Abstract
We hypothesized B-cells are involved in the pathogenesis of idiopathic pulmonary fibrosis (IPF), a progressive, restrictive lung disease that is refractory to glucocorticoids and other nonspecific therapies, and almost invariably lethal. Accordingly, we sought to identify clinically-associated B-cell-related abnormalities in these patients. Phenotypes of circulating B-cells were characterized by flow cytometry. Intrapulmonary processes were evaluated by immunohistochemistry. Plasma B-lymphocyte stimulating factor (BLyS) was assayed by ELISA. Circulating B-cells of IPF subjects were more antigen-differentiated, with greater plasmablast proportions (3.1±0.8%) than in normal controls (1.3±0.3%) (p<0.03), and the extent of this differentiation correlated with IPF patient lung volumes (r=0.44, p<0.03). CD20+ B-cell aggregates, diffuse parenchymal and perivascular immune complexes, and complement depositions were all prevalent in IPF lungs, but much less prominent or absent in normal lungs. Plasma concentrations of BLyS, an obligate factor for B-cell survival and differentiation, were significantly greater (p<0.0001) in 110 IPF (2.05±0.05 ng/ml) than among 53 normal (1.40±0.04 ng/ml) and 90 chronic obstructive pulmonary disease (COPD) subjects (1.59±0.05 ng/ml). BLyS levels were uniquely correlated among IPF patients with pulmonary artery pressures (r=0.58, p<0.0001). The 25% of IPF subjects with the greatest BLyS values also had diminished one-year survival (46±11%), compared to those with lesser BLyS concentrations (81±5%) (HR=4.0, 95%CI=1.8-8.7, p=0.0002). Abnormalities of B-cells and BLyS are common in IPF patients, and highly associated with disease manifestations and patient outcomes. These findings have implications regarding IPF pathogenesis, and illuminate the potential for novel treatment regimens that specifically target B-cells in patients with this lung disease.
Keywords: B-cells, Adaptive Immunity, Interstitial Lung Disease, COPD
Introduction
Idiopathic pulmonary fibrosis (IPF) is a morbid, fibroproliferative disorder characterized by progressive lung restriction and gas exchange abnormalities (1-3). The annual incidence of IPF in the U.S. is approximately 40,000, and afflicted patients have a median survival of about three years (1-3).
Although the pathogenesis of IPF is widely considered to be enigmatic (2), abnormalities of adaptive immunity are common among patients with this disease (4-22, and others). HLA allele frequency perturbations are a typifying feature of immunological disorders, and HLA-DRβ1*15 is over-represented among IPF subjects (20), as well as being linked to dysregulated autoimmune responses in this population (13). Infiltrates of activated T-cells are present in IPF lungs, and the magnitude of these abnormalities is proportionate to disease severity and patient mortality (19,21,22). Circulating T-cells among IPF patients are also antigen-activated, clonally expanded, dysregulated, have augmented productions of myriad proinflammatory and profibrotic mediators, and many characteristics of these lymphocytes are associated with clinical manifestations (9,18,19). One or more antigen(s) in IPF lungs stimulate autologous CD4 T-cells (9), including heat shock protein 70 (HSP70), which induces lymphocyte proliferation and IL-4 production (13).
B-cell studies in IPF are more limited, but examinations of diseased lungs from these patients have shown the presence of highly abnormal intrapulmonary B-cell aggregates (4,5), and over-expressions of immunoglobulin genes (6). Potentially pathogenic immune complexes have been found in the sera, bronchoalveolar lavage, and pulmonary parenchyma of IPF patients (7,8,13). Diverse circulating IgG autoantibodies are present in more than 80% of these subjects (8-16), and some particular immunoglobulin specificities have been linked to disease severity and/or poor prognoses (10-13).
We hypothesized that B-cell characteristics and/or their associated mediators may be correlated with clinical features of IPF. If so, these findings could have considerable importance. To begin, the course of IPF is unpredictable among afflicted individuals (2,3). Thus, facile, valid biomarkers could be very useful to identify patients who are destined for poor near-term outcomes, and thus optimize timings of lung transplantations, and/or aide in selection of high-risk subjects for experimental treatments.
Moreover, the absence of a definitive, mechanistic paradigm of IPF pathogenesis has precluded the rational selection of therapies that are directed specifically at the causal biological process(es) (2,3). Medical treatments used for IPF to date, typically based on glucocorticoids and/or global anti-fibrotic drugs, are ineffectual, and the disease continues to have a worse prognosis than several common malignancies (2,3). Like IPF, many B-cell-mediated lung diseases are also refractory to nonspecific therapy with glucocorticoids. Conversely however, these same syndromes often respond to focused anti-B-cell agents or other treatments that reduce concentrations of pathogenic antibodies (23-29). Hence, finding a compelling link between B-cells and IPF progression might engender considerations for experimental trials of recently developed agents that more specifically target these lymphocytes and/or their functions, and may provide some clinical benefit for these otherwise ill-fated patients.
Accordingly, we conducted investigations to characterize B-cells and examine a critical B-cell mediator in IPF patients that could have therapeutic implications.
Methods
Specimens for Autoantibody Studies
Peripheral blood specimens were obtained from consecutive IPF patients (9,13,19,20) healthy volunteers, and subjects with cigarette smoking-attributable chronic obstructive pulmonary disease (COPD) and/or emphysema (30,31), henceforth collectively denoted as COPD. Plasma was obtained from these specimens by centrifugation, aliquoted, and stored at −80°C prior to use in the BLyS assays. Diagnoses of lung disease were established by expert clinicians, who analyzed all information, and were blinded to the experimental laboratory tests. All IPF subjects fulfilled consensus diagnostic criteria (9,19,20), and had negative conventional autoimmune serological tests (13b). COPD was diagnosed by spirometry (30), and emphysema was detected by chest CT scans (31). Healthy controls were recruited from volunteers among hospital personnel or research registries. Those with tobacco smoking histories had normal spirometry and no radiographic evidence of emphysema.
Subpopulations of IPF and COPD subjects had pulmonary artery (PA) pressures measured by right heart catheterizations during evaluations for possible lung transplantation, or other clinical indications. These catheterizations were performed by cardiologists who were independent and unaware of this study.
All subjects gave written informed consent. This study was approved by the University of Pittsburgh Institutional Review Board.
B-cell Phenotypes
Peripheral blood mononuclear cells (PBMNC) were isolated from venous phlebotomy specimens by density gradient centrifugation (9). B-cells among the PBMNC were stained with panels of mAb and characterized by flow cytometry, as fully detailed previously (32). B-lymphocytes were identified as CD3−/CD14−/CD16−/CD19+ cells, and further stratified for comparisons here as transitional (IgD+/CD38++), IgM+ IgA− IgG− switched memory (IgD+/CD27+), IgG+ switched memory (IgD−/CD27+/-), and plasmablasts (CD19lo/+, CD20−, CD38++, IgD−, CD27++) (Figure 1A).
Figure 1. Phenotypes of circulating B-cells.

A.) Cells were stained with panels of mAb and gated on CD19+, CD20−, CD3−, and IgD− B-cells. Plasmablasts among these (CD27 hi, CD38hi) are denoted in the upper right quadrant of this example. B.) Selected phenotypic characteristics of circulating B-cells among COPD (n=29), IPF (n=25) and normal controls (n=20). The lowest, second lowest, middle, second highest, and highest box points represent 10th, 25th, median, 75th, and 90th percentiles, respectively. Means are represented by squares. Trans = transitional (recent bone marrow emigrant B-cell), and successively differentiated developmental stages: non-isotype switched (IgA−, IgG−) memory (nsMem), isotype (IgG) switched memory (sMEM) and efficient antibody-producing plasmablasts (PB). Absolute sMEM values are 10x those depicted in this figure. P values denote Kruskal-Wallis comparisons of the three cohorts; *denotes the group that is significantly different from the other two. Note increased B-cell differentiation among the disease cohorts (COPD and IPF) relative to healthy controls, and the general similarities between those two lung disease subpopulations. C.) The percentage of plasmablasts (PB) among circulating B-cells was inversely correlated with FVC, as a percentage of predicted values (FVC%p) in IPF subjects. D.) The percentage of plasmablasts (PB) among circulating B-cells was inversely correlated with DLCO%p in COPD subjects.
Lung Specimens
Pulmonary explant specimen processing has been detailed elsewhere (9,13). IPF and COPD explants were obtained during therapeutic transplantations. Normal lungs not used for transplantations were procured from cadaveric donors during harvests of other organs (9,13).
Immunohistochemistry
These studies were performed using methods that have been previously detailed (30). Primary antibodies used on Zn-fixed lung sections were mouse anti-human IgG (Serotec, Raleigh, NC), and rabbit anti-human C4d (LSBio, Seattle, WA). Treatments with these antibodies were followed by successive incubations with species and isotype-specific biotinylated secondary antibodies, and avidin-HRP (30). Mouse anti-human CD20 (Dako, Carpinteria, CA) and anti-Ki-67 (ThermoFisher, Kalamazoo, MI) were analogously used in formalin-fixed, paraffin-embedded lung specimens. Intrapulmonary B-cell aggregates were quantified by blinded observers who counted CD20+ cells in 30 successive, defined rectangular fields in each individual lung tissue specimen (at 10×). These data are expressed as numbers of CD20+ cells/mm2.
B-lymphocyte Stimulating Factor (BLyS)
Plasma was obtained by centrifugation of heparinized phlebotomy specimens and used in BLyS ELISA kits (R&D Systems) according to manufacturer instructions. Optical densities (OD) at 405 nm for replicate specimens were determined in a SpectraMax 190 plate reader (Molecular Devices, Sunnyvale, CA), blanked against untreated wells.
Statistical Analyses
Two and three group comparisons of continuous variables were made by Mann-Whitney or Kruskal-Wallis tests, respectively. Associations between continuous variables were established by linear regression. Logistic regression was used to perform multivariate analyses. Survival analyses were performed using product-limit estimation, with intergroup comparisons by logrank. Hazard ratios (HR) and 95% CI were established by proportional hazard regression. P values <0.05 were considered significant. Unless otherwise denoted, data are depicted as means±SE.
Results
Subjects and Plasma Specimens
Plasma specimens for BLyS assays were collected between December 28, 2005 and December 16, 2011. Characteristics of the aggregate lung disease populations used in these studies are detailed in Table 1. Normal controls (n=53) were 63±1 years old, 64% male, and 57% were former or current smokers.
Table 1.
Demographic and Clinical Characteristics of Lung Disease Subjects in whom BLyS was quantified.
| IPF | COPD | |
|---|---|---|
| n | 110 | 90 |
| Age (yrs) | 69 ± 1 (71, 51-87) | 64 ± 1 (65, 47-83)* |
| Gender (%male) | 73 | 44* |
| FVC%predicted | 62 ± 2 (59, 25-113) | 81 ± 2 (82, 35-136)* |
| FEV1%predicted | 75 ± 2 (73, 31-130) | 53 ± 3 (54, 12-121)* |
| FEV1/FVC | 0.85 ± 0.01 (0.85, 0.71-1.22) | 0.48 ± 0.02 (0.46, 0.15-0.81)* |
| DLCO%predicted | 47 ± 2 (47, 14-110) | 51 ± 2 (48, 12-122) |
| Smoking History (%) | 55 | 100* |
Data are depicted as means ± SE, and in parenthesis: (median, minimum-to-maximum values). FVC%predicted = forced vital capacity, as a percentage of predicted values; FEV1%predicted = forced expiratory volume in the first second of expiration, as a percentage of predicted values; FEV1/FVC = the ratio of forced expiratory volume in the first second to forced vital capacity; DLCO%predicted = diffusing capacity for carbon monoxide, as a percentage of predicted values. Smoking history denotes subjects with ≥5 pack-years of cigarette smoking.
denotes p<0.0001 for nonparametric comparisons between the COPD and IPF cohorts.
B-cell Phenotypes
In order to conduct an initial evaluation for B-cell abnormalities in the respective cohorts, the phenotypes of their circulating lymphocytes were prospectively determined by flow cytometry among recently recruited consecutive subjects. Characteristics of the subjects who provided these specimens are detailed in the Online Supplement.
B-cell phenotype distributions among the IPF patients were abnormal compared to healthy subjects, and similar to those of the subjects with COPD (Figure 1B), a clinically distinct lung disease in which pathogenic autoantibodies have been implicated (30). The magnitude of plasmablast differentiation among the IPF subjects were inversely correlated with forced vital capacities (FVC), a measure of lung restriction (Figure 1C). There was a trend in the COPD cohort for an analogous inverse correlation between the proportion of plasmablasts among B-cells and the ratio of forced expiratory volume in the first second of expiration to FVC (FEV1/FVC), a defining criterion of expiratory airflow obstruction (r=0.32, p=0.09). Plasmablast differentiation among the COPD subjects was significantly correlated with diffusing capacities for carbon monoxide, a correlate of intrapulmonary gas exchange (Figure 1D).
In situ B-cells
Having found evidence of circulating B-cell abnormalities in IPF patients, we performed additional studies to confirm these lymphocytes were also present within patient lungs. Focal aggregates of CD20+ B-cells were present in all IPF lungs examined by immunohistochemistry (n=11), typically proximate to small airways, and to a somewhat lesser extent near small blood vessels (Figure 2A). Fewer, and typically more scattered CD20+ cells were evident in normal lungs (n=9) (Figure 2B). Ki-67 was only infrequently seen among the IPF B-cells (Figure 2A).
Figure 2. Intrapulmonary CD20+B-cells.

A.) Representative images show sections from normal lungs (left panel) and IPF lungs (middle panel) immunostained for CD20. These B-cells are only infrequently positive for Ki-67 (right panel) (×100). B.) Direct counting confirmed CD20+ cells are more numerous in IPF compared to normal lung specimens.
Analogous B-cell aggregates were also evident in COPD lung sections (data not shown), as previously described and illustrated (33).
B Lymphocyte Stimulator (BLyS)
BLyS plasma concentrations were significantly greater among IPF subjects than in both normal and COPD controls (Figure 3A). BLyS concentrations were highest among those IPF subjects who had PA hypertension, defined as PA mean pressure >25 mmHg with PA wedge pressure <15 mmHg, and also among those who died within a year of the specimen acquisitions (Figure 3B). The BLyS values were significantly correlated with PA pressures among individuals with IPF (Figure 3C). Pulmonary function tests were obtained 6.1±0.3 months in 64 of the IPF subjects (those still alive, not transplanted, or not too ill to perform these tests). BLyS concentrations in these patients tended to be inversely associated with subsequent changes in FVC (r = 0.26, p = 0.04).
Figure 3. BLyS levels.

A.) Plasma BLyS concentrations were significantly greater in IPF subjects (n=110) than in either COPD (n=90) or normals (n=53). B.) Among the IPF cohort, BLyS levels were highest among those who died during the next year (n=25), and in those who had pulmonary artery hypertension (PAH) (n=17), compared to those with lesser pulmonary artery (PA) mean pressures (Nl) (n=28). PAH was defined as PA mean pressures >25 mmHg with PA wedge (PAW) pressures <15 mmHg. Subjects with PAW >15 were not tabulated here (n=5). These five excluded subjects had PA mean pressures >25, and their BLyS concentrations were 1.92±0.32 ng/ml). C.) BLyS concentrations were correlated with pulmonary artery pressures among IPF subjects. D.) The quartile (25%) of IPF subjects with greatest BLyS levels (High) had worse outcomes than those subjects with lesser BLyS levels (Low). Cross-hatches and numbers in parentheses denote censored events (end of observation). E.) Absolute mortality was also greater among IPF patients with the highest quartile plasma BLyS concentrations (High) after omission of the subpopulation that had lung transplantations during the observation interval.
To examine for possible associations of BLyS with subsequent outcomes, the IPF patients were stratified into the quartile with highest circulating concentrations of this mediator vs. the 75% of subjects with lower BLyS levels. Actuarial analyses confirmed that subjects with the greatest concentrations of BLyS had worse one-year outcomes (Figure 3D). Post hoc actuarial analyses limited to tabulations of deaths, with omission of those subjects who had transplantations during the year after their specimen acquisitions, also showed greater one-year absolute survival among the IPF subjects with lower BLyS concentrations (Figure 3E).
There were no significant gender differences of BLyS levels among the IPF patients (2.1±0.1 vs. 1.9±0.1 ng/ml, for males and females, respectively, p=0.66), but there was a trend for greater proportions of males within the quartile of subjects with highest BLyS concentrations (Table 2). Otherwise there were no appreciable associations of the clinical/demographic parameters in Table 2 with levels of this mediator. None of these clinical/demographic characteristics were associated with survival independently of BLyS. PA pressure was also not an independent correlate of outcome in this study cohort.
Table 2. Demographic and Clinical Characteristics of IPF Subjects Stratified by Plasma BLyS Levels.
| Lowest BLyS | Highest BLyS | |
|---|---|---|
| n | 82 | 28 |
| Age (yrs) | 70 ± 1 (71, 53-87) | 67 ± 2 (69, 51-82) |
| Gender (%male) | 68 | 86 |
| Lung Biopsy (%) | 51 | 61 |
| FVC%predicted | 63 ± 2 (61, 31-113) | 59 ± 4 (57, 25-102) |
| DLCO%predicted | 49 ± 2 (48, 14-109) | 44 ± 4 (40, 14-91) |
| Smoking History (%) | 59 | 44 |
| Immunosuppressants (%) | 19 | 18 |
Highest BLyS denotes those IPF subjects with mediator levels in the greatest quartile of values, whereas Lowest BlyS denotes the 75% of subjects with lower values. Data are depicted as means ± SE, and in parenthesis: (median, minimum-to-maximum values). Immunosuppressants denote subjects taking any single agent or various permutations of prednisone (5-20 mg/day), azathioprine, interferon-gamma, mycophenolate, or tacrolimus. See Table 1 legend for other explanations. None of these characteristics differed significantly between the subpopulations (p=0.07 for the intergroup gender comparisons).
To date very few of the COPD cohort have died (precluding meaningful analyses of survival correlates). However, BLyS levels in COPD subjects did not significantly correlate with PA pressures (r=0.20, p=0.21) or measures of pulmonary function (data not shown). There were no differences of circulating BLyS concentrations between males (1.56±0.08 ng/ml) and females (1.62±0.06 ng/ml) among the COPD cohort. Proportions of males and females in the highest quartile of BLyS concentrations among COPD subjects were 25% and 24%, respectively.
Intrapulmonary Immune Complexes and Complement
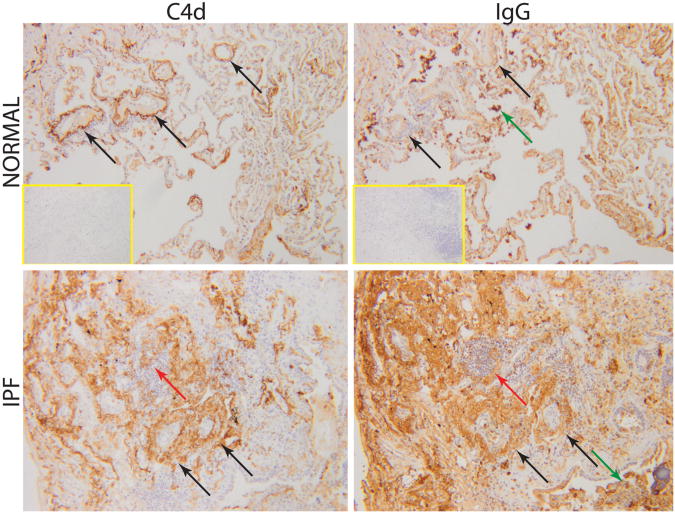
Given the association between BLyS and PA pressures, we examined lung sections for evidence of antibody-mediated processes involving pulmonary blood vessels (Figure 4). Diffuse parenchymal immune complexes and complement depositions were much more extensive in IPF lungs compared to normals, as has been previously described (13). Also in contrast to normal lung specimens, the IPF sections were characterized by thickened blood vessel walls and adventitia that are associated with wider circumferential IgG and C4d staining. These findings were particularly conspicuous in areas proximate to extensive fibrotic abnormalities of lung parenchyma. The presences of intrapulmonary immune complexes and complement depositions in COPD have already been detailed (30).
Figure 4. Intrapulmonary IgG and C4d.
In contrast to normal lungs, the IPF sections are characterized by more intense IgG and C4d staining throughout the parenchyma, and particularly intense staining around blood vessels (black arrows). Staining was also present in lymphoid aggregates (red arrows) and intraluminal cells and debris (green arrows). Inset images show the lack of staining in non-immune controls (magnification ×100).
Discussion
Antigen-stimulated B-cells undergo incremental maturations that result in highly differentiated lymphocytes with increased efficacy for the elaboration of avid, isotype-switched IgG antibodies (34). In comparisons to healthy controls, B-cell phenotypes among patients with recognized autoantibody diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), are more differentiated, with lesser proportions of early B-cells and greater relative proportions of mature lymphocytes, including autoantibody-producing plasmablasts (35-37). Moreover, the magnitude of this B-cell differentiation is a biomarker for the clinical activities of these diseases (35-37). The present data show the extent of B-lymphocyte differentiation among IPF subjects is similarly abnormal (Figure 1B), and is also analogously correlated with the pulmonary function of these lung disease patients (Figure 1C).
Other findings here confirm previous reports of abnormal B-cell aggregates within IPF lungs (4,5) (Figure 2). The B-cell accumulations in these specimens are predominately Ki-67 negative, and thus are almost certainly attributable to trafficking from extrapulmonary compartments rather than local proliferation. This particular finding is also congruent with a previous report (5).
BLyS, also known as B-cell activating factor (BAFF), is a TNF ligand family cytokine that is produced by a variety of leukocytes, and provides essential, non-redundant signals necessary for B-lymphocyte survival, maturation, and antibody production (29,38). Circulating BLyS is increased in patients with many autoantibody-mediated diseases, including SLE and RA, and levels of this mediator among individuals with these diseases are associated with their clinical manifestations (29,38,39). The present data show that plasma concentrations of BLyS are similarly increased in IPF subjects, as well as also being correlated with important clinical features and prognoses of these patients (Figure 3). Pulmonary artery pressures (PAP) were measured here among patient subpopulations that had these determinations during evaluations for lung transplantations and/or other clinical indications (e.g., for disproportionate dyspnea). Accordingly, it is possible the cohorts with these measures were biased, that could have included an enrichment of patients with pulmonary artery hypertension. Thus, the association of BLyS with PAP may not be as rigorous in cross-sectional, unselected IPF populations. Nonetheless, the IPF subpopulation analyzed here also included many patients with normal PAP in whom the BLyS-PAP association was still evident (Figure 3C). Most importantly, circulating BLyS concentrations were also highly associated with patient outcomes (Figures 3D,3E), and the latter analyses are not subject to selection bias.
The findings here are further evidence that B-cell abnormalities are common in IPF subjects (4-16) and linked to clinical manifestations and mortality (10-13). Studies of B-cells in IPF are ongoing, but there are several plausible mechanisms by which these lymphocytes could have pathogenic effects.
Tissue-bound antibodies produced by B-cells can cause cytotoxicities and promote neutrophil recruitment by the formation of antibody-antigen (immune) complexes and complement fixation (Figure 4), and/or by activation of NK cells (17,34,40,41). Observations here confirm previous findings of diffuse immune complex and complement depositions in IPF lungs (13), and additionally show these processes are proximally associated with intrapulmonary blood vessel pathology (Figure 4).
Autoantibodies can also deleteriously alter target cell functions (13,16,42,43) by cross-linking cell surface autoantigen-receptor complexes that transduce and enhance proinflammatory responses, or after gaining access to intracellular autoantigens (44). Previous studies have shown that autoantibodies of IPF patients increase the production of pro-fibrotic TGF-P by alveolar epithelia (16). Anti-heat shock protein 70 IgG isolated from these patients activates monocytes and increases their elaborations of IL-8 (13), a chemokine biomarker of IPF that has been implicated in the pathogenesis of this disease (45).
Although perhaps less widely appreciated, activated B-cells per se also directly elaborate numerous cytokines and other mediators that have vasoactive, pro-inflammatory, and pro-fibrotic effects (34). Intrapulmonary lymphoid aggregates are an abundant source of diverse, highly active mediators, essentially always an abnormal finding, and likely have pathogenic consequences (5,34,46,47). Lymphoid aggregates in proximity to pulmonary blood vessels are associated with anatomic and functional vascular abnormalities among IPF and other disease populations (5,47).
Activated B-cells are also efficient antigen presenting cells for T-lymphocytes (34). In turn, antigen- (or autoantigen-) stimulated T-cells produce myriad pathogenic mediators (48), and have been singularly implicated as the initiators of many disease-associated inflammatory cascades, including those that result in pathological fibrosis (41,48,49). Numerous T-cell abnormalities have been described in IPF patients, including reactivity to autologous lung proteins and intrapulmonary autoantigens, and several characteristics and functions of these lymphocytes are correlated with clinical manifestations and/or patient outcomes (9,13,18,19,21,22).
The present data, in conjunction with other independent evidences in IPF patients (4-22), illuminate several parallels between this lung disease and recognized autoimmune syndromes (34-39,41,42,46,49). Thus, these collective findings may be an impetus to consider innovative experimental trials for this inexorable and highly lethal syndrome. Severe autoantibody-mediated lung diseases in particular (e.g., Goodpasture's disease, anti-Jo-1-associated interstitial lung disease, Wegener's granulomatosis, anti-alloantigen-mediated rejection, etc.) are usually inadequately responsive or even refractory to corticosteroid-based therapy. However, these disorders are often more responsive to treatments with modalities that remove autoantibodies (e.g., plasma exchange [23]) and/or B-cell depleting agents, including rituximab (24-27), and a preliminary report indicates this approach may also possibly benefit IPF patients (50). Alternatively, or in addition, critical B-cell functions can be targeted by interfering with the maturation and survival of these lymphocytes (29,38). A highly specific anti-BLyS agent (belimumab) was recently approved by the FDA for treatment of antibody-mediated SLE, and this drug appears to have efficacy and minimal toxicity in this indication (28).
If humoral autoimmunity plays a pathogenic role in IPF causality or progression, as suggested by the current findings and several other reports (4-17), mechanistic therapies that specifically target B-cells could perhaps benefit patients afflicted with this morbid and heretofore untreatable disease (2,3,50).
Supplementary Material
Footnotes
This research was supported in part by U.S. N.I.H. grants HL107172 and HL084948
References
- 1.Fernández Pérez ER, Daniels CE, Schroeder DR, St Sauver J, Hartman TE, Bartholmai BJ, Yi ES, Ryu JH. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137:129–137. doi: 10.1378/chest.09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borchers AT, Chang C, Keen CL, Gershwin ME. Idiopathic pulmonary fibrosis-an epidemiological and pathological review. Clinic Rev Allerg Immunol. 2011;40:117–134. doi: 10.1007/s12016-010-8211-5. [DOI] [PubMed] [Google Scholar]
- 3.O'Connell OJ, Kennedy MP, Henry MT. Idiopathic pulmonary fibrosis: treatment update. Adv Ther. 2011;28:986–999. doi: 10.1007/s12325-011-0066-5. [DOI] [PubMed] [Google Scholar]
- 4.Campbell DA, Poulte LW, Janossy G, du Bois RM. Immunohistological analysis of lung tissue from patients with cryptogenic fibrosing alveolitis suggesting local expression of immune hypersensitivity. Thorax. 1985;40:405–411. doi: 10.1136/thx.40.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchal-Somme J, Uzunhan Y, Marchand-Adam S, Valeyre D, Soumelis V, Crestani B, Soler P. Cutting edge: non-proliferating mature immune cells form a novel type of organizing lymphoid structure in idiopathic pulmonary fibrosis. J Immunol. 2006;176:5735–5739. doi: 10.4049/jimmunol.176.10.5735. [DOI] [PubMed] [Google Scholar]
- 6.Zuo F, Kaminski N, Eugui E, Allard J, Hakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, Sheppard D, Pardo A, Heller RA. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci (USA) 2002;99:6292–6297. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dall Aglio PP, Pesci A, Bertorelli G, Brianti E, Scarpa S. Study of immune complexes in broncholaveolar lavage fluids. Respiration. 1998;54:36–41. doi: 10.1159/000195495. [DOI] [PubMed] [Google Scholar]
- 8.Dobashi N, Fujita J, Murota M, Ohtsuki Y, Yamadori I, Yoshinouchi T, Ueda R, Bandoh S, Kamei T, Nishioka M, Ishida T, Takahara J. Elevation of anti-cytokeratin 18 antibody and circulating cytokeratin 18: anti-cytokeratin 18 antibody immune complexes in sera of patients with idiopathic pulmonary fibrosis. Lung. 2000;178:171–179. doi: 10.1007/s004080000020. [DOI] [PubMed] [Google Scholar]
- 9.Feghali-Bostwick CA, Tsai CG, Valentine VG, Kantrow S, Stoner MW, Pilewski JM, Gadgil A, George MP, Gibson KF, Choi AM, Kaminski N, Zhang Y, Duncan SR. Cellular and humoral autoreactivity in idiopathic pulmonary fibrosis. J Immunol. 2007;179:2592–2599. doi: 10.4049/jimmunol.179.4.2592. [DOI] [PubMed] [Google Scholar]
- 10.Ogushi F, Tani K, Endo T, Tada H, Kawano T, Asano T, Huang L, Ohmoto Y, Muraguchi M, Moriguchi H, Sone S. Autoantibodies to IL-1α in sera from rapidly progressive idiopathic pulmonary fibrosis. J Med Invest. 2001;48:181–189. [PubMed] [Google Scholar]
- 11.Kurosu K, Takiguchi Y, Okada O, Yumoto N, Sakao S, Tada Y, Kasahara Y, Tanabe N, Tatsumi K, Weiden M, Rom WN, Kuriyama T. Identification of annexin 1 as a novel autoantigen in acute exacerbation of idiopathic pulmonary fibrosis. J Immunol. 2008;181:756–767. doi: 10.4049/jimmunol.181.1.756. [DOI] [PubMed] [Google Scholar]
- 12.Taillé C, Grootenboer-Mignot S, Boursier C, Michel L, Debray MP, Fagart J, Barrientos L, Mailleux A, Cigna N, Tubach F, Marchal-Sommé J, Soler P, Chollet-Martin S, Crestani B. Identification of periplakin as a new target for autoreactivity in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:759–766. doi: 10.1164/rccm.201001-0076OC. [DOI] [PubMed] [Google Scholar]
- 13.Kahloon RA, Xue J, Bhargava A, Csizmadia E, Otterbein L, Kass DJ, Bon J, Soejima M, Levesque MC, Lindell KO, Gibson KF, Kaminski N, Banga G, Oddis CV, Pilewski JM, Sciurba FC, Donahoe M, Zhang Y, Duncan SR. Patients with idiopathic pulmonary fibrosis patients with antibodies to heat shock protein 70 have poor prognoses. Am J Resp Crit Care Med. 2013;187:768–775. doi: 10.1164/rccm.201203-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13b.ibid. Online Data Supplement
- 14.Grigolo B, Mazzetti I, Borzì RM, Hickson ID, Fabbri M, Fasano L, Meliconi R, Facchini A. Mapping of topoisomerase II alpha epitopes recognized by autoantibodies in idiopathic pulmonary fibrosis. Clin Exp Immunol. 1998;114:339–346. doi: 10.1046/j.1365-2249.1998.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y, Fujita J, Bandho S, Ohtsuki Y, Yamadori I, Yoshinouchi T, Ishida T. Detection of antivimentin antibody in sera of patients with idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Clin Exp Immunol. 2002;128:169–174. doi: 10.1046/j.1365-2249.2002.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace WHH, Howie SM. Upregulation of tenascin and TGF-β production in a type II alveolar epithelial cell line by antibody against a pulmonary auto-antigen. J Pathol. 2001;195:251–256. doi: 10.1002/path.916. [DOI] [PubMed] [Google Scholar]
- 17.Magro CM, Waldman WJ, Knight DA, Allen JN, Nadasdy T, Frambach GE, Ross P, Marsh CB. Idiopathic pulmonary fibrosis related to endothelial injury and antiendothelial cell antibodies. Hum Immunol. 2006;67:284–297. doi: 10.1016/j.humimm.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Kotslanidis IE, Nakou E, Bouchliou I, Tzouvelekis A, Spanoudakis E, Steiropoulos P, Sotiriou I, Aidinis V, Margaritis D, Tsatalas C, Bouros D. Global impairment of CD4+CD25+FoxP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Resp Crit Care Med. 2009;179:1121–1130. doi: 10.1164/rccm.200812-1936OC. [DOI] [PubMed] [Google Scholar]
- 19.Gilani SR, Vuga LJ, Lindell KO, Gibson KF, Xue J, Kaminski N, Valentine VG, Lindsay EK, George MP, Steele C, Duncan SR. CD28 down-regulation on circulating CD4 T-cells is associated with poor prognoses of patients with idiopathic pulmonary fibrosis. PLoS One. 2010;5:e8959. doi: 10.1371/journal.pone.0008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue J, Gochuico BR, Alawad AS, Feghali-Bostwick CA, Noth I, Nathan SD, Rosen GD, Rosas IO, Dacic S, Ocak I, Fuhrman CR, Cuenco KT, Smith MA, Jacobs SS, Zeevi A, Morel PA, Pilewski JM, Valentine VG, Gibson KF, Kaminski N, Sciurba FC, Zhang Y, Duncan SR. The HLA Class II allele DRB1*1501 is over-represented in patients with idiopathic pulmonary fibrosis. PLoS One. 2011;6:e14715. doi: 10.1371/journal.pone.0014715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parra ER, Kairalla RA, Ribeiro de Carvalho CR, Eher E, Capelozzi VL. Inflammatory cell phenotyping of the pulmonary interstitium in idiopathic interstitial pneumonia. Respiration. 2007;74:159–69. doi: 10.1159/000097133. [DOI] [PubMed] [Google Scholar]
- 22.Daniil Z, Kitsanta P, Kapotsis G, Mathioudaki M, Kollintza A, Karatza M, Milic-Emili J, Roussos C, Papiris SA. CD8+ T lymphocytes in lung tissue from patients with idiopathic pulmonary fibrosis. Respir Res. 2005;6:81. doi: 10.1186/1465-9921-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erickson SB, Kurtz SB, Donadio JV, Holley KE, Wilson CB, Pineda AA. Use of combined plasmapharesis and immunosuppression in the treatment of Goodpasture’s syndrome. Mayo Clin Proc. 1979;54:714–720. [PubMed] [Google Scholar]
- 24.Sem M, Molberg O, Lund MB, Gran JT. Rheumatology. Vol. 48. Oxford; 2009. Rituximab treatment of the anti-synthetase syndrome: a retrospective case series; pp. 968–971. [DOI] [PubMed] [Google Scholar]
- 25.Martinu T, Howell DN, Palmer SM. Acute cellular rejection and humoral sensitization in lung transplant recipients. Semin Resp Crit Care Med. 2010;31:179–188. doi: 10.1055/s-0030-1249113. [DOI] [PubMed] [Google Scholar]
- 26.Borie R, Debray MP, Laine C, Aubier M, Crestani B. Rituximab therapy in autoimmune pulmonary alveolar proteinosis. Eur Respir J. 2009;33:1503–6. doi: 10.1183/09031936.00160908. [DOI] [PubMed] [Google Scholar]
- 27.Keir GJ, Maher TM, Hansell DM, Denton CP, Ong VH, Singh S, Wells AU, Brezoni EA. Severe interstitial lung disease in connective tissue disease: rituximab as rescue therapy. Eur Resp J. 2012;40:641–648. doi: 10.1183/09031936.00163911. [DOI] [PubMed] [Google Scholar]
- 28.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, Stohl W, Ginzler EM, Hough DR, Zhong ZL, Freimuth W, van Vollenhoven RF BLISS-76 Study Group. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancro MP, D'Cruz DP, Khamashta MA. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest. 2009;119:1066–1073. doi: 10.1172/JCI38010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feghali-Bostwick CA, Gadgil AS, Otterbein LE, Pilewski JM, Stoner MW, Csizmadia E, Zhang Y, Sciurba FC, Duncan SR. Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Resp Critical Care Med. 2008;177:156–163. doi: 10.1164/rccm.200701-014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bon J, Fuhrman CR, Weissfeld JL, Duncan SR, Branch RA, Chang CC, Zhang Y, Leader JK, Gur D, Greenspan SL, Sciurba FC. Radiographic emphysema predicts low bone mineral density in a tobacco-exposed cohort. Am J Respir Crit Care Med. 2011;183:885–890. doi: 10.1164/rccm.201004-0666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levesque MC, Moody MA, Hwang KK, Marshall DJ, Whitesides JF, Amos JD, Gurley TC, Allgood S, Haynes BB, Vandergrift NA, Plonk S, Parker DC, Cohen MS, Tomaras GD, Goepfert PA, Shaw GM, Schmitz JE, Eron JJ, Shaheen NJ, Hicks CB, Liao HX, Markowitz M, Kelsoe G, Margolis DM, Haynes BF. Transmitted/Founder HIV-1 induces B cell polyclonal differentiation and apoptosis with massive gastrointestinal tract germinal center loss in the earliest stages of infection. PLoS Medicine. 6:e1000107. doi: 10.1371/journal.pmed.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 34.Browning JL. B cells move to center stage: novel opportunities for autoimmune disease treatment. Nat Rev. 2006;5:564–575. doi: 10.1038/nrd2085. [DOI] [PubMed] [Google Scholar]
- 35.Jacobi AM, Reiter K, Mackay M, Aranow C, Hiepe F, Radbruch A, Hansen A, Burmester GR, Diamond B, Lipsky PE, Dörner T. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arth Rheum. 2008;58:1762–1773. doi: 10.1002/art.23498. [DOI] [PubMed] [Google Scholar]
- 36.Souto-Carneiro MM, Mahadevan V, Takada K, Fritsch-Stork R, Nanki T, Brown M, Fleisher TA, Wilson M, Goldbach-Mansky R, Lipsky PE. Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumour necrosis factor. Arthritis Res Ther. 2009;11:R84. doi: 10.1186/ar2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anolik JH, Barnard J, Cappione A, Pugh-Bernard AE, Felgar RE, Looney RJ, Sanz I. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 2004;50:3580–3590. doi: 10.1002/art.20592. [DOI] [PubMed] [Google Scholar]
- 38.Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18:263–275. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Dillon SR, Harder B, Lewis KB, Moore MD, Liu H, Bukowski TR, Hamacher NB, Lantry MM, Maurer M, Krejsa CM, Ellsworth JL, Pederson S, Elkon KB, Wener MH, Dall'Era M, Gross JA. B-lymphocyte stimulator/a proliferation-inducing ligand heterotrimers are elevated in the sera of patients with autoimmune disease and are neutralized by atacicept and B-cell maturation antigen-immunoglobulin. Arthrtitis Res Ther. 2010;12:R48. doi: 10.1186/ar2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayadas TN, Tsokos GC, Tsuboi N. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation. 2009;120:2012–2024. doi: 10.1161/CIRCULATIONAHA.108.771170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu SM, Deshmukh US, Gaskin F. Pathogenesis of systemic lupus erythematosus revisited 2011: end organ resistance to damage, autoantibody initiation and diversification, and HLA-DR. J Autoimmunity. 2011;37:104–112. doi: 10.1016/j.jaut.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokota SI, Chiba S, Furuyama H, Fujii N. Cerebrospinal fluids containing anti-HSP70 autoantibodies from multiple sclerosis patients augment HSP70-induced proinflammatory cytokine production in monocytic cells. J Neuroimmunol. 2010;218:129–33. doi: 10.1016/j.jneuroim.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Lu MC, Lai NS, Yu HC, Huang HB, Hsieh SC, Yu CL. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 2010;62:1213–1223. doi: 10.1002/art.27386. [DOI] [PubMed] [Google Scholar]
- 44.Jang JY, Jeong JG, Jun HR, Lee SC, Kim JS, Kim YS, Kwon MH. A nucleic acid-hydrolyzing antibody penetrates into cells via caveolae-mediated endocytosis, localizes in the cytosol and exhibits cytotoxicity. Cell Mol Life Sci. 2009;66:1985–1997. doi: 10.1007/s00018-009-9179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, Klesen M, Zhang Y, Gibson KF. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory disease. Nature Rev. 2006;6:205–211. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 47.Perros F, Dorfmüller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, Humbert M, Lambrecht BN. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:311–21. doi: 10.1164/rccm.201105-0927OC. [DOI] [PubMed] [Google Scholar]
- 48.Monaco C, Andereakos E, Kiriakidis S, Feldman M, Paleolog C. T-cell-mediated signaling in immune, inflammatory and angiogenic processes: the cascade of events leading to inflammatory diseases. Curr Drug Targets Inflamm Allergy. 2004;3:35–42. doi: 10.2174/1568010043483881. [DOI] [PubMed] [Google Scholar]
- 49.Wynn TA. Fibrotic disease and the TH1/TH2 paradigm. Nat Immunol Rev. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donahoe M, Chien N, Raval JS, Gibson KF, Saul M, Zhang Y, Duncan SR. Autoantibody-targeted treatments for acute exacerbations of idiopathic pulmonary fibrosis. Am J Resp Crit Care Med; Presented at the Am Thorac Soc International Meeting; May 2013; 2013. [Accessed June 4, 2013]. Available on: http://cms.psav.com/cPaper2013/ats2013/myitinerary. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.