Abstract
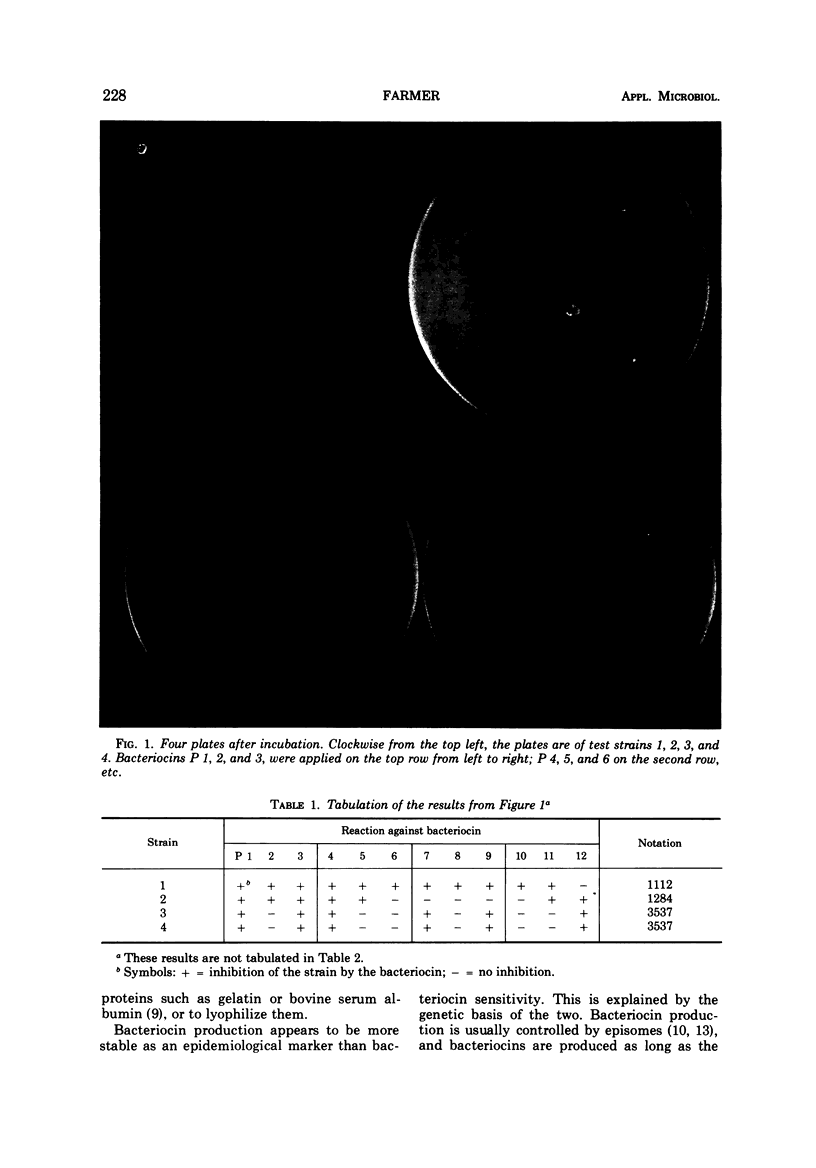
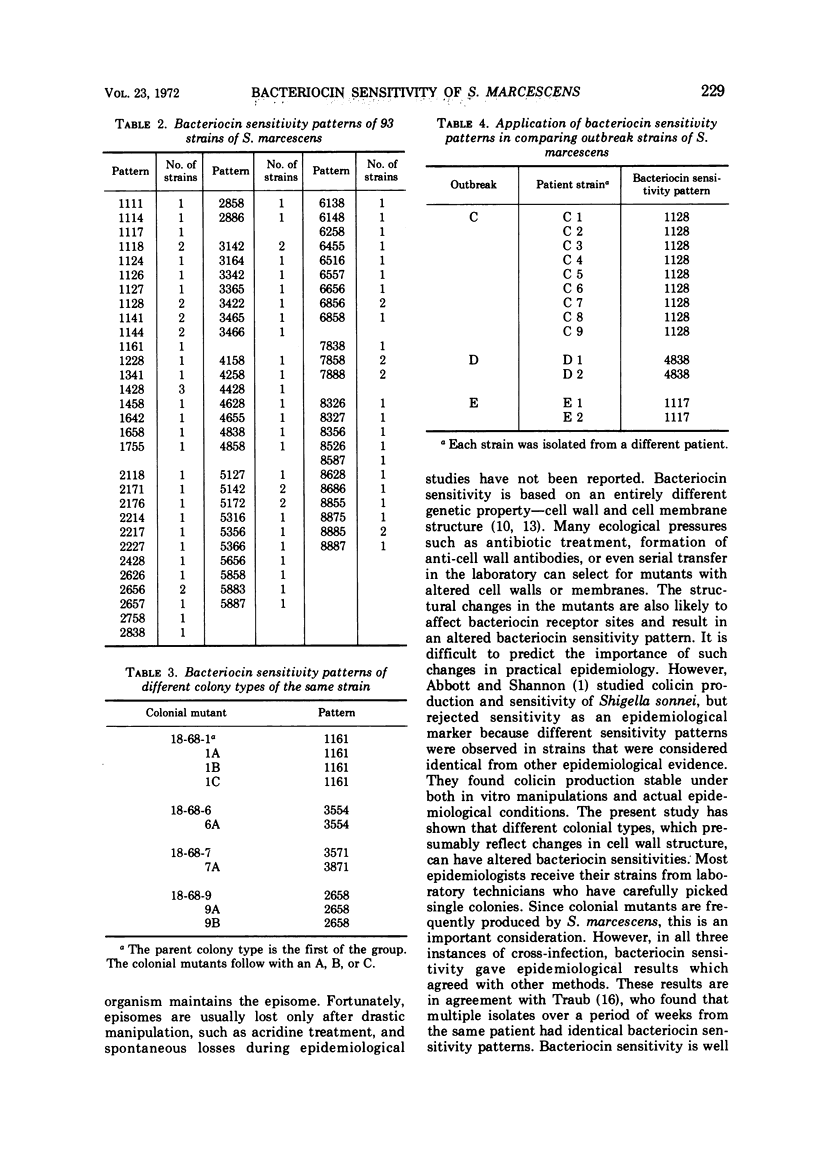
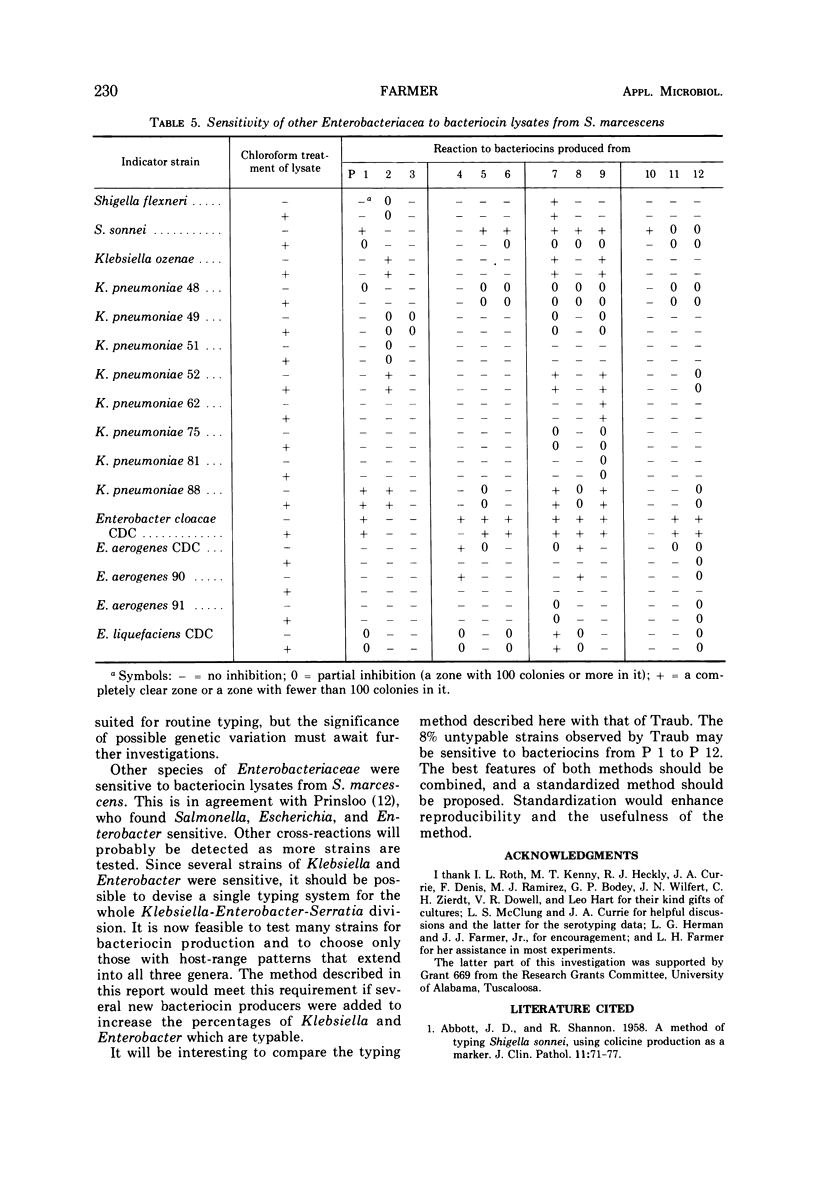
Strains of Serratia marcescens were compared and differentiated by a new method. Bacteriocin lysates were prepared from mitomycin-induced S. marcescens and added to lawns of test strains. From 100 bacteriocin producers, 12 were chosen with the aid of computer analysis as the most useful in differentiation. Uniform drops of the 12 standard bacteriocins were added simultaneously with a bacteriocin-bacteriophage dropper to each strain to be typed. All 93 strains of S. marcescens tested were typable and were differentiated into 79 different sensitivity patterns. One pattern had three strains, 12 patterns had two strains each, and 66 patterns had only one strain. The bacteriocins also inhibited Shigella, Klebsiella, and Enterobacter, but no other Enterobacteriaceae. Bacteriocin sensitivity was less stable as an epidemiological marker than bacteriocin production. Several colonial mutants had sensitivity patterns different from the wild types, but most mutants were identical. In three different instances when cross-infection had been shown by other methods, bacteriocin sensitivity also gave the correct epidemiological results. Until the significance and frequency of genetic variations are known, a more stable epidemiological technique should be used in conjunction with bacteriocin sensitivity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT J. D., SHANNON R. A method for typing Shigella sonnei, using colicine production as a marker. J Clin Pathol. 1958 Jan;11(1):71–77. doi: 10.1136/jcp.11.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd, Herman L. G. Epidemiological fingerprinting of Pseudomonas aeruginosa by the production of and sensitivity of pyocin and bacteriophage. Appl Microbiol. 1969 Nov;18(5):760–765. doi: 10.1128/am.18.5.760-765.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd Improved bacteriophage-bacteriocin applicator. Appl Microbiol. 1970 Sep;20(3):517–518. doi: 10.1128/am.20.3.517-518.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd Mnemonic for reporting bacteriocin and bacteriophage types. Lancet. 1970 Jul 11;1(7663):96–96. doi: 10.1016/s0140-6736(70)92663-2. [DOI] [PubMed] [Google Scholar]
- GILLIES R. R. COLICINE PRODUCTION AS AN EPIDEMIOLOGICAL MARKER OF SHIGELLA SONNEI. J Hyg (Lond) 1964 Mar;62:1–9. doi: 10.1017/s0022172400039711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies R. R., Govan J. R. Typing of Pseudomonas pyocyanea by pyocine production. J Pathol Bacteriol. 1966 Apr;91(2):339–345. doi: 10.1002/path.1700910207. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T. Bacteriocin production by strains of Neisseria meningitidis. J Bacteriol. 1966 May;91(5):1696–1699. doi: 10.1128/jb.91.5.1696-1699.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui E., Mizuno D. Stabilization of colicin E2 by bovine serum albumin. J Bacteriol. 1969 Nov;100(2):1136–1137. doi: 10.1128/jb.100.2.1136-1137.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- OSMAN M. A. PYOCINE TYPING OF PSEUDOMONAS AERUGINOSA. J Clin Pathol. 1965 Mar;18:200–202. doi: 10.1136/jcp.18.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinsloo H. E. Bacteriocins and phages produced by Serratia marcescens. J Gen Microbiol. 1966 Nov;45(2):205–212. doi: 10.1099/00221287-45-2-205. [DOI] [PubMed] [Google Scholar]
- REEVES P. THE BACTERIOCINS. Bacteriol Rev. 1965 Mar;29:24–45. doi: 10.1128/br.29.1.24-45.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopek S., Maresz-Babczyszyn J. A working scheme for typing Klebsiella bacilli by means of pneumocins. Arch Immunol Ther Exp (Warsz) 1967;15(4):525–529. [PubMed] [Google Scholar]
- Stouthamer A. H., Tieze G. A. Bacteriocin production by members of the genus Klebsiella. Antonie Van Leeuwenhoek. 1966;32(2):171–182. doi: 10.1007/BF02097457. [DOI] [PubMed] [Google Scholar]
- Traub W. H., Raymond E. A., Startsman T. S. Bacteriocin (Marcescin) typing of clinical isolates of Serratia marcescens. Appl Microbiol. 1971 May;21(5):837–840. doi: 10.1128/am.21.5.837-840.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosti K. L. Production of and sensitivity to colicins among serologically classified strains of Escherichia coli. J Bacteriol. 1968 Dec;96(6):1947–1952. doi: 10.1128/jb.96.6.1947-1952.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabransky R. J., Day F. E. Pyocine typing of clinical strains of Pseudomonas aeruginosa. Appl Microbiol. 1969 Feb;17(2):293–296. doi: 10.1128/am.17.2.293-296.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



