Abstract
The transcription factor SOX10 is essential for survival and proper differentiation of neural crest cell lineages, where it plays an important role in the generation and maintenance of melanocytes. SOX10 is also highly expressed in melanoma tumors, but a role in disease progression has not been established. Here we report that melanoma tumor cell lines require wild-type SOX10 expression for proliferation, and SOX10 haploinsufficiency reduces melanoma initiation in the metabotropic glutamate receptor 1 (Grm1Tg) transgenic mouse model. Stable SOX10 knockdown in human melanoma cells arrested cell growth, altered cellular morphology, and induced senescence. Melanoma cells with stable loss of SOX10 were arrested in the G1 phase of the cell cycle, with reduced expression in the melanocyte determining factor MITF, elevated expression of p21WAF1 and p27KIP2, hypophosphorylated RB and reduced levels of its binding partner E2F1. Since cell cycle dysregulation is a core event in neoplastic transformation, the role for SOX10 in maintaining cell cycle control in melanocytes suggests a rational new direction for targeted treatment or prevention of melanoma.
Introduction
Melanocytes are melanin-producing cells of neural crest origin located in the dermis and epidermis of the skin, the eye, the inner ear, and mucosal membranes. During development, differentiating melanocytes migrate from the neural tube to these distinct anatomical locations, each with unique cellular environments and potential for UV exposure. Malignancy of melanocytes results in melanoma, an aggressive and often fatal cancer. Moreover, melanoma incidence is increasing worldwide; the American Cancer Society describes melanoma as a disease affecting a wide range of ages, and projects diagnosis of ≈76,600 new cases in 2013 (1). Classification of melanoma subtypes incorporates anatomical melanocyte location, degree of sun exposure, and histopathology in addition to genetic categorization based upon mutations in the MAPK signaling pathway components BRAF, NRAS, KIT, GNA11 and GNAQ (2)(3)(4)(5). While recent targeted therapies to mutant BRAF hold promise, the majority of treated individuals with melanoma ultimately exhibit chemoresistance (6). Given the heterogeneity exhibited in melanoma, not only between tumor subtypes, but also the molecular diversity within individual lesions (7), there is a paramount need to identify and functionally assess additional genes fundamental to the regulation of melanoma growth and metastasis.
Increasing correlations are being discovered between genes that regulate developmental processes and the genes and signaling pathways mediating tumor progression through proliferation, invasion and metastasis. Specification, survival and differentiation of neural crest-derived lineages including melanocytes is dependent on SOX10 (SRY (sex determining region Y)-box 10), a member of the high-mobility-group domain SOX family of transcription factors. The ≈50 known heterozygous germline mutations in SOX10 have been linked to developmental neurocristopathies Waardenburg syndrome (Types II and IV) and PCWH (peripheral demyelinating neuropathy, central dysmyelinating leukodystrophy, Waardenburg syndrome and Hirschsprung disease) (8). SOX10 regulates many targets during neural crest development, including Microphthalmia-associated transcription factor (MITF), which is necessary for normal melanocyte development. SOX10’s regulation of MITF has implications in melanomagenesis, as MITF mutations and amplifications have been identified in a subset of melanomas (9)(10). MITF activates genes involved in melanocyte survival, such as BCL2 (11) and plays a key role in cell cycle via regulation of cyclin-dependent kinase inhibitor 2A (INK4A or CDKN2A), cyclin-dependent kinase inhibitor 1B (p27 or CDKN1B) and p53 (TP53) (12)(13)(14).
In this study we evaluate the role of SOX10 in melanomagenesis. We examine the mutation spectrum of SOX10 in primary and metastatic melanoma tumor samples from multiple histological subtypes and discover a low frequency of mutations. We assess the effects of reduced SOX10 levels on melanoma growth and survival, and we demonstrate a G1 cell cycle arrest results from a loss of SOX10. We also confirm SOX10 expression is fundamental for melanomagenesis in vivo by demonstrating that a reduction in SOX10 expression reduces tumor formation in the Grm1Tg melanoma mouse model. Together these results indicate reducing SOX10 levels has fateful consequences for melanoma formation and expansion, suggesting SOX10 is a promising target for melanoma treatment.
Materials and Methods
PCR, sequencing and mutational analysis
Melanoma genomic DNA samples provided by Dr. Nicola Schönewolf (University Hospital of Zurich) and Dr. Boris Bastian (UCSF). Additional samples from Dr. Nicholas Hayward were sequenced at the Queensland Institute of Medical Research. Cell lines provided by Joanne Hasskamp (Maryland Melanoma Center at MedStar Franklin Square Medical Center) and the University of Arizona Cancer Center (UACC). Genomic DNA isolated using DNeasy Blood & Tissue kits (Qiagen, Valencia, CA). PCR and sequencing primers (Supplemental table 1) designed using NetPrimer (http://www.premierbiosoft.com/netprimer/index.html) and synthesized by Invitrogen (Carlsbad, CA). SOX10 coding regions amplified with TaKaRa LA Taq™ DNA Polymerase (Takara Bio U.S.A., Madison, WI) and sequencing carried out using BigDye® Terminator v3.1 (Applied Biosystems, Carlsbad, CA). Sequence data analyzed with Sequencher 4.9 software (Gene Codes Corporation, Ann Arbor, MI).
Cell Culture, siRNA transfection and generation of stable shRNA lines
UACC melanoma cell line isolations were described previously (15); 0002-ARM-032702 (0002-ARM, IRB protocol number 97-79), 0391-LNA-122304 (0391-LNA, IRB protocol number 2003-131), and 0380-MMU-071802 (0380-MMU) cell lines generated from patients at the Maryland Melanoma Center following standard surgical consent (16). Cell lines maintained at 37°C with 5% CO2 in DMEM (UACC 1022, 3093) or IMDM (0380-MMU, 0391-LNA and 0002-ARM) supplemented with 10% FBS and 2 mM L-glutamine (Invitrogen, Carlsbad, CA). To generate stable shRNA cell lines, melanoma cells were infected with pLKO1 lentiviral shRNA supernatant produced in 293T packaging cell line as described previously (17). Infected cells were selected with dose of puromycin antibiotic, and cultured 3–6 days before knockdown screening and functional assays. For transient transfection of siRNA duplexes, cells treated with siRNA-Lipofectamine 2000 reagent complexes for 72 hours prior to harvesting. RNAi 27mer duplexes targeting SOX10 synthesized by Integrated DNA Technologies (Coralville, IA). siRNA sequences used in this analysis were: SOX10si_1 - 5′ AGACAAAGAAUGAGGUUAUUGGCACAG 3′, SOX10si_2 - 5′ GGUGCAACAGUCAACCUCCUUCUCCUC 3′, and non-silencing control - siGENOME non-targeting siRNA pool #2 (Thermo Scientific, Waltham, MA).
Immunoblotting
Protein gels and Western blots were performed using standard protocols. Primary antibodies were: monoclonal SOX10 (R&D Systems #MAB2864, Minneapolis, MN), monoclonal alpha-Tubulin (Calbiochem #CP06, San Diego, CA), total RB and Phospho-RB Ser807/811 antibody kit (Cell Signaling #9969, Danvers, MA), polyclonal E2F1 (Cell Signaling #3742), monoclonal p16 (F-12 Santa Cruz Biotechnology #sc-1661), monoclonal p21 Waf1/Cip1 (Cell Signaling #2946), monoclonal cyclin D1 (BD Pharmingen #G124-326, Franklin Lakes, NJ), monoclonal p27 (Santa Cruz Biotechnology #sc-1641), polyclonal CDK2, CDK4 and CDK6 (Santa Cruz Biotechnology #sc-163, sc-601, and sc-177, respectively) and monoclonal MITF (generously provided by Heinz Arnheiter). HRP-conjugated secondary antibody from Jackson ImmunoResearch Laboratories (West Grove, PA).
Proliferation Assay
Melanoma cell lines stably expressing non-silencing or SOX10-specific shRNA were examined by seeding into 12-well plates (15,000 cells per well) and culturing 14–16 days. Cells were fed fresh growth media once a week. Samples analyzed every 48–72 hours by Vi-CELL counter (Beckman Coulter, Atlanta, GA). All samples run in duplicate, in two or three independent assays.
Immunohistochemistry
Cells seeded into 8-well chamber CC2 coated slides (Thermo Fisher Scientific) one day before staining. Cells rinsed with 1X PBS, fixed in 4% paraformaldehyde for 10 minutes, rinsed briefly with 1X PBS 0.1% Tween, then permeabilized with 0.1% Triton for 10 minutes. Following 30 minute block in 1mg/mL BSA (Sigma #A3059), cells were incubated 2 hours with primary antibodies in block (anti-SOX10, Santa Cruz, sc-17342; anti-HP1β, Millipore, MAB3448). Cells then rinsed and incubated 20 minutes in Alexa 488 or 568 secondary antibodies (Invitrogen) diluted in block. Cells rinsed before mounting with ProLong Gold mounting media with DAPI (Invitrogen). Cell images taken on Zeiss AxioImager.D2 upright microscope with AxioVision 4.8 software (Carl Zeiss Microscopy, Thornwood, NY).
Senescence-associated β-galactosidase staining
Cultured cells plated into 6-well dishes one day prior to staining. Cells fixed and stained per manufacturer’s protocol (Senescence β-Galactosidase Staining kit, Cell Signaling #9860). Stained cells imaged with a Zeiss Axio Observer inverted scope with differential interference contrast brightfield microscopy (DIC). Ten random images collected and cells counted with ImageJ 1.45s software. Staining performed one week post-infection or 72 hours post-transfection in triplicate.
Fluorescence-activated cell sorting (FACS)
Cultured cells harvested by trypsinization, counted, and 5 × 105 cells were pelleted by centrifugation (1000rpm, 5 minutes). Cell pellets resuspended in 500 μl propidium iodide (PI) then incubated 20 minutes at 37°C (NuCycl protocol; Exalpha Biologicals, Maynard, MA). PI-stained cells were filtered and sorted on a FACS Calibur with 488 nm excitation (BD Biosciences).
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) performed on ABI 7500 (Applied Biosystems, Foster City, CA). RNA isolated with RNeasy mini kits (Qiagen, Valencia, CA), three independent isolations were performed for each sample. cDNA generated with a Super Script III kit (Invitrogen, Carlsbad, CA) per manufacturer’s protocol. Taqman gene expression assays (Applied Biosystems) for SOX10 (Hs00366918_m1), MITF (Hs00165156_m1), RB1 (Hs01078066_m1), p27 (Hs01597588) and E2F1 (Hs00153451_m1) were used with GAPDH (Hs02758991_g1) as reference gene. Alternatively, gene expression was measured with Sybr green I PCR mix (15.2 μl DEPC water, 2.5 μl 10X PCR buffer (10), 2.5 μl 10mM dNTP, 1.5 μl dimethylsulfoxide, 0.5 μl SYBR Green I diluted 1:1000 in DEPC water, 0.5 μl of 50uM forward and reverse primers, 25 μl TaKaRa LA Taq DNA polymerase) for p21 (CDKN1A). Cycling conditions were: 94°C, 2 min; 40 cycles at 94°C, 10 sec; 60°C, 30 sec; and 70°C, 30 sec. Standard curve analysis performed on each gene (ABI 7500 Fast System v1.4.0 software) in triplicate for each biological sample. Primer sequences used in this analysis (5′ to 3′) were: p21 (CDKN1A) forward, ACTCTCAGGGTCGAAAACGG and reverse, CCTCGCGCTTCCAGGACTG (18); GAPDH forward, CATGACCACAGTCCATGCCATCACT and reverse, TGAGGTCCACCACCCTGTTGCTGTA (19).
Mouse husbandry and phenotype scoring
Mice were maintained in NIH animal facilities and all procedures approved by the IACUC in accordance with NIH guidelines. Grm1Tg(p18A4.B)1352Szc mice, hereafter Grm1Tg (20), were originally obtained on a mixed genetic background, then outcrossed repeatedly (>10 generations) to establish a colony on a predominantly C57Bl/6J background. Colonies of Sox10tm1Weg, hereafter named Sox10LacZ (21), Mitfmi-vga9 (hereafter Mitfvga9) (22), and Kittm1Alf mice (hereafter KitLacZ) (23) were also each maintained by repeated outcrossing to C57Bl/6J. Heterozygote carriers from each of the colonies were intercrossed with Grm1Tg/+ heterozygotes, and double heterozygote mice were backcrossed to Grm1Tg/+ heterozygotes to generate mice for analysis.
To quantitate the severity of melanoma progression in Grm1Tg mice, detailed observation and photo-documentation was used to assign numerical scores of 0 to 4 to the ears for 2 independent criteria, pigmentation and raised tumor severity. For scoring pigmentation, 0 indicated no visible pigmented nevi on the ear, and 4 indicated that 90–100% of the ear was covered in continuous pigmentation. For scoring degree to which nevi were raised, 0 indicated that any visible pigmentation was flat with no detectable increased thickness of the skin, and 4 indicated a significant, raised tumor that was becoming necrotic and warranted euthanasia. Ventral spotting was also scored using a scale of 0 to 4 (0=no ventral spotting; 4=severe ventral spotting extending dorsally) as previously described (24).
Results
Assessment of somatic SOX10 mutations across melanoma subtypes
Melanoma can arise in different histological locations, resulting in numerous subtypes with distinct protein expression patterns and genomic aberrations (25). Previously we identified intragenic mutations in the SOX10 locus in a subset of samples from both primary and metastatic melanoma (10). In order to fully evaluate the contribution of SOX10 mutations to melanoma progression we expanded our analysis of SOX10 coding variations with an additional 153 melanoma samples representing various histological subtypes (Table 1). Only one novel SOX10 alteration was observed: a G to T substitution encoding a non-synonymous amino acid change (p.P217L) in a cell line derived from an acral lentiginous melanoma tumor. This altered proline is 3′ of the HMG binding domain, and is evolutionarily conserved from mammals to chicken. Although we were unable to validate that this coding alteration occurred somatically in this sample as normal genomic DNA from this patient was unavailable, query of the dbSNP database indicated the predicted P217L variant has not been observed previously (NCBI, dbSNP Short Genetic Variations, www.ncbi.nlm.nih.gov, accessed August 22, 2012), suggesting this SOX10 DNA change is unique. Overall these findings suggest that maintenance of SOX10 function is required for melanomagenesis, as melanoma cells favor the retention of a wild type SOX10.
Table 1.
Sequencing of SOX10 across various melanoma histologies
| Institution | Sample number | Histology | SOX10 mutations |
|---|---|---|---|
| University of Arizona Cancer Center (UACC) | 12 | Superficial spreading/Cutaneous | None |
| Maryland Melanoma Center at Medstar Franklin Square | 14 | Superficial spreading/Cutaneous | None |
| American Type Culture Collection | 1 | Superficial spreading/Cutaneous | None |
| Queensland Institute of Medical Research | 41 | Superficial spreading/Cutaneous | None |
| University Hospital of Zürich | 26 | Mucosal, Acral lentiginous | 1/26 (3.8%) |
| University of California, San Fransisco | 59 | Acral, Mucosal, Lentigo, Desmoplastic | None |
|
| |||
| TOTAL: | 153 | 1/153 (0.65%) | |
SOX10 knockdown in melanoma cells results in G1 arrest and cell senescence
To assess if SOX10 is required for melanoma cells in vitro, we performed RNAi-mediated knockdown. Three melanoma cell lines were transduced with two different SOX10-specific lentiviral RNAi hairpins (shSOX10) and compared with lines transduced with a non-silencing control hairpin. One week post-infection, the shSOX10 lines showed reduced SOX10 protein levels and the cells became enlarged and translucent (flat) (Figure 1A–B). These cells also showed arrested or slowed growth (Figure 1C). Analysis of five additional melanoma lines showed consistent results, with six of the eight lines displaying altered cellular morphology and slowed growth resulting from SOX10 knockdown (data not shown). Three of these six lines were selected for further functional analysis: the 0002-ARM and UACC 1022 cell lines, which contain V600E and L597S BRAF mutations, respectively, and the 0380-MMU cell line, which harbors an NRAS Q61K mutation.
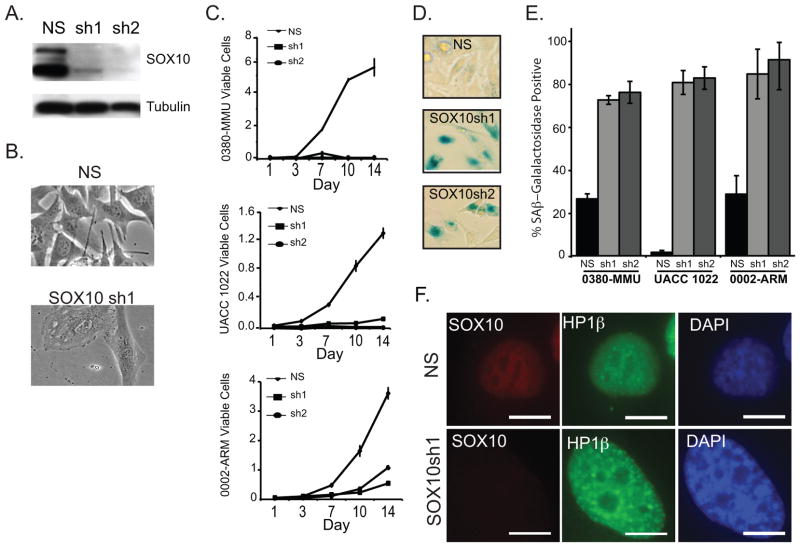
Figure 1.
SOX10 depletion triggers arrested growth and melanoma cell senescence
A. Western blot analysis of SOX10 expression in 0380-MMU cell lysates stably transduced with a non-silencing control hairpin (NS) or SOX10-specific lentiviral RNAi hairpins (sh1 and sh2). B. Phase-contrast images of 0380-MMU stable cell lines, 1 week post-infection with NS or SOX10sh1. C. Proliferation assays for 0380-MMU, UACC 1022, and 0002-ARM cell lines transduced with NS, sh1, or sh2. Cells were counted in triplicate 1 to 14 days after plating, and proliferation assays were performed at least twice with subsequent infections. D. Representative images of 0380-MMU cells stained for SA-β-Gal activity one week post-infection with NS, SOX10sh1 and SOX10sh2. E. Significant increases in the percentage of SA-β-Gal positive cells were seen in SOX10sh lines relative to NS lines, as determined by t-test (for 0380-MMU, sh1 p<7.32×10−5, sh2 p<7.95×10−3; for UACC 1022, sh1 p<5.49×10−11, sh2 p<2.17×10−8; for 0002-ARM, sh1 p<2.90×10−5, sh2 p<8.80×10−5). F. Stably transduced UACC 1022 cell lines stained with anti-SOX10, anti-HP1β antibodies and DAPI nuclei counterstain. HP1β staining indicated the presence of senescence-associated heterochromatin foci in SOX10sh1 cells. Scale bar = 10 μm.
To further characterize the arrested growth we observed in the shSOX10 lines, we stained for β-galactosidase (SA-β-Gal), a biomarker of cellular senescence (Figure 1D). Significant increases in SA-β-Gal activity were seen in all three lines when stably transduced with shSOX10 hairpins (Figure 1E), suggesting that loss of SOX10 induces senescence in melanoma cells. Another hallmark of cellular senescence, senescence-associated heterochromatin foci (SAHF), was observed upon chromatin binding protein HP1β immunostaining (Figure 1F and Supplemental Figure 1). Upon loss of SOX10 protein, the cells show more severe punctate staining of HP1β as a result of chromatin rearrangements brought on by senescent fate.
The shSOX10 cells showed no increase in APC-annexin V staining as compared to controls, indicating no increase in apoptosis (data not shown). Quantitation of the number of cells in each phase of the cell cycle by FACS analysis demonstrated that SOX10 knockdown cells consistently showed a significant increase in the population of cells in G1-phase (Figure 2A), indicative of G1 arrest and decreased cell cycle progression. Taken together, these data suggest SOX10 is required for proliferation of melanoma cells.
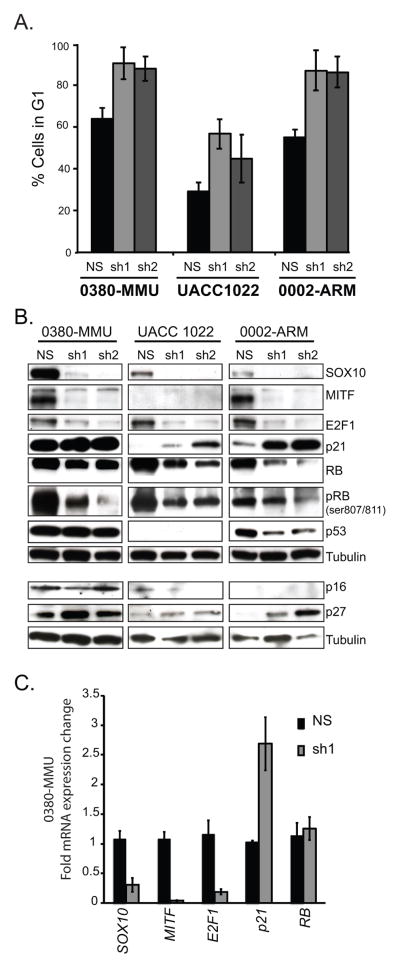
Figure 2.
Cell cycle arrest and expression changes in cell cycle regulators are observed upon stable loss of SOX10
A. Significant increases in G1 cell populations were observed in 0380-MMU, UACC 1022, and 0002-ARM lines stably transduced with SOX10-specific hairpins (sh1 and sh2) relative to lines stably transduced with a non-silencing control hairpin (NS) (t-test p values were: 0380-MMU, sh1 = p<0.007, sh2 = p<0.026; UACC 1022 sh1 = p<0.026, sh2 = 0.17; 0002-ARM sh1 = p<0.023, sh2 = p<0.03). FACS analysis on each line was repeated twice with subsequent infections. B. Western blot analysis of the effects of NS, sh1 and sh2 stable transduction in each line on important cell cycle regulatory proteins. C. Quantitative RT-PCR of RNA isolated from 0380-MMU NS and sh1 stable lines showed significant changes in SOX10 (p<0.006), MITF (p<0.0005), E2F1 (p<0.005) and p21 (p<0.016) expression as determined by t-test. Data are fold change normalized to control, and the average of three replicate assays.
SOX10 knockdown results in reduced E2F1 and RB levels
The RB-E2F1 complex gates a fundamental checkpoint regulating progression through the G1-phase of the cell cycle (7). Phosphorylation releases RB from E2F1 during G1 and is required for the accumulation of transcriptionally active E2F1 (26). Thus we evaluated E2F1 and RB protein levels by Western blot in SOX10 knockdown cells. In all three melanoma lines, SOX10 knockdown led to reduced levels of E2F1 protein and total RB protein (Figure 2B). Consistent with these results, we found E2F1 transcript levels decreased upon SOX10 knockdown (Figure 2C), however no change in RB mRNA levels were seen (Figure 2C) despite seeing less RB protein. When the degree of RB phosphorylation was evaluated with an antibody specific for RB serine residues Ser807 and Ser811, we observed a decrease in levels of phosphorylated RB (Figure 2B). These data suggest that the total RB protein level decreases as a result of a loss of stable, phosphorylated RB and are consistent with the G1 arrest observed. A similar destabilization of RB protein leading to G1 arrest has been reported in PAX8 knockdown experiments (27).
shSOX10-induced G1 arrest is not mediated by p53 signaling
The p53 pathway regulates the G1 cell cycle checkpoint, and p53 activation has been shown to result in melanoma cell cycle arrest (28). Therefore we measured p53 protein levels in SOX10 knockdown cells. In the 0002-ARM cell line, p53 levels decreased with reduced SOX10 expression, however no change in p53 levels was observed in 0380-MMU cells (Figure 2B). For the UACC 1022 line, p53 was undetectable in both the control cells and shSOX10 lines (Figure 2B). Sequencing of the TP53 locus in the UACC 1022 line identified a premature stop codon along with loss of heterozygosity (R195X) (data not shown), indicating UACC 1022 is null for p53 protein signaling. As growth arrest was observed in all three lines, independent of p53 status, the G1 arrest observed in shSOX10 cells is not solely mediated through the p53 signaling pathway.
SOX10 knockdown results in decreased MITF and increased CDK inhibitors p21 and p27
As knockdown of MITF in melanoma cell lines has been shown to produce a similar decrease in growth rate and G1 cell cycle arrest (29), and MITF is a well-established downstream transcriptional target of SOX10 (30), we assessed MITF expression upon shSOX10 knockdown. Reduced MITF protein was observed upon shSOX10 knockdown, in both 0380-MMU and 0002-ARM cells (Figure 2B), while MITF protein expression was undetectable by Western blot in UACC 1022 control cells (Figure 2B).
Given these altered MITF expression levels, we looked for consistent alterations in G1 cell cycle regulators across the three lines. CDK2, itself a downstream target of MITF transactivation (31), decreased in both the 0380-MMU and UACC 1022 shSOX10 cells, and increased in the 0002-ARM shSOX10 cells (Supplemental Figure 2). However, no consistent change in total expression level for CDK4, CDK6, or Cyclin D1 proteins was observed (Supplemental Figure 2), thus CDK protein expression levels did not provide a unified mechanism to explain the loss of phosphorylated RB protein observed in shSOX10 cells.
We next evaluated protein levels of p16 (CDKN2A), p21 (CDKN1A) and p27 (CDKN1B) following SOX10 knockdown (Figure 2B). Lowered MITF levels leading to G1 arrest have been correlated with an increase in p27 levels (12) and with decreased levels of p21, as MITF directly regulates p21 transcription (32)(33). We observed moderate p16 expression in 0380-MMU cells, however p16 levels remained unchanged following SOX10 knockdown. We consistently observed increases in p27 levels in all three cell lines as a result of SOX10 knockdown (Figure 2B), similar to previous MITF knockdown studies (12). However, SOX10 knockdown caused an increase in p21 protein in all three cell lines (Figure 2B), thus showing a distinct difference from the MITF knockdown results.
Altered MITF and p27 gene expression occur prior to G1 arrest
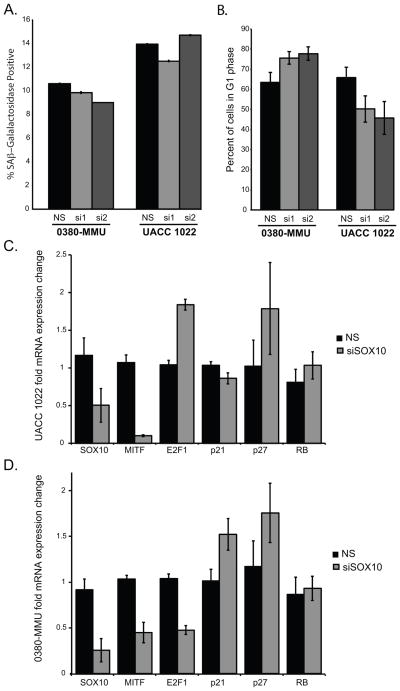
To assess changes in transcript levels prior to G1 arrest, we performed a transient, 72 hour siRNA knockdown of SOX10. In both 0380-MMU and UACC 1022 cell lines, siSOX10 knockdown led to decreased SOX10 protein (Supplemental Figure 3), however siSOX10 knockdown did not increase SA β-gal activity or the G1 cell population (Figure 3A–B). Consistent with the stable shSOX10 knockdown results, we found transient siSOX10 treatment reduced MITF mRNA in both cell lines, increased p27 mRNA in both cell lines, increased p21 mRNA in 0380-MMU cells but had no effect on p21 in UACC1022 cells, and had no effect on RB mRNA levels in both lines (Figure 3C–D), E2F1 message changes appeared to be cell type dependent (Figure 3C–D).
Figure 3.
Altered MITF, p21 and E2F1 expression precedes G1 cell cycle arrest
A. Quantification of the percentages of SA-β-Gal-positive cells in 0380-MMU and UACC 1022 cells transiently transfected with non-silencing (NS) or SOX10-specific (si1 and si2) siRNA showed no significant differences. B. No significant differences were seen in G1 cell populations in 0380-MMU and UACC 1022 melanoma cell lines transiently transfected with NS, si1, or si2. FACS analysis on independent cell lines was repeated twice with subsequent transfections. C–D. Quantitative RT-PCR analysis of (C) UACC 1022 and (D) 0380-MMU cell lines transiently transfected with NS or siSOX10. Data are fold change normalized to NS control. siSOX10-transfected UACC 1022 cells showed significant changes in expression of SOX10 (p<0.05), MITF (p<0.0004), and E2F1 (p<0.001). siSOX10-transfected 0380-MMU cells showed significant changes in the expression of SOX10 (p<0.01), MITF (p<0.007), E2F1 (p<0.001); p21 and p27 showed increased expression that did not achieve statistical significance. Data analyzed by Student’s t-test.
Reduction of SOX10 expression in vivo suppresses melanomagenesis
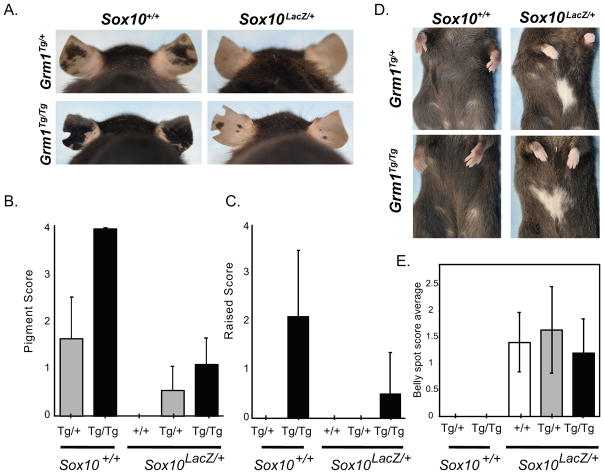
To assess the role of SOX10 in melanoma progression in vivo, we utilized a mouse model of Sox10 haploinsufficiency (Sox10LacZ/+) (21) crossed with a Grm1Tg mouse melanoma model (20). In human melanomas, glutamate receptor pathway activation is observed in tumors harboring recurrent mutations in the glutamate receptor genes GRM3 (34) and GRIN2A (35). Similarly, Grm1Tg mice harbor a transgene insertion that causes overexpression of Grm1, activates the glutamatergic pathway and causes numerous, pigmented nevi at weaning in heterozygous and homozygous mice (Figure 4A, left two panels). Nevi in homozygotes (Grm1Tg/Tg) are raised by 2–3 months of age and typically necessitate euthanasia by 5 months of age. We crossed Grm1Tg/Tg mice with Sox10LacZ/+ mice, then double heterozygotes were backcrossed to Grm1Tg/Tg mice, thus generating Grm1Tg/+ and Grm1Tg/Tg offspring that were also haploinsufficient for Sox10 (Grm1Tg/+;Sox10LacZ/+ and Grm1Tg/Tg;Sox10LacZ/+, respectively) along with Grm1Tg/+ and Grm1Tg/Tg littermates. The extent of pigmentation and severity of raised tumors visible on the ears of 3–5 month old mice was quantitatively scored and compared among genotypes (Figure 4A–C). Sox10 haploinsufficiency significantly reduced the number of pigmented nevi in both Grm1Tg/+ and Grm1Tg/Tg mice (p<0.005), and also the severity of raised nevi in Grm1Tg/Tg mice (p<0.05). An extended tumor latency was also observed, with raised tumors first appearing in Grm1Tg/Tg;Sox10LacZ/+ mice at 5–6 months vs 2–3 months in Grm1Tg/Tg mice (data not shown).
Figure 4.
SOX10 expression is required for melanoma progression in vivo
A. Grm1Tg/+;Sox10+/+ mice show moderate ear hyperpigmentation (top left panel). This phenotype is exaggerated in Grm1Tg/Tg;Sox10+/+ mice, where the pigmented lesions are raised (bottom left panel). Haploinsufficiency for SOX10 (Sox10LacZ/+) in conjunction with one or two Grm1Tg alleles reduces pigmented ear lesions (right two panels). B,C. Grm1Tg phenotypic severity either alone or in the context of Sox10 haploinsufficiency was quantitatively measured by scoring each individual animal on a scale of 1–4 for (B) the extent of pigmentation and (C) burden of raised tumors. Sox10 haploinsufficiency significantly reduced pigmented nevi (p<0.005) in both Grm1Tg/+ and Grm1Tg/Tg mice, and also the extent to which they were raised (p<0.05) in Grm1Tg/Tg mice (Mann-Whitney test with Bonferonni correction). D. No ventral spotting was observed in Grm1Tg/+ or Grm1Tg/Tg mice that were also Sox10+/+ (left two panels), and the presence of one or two Grm1Tg/+ alleles did not affect ventral spotting caused by the Sox10LacZ allele. (right two panels; mice shown received a spotting severity score of two). E. Quantitative scoring of belly spots in Sox10LacZ; Grm1+/+, Sox10LacZ; Grm1Tg/+, and Sox10LacZ; Grm1Tg/Tg mice showed no significant differences (p=0.3342; Kruskal-Wallis nonparametric one-way ANOVA).
Interestingly, the Grm1Tg transgene did not suppress the hypopigmentation (belly spots) that is normally observed in Sox10LacZ/+ mice due to a developmental reduction in melanoblast number (Figure 4D–E). This indicates that Grm1Tg does not cause a developmental expansion of melanoblasts and suggests that SOX10’s role in melanoma suppression is independent of its effect on embryonic melanoblast number. Another white spotting allele with a developmental reduction in melanoblasts, KitLacZ, was not sufficient to suppress melanoma in Grm1Tg (Supplemental Figure 4), which is consistent with the separation of SOX10’s roles in developmental melanoblast number and melanoma formation in the Grm1Tg model.
To understand the role of Mitf in the Sox10LacZ suppression of Grm1Tg mouse melanoma lesions, Grm1Tg/Tg mice were crossed to mice that harbor a transgene disruption that results in a functionally null allele of Mitf. In contrast to the Grm1Tg/+;Sox10LacZ/+ and Grm1Tg/Tg;Sox10LacZ/+ mice, heterozygosity for the Mitfvga9 allele did not suppress the development of hyperpigmented, raised lesions in the Grm1Tg/+;Mitfvga/+ and Grm1Tg/Tg;Mitfvga/+ mice (Supplemental Figure 4). Of note, Mitfvga/vga homozygotes lack melanocytes, and thus could not be analyzed (22)(36). In summary, Sox10 haploinsufficiency is sufficient to suppress the phenotype of Grm1Tg mice indicating an essential role of SOX10 in melanoma progression.
Discussion
Melanoma is an aggressive tumor type that progresses rapidly and exhibits resistance to most current therapeutic interventions. Although targeted therapies to known tumor suppressor and oncogenic players in melanoma cells may achieve responses, patients are subject to relapse (37)(38). This study focuses on the role of SOX10 in melanoma, and supports mounting evidence suggesting SOX10 is an important lineage-specific target for patient intervention. Previous studies have detailed broad expression of SOX10 in melanoma tumor samples across various stages of disease, further suggesting a necessity for this protein in melanoma (39)(40)(41)(42).
Our sequencing analysis reveals a low frequency of intragenic mutations in SOX10, suggesting a preference for wild type SOX10 protein in melanoma formation and maintenance. Recent research has demonstrated the rate of “passenger” mutations, propagated by clonal expansion without any growth advantage, is higher in cutaneous melanoma than in solid tumors (43). This high passenger mutation rate was exemplified in genomic sequencing of the 121 melanoma samples, where 518 genes were found to have mutations in > 10% of melanoma samples (5). Previously, we identified nine SOX10 mutations from two small, distinct melanoma patient cohorts. We identified 3/50 mutations in metastatic samples from individuals enrolled in a single clinical trial, and 7/55 mutations from primary samples for a combined mutation rate of 8.5%. Of these, only the three mutations identified from samples obtained from the single metastatic cohort of patients were characterized by either an early truncation of SOX10 or an alteration leading to a C-terminal extension of SOX10 and were found to have reduced SOX10 functional activity (10). Given that the combined mutation rate (8.5%) falls within the range anticipated for melanoma, and that all of the confirmed functional mutations were ascertained from one patient cohort, we felt further analysis of the SOX10 locus was warranted.
In our current analysis, we increased sample numbers of mucosal and lentiginous melanoma subtypes and increased the diversity of clinical sources from which samples were collected, to reduce the potential for ascertainment bias. From this diverse sample cohort we found only 1/153 (<1%) of melanoma samples mutated for SOX10. This rate is consistent with the SOX10 mutation rate identified from recent large scale sequencing of metastatic melanoma (5). These data show that the SOX10 mutation rate is lower than the passenger mutation rate, suggesting the potential preference for wild type SOX10 protein in melanoma formation and maintenance.
Consistent with this finding, we hypothesize that SOX10 regulates melanoma cell proliferation and cell cycle regulation via multiple, convergent pathways regulating RB and E2F1 levels. Reduction in SOX10 levels results in increased expression of p21 and p27, inhibitors of CDKs which regulate multiple, sequential phosphorylation events on RB necessary for cell cycle progression. The E2F1-RB complex provides a cellular checkpoint for cell cycle progression out of G1. RB in its hypophosphorylated state prevents cells from entering S-phase and arrests them at G1. E2F1 level regulation is a key mediator of progression through G1, as E2F1 functions to activate downstream targets required for entry into S phase. Since we observed a change in E2F1 RNA levels combined with a senescent cellular phenotype when SOX10 expression was decreased in melanoma cell lines, we postulate that SOX10, or a downstream target, is required for proper regulation of E2F1 and subsequent control of cell proliferation through RB-E2F1 signaling. This is in contrast to what is observed for RB, where RB protein is reduced but transcript expression is unchanged following SOX10 reduction. We cannot exclude the possibility that SOX10 could indirectly stabilize the RB protein to prevent proteasomal degradation. In fact, the reduction in total RB protein may be a result of SOX10-mediated reduction in E2F1 levels, as reduced levels of active RB have been shown to be a direct consequence of decreased E2F1 expression (44) as well as increased p27 and p21 levels, ultimately resulting in more unstable hypophosphorylated RB protein (45).
Studies involving human melanoma cell lines can be limited due to the heterogeneity and phenotypic variations resulting from context-dependent effects on the genome. The melanoma cell lines described in this study mirror that heterogeneity, showing differences in p53 status, p16ARF and MITF expression levels, and mutation status of the major oncogenes NRAS and BRAF. Despite these notable variations, a dysregulated cell cycle with altered RB and E2F1 levels was consistently observed upon loss of SOX10 protein. Initially we hypothesized that MITF, which itself is a direct transcriptional target of SOX10, was appropriately poised to be the responsible transcription factor. Although RNAi-mediated reduction of MITF also leads to a senescent morphology and G1 cell arrest (13), this occurred in conjunction with a decrease in p21 expression, which was not observed in our study of SOX10 knockdown. These results may have potential implications clinically given the heterogeneity that exists within melanoma tumors. Importantly, the consistent observation that SOX10 is required for proliferation of melanoma cells suggests that SOX10 or its downstream targets are involved in the regulation of cell cycle.
The frequent acquired resistance to current BRAF inhibitors observed in recent clinical trials is mediated by re-acquisition of increased MAPK signaling (46). These results highlight the need for a more comprehensive understanding of all aspects of cell cycle dysregulation that occurs as a result of increased MAPK signaling in tumors, and the need to evaluate additional mechanisms that have the capacity to reduce cell proliferation and tumor formation. We find that SOX10 has a pivotal role in melanoma cell proliferation not only in cell culture, but also in vivo, as Sox10 haploinsufficiency prevents the formation of hyperpigmented lesions observed in the Grm1Tg mouse melanoma model. A recent study by Shakhova et al. reported a similar reduction in tumor formation when SOX10 levels were reduced in the context of the NrasQ61K-driven mouse melanoma model (47). Together these studies show that distinct melanoma drivers require SOX10 for tumor formation and maintenance. Both NRAS and GRM1 activate the MAPK and PI3K/AKT signaling pathways, which are fundamental to melanomagenesis. Given that both the NrasQ61K and the Grm1Tg mouse melanoma models are rescued with Sox10 haploinsufficiency, SOX10 may occupy an important position of regulation of oncogenic cell cycle progression downstream of one or both of these signaling pathways. Interestingly, the Grm1Tg mouse melanoma model is not rescued with the Mitfvga9 mouse, suggesting that either this cross did not result in a sufficient loss in Mitf expression, as Mitfvga/+ mice have been demonstrated to exhibit a 50% reduction in melanocyte MITF levels (22), or that SOX10 is involved in key melanoma pathways independent of its regulation of Mitf levels. When these data are combined with the extensive occurrence of SOX10-positive cells in both primary and metastatic tumors (41), the frequent tumor retention of wild-type SOX10 protein, and the effect of reduced SOX10 levels on RB and E2F1 levels leading to decreased cell cycle progression and melanoma cell proliferation, SOX10 is revealed as an notable molecule for further assessment as a therapeutic target.
Supplementary Material
Acknowledgments
We thank Anand K. Ganesan for generously providing pLKO1 shRNA and lentiviral packaging plasmid, Laura L. Baxter and Ashani T. Weeraratna for manuscript assistance, Amy M. Avergas and John L. Zapas for communications regarding cell lines acquired from the Maryland Melanoma Center at Medstar Franklin Square Medical Center, and Ken Dutton-Regester for assistance with cell culture and DNA extraction. This work was funded by the National Human Genome Research Institute, National Institutes of Health and the National Health and Medical Research Council of Australia (NHMRC). NKH is supported by a Senior Principal Research Fellowship from the NHMRC.
Footnotes
“The authors disclose no potential conflicts of interest”
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013 Jan;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Broekaert SMC, Roy R, Okamoto I, van den Oord J, Bauer J, Garbe C, et al. Genetic and morphologic features for melanoma classification. Pigment Cell & Melanoma Research. 2010 Dec;23(6):763–770. doi: 10.1111/j.1755-148X.2010.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiteman DC, Pavan WJ, Bastian BC. The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell & Melanoma Research. 2011 Oct;24(5):879–897. doi: 10.1111/j.1755-148X.2011.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012 Jul 29;44(9):1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat J-P, et al. A landscape of driver mutations in melanoma. Cell. 2012 Jul 20;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pérez-Lorenzo R, Zheng B. Targeted inhibition of BRAF kinase: opportunities and challenges for therapeutics in melanoma. Biosci Rep. 2012 Feb;32(1):25–33. doi: 10.1042/BSR20110068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narita M, Nuñez S, Heard E, Narita M, Lin AW, Hearn SA, et al. Rb-Mediated Heterochromatin Formation and Silencing of E2F Target Genes during Cellular Senescence. Cell. 2003 Jun;113(6):703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 8.Chaoui A, Watanabe Y, Touraine R, Baral V, Goossens M, Pingault V, et al. Identification and functional analysis of SOX10 missense mutations in different subtypes of Waardenburg syndrome. Hum Mutat. 2011 Dec;32(12):1436–1449. doi: 10.1002/humu.21583. [DOI] [PubMed] [Google Scholar]
- 9.Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature. 2005 Jul 7;436(7047):117–122. doi: 10.1038/nature03664. [DOI] [PubMed] [Google Scholar]
- 10.Cronin JC, Wunderlich J, Loftus SK, Prickett TD, Wei X, Ridd K, et al. Frequent mutations in the MITF pathway in melanoma. Pigment Cell & Melanoma Research. 2009 Aug;22(4):435–444. doi: 10.1111/j.1755-148X.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGill GG, Horstmann M, Widlund HR, Du J, Motyckova G, Nishimura EK, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002 Jun 14;109(6):707–718. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- 12.Cheli Y, Giuliano S, Guiliano S, Botton T, Rocchi S, Hofman V, et al. Mitf is the key molecular switch between mouse or human melanoma initiating cells and their differentiated progeny. Oncogene. 2011 May 19;30(20):2307–2318. doi: 10.1038/onc.2010.598. [DOI] [PubMed] [Google Scholar]
- 13.Giuliano S, Cheli Y, Ohanna M, Bonet C, Beuret L, Bille K, et al. Microphthalmia-associated transcription factor controls the DNA damage response and a lineage-specific senescence program in melanomas. Cancer Research. 2010 May 1;70(9):3813–3822. doi: 10.1158/0008-5472.CAN-09-2913. [DOI] [PubMed] [Google Scholar]
- 14.Loercher AE, Tank EMH, Delston RB, Harbour JW. MITF links differentiation with cell cycle arrest in melanocytes by transcriptional activation of INK4A. J Cell Biol. 2005 Jan 3;168(1):35–40. doi: 10.1083/jcb.200410115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trent JM, Stanbridge EJ, McBride HL, Meese EU, Casey G, Araujo DE, et al. Tumorigenicity in human melanoma cell lines controlled by introduction of human chromosome 6. Science. 1990 Feb 2;247(4942):568–571. doi: 10.1126/science.2300817. [DOI] [PubMed] [Google Scholar]
- 16.Sharma BK, Manglik V, O’Connell M, Weeraratna A, McCarron EC, Broussard JN, et al. Clonal dominance of CD133+ subset population as risk factor in tumor progression and disease recurrence of human cutaneous melanoma. Int J Oncol. 2012 Nov;41(5):1570–1576. doi: 10.3892/ijo.2012.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Root DE, Hacohen N, Hahn WC, Lander ES, Sabatini DM. Genome-scale loss-of-function screening with a lentiviral RNAi library. Nat Methods. 2006 Sep;3(9):715–719. doi: 10.1038/nmeth924. [DOI] [PubMed] [Google Scholar]
- 18.Narla G, Difeo A, Reeves HL, Schaid DJ, Hirshfeld J, Hod E, et al. A germline DNA polymorphism enhances alternative splicing of the KLF6 tumor suppressor gene and is associated with increased prostate cancer risk. Cancer Research. 2005 Feb 15;65(4):1213–1222. doi: 10.1158/0008-5472.CAN-04-4249. [DOI] [PubMed] [Google Scholar]
- 19.Jang YK, Park JJ, Lee MC, Yoon BH, Yang YS, Yang SE, et al. Retinoic acid-mediated induction of neurons and glial cells from human umbilical cord-derived hematopoietic stem cells. J Neurosci Res. 2004 Feb 15;75(4):573–584. doi: 10.1002/jnr.10789. [DOI] [PubMed] [Google Scholar]
- 20.Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003 May;34(1):108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 21.Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001 Jan 1;15(1):66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodgkinson CA, Moore KJ, Nakayama A, Steingrímsson E, Copeland NG, Jenkins NA, et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993 Jul;74(2):395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- 23.Bernex F, De Sepulveda P, Kress C, Elbaz C, Delouis C, Panthier JJ. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development. 1996 Oct;122(10):3023–3033. doi: 10.1242/dev.122.10.3023. [DOI] [PubMed] [Google Scholar]
- 24.Matera I, Watkins-Chow DE, Loftus SK, Hou L, Incao A, Silver DL, et al. A sensitized mutagenesis screen identifies Gli3 as a modifier of Sox10 neurocristopathy. Hum Mol Genet. 2008 Jul 15;17(14):2118–2131. doi: 10.1093/hmg/ddn110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houghton AN, Real FX, Davis LJ, Cordon-Cardo C, Old LJ. Phenotypic heterogeneity of melanoma. Relation to the differentiation program of melanoma cells. Journal of Experimental Medicine. 1987 Mar 1;164(EP0110716A2):812–829. doi: 10.1084/jem.165.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halaban R. Rb/E2F: a two-edged sword in the melanocytic system. Cancer Metastasis Rev. 2005 Jun;24(2):339–356. doi: 10.1007/s10555-005-1582-z. [DOI] [PubMed] [Google Scholar]
- 27.Li CG, Nyman JE, Braithwaite AW, Eccles MR. PAX8 promotes tumor cell growth by transcriptionally regulating E2F1 and stabilizing RB protein. Oncogene. 2011 Dec 1;30(48):4824–4834. doi: 10.1038/onc.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenouille N, Robert G, Tichet M, Puissant A, Dufies M, Rocchi S, et al. The p53/p21Cip1/_Waf1 pathway mediates the effects of SPARC on melanoma cell cycle progression. Pigment Cell & Melanoma Research. 2011 Feb;24(1):219–232. doi: 10.1111/j.1755-148X.2010.00790.x. [DOI] [PubMed] [Google Scholar]
- 29.Carreira S, Goodall J, Denat L, Rodriguez M, Nuciforo P, Hoek KS, et al. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev. 2006 Dec 15;20(24):3426–3439. doi: 10.1101/gad.406406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potterf SB, Furumura M, Dunn KJ, Arnheiter H, Pavan WJ. Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum Genet. 2000 Jul;107(1):1–6. doi: 10.1007/s004390000328. [DOI] [PubMed] [Google Scholar]
- 31.Du J, Widlund HR, Horstmann MA, Ramaswamy S, Ross K, Huber WE, et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell. 2004 Dec;6(6):565–576. doi: 10.1016/j.ccr.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 32.Carreira S, Goodall J, Aksan I, La Rocca SA, Galibert M-D, Denat L, et al. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature. 2005 Feb 17;433(7027):764–769. doi: 10.1038/nature03269. [DOI] [PubMed] [Google Scholar]
- 33.Liu F, Singh A, Yang Z, Garcia A, Kong Y, Meyskens FL. MiTF links Erk1/2 kinase and p21CIP1/WAF1 activation after UVC radiation in normal human melanocytes and melanoma cells. Mol Cancer. 2010;9(1):214. doi: 10.1186/1476-4598-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prickett TD, Wei X, Cardenas-Navia I, Teer JK, Lin JC, Walia V, et al. Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma. Nat Genet. 2011 Nov;43(11):1119–1126. doi: 10.1038/ng.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei X, Walia V, Lin JC, Teer JK, Prickett TD, Gartner J, et al. Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat Genet. 2011 May;43(5):442–446. doi: 10.1038/ng.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hornyak TJ, Jiang S, Guzmán EA, Scissors BN, Tuchinda C, He H, et al. Mitf dosage as a primary determinant of melanocyte survival after ultraviolet irradiation. Pigment Cell & Melanoma Research. 2009 Jun;22(3):307–318. doi: 10.1111/j.1755-148X.2009.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paraiso KHT, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Research. 2011 Apr 1;71(7):2750–2760. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alcala AM, Flaherty KT. BRAF Inhibitors for the Treatment of Metastatic Melanoma: Clinical Trials and Mechanisms of Resistance. Clinical Cancer Research. 2012 Jan 2;18(1):33–39. doi: 10.1158/1078-0432.CCR-11-0997. [DOI] [PubMed] [Google Scholar]
- 39.Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008 Sep;32(9):1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 40.Bakos RM, Maier T, Besch R, Mestel DS, Ruzicka T, Sturm RA, et al. Nestin and SOX9 and SOX10 transcription factors are coexpressed in melanoma. Exp Dermatol. 2010 Aug;19(8):e89–94. doi: 10.1111/j.1600-0625.2009.00991.x. [DOI] [PubMed] [Google Scholar]
- 41.Agnarsdóttir M, Sooman L, Bolander A, Strömberg S, Rexhepaj E, Bergqvist M, et al. SOX10 expression in superficial spreading and nodular malignant melanomas. Melanoma Research. 2010 Dec;20(6):468–478. doi: 10.1097/CMR.0b013e3283403ccd. [DOI] [PubMed] [Google Scholar]
- 42.Flammiger A, Besch R, Cook AL, Maier T, Sturm RA, Berking C. SOX9 and SOX10 but Not BRN2 Are Required for Nestin Expression in Human Melanoma Cells. Journal of Investigative Dermatology. 2008 Oct 16;129(4):945–953. doi: 10.1038/jid.2008.316. [DOI] [PubMed] [Google Scholar]
- 43.Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012 May 24;485(7399):502–506. doi: 10.1038/nature11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofmann F, Martelli F, Livingston DM, Wang Z. The retinoblastoma gene product protects E2F-1 from degradation by the ubiquitin-proteasome pathway. Genes Dev. 1996 Dec 1;10(23):2949–2959. doi: 10.1101/gad.10.23.2949. [DOI] [PubMed] [Google Scholar]
- 45.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Research. 1996 Oct 15;56(20):4620–4624. [PubMed] [Google Scholar]
- 46.Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013 Feb 1;19(3):598–609. doi: 10.1158/1078-0432.CCR-12-2731. [DOI] [PubMed] [Google Scholar]
- 47.Shakhova O, Zingg D, Schaefer SM, Hari L, Civenni G, Blunschi J, et al. Sox10 promotes the formation and maintenance of giant congenital naevi and melanoma. Nat Cell Biol. 2012 Aug;14(8):882–890. doi: 10.1038/ncb2535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.