Abstract
Objectives
The aim of this study was to investigate whether patients with systolic heart failure (HF) and abnormal thyroid function are at increased risk for death.
Background
Thyroid hormone homeostasis is vital to the optimal functioning of the cardiovascular system, but an independent prognostic effect of thyroid abnormalities in patients with HF has not been established.
Methods
In SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial), which randomized patients with ischemic or nonischemic HF to placebo or amiodarone or implantable cardioverter-defibrillator therapy, thyroid-stimulating hormone (TSH) was measured at baseline and at 6-month intervals throughout the 5-year study.
Results
Of 2,225 patients, the majority (87%) had normal TSH levels (0.3 to 5.0 μU/ml) at baseline, 12% had values suggestive of hypothyroidism, and 1% had values consistent with hyperthyroidism. Compared with euthyroid patients, those hypothyroid at baseline were older and included more women and Caucasians (all p values <0.05). Over the median follow-up period of 45.5 months, among patients euthyroid at baseline, 89 developed abnormally low TSH levels, and 341 developed abnormally high values. Patients randomized to amiodarone (median dose 300 mg) had an elevated risk for developing abnormal TSH levels compared with implantable cardioverter-defibrillator therapy or placebo (p < 0.0001). Patients with baseline or new-onset abnormal thyroid function had a higher mortality than those with normal thyroid function, even after controlling for other known mortality predictors (hazard ratio: 1.58; 95% confidence interval: 1.29 to 1.94; p < 0.0001 for hypothyroid; hazard ratio: 1.85; 95% confidence interval: 1.21 to 2.83; p = 0.0048 for hyperthyroid). Implantable cardioverter-defibrillator benefit did not vary with thyroid function.
Conclusions
Abnormal thyroid function in patients with symptomatic HF and ejection fractions ≤35% is associated with significantly increased risk for death, even after controlling for known mortality predictors. (Sudden Cardiac Death in Heart Failure Trial [SCD-HeFT]; NCT00000609)
Keywords: amiodarone, heart failure, ICD, thyroid disease
Thyroid hormone influences every cell, tissue, and organ in the body, and its homeostasis is essential to the optimal functioning of the heart. Thyroid dysfunction is present in 5% to 10% of the population, with a higher prevalence in women and patients over 60 years of age (1,2). Heart failure (HF) is currently diagnosed in approximately 5.8 million patients in the United States (3–5). Studies examining cardiovascular risk associated with thyroid abnormality have produced conflicting evidence (1,2,6–11). Although study results support an association of hyperthyroidism with atrial fibrillation (AF) (6,10,11), hypothyroidism’s connection to an increased risk for HF or other cardiovascular diseases and mortality spans the range from nonexistent to an increased number of HF events and mortality (6–9,15). The purpose of the present study was to examine the association of an abnormal level of serum thyrotropin, a thyroid-stimulating hormone (TSH), with prognosis in patients with systolic HF enrolled in SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial).
Methods
Study design, population, and primary clinical results
As previously described (12), SCD-HeFT was a National Heart, Lung, and Blood Institute–sponsored, multicenter, multinational, randomized trial that enrolled 2,521 patients from 148 centers in the United States, Canada, and New Zealand. Key inclusion criteria were age >18 years with ischemic or nonischemic New York Heart Association (NYHA) functional class II or III HF and an ejection fraction (EF) ≤35%. Baseline EF was measured using the following methods: echocardiography (58%), multigated acquisition (25%), and catheterization (17%). Patients were randomized to receive medical therapy plus amiodarone, amiodarone placebo, or a shock-only, single-lead implantable cardioverter-defibrillator (ICD). The investigational review board of each participating institution approved the protocol.
The primary endpoint was all-cause mortality. The median length of follow-up was 45.5 months, and vital status was known for all 2,521 patients at last follow-up within 3 months of trial end. Overall, ICD therapy reduced all-cause mortality by 23% relative to medical therapy alone (p = 0.007). Amiodarone had no effect on survival (hazard ratio [HR]: 1.06; p = 0.529). No patient was on amiodarone before randomization.
TSH values and thyroid replacement therapy (TRT) status were recorded at baseline and at 6-month intervals throughout the study. TSH ranges were defined as euthyroid (0.3 to 5.0 μU/ml), hyperthyroid (<0.3 μU/ml), or hypothyroid (>5.0 μU/ml). Patients were classified at baseline as euthyroid if their TSH values were euthyroid and TRT use was not reported, as hypothyroid if TRT use or a hypothyroid TSH level was reported, or as hyperthyroid if their TSH values were hyperthyroid and TRT was not reported. Baseline euthyroid patients were further classified as euthyroid at all times if all follow-up TSH values were euthyroid and TRT was not reported at any time, as new-onset hypothyroid if a hypothyroid TSH value or TRT use was reported during follow-up, or as new-onset hyperthyroid if a hyperthyroid TSH value was reported during follow-up, with no report of TRT use at any time. For patients missing baseline TSH values (5%), the first available follow-up TSH value was considered as baseline for the purposes of classification and summaries.
There were 2,225 patients in this substudy. Patients were omitted if they were not classifiable because of missing TSH data (n = 296).
Statistical analysis
Baseline characteristics were compared between hypothyroid and euthyroid patients, and between hyperthyroid and euthyroid patients, using Wilcoxon rank sum tests for continuous variables and likelihood ratio chi-square tests for categorical variables. Continuous variables are presented as mean ± SD.
Risk for new-onset abnormal thyroid function among patients euthyroid at baseline was compared among randomized treatment groups using an unadjusted Cox proportional hazards model (13). Rates of new TRT were compared between treatment arms using a likelihood ratio chi-square test.
In addition, the association between actual amiodarone dose (both randomized study drug and open-label use) and risk for new-onset abnormal thyroid function was assessed using Cox proportional hazards models. In these models, amiodarone dose was included as a time-dependent covariate. Both total daily dose and weight-adjusted daily dose were considered.
The relationship of thyroid function to change in EF at 12 and 30 months was assessed by comparing the proportion of patients who had increases of at least 10 points in EF from baseline, between hypothyroid or hyperthyroid patients and euthyroid patients, using likelihood ratio chi-square tests. Patients were classified by their thyroid status at those particular time points. A similar comparison was made for a decrease of 10 points in EF.
Factors associated with cardiovascular disease—diabetes, hyperlipidemia, weight, renal function, and blood pressure—were compared between baseline hypothyroid and euthyroid groups, and between baseline hyperthyroid and euthyroid groups, using Wilcoxon rank sum tests for continuous variables and likelihood ratio chi-square tests for categorical variables. Associations of these risk factors with new onset hypothyroidism or hyperthyroidism were tested using multivariate Cox proportional hazards models that considered all 5 risk factors simultaneously for each outcome. The associations of continuous variables with outcomes were assessed for linearity, and when not strictly linear, modifications (usually truncations) were made.
To examine the association of abnormal thyroid function with outcomes (mortality and AF), Cox proportional hazards models were used. In each model, hypothyroid and hyperthyroid state were included as time-dependent covariates. Hypothyroid and hyperthyroid status were each tested against euthyroid status. Each model contained covariates previously identified as being associated with that end point in the full SCD-HeFT cohort. For mortality, the model covariates were randomized treatment, HF etiology, NYHA class, age, sex, diabetes, mitral regurgitation, renal insufficiency, substance abuse, systolic blood pressure, EF, time since HF diagnosis, electrocardiographic measures, 6-min walk distance, Duke Activity Status Index, and angiotensin-converting enzyme inhibitor or digoxin use. For AF, the model covariates were randomized treatment, age, sex, EF, history of AF, and absence of beta-blocker use. To determine whether thyroid function affected the relationship of randomized therapy with mortality risk, interaction tests were used in a simplified mortality model, in which all patients with abnormal thyroid status were considered as a single group (on the basis of the similarity of parameter estimates for hypothyroidism and hyperthyroidism in the main mortality model). The role of TRT was assessed by considering 2 different HRs for hypothyroidism, 1 treated and 1 untreated, and testing for a difference between them. For all Cox models, risk relationships are expressed as HRs and 95% confidence intervals (CIs).
Results
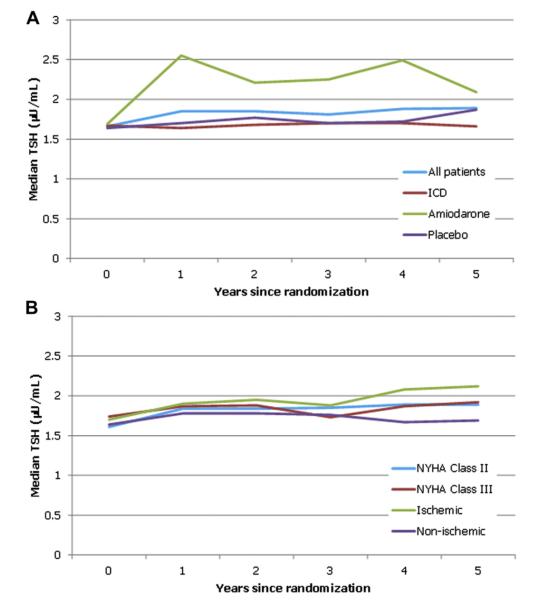
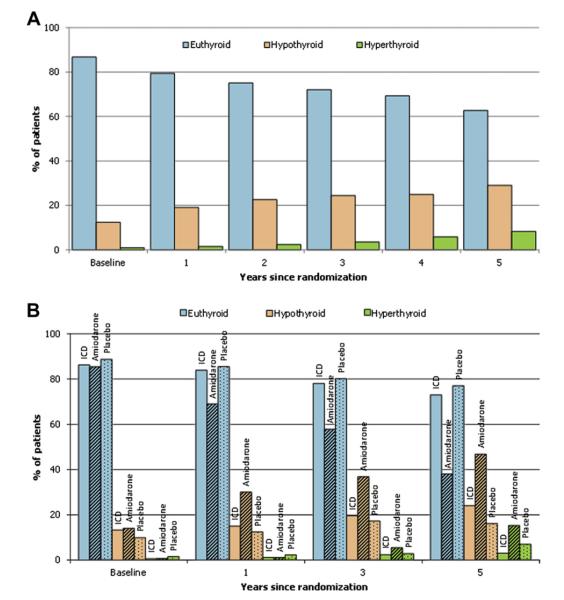
The majority of patients, 87% (n = 1,930), had euthyroid TSH values (0.3 to 5.0 μU/ml) at baseline (Fig. 1). Twelve percent (n = 275) had values suggestive of hypothyroidism, and 1% (20) had values consistent with hyperthyroidism. Only a minuscule percent of these patients had clinically overt hypothyroidism (TSH >20 μU/ml) (0.5%) or overt hyperthyroidism (TSH <0.1 μU/ml) (0.4%) (Fig. 1). Over time, median TSH values for patients randomized to ICD or placebo, or patients categorized by NYHA functional class (II or III) or grouped by HF etiology (ischemic or nonischemic), remained generally consistent. However, in those patients randomized to amiodarone, there was a marked increase (Fig. 2). Between baseline and the conclusion of the follow-up period, the total percent of hypothyroid patients (hypothyroid at baseline plus new-onset hypothyroid) increased from 12% (n = 275) to 28% (n = 616) (Table 1, Fig. 3). The percent of hyperthyroid patients (baseline plus new onset) increased from 1.0% (n = 20) to 5.0% (n = 109).
Figure 1. Distribution of Baseline TSH Values.

Intervals on the right side of the graph include a wider range of values due to sparseness of the data. Very low thyroid-stimulating hormone (TSH) values include hypothyroid patients on thyroid replacement therapy as well as true hyperthyroid patients.
Figure 2. Median TSH Values Over Time.
(A) All patients and by randomized treatment. (B) By New York Heart Association (NYHA) functional class and heart failure etiology subgroups. ICD = implantable cardioverter-defibrillator; TSH = thyroid-stimulating hormone.
Table 1.
Baseline Characteristics by Thyroid Function Group
| Baseline Thyroid Group |
Overall Thyroid Group |
|||||
|---|---|---|---|---|---|---|
| Baseline Characteristic |
Hyperthyroid at Baseline n = 20 (0.9%) |
Euthyroid at Baseline n = 1,930 (86.7%) |
Hypothyroid at Baseline n = 275 (12.4%) |
Hyperthyroid (Baseline + New Onset) n = 109 (4.9%) |
Euthyroid (at All Times) n = 1,500 (67.4%) |
Hypothyroid (Baseline + New Onset) n = 616 (27.7%) |
| Age (yrs) | 56.8 ± 14.7 | 59.2 ± 11.8 | 61.7 ± 12.0* | 54.5 ± 12.0† | 59.1 ± 11.7 | 61.5 ± 11.8† |
| Age >60 yrs | 40% (8) | 49% (947) | 60% (166)* | 32% (35)† | 48% (723) | 59% (363)† |
| Women | 35% (7) | 22% (417) | 41% (113)* | 22% (24) | 20% (296) | 35% (217)† |
| Nonwhite race | 25% (5) | 24% (454) | 18% (50)* | 26% (28) | 25% (377) | 17% (104)† |
| NYHA class III | 20% (4) | 29% (551) | 32% (88) | 27% (29) | 29% (428) | 30% (186) |
| Ischemic HF etiology |
40% (8) | 52% (996) | 51% (140) | 42% (46) | 52% (776) | 52% (322) |
| Ejection fraction (%) | 23.1 ± 6.4 | 23.9 ± 6.8 | 24.7 ± 6.9 | 24.2 ± 6.2 | 23.9 ± 6.9 | 24.2 ± 6.8 |
| 6-min walk distance (ft) |
1,080.4 ± 396.6 | 1,122.2 ± 398.5 | 1,091.0 ± 409.3 | 1,140.2 ± 395.1 | 1,126.6 ± 405.5 | 1,093.3 ± 385.8† |
Values are mean ± SD or % (n). Comparisons are with the euthyroid group within each classification, using Wilcoxon rank sum tests for continuous variables and likelihood ratio chi-square tests for categorical variables.
p < 0.05 for baseline group comparisons.
†p < 0.05 for overall group comparisons.
HF = heart failure; NYHA = New York Heart Association.
Figure 3. Distribution of Thyroid Function Groups Over Follow-Up.
Abnormal thyroid groups at each point include patients in those groups at baseline as well as new-onset patients whose onset occurred before the end of that year. (A) All patients. (B) By randomized treatment. ICD = implantable cardioverter-defibrillator.
Demographics and baseline clinical data
Compared with the euthyroid group, patients who were hypothyroid at baseline or at any time during the course of the trial included more women, Caucasians, and patients over 60 years of age (Table 1). Hypothyroidism at baseline was linked to a significantly lower body weight (mean 184.9 ± 44.3 lb) compared with the euthyroid state (mean 195.7 ± 44.9 lb) (p < 0.0001). Patients with diabetes had a 49% increase in relative risk (HR: 1.49; 95% CI: 1.19 to 1.86; p = 0.0006) of hypothyroidism compared with those without diabetes.
There were no significant differences between hyperthyroid and euthyroid patients at baseline. There were no significant differences in NYHA functional class or exercise tolerance (as measured by the 6-minute walk test) between either hypothyroid or hyperthyroid and euthyroid groups.
Randomized therapy and the development of abnormal thyroid function
Among patients with baseline euthyroid function, 40.9% of those randomized to amiodarone developed new-onset abnormal (hyperthyroid or hypothyroid) thyroid function during follow-up, compared with 13.8% of placebo patients and 12.3% of ICD patients (Table 2). Patients in the amiodarone arm had a significantly greater risk for new-onset abnormal thyroid function than patients in the placebo arm (HR: 3.65; 95% CI: 2.88 to 4.63; p < 0.0001) or ICD arm (HR: 4.18; 95% CI: 3.24 to 5.39; p < 0.0001) (Table 2). However, among all patients who were taking amiodarone, higher amiodarone dose (total daily or weight adjusted daily) did not convey additional risk (p = NS for all) for the development of abnormal thyroid function compared with lower doses. Over the course of the trial, the percent of patients on TRT increased from 6.8% at baseline to 16.1% reported at any time. Patients randomized to amiodarone had a higher rate of new TRT during follow-up (19.7%) than patients on placebo (4.8%) or with ICDs (5.7%) (p < 0.0001).
Table 2.
Randomized Therapy and Development of Abnormal Thyroid Function
| Randomized Therapy |
|||
|---|---|---|---|
| Amiodarone | ICD | Placebo | |
| Euthyroid at baseline | 639 | 626 | 665 |
| Hyperthyroid, new onset | 8.0% (51) | 2.4% (15) | 3.5% (23) |
| Hypothyroid, new onset | 32.9% (210) | 9.9% (62) | 10.4% (69) |
| Any abnormal thyroid function, new onset | 40.9% (261) | 12.3% (77) | 13.8% (92) |
| Relative risk for new-onset abnormal thyroid function, vs. placebo |
3.65 (2.88–4.63) (p < 0.0001) | 0.87 (0.65–1.18) (p = 0.38) | — |
| Relative risk for new-onset abnormal thyroid function, vs. ICD |
4.18 (3.24–5.39) (p < 0.0001) | — | — |
Values are n, % (n), or hazard ratio (95% confidence interval).
CI = confidence interval; HR = hazard ratio; ICD = implantable cardioverter-defibrillator.
EF and thyroid status
Mean baseline EF was similar across thyroid groups at baseline (Table 1). Repeat EF assessments at 12 and 30 months revealed an increase in the mean EF (31.1 ± 11.9% and 33.0 ± 12.9%, respectively), but there were no differences between hypothyroid or hyperthyroid and euthyroid patients in the change in EF from baseline to either time point (all p > 0.10).
Thyroid status and outcomes
Abnormal thyroid status was associated with increased risk of mortality, even after controlling for known mortality predictors. Hypothyroid and hyperthyroid status were associated with 58% and 85% increases, respectively, in the relative risk for death compared with euthyroid patients (HR: 1.58; 95% CI: 1.29 to 1.94; p < 0.0001 for hypothyroid vs. euthyroid; HR: 1.85; 95% CI: 1.21 to 2.83; p = 0.0048 for hyperthyroid vs. euthyroid). The association of hypothyroidism with mortality risk did not differ for patients on TRT compared with those who were untreated (p 0.38).
There was no interaction between abnormal thyroid function and either amiodarone or ICD therapy (p = 0.49 and p = 0.50, respectively), indicating that the beneficial effect of ICD therapy and the lack of benefit of amiodarone with respect to mortality were the same regardless of whether patients had normal or abnormal thyroid function.
Mode of death (sudden cardiac death, progressive HF or other cardiac deaths, noncardiac or unknown cause of death) was similar across thyroid status groups (data not shown).
Neither hypothyroidism nor hyperthyroidism was associated with the risk for developing AF (p = 0.60 and p = 0.16, respectively).
Discussion
The most important finding of our study is that abnormal thyroid function, whether present at baseline or developing in follow-up, is a significant independent prognostic factor in patients with moderately symptomatic HF and EFs ≤35%. Treatment for hypothyroidism, which was present in almost 11% of the patients at baseline, did not reduce or eliminate the adverse prognostic effects of this abnormality. Abnormal thyroid function was not associated with the severity of HF and did not have any discernible influence on left ventricular function over time.
The American College of Cardiology and American Heart Association HF guidelines (3) support performing thyroid function tests in patients with HF, especially measurement of TSH, in recognition that both hyperthyroidism and hypothyroidism can be primary or contributory causes of HF. The Heart Failure Society of America guideline (14) recommends that for patients suspected of having HF, evaluation of thyroid disorder should be part of the standard laboratory tests. They further note that because up to 20% of patients hospitalized for decompensated HF are already being treated for thyroid disease, evaluation of their thyroid therapy is recommended.
However, both the American College of Cardiology and American Heart Association and the Heart Failure Society of America recommendations are based largely on consensus agreement rather than from evidence from large randomized placebo-controlled clinical trials.
Studies tracking outcome as a function of thyroid status in cardiovascular disease have reported vastly disparate results. An observational study, the Framingham Heart Study, examining 4,331 patients, found no link between subclinical abnormal TSH levels and an increased risk for cardiovascular disease or mortality (7). Different findings were observed in another community-based prospective 4-year study of 2,730 men and women aged 70 to 79 years (8). Compared with euthyroid adults, this study found that people with moderate or severe subclinical hypothyroidism had a 2-fold to 3-fold increased risk for congestive HF events, both incident and recurrent. This association between significant thyroid abnormality and HF events was supported by a study performed by Gencer et al. (15). The individual data analysis of 25,390 participants from 6 prospective cohorts from the United States and Europe showed that the risks for HF events, mainly related to hospitalizations, were increased with both higher and lower TSH values, particularly for TSH >10 mIU/l and TSH <0.10 mIU/l.
Also demonstrating that patients with subclinical hypothyroidism differed from euthyroid subjects, Tseng et al. (16) found that subclinical hypothyroidism was associated with an increased risk for all-cause and cardiovascular mortality in adults. They collected data on 115,746 adult Taiwanese with subclinical hypothyroidism from 4 private nationwide health screening centers in Taiwan from 1998 to 1999. They showed that after adjustment of cardiovascular disease risk factors, the subjects had a 30% increase in the relative risk for all-cause mortality and a 68% increase in the relative risk for cardiovascular disease death.
Examining the prevalence of coronary heart disease in subjects with and without subclinical thyroid dysfunction, Walsh et al. (17) found an increased risk for coronary artery disease in patients with subclinical hypothyroidism, even after controlling for age and sex. The relationship remained after further adjustment for standard cardiovascular risk factors. Because coronary artery disease is arguably the most common etiology of HF, the potential contribution of thyroid abnormality to the development of HF is evident.
Probably the most consistent and tightest link between thyroid abnormality and cardiovascular disease is found between hyperthyroidism and the development of AF. Heeringa et al. (10) found in a population-based study in the elderly that those in the lowest quartile of the normal range of serum TSH had an almost 2-fold increased risk for AF compared with those who were in the highest quartile.
There are a couple of possible reasons why our study did not show a link between thyroid abnormality and AF. First, the percent of our population with evidence of hyperthyroidism, at baseline plus new onset, was small (5.0%), and among these patients, AF was a relatively rare occurrence (18 cases). Second, clinically overt hyperthyroidism, a recognized prominent risk factor for AF (6,10,11), was present in only 0.4% of our population.
One arm of SCD-HeFT was treatment with amiodarone. This therapy is well known to be associated with the development of thyroid abnormality because of its high iodine content. It can be the cause of primary hypothyroidism, which occurs more frequently in women than in men (in a 1.5:1.0 ratio) or can cause hyperthyroidism. In the United States, approximately 2% of patients receiving long-term treatment with amiodarone develop hyperthyroidism, and about 22% develop hypothyroidism (18). Despite this increased incidence of biochemical thyroid abnormality, patients are usually clinically euthyroid. However, because certain signs of hypothyroidism, such as bradycardia, are among the features expected secondary to the pharmacological action of the drug, clinically, we may be underrecognizing evidence of thyroid disease. With the use of amiodarone, periodic surveillance over the course of treatment for potential side effects, including thyroid abnormality, is recommended. It is important to state that although thyroid abnormality was more likely to be associated with patients taking amiodarone, it was the state (in particular hypothyroidism) that portended a worse prognosis. Hypothyroidism in our study also carried a higher relative risk for death when present in patients not on the drug.
The “low-T3 syndrome” (19) is a profile of low serum triiodothyronine (T3), normal thyroxine (T4), and normal TSH that can be seen in acute or chronic illness and can be induced by weight loss due to chronic caloric restriction. In experimental animal studies (19), the low-T3 syndrome leads to the same changes in cardiac function (decreased maximal rate of contraction and relaxation) and gene expression (alteration in myosin heavy chain isoform expression) as does primary hypothyroidism. Although hypothesized as a potentially beneficial response by way of energy conservation in patients with nonthyroidal illnesses, 1 study (20) found the syndrome to be a strong predictor of death in patients with cardiac disease. In this study, the findings were independent of treatment with amiodarone, a drug with a known ability to reduce T3 serum concentration. Only TSH levels were collected in SCD-HeFT, so the prevalence of the low-T3 syndrome and its effects could not be examined. We may have underestimated the potential effect of thyroid abnormality in our cohort by not considering this “hypothyroid-like equivalent.”
Patients on TRT did not fare better than those not on TRT, although caution must be used in interpreting results of any nonrandomized therapy comparison, especially in a small subgroup. Whether treatment of hypothyroidism will affect mortality risk or HF events in patients with HF requires a prospective study. To date, no such large randomized controlled trial has been conducted. A similar call for an appropriately powered randomized controlled trial of replacement therapy was echoed by Razvi et al. (21), who, using the United Kingdom General Practitioner Database, recently found that treatment of subclinical hypothyroidism was associated with fewer ischemic heart disease events and all-cause mortality in younger (age ≤70 years) patients. Gencer et al. (15) believed that their study showing the values associated with the highest risk for HF events may be useful in defining the threshold for treatment among patients with thyroid dysfunction. Nevertheless, they pointed out the limitations of using this approach and recommended caution in making clinical decisions based only on observational studies.
The single-lead ICD arm of SCD-HeFT had significantly lower mortality compared with the placebo and amiodarone arms. The benefits experienced by patients with ICDs were not affected by thyroid dysfunction.
Last, and not unexpectedly, diabetes was associated with increased risk for new hypothyroidism in our study. This well-established cardiovascular risk factor has been proposed as possibly playing a role in the mechanism of the increased cardiovascular disease risk linked to this subset (15). Our analysis agrees with a recent study of Tseng et al. (16) showing that even after adjusting for known mortality predictors, including diabetes, the increased relative risk for death associated with thyroid abnormality persisted.
Study limitations
Study limitations include the relatively small percent of patients with hyperthyroidism. Variation in TSH values, related to the time the sample is obtained and the type of assay used, has been reported. Neither was standardized across centers or countries in SCD-HeFT. The low-T3 syndrome, defined after SCD-HeFT was completed, could not be identified with the available data from this trial, because only TSH values were recorded. We may have underestimated the mortality associated with thyroid abnormality.
Only the first abnormal thyroid change during the trial was analyzed. We therefore cannot comment on the number of patients whose thyroid function may have normalized spontaneously or the number who progressed to overt hypothyroidism or hyperthyroidism. Changes from hypothyroid state to hyperthyroid state and vice versa over time can be seen with continued use of amiodarone. In addition, drug-induced hyperthyroidism can vary directly with the duration of amiodarone treatment (22). The length of follow-up, although extensive, may have limited the number of patients with hyperthyroidism observed. The effort, by trial design, to provide consistent dosing for all amiodarone patients in the study may have limited our ability to find any relationship between amiodarone dose and abnormal thyroid function.
Clinical implications
Our findings in patients with moderately symptomatic HF with EFs ≤35% highlight the need for careful consideration and monitoring of these patients with abnormal thyroid function. They have a worse prognosis. A recent study found that treatment improved outcomes in ischemic heart disease events and all-cause mortality in younger patients with subclinical hypothyroidism (21). However, prospective randomized controlled clinical trials in patients with HF and abnormal thyroid function are lacking.
Conclusions
Patients in SCD-HeFT with moderately symptomatic HF and abnormal thyroid function, either present at baseline or developed during the trial, had an approximate 60% increase in the relative risk for death. The risks associated with hyperthyroid and hypothyroid states were similar. This significantly higher relative risk for death over the course of the study was irrespective of randomization to the amiodarone, ICD, or placebo treatment group. Importantly, the benefits experienced by patients with ICDs were not affected by thyroid dysfunction.
Acknowledgments
This study was supported by grants UO1 HL55766, UO1 HL55297, and UO1 HL55496 from the National Heart, Lung, and Blood Institute, National Institutes of Health. Additional support was provided by Medtronic, Inc., and Wyeth-Ayerst Laboratories. Dr. Mark has received grant funding from Eli Lilly & Company, Medtronic, Inc., Gilead Sciences, Inc., and AstraZeneca. Dr. Poole is an educational speaker for Medtronic, Inc., St. Jude Medical, Boston Scientific Corporation, and Biotronik and is a member of the scientific advisory board of Boston Scientific Corporation. Dr. Lee is a consultant for Medtronic, Inc.
Abbreviations and Acronyms
- AF
atrial fibrillation
- CI
confidence interval
- EF
ejection fraction
- HF
heart failure
- HR
hazard ratio
- ICD
implantable cardioverter-defibrillator
- NYHA
New York Heart Association
- T3
triiodothyronine
- TRT
thyroid replacement therapy
- TSH
thyroid-stimulating hormone
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Helfand M. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140:128–41. doi: 10.7326/0003-4819-140-2-200401200-00015. [DOI] [PubMed] [Google Scholar]
- 2.Canaris GJ, Manowitz NR, Mayor G, Ridgway C. The Colorado Thyroid Disease Prevalence Study. Arch Intern Med. 2000;160:526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 3.Hunt SA, Abraham WT, Chin MH, et al. for the American College of Cardiology, American Heart Association Task Force on Practice Guidelines, American College of Chest Physicians, International Society for Heart and Lung Transplantation, Heart Rhythm Society ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:1343–82. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Writing Group Members. Lloyd-Jones D, Adams RJ, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 6.Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–41. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearce EN, Yang Q, Sawin CT, Vasan RS. [Accessed April 20, 2012];Subclinical thyroid dysfunction not linked to CVD, mortality. Available at: http://www.endo-society.org/media/upload/RSB_FINAL.doc.
- 8.Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165:2460–6. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 9.Rodondi N, Bauer DC, Cappola AR, et al. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure. J Am Coll Cardiol. 2008;52:1152–9. doi: 10.1016/j.jacc.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heeringa J, Hoogendoorn EH, van der Deure WM, et al. High-normal thyroid function and risk of atrial fibrillation. Arch Intern Med. 2008;1168:2219–24. doi: 10.1001/archinte.168.20.2219. [DOI] [PubMed] [Google Scholar]
- 11.Collett T-H, Gussekloo J, Bauer DC, et al. for the Thyroid Studies Collaboration Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172:799–809. doi: 10.1001/archinternmed.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bardy GH, Lee KL, Mark DB, et al. for the SCD-HeFT Investigators Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 13.Cox DR. Regression models and life-tables. J R Stat Soc Ser B Methodol. 1972;34:187–220. [Google Scholar]
- 14.Heart Failure Society of America Executive summary: HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:475–539. [Google Scholar]
- 15.Gencer B, Collet T-H, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from six prospective cohorts. Circulation. 2012;126:1040–9. doi: 10.1161/CIRCULATIONAHA.112.096024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng F-U, Lin W-Y, Lin C-C, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730–7. doi: 10.1016/j.jacc.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 17.Walsh JP, Bremner AP, Bulsara MK, et al. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med. 2005;165:2467–72. doi: 10.1001/archinte.165.21.2467. [DOI] [PubMed] [Google Scholar]
- 18.Goldschlager N, Epstein AE, Naccarelli G, Olshansky B, Singh Bfor the Practice Guidelines Subcommittee North American Society of Pacing and Electrophysiology. Practical guidelines for clinicians who treat patients with amiodarone. Arch Intern Med. 2001;160:1741–8. doi: 10.1001/archinte.160.12.1741. [DOI] [PubMed] [Google Scholar]
- 19.Katzeff HL, Powell SR, Ojamaa K. Alterations in cardiac contractility and gene expression during low-T3 syndrome: prevention with T3. Am J Physiol. 1997;273:E951–6. doi: 10.1152/ajpendo.1997.273.5.E951. [DOI] [PubMed] [Google Scholar]
- 20.Iervasi G, Pingitore A, Landi P, et al. Low-T3 syndrome—a strong prognostic predictor of death in patients with heart disease. Circulation. 2003;107:708–13. doi: 10.1161/01.cir.0000048124.64204.3f. [DOI] [PubMed] [Google Scholar]
- 21.Razvi S, Weaver JU, Butler TJ, Pearce SHS. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811–7. doi: 10.1001/archinternmed.2012.1159. [DOI] [PubMed] [Google Scholar]
- 22.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–35. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]