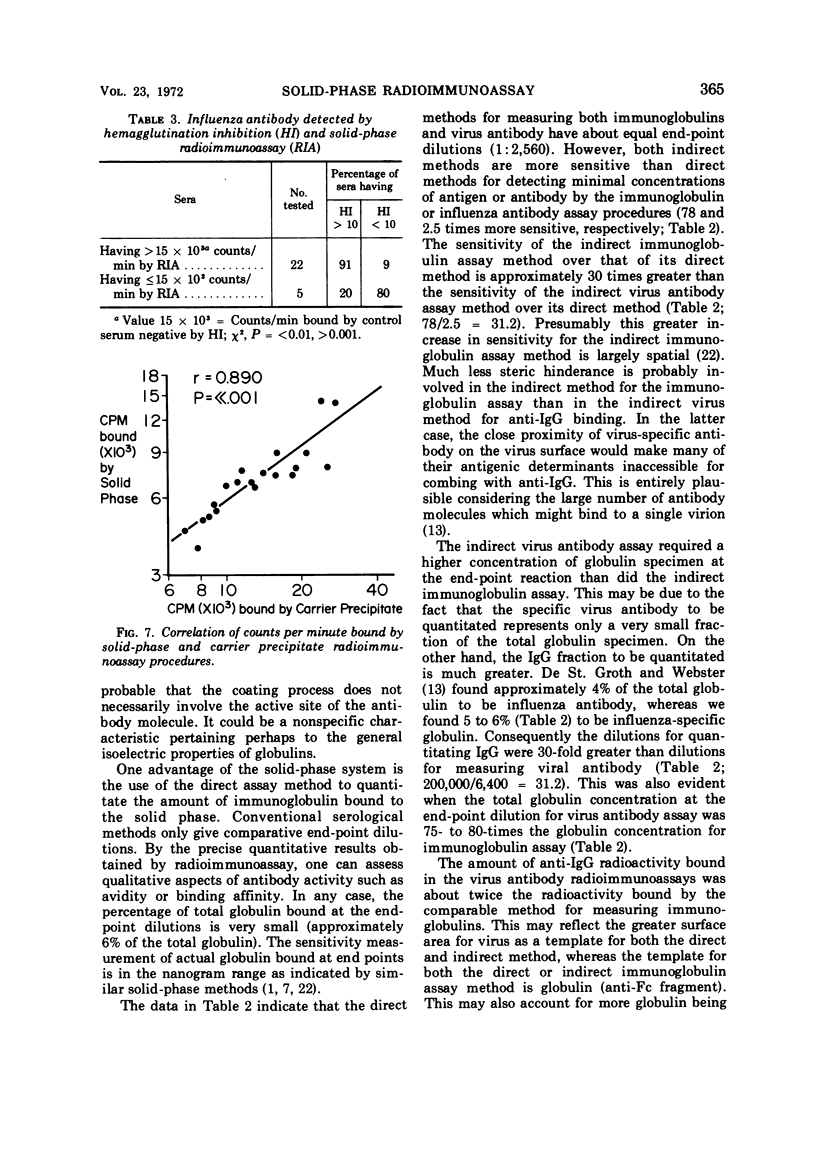
Abstract
An antigen-antibody system of polystyrene tubes coated with immunoglobulin antibody was used for quantitating immunoglobulins. A similar radioimmunoassay method was adapted for a viral antigen-antibody system. The viral system can be used for quantitating viruses and for measuring virus-specific antibodies by reacting with 125iodine-labeled anti-immunoglobulin G (IgG). Optimal conditions for coating the solid phase, specificity of the immune reaction, and other kinetics and sensitivities of the assay method were investigated. Comparison of direct and indirect methods of assaying for immunoglobulins or viral antibody indicates that the indirect method is more sensitive and can quantitate a minimum of 0.037 μg of IgG per ml. Results of solid-phase radioimmunoassay for influenza antibody correlate well with hemagglutinin antibody titers but not with complement-fixing antibody titers. Radioimmunoassay results for influenza antibody by solid phase are likewise in agreement with results by the carrier precipitate radioimmunoassay method. The simplicity, reproducibility, and versatility of the solid-phase procedure make it diagnostically useful.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenase P. W., Leonard E. J. Solid phase radioimmunoassay of human beta 1C globulin. Immunochemistry. 1970 Jan;7(1):29–41. doi: 10.1016/0019-2791(70)90028-5. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Bandilla K. K., McDuffie F. C., Gleich G. J. Immunoglobulin classes of antibodies produced in the primary and secondary responses in man. Clin Exp Immunol. 1969 Dec;5(6):627–641. [PMC free article] [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. General principles of radioimmunoassay. Clin Chim Acta. 1968 Sep;22(1):51–69. doi: 10.1016/0009-8981(68)90247-7. [DOI] [PubMed] [Google Scholar]
- Boche R. D., Quilligan J. J., Jr Adsorption to glass and specific antibody inhibition of iodine-125 labeled influenza virus. J Immunol. 1966 Dec;97(6):942–950. [PubMed] [Google Scholar]
- Bombardieri S., Christian C. L. A technique for immunoassay of human IgG. Proc Soc Exp Biol Med. 1970 Apr;133(4):1366–1369. doi: 10.3181/00379727-133-34691. [DOI] [PubMed] [Google Scholar]
- Cheng W. C., Talmage D. W. The reaction of immunoglobulin-coated bentonite and radioiodinated antigen as a basis for antibody detection and antigen binding. J Immunol. 1969 Dec;103(6):1385–1394. [PubMed] [Google Scholar]
- Daniels C. A., Mergenhagen S. E., Notkins A. L. Measurement of antibody against human IgG by the neutralization of IgG-sensitized herpes simplex virus. J Immunol. 1970 Feb;104(2):470–474. [PubMed] [Google Scholar]
- Daugharty H. Immunoprecipitin reaction of influenza virus-antibody complex with anti-IgG. J Immunol. 1971 Sep;107(3):802–809. [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PORTER R. R., PRESS E. M. THE ARRANGEMENT OF THE PEPTIDE CHAINS IN GAMMA-GLOBULIN. Biochem J. 1963 Aug;88:220–228. doi: 10.1042/bj0880220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochwa S., Brownell M., Rosenfield R. E., Wasserman L. R. Adsorption of proteins by polystyrene particles. I. Molecular unfolding and acquired immunogenicity of IgG. J Immunol. 1967 Nov;99(5):981–986. [PubMed] [Google Scholar]
- Mann D., Granger H., Fahey J. L. Use of insoluble antibody for quantitative determination of small amounts of immunoglobulin. J Immunol. 1969 Mar;102(3):618–624. [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., Haimovich J., Sela M. Purification of antibodies with immunoadsorbents prepared using bromoacetyl cellulose. Immunochemistry. 1967 Jan;4(1):11–22. doi: 10.1016/0019-2791(67)90192-9. [DOI] [PubMed] [Google Scholar]
- SVEHAG S. E., MANDEL B. THE FORMATION AND PROPERTIES OF POLIOVIRUS-NEUTRALIZING ANTIBODY. I. 19S AND 7S ANTIBODY FORMATION: DIFFERENCES IN KINETICS AND ANTIGEN DOSE REQUIREMENT FOR INDUCTION. J Exp Med. 1964 Jan 1;119:1–19. doi: 10.1084/jem.119.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVEHAG S. E., MANDEL B. THE FORMATION AND PROPERTIES OF POLIOVIRUS-NEUTRALIZING ANTIBODY. II. 19S AND 7S ANTIBODY FORMATION: DIFFERENCES IN ANTIGEN DOSE REQUIREMENT FOR SUSTAINED SYNTHESIS, ANAMNESIS, AND SENSITIVITY TO X-IRRADIATION. J Exp Med. 1964 Jan 1;119:21–39. doi: 10.1084/jem.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon S. E., Mackey G., Fudenberg H. H. "Sandwich" solid phase radioimmunoassay for the quantitative deterimination of human immunoglobulins. J Immunol. 1969 Jul;103(1):129–137. [PubMed] [Google Scholar]
- Spiegelberg H. L., Weigle W. O. The immune response to human gamma-G-immunoglobulin in rabbits unresponsive to Fc fragment and H chain protein. J Immunol. 1967 May;98(5):1020–1027. [PubMed] [Google Scholar]


