Abstract
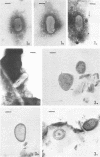
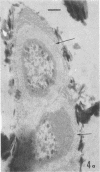
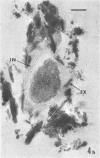
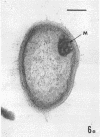
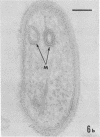
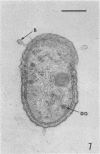
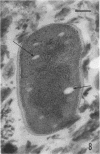
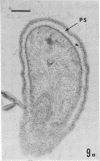
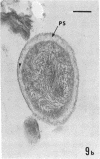
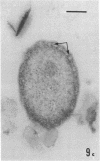
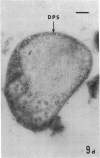
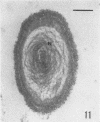
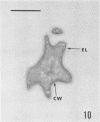
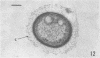
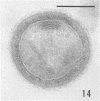
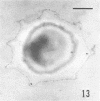
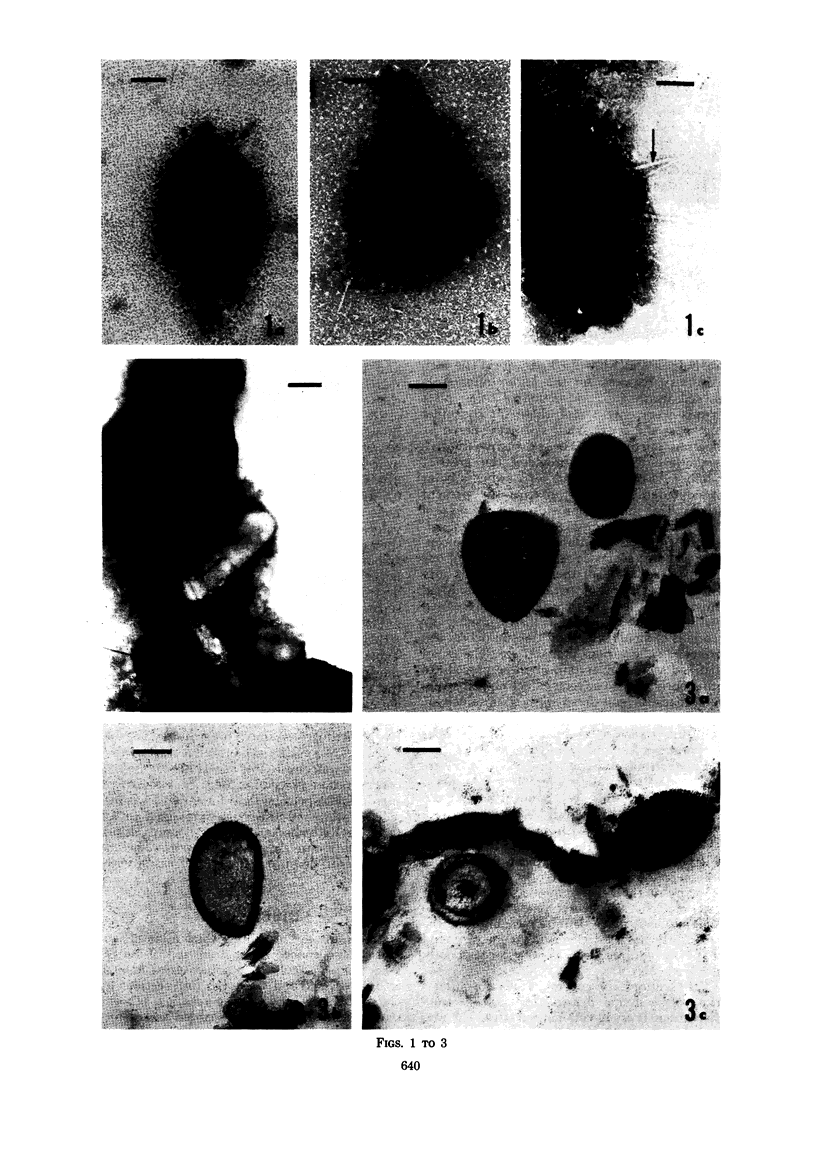
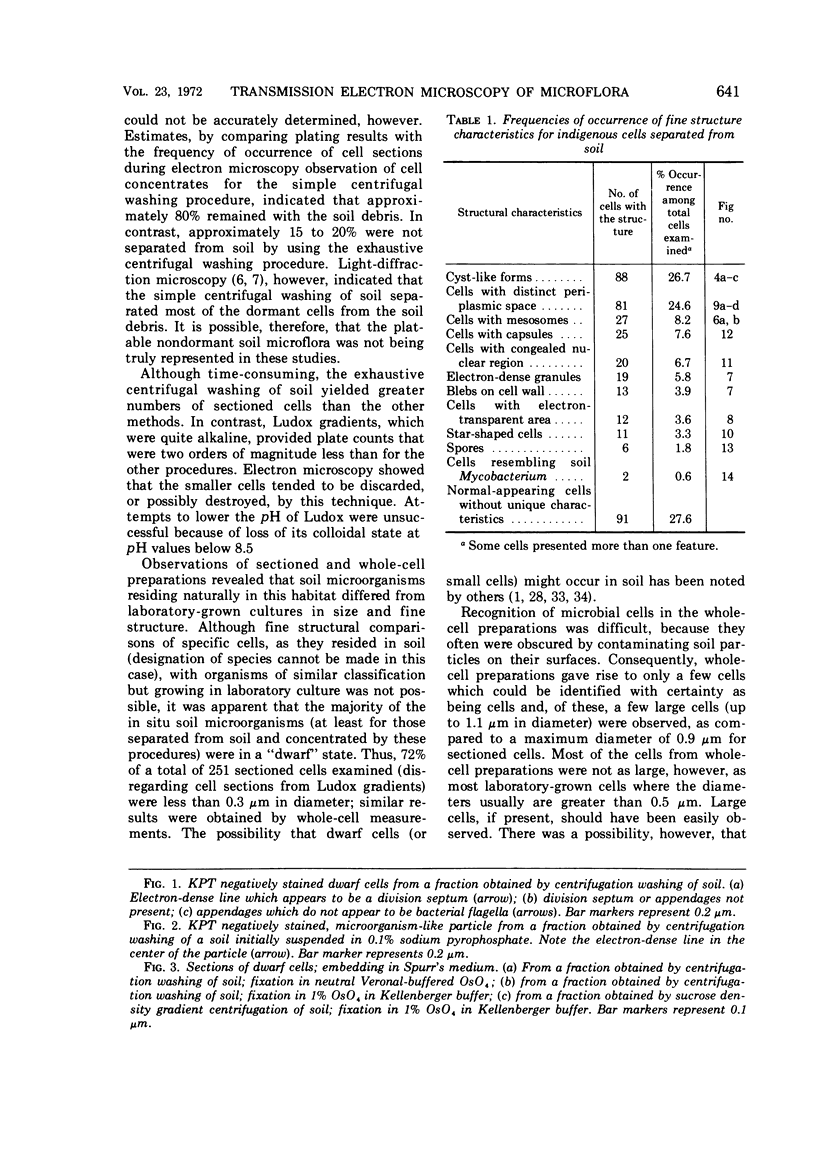
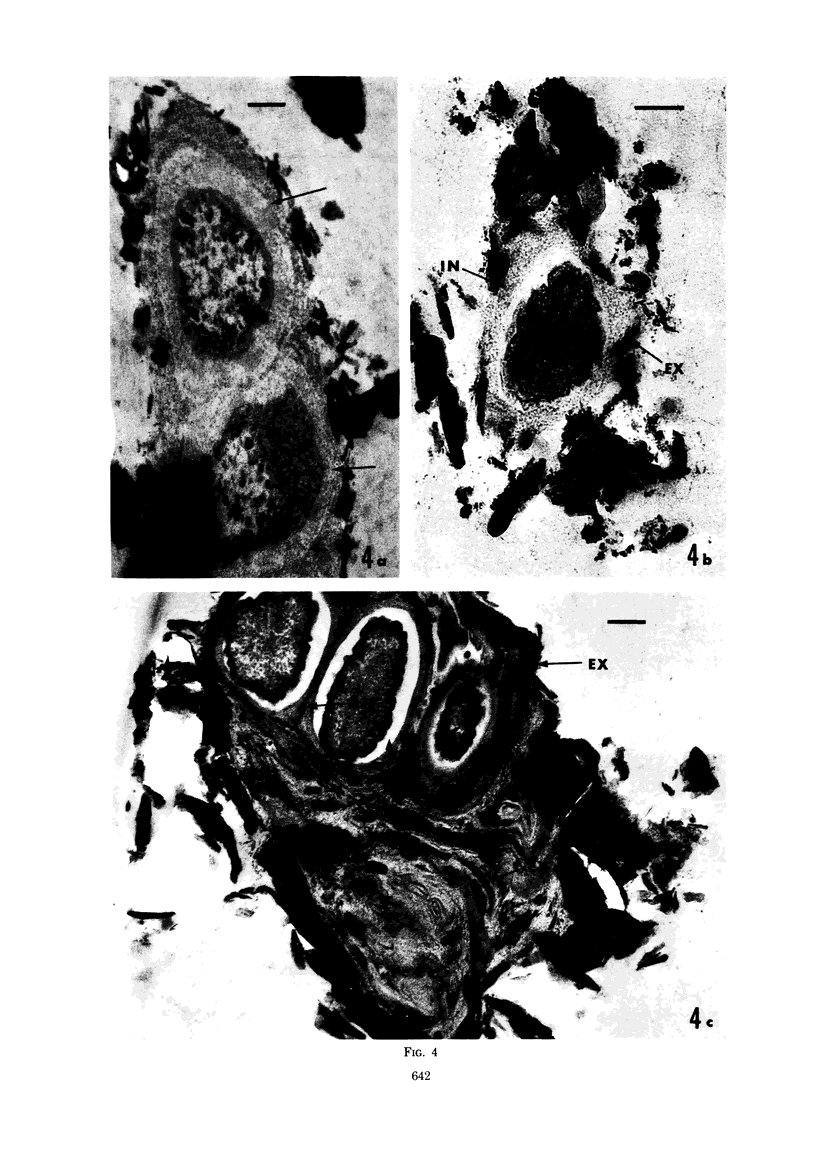
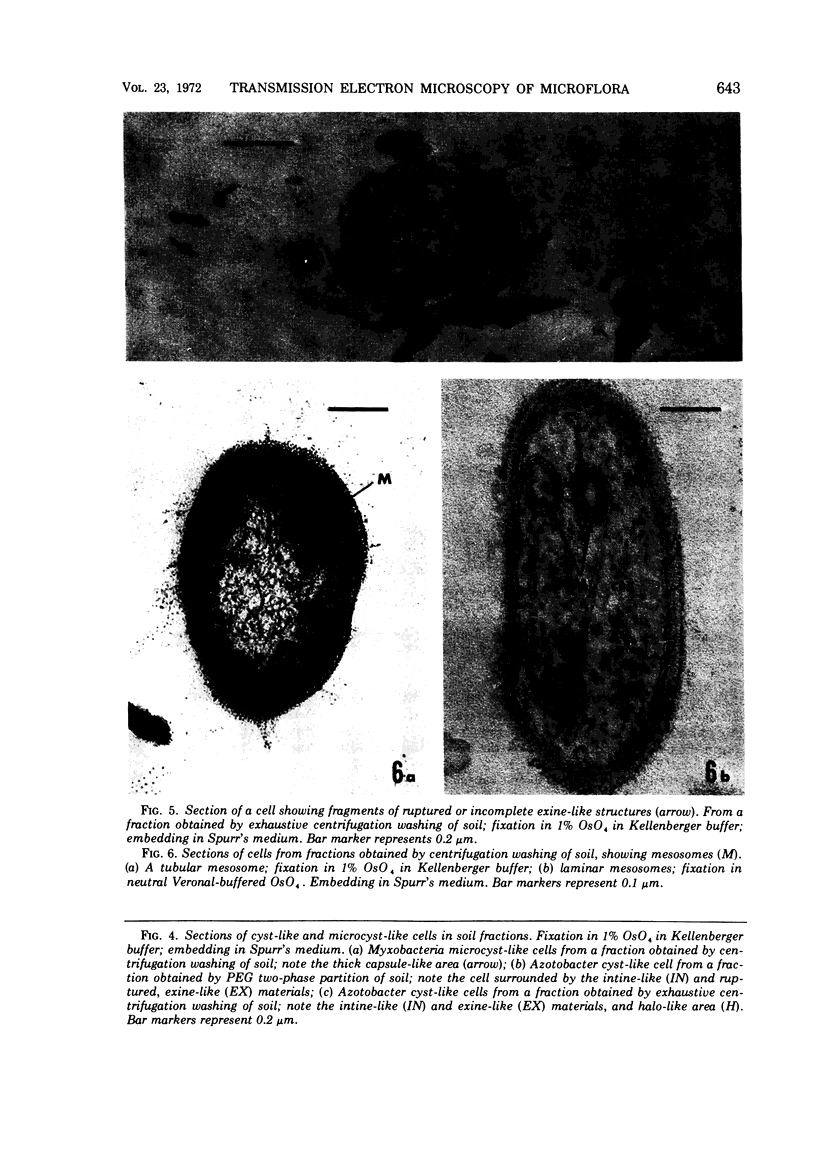
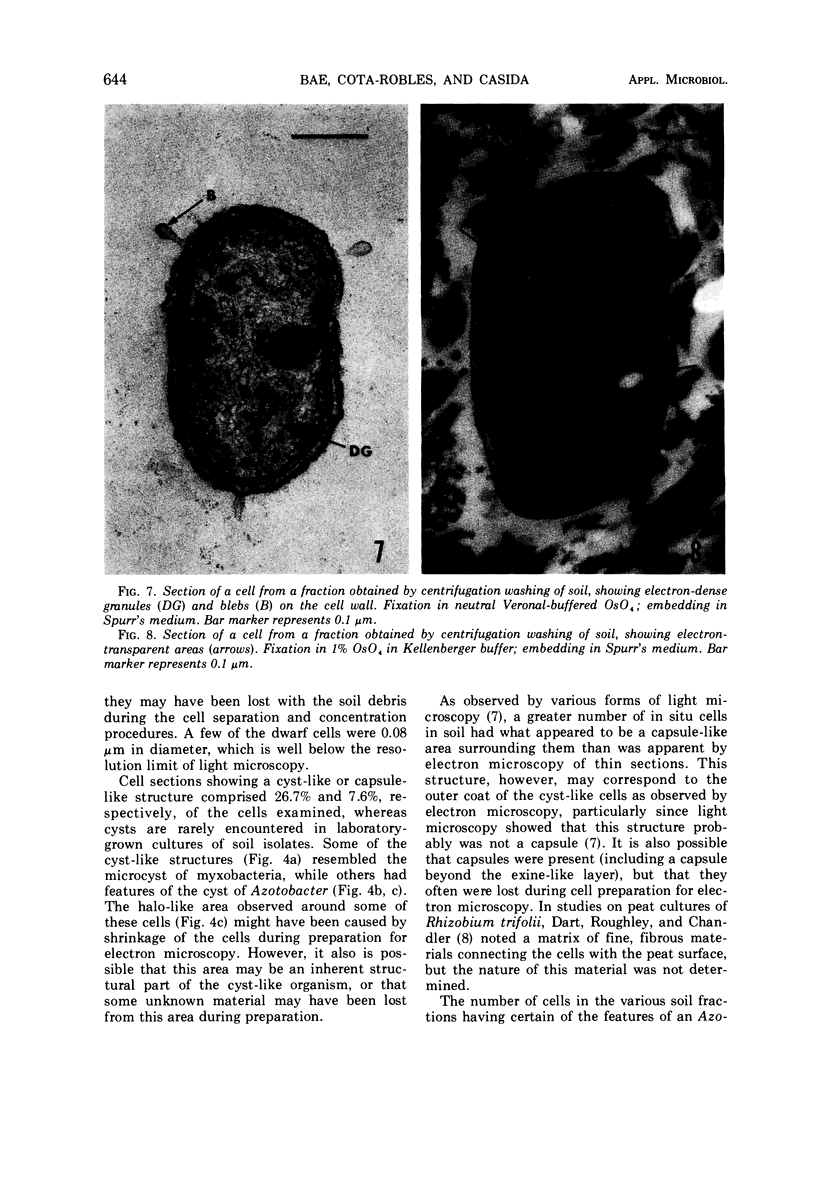
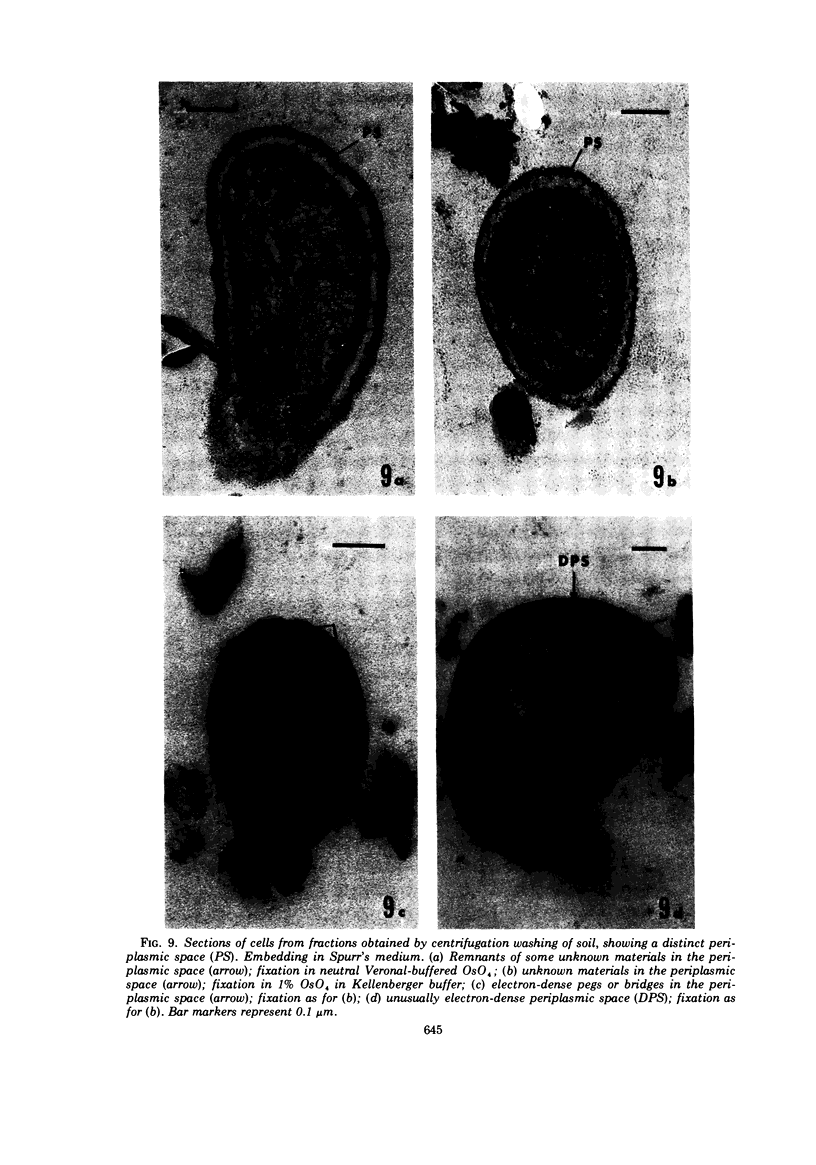
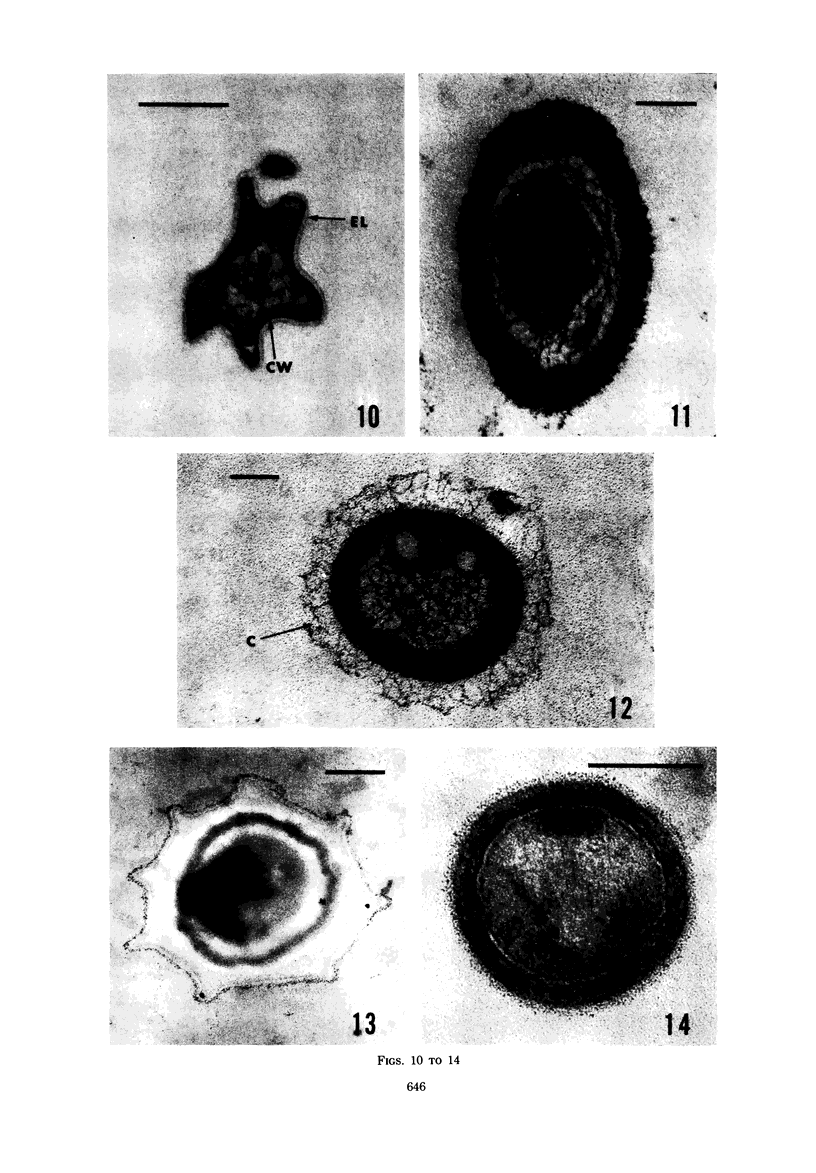
Several procedures were evaluated for separating and concentrating indigenous microorganisms from soil without the occurrence of growth. Electron microscopy of nontangential, thin sections through these cells revealed that all of the cells examined were less than 0.9 μm in diameter, and up to 72% were „dwarf” cells less than 0.3 μm in diameter. Some were small enough that they should not be resolved with the light microscope. Approximately 27% had a fine structure bearing some resemblance to that of a bacterial cyst or microcyst, but this value may be low because cells having their outer layers partially stripped off were not included in the count. Approximately 25% showed a distinct periplasmic space, which often contained stainable material. Other fine structure features are presented together with frequencies of occurrence for the populations examined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz J. V. Sheathed cells in cultures of Clostridium sporogenes. J Bacteriol. 1970 Sep;103(3):814–825. doi: 10.1128/jb.103.3.814-825.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASIDA L. E., Jr ABUNDANT MICROORGANISM IN SOIL. Appl Microbiol. 1965 May;13:327–334. doi: 10.1128/am.13.3.327-334.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida L. E., Jr Microorganisms in unamended soil as observed by various forms of microscopy and staining. Appl Microbiol. 1971 Jun;21(6):1040–1045. doi: 10.1128/am.21.6.1040-1045.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida L. E., Jr Observation of microorganisms in soil and other natural habitats. Appl Microbiol. 1969 Dec;18(6):1065–1071. doi: 10.1128/am.18.6.1065-1071.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fine structure of Listeria monocytogenes in relation to protoplast formation. J Bacteriol. 1967 Jan;93(1):411–426. doi: 10.1128/jb.93.1.411-426.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gledhill W. E., Casida L. E., Jr Predominant catalase-negative soil bacteria. I. Streptococcal population indigenous to soil. Appl Microbiol. 1969 Feb;17(2):208–213. doi: 10.1128/am.17.2.208-213.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt M. C., Wyss O. Chelation effects on Azotobacter cells and cysts. J Bacteriol. 1966 Jan;91(1):120–124. doi: 10.1128/jb.91.1.120-124.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., WATSON S. W. STRUCTURE OF NITROSOCYSTIS OCEANUS AND COMPARISON WITH NITROSOMONAS AND NITROBACTER. J Bacteriol. 1965 Jun;89:1594–1609. doi: 10.1128/jb.89.6.1594-1609.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenski S. W., Bystricky V., Maramorosch K. The occurrence of microbial forms of unusual morphology in European and Asian soils. Can J Microbiol. 1966 Dec;12(6):1291–1292. doi: 10.1139/m66-174. [DOI] [PubMed] [Google Scholar]
- Parker L. T., Socolofsky M. D. Central body of the Azotobacter cyst. J Bacteriol. 1966 Jan;91(1):297–303. doi: 10.1128/jb.91.1.297-303.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E. L'inclusion au polyester pour l'ultramicrotomie. J Ultrastruct Res. 1958 Dec;2(2):200–214. doi: 10.1016/s0022-5320(58)90018-2. [DOI] [PubMed] [Google Scholar]
- Remsen C. C., Watson S. W., Waterbury J. B., Trüper H. G. Fine structure of Ectothiorhodospira mobilis Pelsh. J Bacteriol. 1968 Jun;95(6):2374–2392. doi: 10.1128/jb.95.6.2374-2392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACKS L. E., ALDERTON G. Behavior of bacterial spores in aqueous polymer two-phase systems. J Bacteriol. 1961 Sep;82:331–341. doi: 10.1128/jb.82.3.331-341.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socolofsky M. D., Wyss O. CYSTS OF AZOTOBACTER. J Bacteriol. 1961 Jun;81(6):946–954. doi: 10.1128/jb.81.6.946-954.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- VELVA G. R., WYSS O. RADIATION RESISTANCE OF SOIL AZOTOBACTER. J Bacteriol. 1965 May;89:1280–1285. doi: 10.1128/jb.89.5.1280-1285.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]