273–5279. doi: 10.1523/JNEUROSCI.4893-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Westwood SC, Hanson GR. Effects of stimulants of abuse on extrapyramidal and limbic neuropeptide Y systems. J Pharmacol Exp Ther. 1999;288:1160–1166. [PubMed] [Google Scholar]
- 129.Horner KA, Westwood SC, Hanson GR, Keefe KA. Multiple high doses of methamphetamine increase the number of preproneuropeptide Y mRNA-expressing neurons in the striatum of rat via a dopamine D1 receptor-dependent mechanism. J Pharmacol Exp Ther. 2006;319:414–421. doi: 10.1124/jpet.106.106856. [DOI] [PubMed] [Google Scholar]
- 130.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 131.Fujiyama K, Kajii Y, Hiraoka S, Nishikawa T. Differential regulation by stimulants of neocortical expression of mrt1, arc, and homer1a mRNA in the rats treated with repeated methamphetamine. Synapse. 2003;49:143–149. doi: 10.1002/syn.10220. [DOI] [PubMed] [Google Scholar]
- 132.Kodama M, Akiyama K, Ujike H, Shimizu Y, Tanaka Y, Kuroda S. A robust increase in expression of arc gene, an effector immediate early gene, in the rat brain after acute and chronic methamphetamine administration. Brain Res. 1998;796:273–283. doi: 10.1016/s0006-8993(98)00349-7. [DOI] [PubMed] [Google Scholar]
- 133.Kuppers E, Beyer C. Dopamine regulates brain-derived neurotrophic factor (BDNF) expression in cultured embryonic mouse striatal cells. Neuroreport. 2001;12:1175–1179. doi: 10.1097/00001756-200105080-00025. [DOI] [PubMed] [Google Scholar]
- 134.Williams SN, Undieh AS. Dopamine D1-like receptor activation induces brain-derived neurotrophic factor protein expression. Neuroreport. 2009;20:606–610. doi: 10.1097/WNR.0b013e32832a0a98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- 136.Zhang X, Zhou Z, Wang D, Li A, Yin Y, Gu X, Ding F, Zhen X, Zhou J. Activation of phosphatidylinositol-linked D1-like receptor modulates FGF-2 expression in astrocytes via IP3-dependent Ca2+ signaling. J Neurosci. 2009;29:7766–7775. doi: 10.1523/JNEUROSCI.0389-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xi ZX, Kleitz HK, Deng X, Ladenheim B, Peng XQ, Li X, Gardner EL, Stein EA, Cadet JL. A single high dose of methamphetamine increases cocaine self-administration by depletion of striatal dopamine in rats. Neuroscience. 2009;161:392–402. doi: 10.1016/j.neuroscience.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Butcher SP, Fairbrother IS, Kelly JS, Arbuthnott GW. Amphetamine-induced dopamine release in the rat striatum: an in vivo microdialysis study. J Neurochem. 1988;50:346–355. doi: 10.1111/j.1471-4159.1988.tb02919.x. [DOI] [PubMed] [Google Scholar]
- 139.Hattori A, Luo Y, Umegaki H, Munoz J, Roth GS. Intrastriatal injection of dopamine results in DNA damage and apoptosis in rats. Neuroreport. 1998;9:2569–2572. doi: 10.1097/00001756-199808030-00026. [DOI] [PubMed] [Google Scholar]
- 140.Luo Y, Hattori A, Munoz J, Qin ZH, Roth GS. Intrastriatal dopamine injection induces apoptosis through oxidation- involved activation of transcription factors AP-1 and NF-kappaB in rats. Mol Pharmacol. 1999;56:254–264. doi: 10.1124/mol.56.2.254. [DOI] [PubMed] [Google Scholar]
- 141.Cheng N, Maeda T, Kume T, Kaneko S, Kochiyama H, Akaike A, Goshima Y, Misu Y. Differential neurotoxicity induced by L-DOPA and dopamine in cultured striatal neurons. Brain Res. 1996;743:278–283. doi: 10.1016/s0006-8993(96)01056-6. [DOI] [PubMed] [Google Scholar]
- 142.Shinkai T, Zhang L, Mathias SA, Roth GS. Dopamine induces apoptosis in cultured rat striatal neurons; possible mechanism of D2-dopamine receptor neuron loss during aging. J Neurosci Res. 1997;47:393–399. [PubMed] [Google Scholar]
- 143.McLaughlin BA, Nelson D, Erecinska M, Chesselet MF. Toxicity of dopamine to striatal neurons in vitro and potentiation of cell death by a mitochondrial inhibitor. J Neurochem. 1998;70:2406–2415. doi: 10.1046/j.1471-4159.1998.70062406.x. [DOI] [PubMed] [Google Scholar]
- 144.Iwatsubo K, Suzuki S, Li C, Tsunematsu T, Nakamura F, Okumura S, Sato M, Minamisawa S, Toya Y, Umemura S, Ishikawa Y. Dopamine induces apoptosis in young, but not in neonatal, neurons via Ca2+-dependent signal. Am J Physiol Cell Physiol. 2007;293:C1498–1508. doi: 10.1152/ajpcell.00088.2007. [DOI] [PubMed] [Google Scholar]
- 145.Rosenberg PA. Catecholamine toxicity in cerebral cortex in dissociated cell culture. J Neurosci. 1988;8:2887–2894. doi: 10.1523/JNEUROSCI.08-08-02887.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tanaka M, Sotomatsu A, Kanai H, Hirai S. Dopa and dopamine cause cultured neuronal death in the presence of iron. J Neurol Sci. 1991;101:198–203. doi: 10.1016/0022-510x(91)90046-a. [DOI] [PubMed] [Google Scholar]
- 147.Mytilineou C, Han SK, Cohen G. Toxic and protective effects of L-dopa on mesencephalic cell cultures. J Neurochem. 1993;61:1470–1478. doi: 10.1111/j.1471-4159.1993.tb13642.x. [DOI] [PubMed] [Google Scholar]
- 148.Hoyt KR, Reynolds IJ, Hastings TG. Mechanisms of dopamine-induced cell death in cultured rat forebrain neurons: interactions with and differences from glutamate-induced cell death. Exp Neurol. 1997;143:269–281. doi: 10.1006/exnr.1996.6374. [DOI] [PubMed] [Google Scholar]
- 149.Luo Y, Umegaki H, Wang X, Abe R, Roth GS. Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. J Biol Chem. 1998;273:3756–3764. doi: 10.1074/jbc.273.6.3756. [DOI] [PubMed] [Google Scholar]
- 150.Jones DC, Gunasekar PG, Borowitz JL, Isom GE. Dopamine-induced apoptosis is mediated by oxidative stress and Is enhanced by cyanide in differentiated PC12 cells. J Neurochem. 2000;74:2296–2304. doi: 10.1046/j.1471-4159.2000.0742296.x. [DOI] [PubMed] [Google Scholar]
- 151.Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- 152.Graham DG, Tiffany SM, Bell WR, Jr, Gutknecht WF. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol. 1978;14:644–653. [PubMed] [Google Scholar]
- 153.Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int. 1998;32:117–131. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- 154.Wersinger C, Chen J, Sidhu A. Bimodal induction of dopamine-mediated striatal neurotoxicity is mediated through both activation of D1 dopamine receptors and autoxidation. Mol Cell Neurosci. 2004;25:124–137. doi: 10.1016/j.mcn.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 155.Chen J, Wersinger C, Sidhu A. Chronic stimulation of D1 dopamine receptors in human SK-N-MC neuroblastoma cells induces nitric-oxide synthase activation and cytotoxicity. J Biol Chem. 2003;278:28089–28100. doi: 10.1074/jbc.M303094200. [DOI] [PubMed] [Google Scholar]
- 156.Chen J, Rusnak M, Luedtke RR, Sidhu A. D1 dopamine receptor mediates dopamine-induced cytotoxicity via the ERK signal cascade. J Biol Chem. 2004;279:39317–39330. doi: 10.1074/jbc.M403891200. [DOI] [PubMed] [Google Scholar]
- 157.Deng X, Cadet JL. Methamphetamine administration causes overexpression of nNOS in the mouse striatum. Brain Res. 1999;851:254–257. doi: 10.1016/s0006-8993(99)02087-9. [DOI] [PubMed] [Google Scholar]
- 158.Zhu JP, Xu W, Angulo JA. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience. 2006;140:607–622. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18:238–251. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- 160.Aramburu J, Heitman J, Crabtree GR. Calcineurin: a central controller of signalling in eukaryotes. EMBO Rep. 2004;5:343–348. doi: 10.1038/sj.embor.7400133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Asai A, Qiu J, Narita Y, Chi S, Saito N, Shinoura N, Hamada H, Kuchino Y, Kirino T. High level calcineurin activity predisposes neuronal cells to apoptosis. J Biol Chem. 1999;274:34450–34458. doi: 10.1074/jbc.274.48.34450. [DOI] [PubMed] [Google Scholar]
- 162.O’Sullivan GJ, Clifford JJ, Tomiyama K, Koshikawa N, Drago J, Sibley DR, Croke DT, Waddington JL. D1-like dopamine receptor-mediated function in congenic mutants with D1 vs. D5 receptor “knockout”. J Recept Signal Transduct Res. 2004;24:107–116. doi: 10.1081/rrs-200032078. [DOI] [PubMed] [Google Scholar]