Abstract
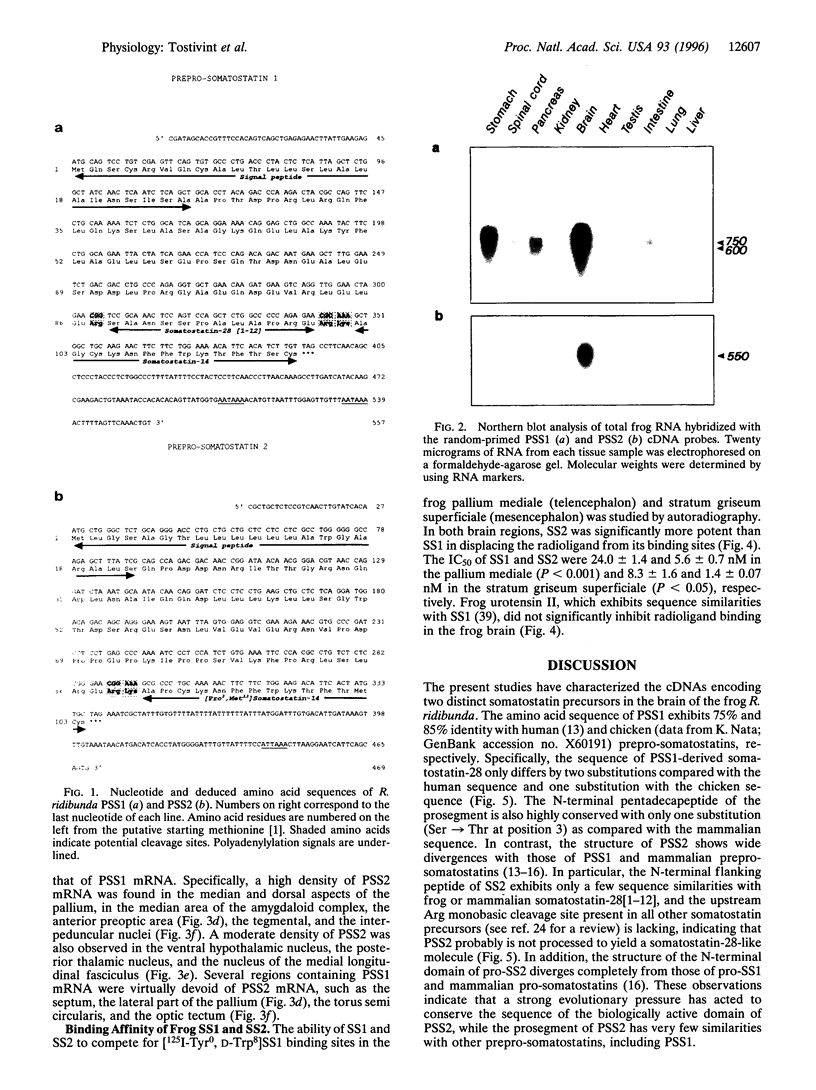
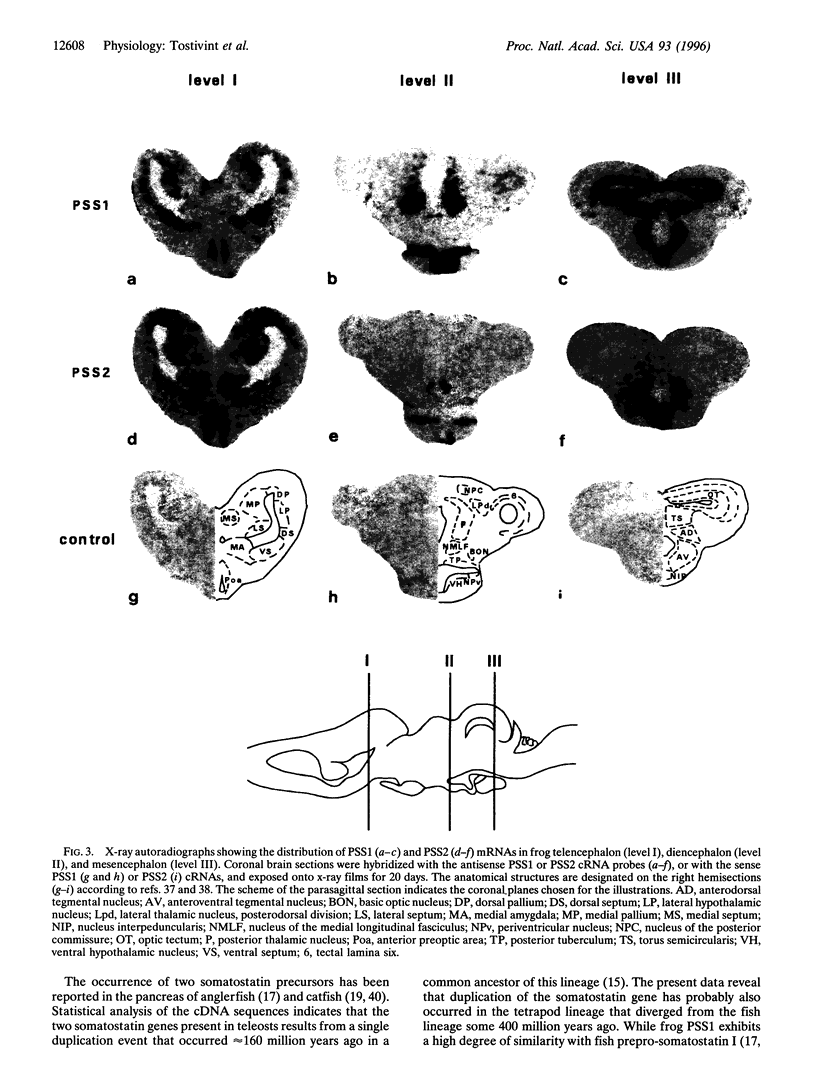
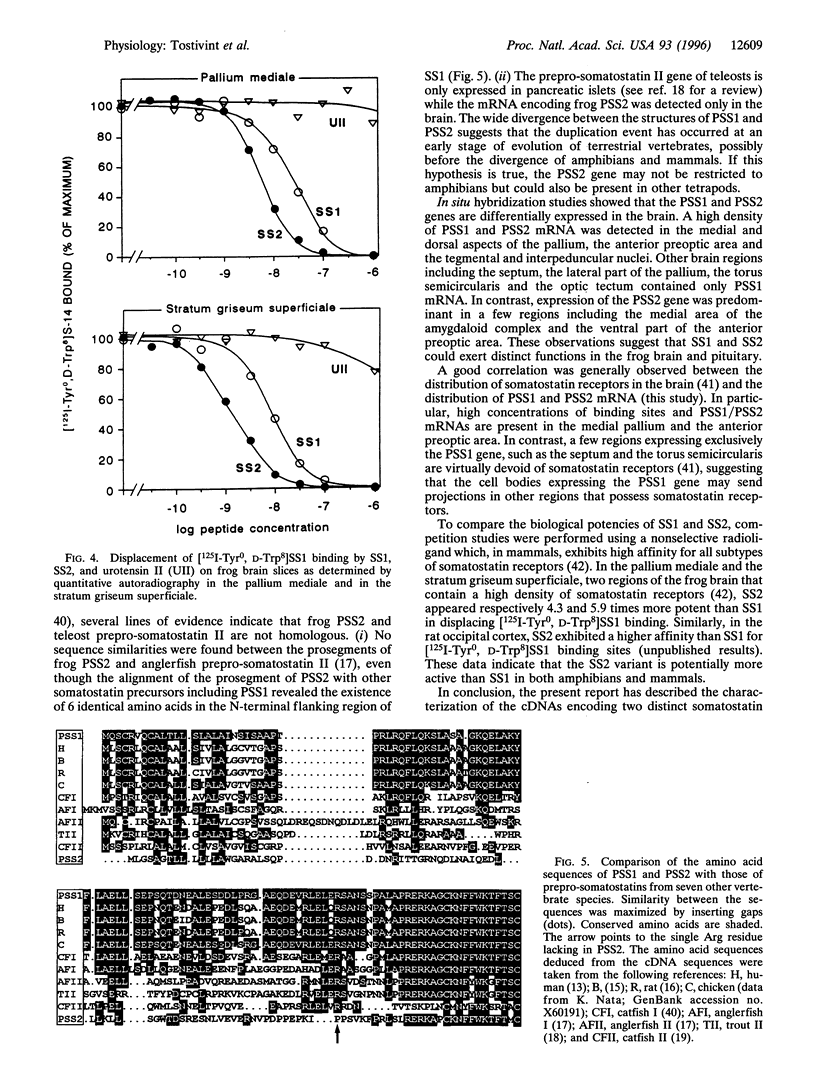
In tetrapods, only one gene encoding a somatostatin precursor has been identified so far. The present study reports the characterization of the cDNA clones that encode two distinct somatostatin precursors in the brain of the frog Rana ridibunda. The cDNAs were isolated by using degenerate oligonucleotides based on the sequence of the central region of somatostatin to screen a frog brain cDNA library. One of the cDNAs encodes a 115-amino acid protein (prepro-somatostatin-14; PSS1) that exhibits a high degree of structural similarity with the mammalian somatostatin precursor. The other cDNA encodes a 103-amino acid protein (prepro-[Pro2, Met13]somatostatin-14; PSS2) that contains the sequence of the somatostatin analog (peptide SS2) at its C terminus, but does not exhibit appreciable sequence similarity with PSS1 in the remaining region. In situ hybridization studies indicate differential expression of the PSS1 and PSS2 genes in the septum, the lateral part of the pallium, the amygdaloid complex, the posterior nuclei of the thalamus, the ventral hypothalamic nucleus, the torus semicircularis and the optic tectum. The somatostatin variant SS2 was significantly more potent (4-6 fold) than somatostatin itself in displacing [125I-Tyr0, D-Trp8] somatostatin-14 from its specific binding sites. The present study indicates that the two somatostatin variants could exert different functions in the frog brain and pituitary. These data also suggest that distinct genes encoding somatostatin variants may be expressed in the brain of other tetrapods.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. C., Pollock H. G., Elliott W. M., Youson J. H., Plisetskaya E. M. Isolation and characterization of a variant somatostatin-14 and two related somatostatins of 34 and 37 residues from lamprey (Petromyzon marinus). J Biol Chem. 1988 Oct 25;263(30):15809–15814. [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Chartrel N., Conlon J. M., Danger J. M., Fournier A., Tonon M. C., Vaudry H. Characterization of melanotropin-release-inhibiting factor (melanostatin) from frog brain: homology with human neuropeptide Y. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3862–3866. doi: 10.1073/pnas.88.9.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Agoston D. V., Thim L. An elasmobranchian somatostatin: primary structure and tissue distribution in Torpedo marmorata. Gen Comp Endocrinol. 1985 Dec;60(3):406–413. doi: 10.1016/0016-6480(85)90074-7. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Askensten U., Falkmer S., Thim L. Primary structures of somatostatins from the islet organ of the hagfish suggest an anomalous pathway of posttranslational processing of prosomatostatin-1. Endocrinology. 1988 May;122(5):1855–1859. doi: 10.1210/endo-122-5-1855. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Bondareva V., Rusakov Y., Plisetskaya E. M., Mynarcik D. C., Whittaker J. Characterization of insulin, glucagon, and somatostatin from the river lamprey, Lampetra fluviatilis. Gen Comp Endocrinol. 1995 Oct;100(1):96–105. doi: 10.1006/gcen.1995.1138. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Deacon C. F., Hazon N., Henderson I. W., Thim L. Somatostatin-related and glucagon-related peptides with unusual structural features from the European eel (Anguilla anguilla). Gen Comp Endocrinol. 1988 Nov;72(2):181–189. doi: 10.1016/0016-6480(88)90201-8. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Hicks J. W. Isolation and structural characterization of insulin, glucagon and somatostatin from the turtle, Pseudemys scripta. Peptides. 1990 May-Jun;11(3):461–466. doi: 10.1016/0196-9781(90)90043-5. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., Nielsen P. F., Youson J. H., Potter I. C. Proinsulin and somatostatin from the islet organ of the southern-hemisphere lamprey Geotria australis. Gen Comp Endocrinol. 1995 Dec;100(3):413–422. doi: 10.1006/gcen.1995.1172. [DOI] [PubMed] [Google Scholar]
- Conlon J. M., O'Harte F., Smith D. D., Tonon M. C., Vaudry H. Isolation and primary structure of urotensin II from the brain of a tetrapod, the frog Rana ridibunda. Biochem Biophys Res Commun. 1992 Oct 30;188(2):578–583. doi: 10.1016/0006-291x(92)91095-8. [DOI] [PubMed] [Google Scholar]
- Conlon J. M. Somatostatin: aspects of molecular evolution. Prog Clin Biol Res. 1990;342:10–15. [PubMed] [Google Scholar]
- Conlon J. M. [Ser5]-somatostatin-14: isolation from the pancreas of a holocephalan fish, the Pacific ratfish (Hydrolagus colliei). Gen Comp Endocrinol. 1990 Nov;80(2):314–320. doi: 10.1016/0016-6480(90)90175-l. [DOI] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988 Nov 25;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funckes C. L., Minth C. D., Deschenes R., Magazin M., Tavianini M. A., Sheets M., Collier K., Weith H. L., Aron D. C., Roos B. A. Cloning and characterization of a mRNA-encoding rat preprosomatostatin. J Biol Chem. 1983 Jul 25;258(14):8781–8787. [PubMed] [Google Scholar]
- Gonzalez B., Leroux P., Lamacz M., Bodenant C., Balazs R., Vaudry H. Somatostatin receptors are expressed by immature cerebellar granule cells: evidence for a direct inhibitory effect of somatostatin on neuroblast activity. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9627–9631. doi: 10.1073/pnas.89.20.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobart P., Crawford R., Shen L., Pictet R., Rutter W. J. Cloning and sequence analysis of cDNAs encoding two distinct somatostatin precursors found in the endocrine pancreas of anglerfish. Nature. 1980 Nov 13;288(5787):137–141. doi: 10.1038/288137a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987 Aug 20;196(4):947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Laquerriere A., Leroux P., Gonzalez B. J., Bodenant C., Benoit R., Vaudry H. Distribution of somatostatin receptors in the brain of the frog Rana ridibunda: correlation with the localization of somatostatin-containing neurons. J Comp Neurol. 1989 Feb 15;280(3):451–467. doi: 10.1002/cne.902800310. [DOI] [PubMed] [Google Scholar]
- Leroux P., Gonzalez B. J., Laquerrière A., Bodenant C., Vaudry H. Autoradiographic study of somatostatin receptors in the rat hypothalamus: validation of a GTP-induced desaturation procedure. Neuroendocrinology. 1988 Jun;47(6):533–544. doi: 10.1159/000124966. [DOI] [PubMed] [Google Scholar]
- Lihrmann I., Plaquevent J. C., Tostivint H., Raijmakers R., Tonon M. C., Conlon J. M., Vaudry H. Frog diazepam-binding inhibitor: peptide sequence, cDNA cloning, and expression in the brain. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6899–6903. doi: 10.1073/pnas.91.15.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazin M., Minth C. D., Funckes C. L., Deschenes R., Tavianini M. A., Dixon J. E. Sequence of a cDNA encoding pancreatic preprosomatostatin-22. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5152–5156. doi: 10.1073/pnas.79.17.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minth C. D., Taylor W. L., Magazin M., Tavianini M. A., Collier K., Weith H. L., Dixon J. E. The structure of cloned DNA complementary to catfish pancreatic somatostatin-14 messenger RNA. J Biol Chem. 1982 Sep 10;257(17):10372–10377. [PubMed] [Google Scholar]
- Moore C. A., Kittilson J. D., Dahl S. K., Sheridan M. A. Isolation and characterization of a cDNA encoding for preprosomatostatin containing [Tyr7, Gly10]-somatostatin-14 from the endocrine pancreas of rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol. 1995 Jun;98(3):253–261. doi: 10.1006/gcen.1995.1067. [DOI] [PubMed] [Google Scholar]
- Neary T. J., Northcutt R. G. Nuclear organization of the bullfrog diencephalon. J Comp Neurol. 1983 Jan 20;213(3):262–278. doi: 10.1002/cne.902130303. [DOI] [PubMed] [Google Scholar]
- Nishii M., Movérus B., Bukovskaya O. S., Takahashi A., Kawauchi H. Isolation and characterization of [Pro2]somatostatin-14 and melanotropins from Russian sturgeon, Acipenser gueldenstaedti Brandt. Gen Comp Endocrinol. 1995 Jul;99(1):6–12. doi: 10.1006/gcen.1995.1078. [DOI] [PubMed] [Google Scholar]
- Patel Y. C., Srikant C. B. Subtype selectivity of peptide analogs for all five cloned human somatostatin receptors (hsstr 1-5). Endocrinology. 1994 Dec;135(6):2814–2817. doi: 10.1210/endo.135.6.7988476. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin (second of two parts). N Engl J Med. 1983 Dec 22;309(25):1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. P., Pictet R. L., Rutter W. J. Human somatostatin I: sequence of the cDNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4575–4579. doi: 10.1073/pnas.79.15.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sower S. A., Chiang Y. C., Conlon J. M. Polygenic expression of somatostatin in lamprey. Peptides. 1994 Jan;15(1):151–154. doi: 10.1016/0196-9781(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Spiess J., Rivier J. E., Rodkey J. A., Bennett C. D., Vale W. Isolation and characterization of somatostatin from pigeon pancreas. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2974–2978. doi: 10.1073/pnas.76.6.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., White J. W., Li W. H., Luo C. C., Frazier M. L., Saunders G. F., Chan L. Structure and evolution of somatostatin genes. Mol Endocrinol. 1988 Mar;2(3):209–216. doi: 10.1210/mend-2-3-209. [DOI] [PubMed] [Google Scholar]
- Takami M., Reeve J. R., Jr, Hawke D., Shively J. E., Basinger S., Yamada T. Purification of somatostatin from frog brain: coisolation with retinal somatostatin-like immunoreactivity. J Neurochem. 1985 Dec;45(6):1869–1874. doi: 10.1111/j.1471-4159.1985.tb10545.x. [DOI] [PubMed] [Google Scholar]
- Travis G. H., Sutcliffe J. G. Phenol emulsion-enhanced DNA-driven subtractive cDNA cloning: isolation of low-abundance monkey cortex-specific mRNAs. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1696–1700. doi: 10.1073/pnas.85.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry H., Chartrel N., Conlon J. M. Isolation of [Pro2,Met13]somatostatin-14 and somatostatin-14 from the frog brain reveals the existence of a somatostatin gene family in a tetrapod. Biochem Biophys Res Commun. 1992 Oct 15;188(1):477–482. doi: 10.1016/0006-291x(92)92409-q. [DOI] [PubMed] [Google Scholar]
- Wang Y., Conlon J. M. Neuroendocrine peptides (NPY, GRP, VIP, somatostatin) from the brain and stomach of the alligator. Peptides. 1993 May-Jun;14(3):573–579. doi: 10.1016/0196-9781(93)90147-9. [DOI] [PubMed] [Google Scholar]
- Wang Y., Youson J. H., Conlon J. M. Prosomatostatin-I is processed to somatostatin-26 and somatostatin-14 in the pancreas of the bowfin, Amia calva. Regul Pept. 1993 Aug 13;47(1):33–39. doi: 10.1016/0167-0115(93)90270-i. [DOI] [PubMed] [Google Scholar]
- de Keyzer Y., Lenne F., Auzan C., Jégou S., René P., Vaudry H., Kuhn J. M., Luton J. P., Clauser E., Bertagna X. The pituitary V3 vasopressin receptor and the corticotroph phenotype in ectopic ACTH syndrome. J Clin Invest. 1996 Mar 1;97(5):1311–1318. doi: 10.1172/JCI118547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L., Criado J. R., Prospero-Garcia O., Gautvik K. M., Schweitzer P., Danielson P. E., Dunlop C. L., Siggins G. R., Henriksen S. J., Sutcliffe J. G. A cortical neuropeptide with neuronal depressant and sleep-modulating properties. Nature. 1996 May 16;381(6579):242–245. doi: 10.1038/381242a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]





