Abstract
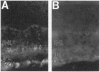
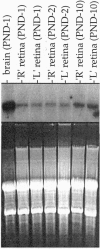
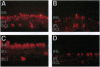
trkB is the high-affinity receptor for brain-derived neurotrophic factor (BDNF), a trophic molecule with demonstrated effects on the survival and differentiation of a wide variety of neuronal populations. In the mammalian retina, trkB is localized to both ganglion cells and numerous cells in the inner nuclear layer. Much information on the role of BDNF in neuronal development has been derived from the study of trkB- and BDNF-deficient mutant mice. This includes an attenuation of the numbers of cortical neurons immunopositive for the calcium-binding proteins, parvalbumin, and calbindin. Unfortunately, these mutant animals typically fail to survive for > 24-48 hr after birth. Since most retinal neuronal differentiation occurs postnatally, we have devised an alternative scheme to suppress the expression of trkB in the retina to examine the role of BDNF on the postnatal development of neurons of the inner retina. Neonatal rats were treated with intraocular injection of an antisense oligonucleotide (1-2 microliters of 10-100 microM solution) targeted to the trkB mRNA. Immunohistochemistry with a polyclonal antibody to trkB showed that the expression of trkB in retinal neurons was suppressed 48-72 hr following a single injection. Northern blot analysis demonstrated that antisense treatment had no effect on the level of trkB mRNA, even after multiple injections. This suggests an effect of trkB antisense treatment on protein translation, but not on RNA transcription. No alterations were observed in the thickness of retinal cellular or plexiform layers, suggesting that BDNF is not the sole survival factor for these neurons. There were, however, alterations in the patterns of immunostaining for parvalbumin, a marker for the narrow-field, bistratified AII amacrine cell-a central element of the rod (scotopic) pathway. This was evidenced by a decrease in both the number of immunostained somata (> 50%) and in the intensity of immunolabeling. However, the immunostaining pattern of calbindin was not affected. These studies suggest that the ligands for trkB have specific effects on the neurochemical phenotypic expression of inner retinal neurons and in the development of a well-defined retinal circuit.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boos R., Schneider H., Wässle H. Voltage- and transmitter-gated currents of all-amacrine cells in a slice preparation of the rat retina. J Neurosci. 1993 Jul;13(7):2874–2888. doi: 10.1523/JNEUROSCI.13-07-02874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunt A. H., Lund R. D., Lund J. S. Retrograde axonal transport of horseradish peroxidase by ganglion cells of the albino rat retina. Brain Res. 1974 Jun 20;73(2):215–228. doi: 10.1016/0006-8993(74)91045-2. [DOI] [PubMed] [Google Scholar]
- Carmignoto G., Comelli M. C., Candeo P., Cavicchioli L., Yan Q., Merighi A., Maffei L. Expression of NGF receptor and NGF receptor mRNA in the developing and adult rat retina. Exp Neurol. 1991 Mar;111(3):302–311. doi: 10.1016/0014-4886(91)90097-v. [DOI] [PubMed] [Google Scholar]
- Castillo B., Jr, del Cerro M., Breakefield X. O., Frim D. M., Barnstable C. J., Dean D. O., Bohn M. C. Retinal ganglion cell survival is promoted by genetically modified astrocytes designed to secrete brain-derived neurotrophic factor (BDNF). Brain Res. 1994 May 30;647(1):30–36. doi: 10.1016/0006-8993(94)91395-1. [DOI] [PubMed] [Google Scholar]
- Chun M. H., Han S. H., Chung J. W., Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993 Jun 22;332(4):421–432. doi: 10.1002/cne.903320404. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S., Fraser S. E. BDNF in the development of the visual system of Xenopus. Neuron. 1994 Apr;12(4):747–761. doi: 10.1016/0896-6273(94)90328-x. [DOI] [PubMed] [Google Scholar]
- Cohen A., Bray G. M., Aguayo A. J. Neurotrophin-4/5 (NT-4/5) increases adult rat retinal ganglion cell survival and neurite outgrowth in vitro. J Neurobiol. 1994 Aug;25(8):953–959. doi: 10.1002/neu.480250805. [DOI] [PubMed] [Google Scholar]
- Dixon J. E., McKinnon D. Expression of the trk gene family of neurotrophin receptors in prevertebral sympathetic ganglia. Brain Res Dev Brain Res. 1994 Feb 18;77(2):177–182. doi: 10.1016/0165-3806(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Eide F. F., Lowenstein D. H., Reichardt L. F. Neurotrophins and their receptors--current concepts and implications for neurologic disease. Exp Neurol. 1993 Jun;121(2):200–214. doi: 10.1006/exnr.1993.1087. [DOI] [PubMed] [Google Scholar]
- Endo T., Kobayashi M., Kobayashi S., Onaya T. Immunocytochemical and biochemical localization of parvalbumin in the retina. Cell Tissue Res. 1986;243(1):213–217. doi: 10.1007/BF00221870. [DOI] [PubMed] [Google Scholar]
- Ernfors Patrik, Merlio Jean-Phillipe, Persson Håkan. Cells Expressing mRNA for Neurotrophins and their Receptors During Embryonic Rat Development. Eur J Neurosci. 1992 Oct;4(11):1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Kolb H. A bistratified amacrine cell and synaptic cirucitry in the inner plexiform layer of the retina. Brain Res. 1975 Feb 7;84(2):293–300. doi: 10.1016/0006-8993(75)90983-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Guo Q. X., Yu M. C., Garey L. J., Jen L. S. Development of parvalbumin immunoreactive neurons in normal and intracranially transplanted retinas in the rat. Exp Brain Res. 1992;90(2):359–368. doi: 10.1007/BF00227249. [DOI] [PubMed] [Google Scholar]
- Götz R., Köster R., Winkler C., Raulf F., Lottspeich F., Schartl M., Thoenen H. Neurotrophin-6 is a new member of the nerve growth factor family. Nature. 1994 Nov 17;372(6503):266–269. doi: 10.1038/372266a0. [DOI] [PubMed] [Google Scholar]
- Hamano K., Kiyama H., Emson P. C., Manabe R., Nakauchi M., Tohyama M. Localization of two calcium binding proteins, calbindin (28 kD) and parvalbumin (12 kD), in the vertebrate retina. J Comp Neurol. 1990 Dec 8;302(2):417–424. doi: 10.1002/cne.903020217. [DOI] [PubMed] [Google Scholar]
- Hofer M., Pagliusi S. R., Hohn A., Leibrock J., Barde Y. A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990 Aug;9(8):2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. E., Barde Y. A., Schwab M., Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J Neurosci. 1986 Oct;6(10):3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. R., Fariñas I., Backus C., Reichardt L. F. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994 Mar 25;76(6):989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Lamballe F., Bryant S., Barbacid M. The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron. 1992 May;8(5):947–956. doi: 10.1016/0896-6273(92)90209-v. [DOI] [PubMed] [Google Scholar]
- Klein R., Nanduri V., Jing S. A., Lamballe F., Tapley P., Bryant S., Cordon-Cardo C., Jones K. R., Reichardt L. F., Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991 Jul 26;66(2):395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Smeyne R. J., Wurst W., Long L. K., Auerbach B. A., Joyner A. L., Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993 Oct 8;75(1):113–122. [PubMed] [Google Scholar]
- Kondo Y., Takada M., Honda Y., Mizuno N. Bilateral projections of single retinal ganglion cells to the lateral geniculate nuclei and superior colliculi in the albino rat. Brain Res. 1993 Apr 16;608(2):204–215. doi: 10.1016/0006-8993(93)91460-a. [DOI] [PubMed] [Google Scholar]
- Lamballe F., Klein R., Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991 Sep 6;66(5):967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Lauterborn J. C., Isackson P. J., Gall C. M. Cellular localization of NGF and NT-3 mRNAs in postnatal rat forebrain. Mol Cell Neurosci. 1994 Feb;5(1):46–62. doi: 10.1006/mcne.1994.1005. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Squinto S., Ip N. Y., Furth M. E., Lindsay R. M., Yancopoulos G. D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990 Mar 23;247(4949 Pt 1):1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Mansour-Robaey S., Clarke D. B., Wang Y. C., Bray G. M., Aguayo A. J. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- McAllister A. K., Lo D. C., Katz L. C. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995 Oct;15(4):791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Mey J., Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993 Feb 5;602(2):304–317. doi: 10.1016/0006-8993(93)90695-j. [DOI] [PubMed] [Google Scholar]
- Pasteels B., Rogers J., Blachier F., Pochet R. Calbindin and calretinin localization in retina from different species. Vis Neurosci. 1990 Jul;5(1):1–16. doi: 10.1017/s0952523800000031. [DOI] [PubMed] [Google Scholar]
- Perez M. T., Caminos E. Expression of brain-derived neurotrophic factor and of its functional receptor in neonatal and adult rat retina. Neurosci Lett. 1995 Jan 2;183(1-2):96–99. doi: 10.1016/0304-3940(94)11123-z. [DOI] [PubMed] [Google Scholar]
- Rickman D. W., Brecha N. C. Expression of the proto-oncogene, trk, receptors in the developing rat retina. Vis Neurosci. 1995 Mar-Apr;12(2):215–222. doi: 10.1017/s0952523800007896. [DOI] [PubMed] [Google Scholar]
- Ross A. H. Identification of tyrosine kinase Trk as a nerve growth factor receptor. Cell Regul. 1991 Sep;2(9):685–690. doi: 10.1091/mbc.2.9.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna P. P., Keyser K. T., Celio M. R., Karten H. J., Bloom F. E. Distribution of parvalbumin immunoreactivity in the vertebrate retina. Brain Res. 1993 Jan 8;600(1):141–150. doi: 10.1016/0006-8993(93)90412-g. [DOI] [PubMed] [Google Scholar]
- Schecterson L. C., Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992 Sep;9(3):449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Snider W. D. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994 Jun 3;77(5):627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Sterling P. Microcircuitry of the cat retina. Annu Rev Neurosci. 1983;6:149–185. doi: 10.1146/annurev.ne.06.030183.001053. [DOI] [PubMed] [Google Scholar]
- Strettoi E., Raviola E., Dacheux R. F. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992 Nov 8;325(2):152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- Takahashi J. B., Hoshimaru M., Kikuchi H., Hatanaka M. Developmental expression of trkB and low-affinity NGF receptor in the rat retina. Neurosci Lett. 1993 Mar 19;151(2):174–177. doi: 10.1016/0304-3940(93)90014-c. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C. Antisense oligonucleotide strategies in neuropharmacology. Trends Pharmacol Sci. 1994 Feb;15(2):42–46. doi: 10.1016/0165-6147(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Wässle H., Grünert U., Röhrenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J Comp Neurol. 1993 Jun 22;332(4):407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- Zanellato A., Comelli M. C., Dal Toso R., Carmignoto G. Developing rat retinal ganglion cells express the functional NGF receptor p140trkA. Dev Biol. 1993 Sep;159(1):105–113. doi: 10.1006/dbio.1993.1224. [DOI] [PubMed] [Google Scholar]