Abstract
A previous study in this laboratory demonstrated, for the first time, that neonatal lesions of the hippocampus impair monitoring working memory, as measured by a self-order task, but spare recency memory, as measured by the session-unique delayed nonmatching task. To substantiate and extend this novel finding, we assessed working memory in these same animals using a serial order memory task. In humans and non-human primates the serial order memory task has been shown to be dependent upon the integrity of the dorsolateral prefrontal cortex. Additionally, the serial order task has the ability to examine the integrity of non-dorsolateral dependent working memory functions, providing specificity to conclusions drawn from this task. Thus, monkeys with neonatal lesions of the hippocampus and sham-operated control subjects were tested on two versions of the serial order memory task (3 and 4 object). The results of this study demonstrated that neonatal hippocampal lesions did not impair performance on the 3-object version of the task, confirming our previous finding of intact non-dlPFC dependent working memory. In contrast, these same animals showed a significant impairment on the dlPFC dependent phase of the 4-object serial order task. This finding was further confirmed through a series of probe trials. These results, in combination with our earlier finding, suggest that early lesions of the hippocampus may have impacted the function of the dlPFC or its interactions with the hippocampus.
Keywords: dlPFC, schizophrenia, hippocampus, monkey
1. Introduction
A recent study in monkeys demonstrated that neonatal lesions of the hippocampal formation yielded abnormal functioning of the lateral prefrontal cortex [1]. Adult monkeys that had received neonatal lesions of the hippocampus between 10–12 days of age and their age-matched sham-operated controls were trained on two tasks of working memory to assess functioning of the prefrontal cortex [2, 3]. The Session-Unique Delayed Nonmatch-to-Sample (SU-DNMS) measures recency memory, i.e. the ability to maintain online information in a memory buffer, and is known to depend on the integrity of the ventrolateral prefrontal cortex. The object self-ordered (Obj-SO) task measures the monitoring of mental representations and is known to depend on the integrity of the dorsolateral prefrontal cortex. Monkeys with neonatal hippocampal lesions were severely impaired in the Obj-SO but not the SU-DNMS, suggesting for the first time in primates that early damage to the hippocampus selectively impacted working memory processes also known to depend on the normal functioning of the dorsolateral prefrontal cortex. These findings not only confirmed earlier reports in rodents demonstrating that neonatal damage to the ventral hippocampus disrupted the normal functional development of the medial prefrontal cortex [4–8] but also extended these findings by demonstrating that in the primate the impact of early hippocampal damage on the prefrontal cortex appeared to be restricted to a specific prefrontal region, namely the dorsolateral prefrontal cortex. Given the growing evidence of critical interactions between the hippocampus and the dorsolateral prefrontal cortex in working memory processes [9–11] and the relevance of the primate findings for developmental psychiatric disorders in humans such as schizophrenia [12], it was important to confirm the specificity of the working memory impairment following the neonatal hippocampal lesions given that recent studies have demonstrated that direct damage to the hippocampus may by itself impaired working memory processes. Thus, in the present study, we re-tested the same animals [1] in another working memory task, the serial order memory task that measures working memory for the temporal order of stimuli.
Although both the self-order and the serial-order memory tasks assess the active monitoring of neural representations held in memory buffer, the main difference between the two tasks lies on the control of stimulus order, which is made by the subject in the self-order task but by the experimenter in the serial order task. The serial order memory task (SOMT) was originally designed by Petrides [3] as a method to confirm findings obtained from the self-ordered working memory task and requires the animal to remember the temporal order of a series of stimuli on a trial-by-trial basis. Thus, on each trial, the animal is presented with a list of objects (1, 2, 3, and 4) and then with a choice test containing 2 objects of the list and is rewarded for selecting the stimuli occurring earlier in the list. Selective damage to the dorsolateral prefrontal cortex impairs performance on recency discrimination problems that include stimuli from the middle items of the lists (i.e. 2 and 3), but not on problems that contain either the first or the last items of the list (for example, 1 vs 3 and 2 vs 4). One advantage of the serial order task is that it may help dissociating the working memory processes dependent upon the integrity of the dorsolateral prefrontal cortex and those that are not.
In fact, in rodents, memory for temporal order as measured by serial order tasks has also been attributed to functioning of the hippocampus and is severely impaired by hippocampal lesions, which has been observed for both the temporal order of spatial locations and objects [13– 19]. Thus, one potential impediment for using the serial order memory task in the present study is that it may not permit to conclude whether poor performance on the task could be ascribed specifically to dysfunction of the dorsolateral prefrontal cortex, the hippocampus or both. This problem may, however, be overcome when investigating the patterns of impairment seen on the recency discriminations by the two types of lesions. That is, as described above, lesions of the dorsolateral prefrontal cortex in monkeys do not lead to a general impairment in discriminating the temporal order of all two items of the lists (i.e. 1–2, 1–3, 2–4 and so one), rather they yield a specific impairment in discriminating the temporal order of only the middle items (2 and 3) of the list [3]. By contrast, lesions of the hippocampus, at least in rodents, impaired the discrimination of the temporal order, irrespective of the temporal position of the items [14, 15]. In addition, rats have been shown to be unable to discriminate between adjacent stimuli in the serial order task, suggesting that the serial order task in rodents, may be measuring something other than prefrontal-dependent working memory processes [14, 15, 17]. Thus, the SOMT task could be used to test whether neonatal hippocampal lesions in monkeys have impacted all discrimination problems or rather only the discriminations including the two middle items of the list. Our results in fact demonstrate that monkeys with neonatal hippocampal lesions were impaired only when temporal order of the middle items of the list had to be discriminated, suggesting a specific dysfunction of the dorsolateral prefrontal cortex and its interactions with the hippocampus. Preliminary reports of this work has already appeared in abstract form [20].
2. Methods
2.1 Subjects
Eleven adult rhesus macaques (Macaca mulatta) between 6 to 8 years of age and weighing between 5 to 10kg were utilized in the current experiment. They were obtained as newborn monkeys from the University of Texas, M.D. Anderson Cancer Research Center (Bastrop, TX) and were subsequently surrogate nursery-reared with age-matched peers in cohorts of 4 animals in the primate nursery of the M.D. Anderson Cancer Center (Houston, TX). At the age of 10 – 12 days, five infants received sham lesions (Neo-C, 2 males, 3 females) and five received neurotoxic lesions of the hippocampus (Neo-H-ibo, 4 males, 2 females).
Full details of their rearing experience have been previously published [21] and will be briefly summarized here. Upon arrival to the primate nursery, the infants were individually housed in cages that permitted visual, auditory and social contacts with other animals located in adjacent cages until they were one month of age. A plush surrogate (30cm in length) was provided to the animals and a principal human caregiver spent roughly 6 h/day, 5 days/week in the nursery with the infant monkeys. On weekends, the infants were handled 2–3 times per day and received a total of 2–4 hours of social interactions with familiar human experimenters. From three to nine months of age infants received in addition daily social interactions (3–4 hours/day, 5 days/week) with age- and sex-matched peers and from 1 – 3 years, each cohort was placed in a large social cage that allowed them to socialize 24-hrs per day. Finally, at approximately 3 years of age they were then moved to the Yerkes National Primate Research Center (Atlanta, GA) where they were individually housed and maintained in dyads on a continual 12 hour, light-day cycle (7am–7pm).
All animals were fed a diet of Purina Old World Monkey Chow, which was supplemented with fresh fruit daily and were permitted unrestricted access to water throughout the duration of the experiment. During behavioral testing, access to food was minimally restricted to provide sufficient motivation to complete the behavioral paradigm. Monkeys’ weights were monitored weekly and maintained 85% or above their full feed weight.
All procedures were approved and used in full compliance with the Institutional Animal Care and Use Committees of both the University of Texas at Houston and Emory University, and were in line with the policies outlined in the NIH Guide for the care and use of Laboratory Animals.
2.2 Magnetic Resonance Imaging and Surgical Procedures
Details of all procedures were already reported in several recent publications [1, 21–23] and will be only briefly summarized below.
2.2.1 Magnetic Resonance Imaging
All animals received three Magnetic Resonance Imaging scans (MRI’s). The initial MRI was taken just prior to the surgery for all animals in both groups and served to select the injection sites of the neurotoxin injections in the hippocampus (see below). Two additional MR scans were performed after surgery and used to estimate the lesion location and extent. One was performed 5–8 days after the neurotoxin injections and allowed the visualization of edema caused by cell death and the other was performed one year post-surgery and permitted the measurement of hippocampal volume reduction that followed cell death. Both post-surgical MRI procedures provide a reasonable estimate of the lesion size as well as damage to structures adjacent to the target site [24]. Some animals in the current study received an additional MR scan as adults (6–8 years of age), which was used here to illustrate the lesion extent (Figure 1).
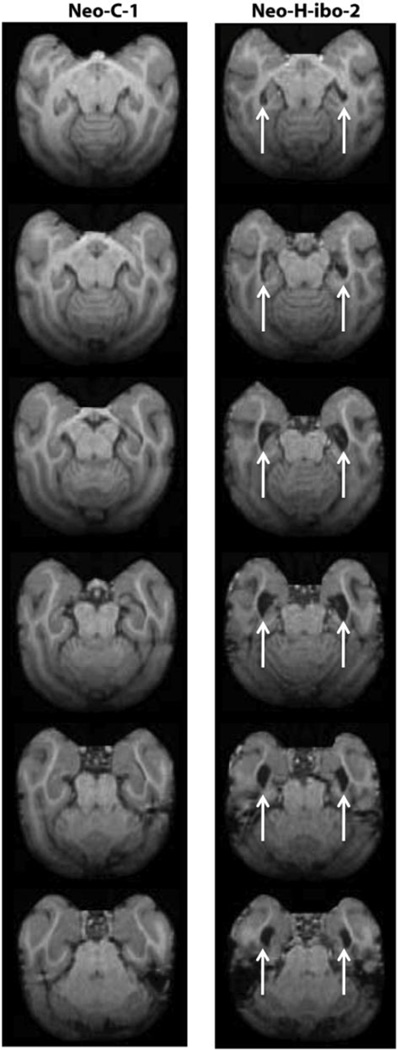
Figure 1.
Transverse T1-weighed images through the brain of a normal control monkey (Neo-C-1) on the left and a monkey with a neonatal lesion of the hippocampus (Neo-H-ibo-2) on the right. Comparisons between the two series of images clearly illustrate the 67% reduction in hippocampal volume in case Neo-Hibo-2, estimated from the one year post-surgical scan (see Table 2). Arrows point to increased lateral ventricle due to volume reduction of the hippocampus.
Prior to the pre-surgical MRI procedure, the animal was sedated and maintained under isoflurane gas (1.0–3.0%, v/v, to effect) until the end of the surgical procedure. The animal’s head was secured in a non-ferromagnetic stereotaxic apparatus (Crist Instruments, Damascus, MD). The MR images were acquired with a GE Signa 1.5 Tesla Echo Speed scanner (GE Medical Systems, Milwaukee, WI) using a 7.5-cm circular surface coil. For the pre-surgical and first post-surgical scans, two types of MR images were obtained in the coronal plane. A series of high-resolution T1-weighted MR images (echo time (TE) = 11ms, repetition time (TR) = 450ms in contiguous 1mm sections, 12cm field of view (FOV), 256 × 256 matrix) followed by a series of three Fluid Attenuated Inversion Recovery (FLAIR) images (Parameters: TE = 140ms, TR = 10,000ms, inversion time (TI) = 2200ms, contiguous 3mm sections, 14cm FOV, 256 × 256 matrix), which were offset of 1 mm in the anterior-posterior axis. The one-year post-surgical MR scans included only acquisition of the high-resolution T1-weighed images.
2.2.2 Surgical Procedures
After the pre-surgical MRI, the infant monkey was immediately transported to the surgical suite whilst maintaining anesthesia and its head fixed in the stereotaxic apparatus. Through the duration of the surgery, the monkey received supplemental i.v. fluids (5% dextrose and 0.5% sodium chloride) to maintain hydration. Prior to incision and after the procedure was completed the scalp was thoroughly disinfected. In order to minimize the potential risk of infection, all animals received Cefazolin (25mg/kg, i.m), swelling was monitored with dexamethasone sodium phosphate (0.3mg/kg, i.m.) and pain was alleviated with acetaminophen treatment.
After the skull was disinfected, Marcaine (25%, 1.5m, s.c.) was injected along the midline of the scalp and a skin incision was made to expose the underlying tissue and skull. The subcutaneous tissue was dissected and retracted laterally, two small crainiotomies were made bilaterally above the hippocampus, and small cuts were made in the dura to expose the brain.
The neurotoxin injections for the hippocampal lesions were made simultaneously in both hemispheres with two 10µl Hamilton syringes attached to two Kopf manipulators (David Kopf Instruments Tujunga, CA). Ibotenic acid (Biosearch Technologies Novato, CA, 10mg/ml in PBS, pH 7.4) was injected at 7–8 sites along the axis of the hippocampus (0.4–0.6µl/site delivered at a rate of 0.2 µl/30 s and for a total of 2.8–4.2µl/hemisphere). At the end of each injection, the needles were left in place for 3 minutes to allow for the ibotenic acid to diffuse and minimize it leaking back as the needle was removed. The sham surgeries followed the same procedures than those used for the hippocampal lesion with the exception that no needle was inserted into the brain.
2.3 Lesion Assessment
Extent of lesion for animals in Neo-H-ibo was estimated with the pre- and post-surgical scans since all animals are still undergoing behavioral testing. Extent of hypersignals resulting from edema caused by cell death was evaluated using the pre- and post-surgical coronal FLAIR images, which were first matched to drawings of histological coronal images of a normal 1-week-old infant monkeys (J. Bachevalier, unpublished data) onto which the extent of the hypersignal seen in each post-surgical FLAIR were drawn. Using the image analysis software program (ImageJ® http://rsb.info.nih.gov/ij), the total volume of damage on each slice for the intended target (hippocampus) and any unintended targets (perirhinal and entorhinal cortex, parahippocampal cortical areas TH/TF and amygdala) was calculated by measuring the surface area of the hypersignals on each slice and summing them. For a given structure, the sum of hypersignals calculated from the left and right hemispheres was multiplied by the slice thickness (1 mm), then divided by the total volume of the structure, and finally multiplied by 100 to provide a percent of extent of damage to each structure[25].
A second estimate of hippocampal damage was given by calculating the total hippocampal volume reduction using T-1 weighted scans taken 1 year post-surgery. The MR image, for each subject, through the full extent of the hippocampus was imported into the image analysis program ImageJ® (http://rsb.info.nih.gov/ij). On each image surface area of the hippocampus was recorded for the left and right hemispheres separately using the specific borders previously described [26]. The volume of the hippocampal formation included the CA fields, dentate gyrus, subicular complex and fimbria, but excluded the entorhinal, perirhinal, and parahippocampal cortices. For each hemisphere (separately), the hippocampal volume (in mm3) was calculated by summing hippocampal surface areas on each image and multiplying by the distance between the images (i.e. 1 mm), using Cavalieri’s principle [25]. For each animal in Group Neo-H-ibo, the hippocampal volume in each hemisphere was then compared to the averaged hippocampal volume from 6 normal male (n=3) and female (n = 3) monkeys of the same age (approximately 1 year of age). Percent volume reduction was then calculated using the following formula: [100-total H volume remaining/average H volume in normal subject]*100). Two trained observers measured the volume of hippocampal formation in normal animals and animals of Group Neo-H-ibo (Cronbach’s alpha; p < 0.01 for all inter- and intra-observer reliabilities).
2.4 Behavioral Procedures
Before participating in the current investigation the subjects have had extensive, albeit identical testing histories. This testing consisted of: Item-specific Visual Paired Comparison (1.5, 6, 18 and 48 months; [27]), Oddity (3 and 15 months), Spatial Visual Paired Comparison (8, 24 and 54 months), Object discrimination reversals (48–60 months), Food Preference and Discrimination Devaluation task (60 months), dyadic social interactions (3, 6, and 36 months), emotional reactivity to human intruder (2 and 4.5 months), social attachment to caregiver (9 months; [21]), trial unique delayed nonmatching-to-sample (DNMS), object/spatial memory span tasks [23] and finally session-unique DNMS and self-order task (5–6 years) [1].
All animals were trained on two versions of the serial order task. The initial version consisted of lists of three objects (3-SOMT) and the second one consisting of lists of four objects (4-SOMT). Both versions of the task were run in a manner identical to that described by Petrides (1991). In both the earlier study [3] and this one, animals had already been trained on the Trial-Unique Delayed Non-Matching-to-Sample prior to begin testing on the serial order task.
2.4.1 Apparatus and Stimuli
A Wisconsin General Testing Apparatus (WGTA) located in a dark room containing a white noise generator to mask distracting extraneous noise was used for behavioral testing. A testing board containing three recessed food wells (2cm in diameter, 1 cm deep and 13 centimeters apart, on center) served to display the stimuli, which were selected from a pool of 1000 junk objects that had been used to train all animals in the Trial Unique-Delayed Non-Match to sample Task. Therefore, animals were familiar with all objects and care was taken to run through the entire series before re-using objects to preclude the subject from building associations between objects. In addition for each list of 3 or 4 objects care was taken to select objects that were easily discriminable from one another, so that task performance was not confounded by stimulus ambiguity. Rewards provided for correct choices were unsalted peanuts (presentation of single objects) and miniature M&M’s (recency discrimination).
2.4.2 3-Object Serial Order Memory Task (3- SOMT)
Ten trials were administered in each daily session. Each trial used three objects and consisted of the successive presentations of the 3 objects one at a time over the central baited well at 10-s intervals, followed 10-s later by a recency judgment between 2 objects of the list positioned on the lateral wells of the tray. After a 30-s interval, a list consisted of three new objects was presented and so on for each following trials. Phase 1 of the 3-SOMT consisted of 10 trials in which recency judgments were between Object 1 and Object 3 of the list, and the animal was rewarded for selecting the object appearing earlier in the list (i.e. Object 1 in this case). Left-right position of the rewarded object varied pseudo-randomly. After reaching a criterion of 80% correct or better in a single daily session, Phase 2 was introduced in which 10 daily trials were given as for Phase 1 but the recency judgments were between Object 1 and Object 2. Once monkeys scored 80% correct or better on Phase 2, they were moved to Phase 3 in which recency judgments were now between Object 2 and Object 3 and testing continued until animals again reached a criterion of 80% correct or better. A titration procedure was used throughout testing as described by Petrides (1991). Thus, if the animal scored 70% correct on any given test day of a Phase, this Phase was repeated on the following day but if the animal scored 60% correct or poorer, they were moved back to the previous Phase. Thus, if failing Phase 3, animals were moved back to Phase 2, if failing Phase 2, they were moved back to Phase 1, and if failing Phase 1, they were moved back to trial-unique DNMS. This titration procedure was implemented to prevent the animal from being frustrated with continual poor performance on a Phase and to minimize their fixation on any single object in a series [2, 3]. Testing was discontinued if an animal required 20 daily sessions at any phase of the task.
Each Phase was scored independently using the number of daily sessions required to achieve the criterion (80% or better in one session). It should be noted that, when a phase was repeated and the animal performed below 60%, this session was not included in the total number of daily sessions for that phase.
2.4.3 4 Object Serial Order Memory Task (4-SOMT)
The 4-Object version of the SOMT was run identically to the 3-object version with the difference that each series consisted of four objects. Parameters of the task were held constant (i.e. 10-s intervals between each object presentation as well as between the end of the list and the recency judgments, and 30-s delays between each series). The addition of a fourth stimulus added recency judgments and resulted in six training phases as follows: Phase 1 (Objects 1– 4), Phase 2 (Objects 1–3), Phase 3 (Objects 1–2), Phase 4 (Objects 2– 4), Phase 5 (Objects 3–4), and Phase 6 (Objects 2–3). As was the case with 3-SOMT, the animals progressed through the different phases of the task by achieving 80% or better on any one day of testing, repeated the phase if they achieved 70%, and moved back one phase if they score 60% or poorer. The number of daily sessions for each phase, excluding the repeat phase, served to measure performance.
2.4.4 4-SOMT Probe Trials
One potential problem with the current version of the SOMT is that, because animals had to learn each discrimination (i.e. 1–4, 1–3, etc.) separately to criterion, they only had to track the order of a single object in a daily session of any given phase. To account for this possibility, the task was manipulated by a set of probe trials designed to require the animal to monitor the order of all objects within a single session. The task was administered in the same way as the 4-SOMT, except that half of the trials (5 trials) in the daily session were temporal judgments between Object 1 and Object 4 (as in Phase 1) and in the other half (5 trials) temporal judgments was between Object 2 and Object 3 (as in Phase 6). These two types of temporal judgments were randomized within the trials of a daily session so that the monkey would not know which discrimination problem would be presented on any one trial, requiring them to track all stimuli in each list. Probe trials were run for three consecutive days giving a total of 30 trials, 15 of each discrimination types. To control for performance differences between animals, cumulative number of correct choices for each type of discrimination was converted to a ratio score in which the total number of correct responses on Problems 2–3 was divided by the total number of correct responses on Problems1–4. Thus, a ratio score of 1 or near 1 would indicate equivalent performance on both problem types, whereas a ratio less, or greater, than 1 would indicate better performance on one type of discrimination than the other.
2.4.5 Behavioral Analysis
Given that the scores obtained for the two groups on the 3-SOMT and 4-SOMT tasks did not follow a normal distribution, nonparametric analyses were used. First, performance of the animals in the two groups varied depending of the pairings of the objects of the list, to test whether this variation was reliable we initially performed a Friedman analysis on each group separately. . When Friedman tests reached significance, post-hoc analysis (Wilcoxon rank sum test) were performed on each group separately to assess between which phases animals’ performance differed. Finally, group comparisons were made using a Mann-Whitney U test at each phase of the 3-SOMT and 4-SOMT tasks to determine if neonatal hippocampal lesions impaired the performance on any phase of both tasks.
3. Results
3.1 Lesion Extent
The results of the lesion reconstruction as evaluated with the FLAIR images are summarized in Table 1 for all cases. Cases Neo-H-ibo-2 and 3 had the most complete hippocampal lesions, averaging 67% and 87% damage to both hemispheres, respectively. Cases Neo-H-ibo-1, −4 and −5 had significant damage in one hemisphere (64%, 67% and 84%, respectively) but milder hippocampal damage in the other hemisphere (3%, 20.3% and 20.7%, respectively). Finally, the last case (Neo-H-ibo-6) had the smallest lesions, specifically located in the anterior portion of the hippocampus. As shown on Table 1, none of the cases had significant unintended damage to adjacent structures. This lesion estimate was also confirmed by measuring the percent hippocampal volume reduction measured on T1-W MR images taken 1 year after surgery (Table 2). Correlation between the two methods was r = 0.805, p = 0.05. The full extent of the hippocampal lesion in case Neo-H-ibo-2 is illustrated on axial MR images through the entire brain in Figure 1 (right column).
Table 1.
Lesion Extent as assessed from post-surgical FLAIR images
| Group |
Intended Damage |
Unintended Damage |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects |
Hippocampus |
Amygdala |
TH/TF |
|||||||||
| Neo-H-ibo | L% | R% | X% | W% | L% | R% | X% | W% | L% | R% | X% | W% |
| Neo-H-ibo-1 | 63.8 | 2.9 | 33.2 | 1.8 | 14.0 | 0.0 | 7.0 | 0.0 | 3.1 | 0.5 | 1.8 | 0.0 |
| Neo-H-ibo-2 | 54.4 | 80.9 | 67.6 | 44.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21.4 | 2.7 | 12.1 | 0.6 |
| Neo-H-ibo-3 | 78.5 | 96.3 | 87.4 | 75.6 | 1.7 | 0.0 | 0.8 | 0.0 | 6.1 | 5.5 | 5.8 | 0.3 |
| Neo-H-ibo-4 | 20.3 | 67.3 | 43.8 | 13.6 | 0.0 | 4.7 | 2.4 | 0.0 | 15.3 | 0.0 | 7.6 | 0.0 |
| Neo-H-ibo-5 | 20.7 | 84.0 | 52.6 | 17.5 | 0.0 | 4.9 | 2.5 | 0.0 | 6.1 | 4.0 | 5.1 | 0.2 |
| Neo-H-ibo-6 | 7.9 | 0.0 | 3.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mean | 40.9 | 55.2 | 48.0 | 25.4 | 2.6 | 1.6 | 2.1 | 0.0 | 8.6 | 2.1 | 5.4 | 0.1 |
Note: L% percent damage in the left hemisphere; R%: percent damage in the right hemisphere; X% average damage to both hemispheres; W%: weighted average damage to both hemispheres (W% = L% x R%)/100; weighted index as defined by Hodos and Bobko [50]). Mean: average damage per group; TH/TF: ventral cortical area of the temporal lobe as defined by Bonin and Bailey, 1947)
Table 2.
Volumetric Reduction of the Hippocampus
| Group |
Volume Reduction |
|||
|---|---|---|---|---|
| Subjects |
Hippocampus |
|||
| Neo-H-ibo | L% | R% | X% | % Remaining |
| Neo-H-ibo-1 | 27.6 | 10.6 | 19.1 | 80.8 |
| Neo-H-ibo-2 | 61.1 | 72.8 | 67.0 | 33.0 |
| Neo-H-ibo-3 | 54.7 | 47.8 | 51.3 | 48.7 |
| Neo-H-ibo-4 | 33.6 | 61.6 | 47.6 | 52.3 |
| Neo-H-ibo-5 | 49.1 | 64.0 | 56.6 | 43.4 |
| Neo-H-ibo-6 | 21.3 | 8.3 | 14.8 | 85.2 |
| Mean | 41.2 | 44.1 | 42.7 | 57.2 |
L% = Percent reduction of hippocampal volume and left hemisphere, R% = Percent reduction of hippocampal volume and right hemisphere, X% = Average percent reduction in both hemispheres and % Remaining = tissue still visible in the T1 despite hippocampal lesion
3.2 Behavioral Measures
3.2.1 3-SOMT
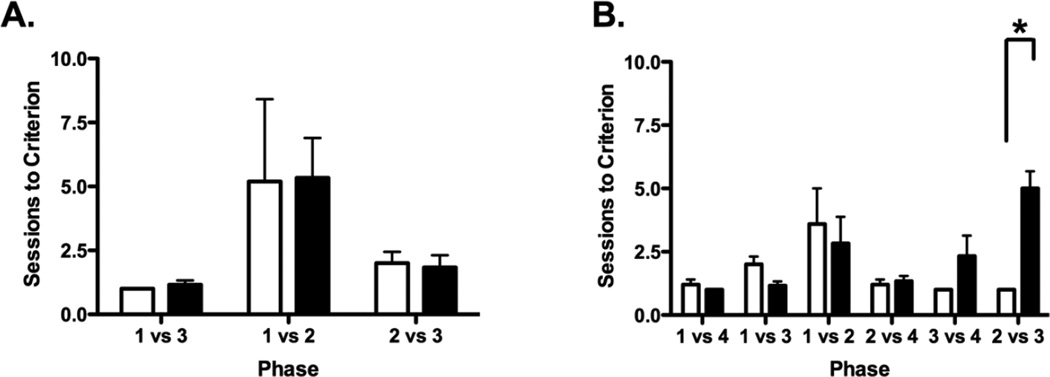
The results of the 3-SOMT for monkeys with neonatal lesions of the hippocampus and sham-operated controls are displayed in Figure 2A. All subjects readily met criterion within the allotted number of sessions (20 per phase), averaging 1, 5 and 2 sessions to criterion across the three phases of the 3-SOMT, respectively. Performance of both groups varied significantly across the three phases (Friedman: χ2(2) = 6.30, p < .05 and χ2(2) = 6.00, p < .05 for Groups Neo-C and Neo-H, respectively). Post-hoc analyses indicated that both Groups Neo-C and Neo-H-ibo took more sessions to reach criterion in Phase 2 than in Phase 1 (Wilcoxon: Z = 2.04, p < .05 and Z = 2.023, p < .05, respectively), although the performance difference between Phase 2 and Phase 3 did not reach significance for either group (all ps > .05). . In addition, group comparisons (Mann-Whitney U test) performed at each phase confirmed that animals with neonatal hippocampal lesions performed as well as controls in all three phases of the 3-SOMT (U = 18, 15, 22, all ps > .05 for Phases 12 and 3, respectively).
Figure 2.
Scores are Mean number of sessions (± SEM) to reach criterion (A) on the three phases (1 vs. 3, 1 vs. 2 and 2 vs 3) of the 3-SOMT and (B) the six phases (1 vs. 4, 1 vs. 3, 1 vs. 2, 2 vs. 4, 3 vs. 4, and 2 vs. 3) of the 4-SOMT for sham-operated controls (white bars) and monkeys with neonatal hippocampal lesions (black bars). * indicates p = 0.004.
3.2.2 4-SOMT
The results of the 4-SOMT for animals with neonatal lesions of the hippocampus and sham-operated controls are displayed in Figure 2B. As for the 3-SOMT, animals’ performance varied depending on the pairings used in the test phase and this variation was true for both groups [χ2(5) = 12.336, p = 0.03 and χ 2(4) = 17.991, p = 0.003 for Groups Neo-C and Neo-H-ibo, respectively]. Thus, control animals took more sessions to reach criterion on Phase 3 (Objects 1–2) as compared to all other phases, although this difference reached significance only for Phases 1, 2, and 6 [Z = 2.06, p < .04, Z = 1.84, p = .06 and Z = 2.06, p < .04, respectively]. This pattern of results shows that overall Phase 3, which includes pairings between the first 2 objects of the list, was the most difficult phase for the control group to complete. As control animals, animals with neonatal hippocampal lesions required more sessions to reach criterion on Phase 3, but they also took more trials to complete Phases 5 and 6 (see Fig. 2B). However, this difference in performance reached significance only for Phase 6 in which animals in Group Neo-H-ibo took more sessions to complete than Phases 1, 2, 4 and 5 [Z = 2.23, p < .03; Z = 2.23, p < .03; Z = 2.21, p < .03 and Z = 1.89, p = .058, respectively]. The greater difficulty animals with neonatal hippocampal lesions displayed in learning the pairings between the two inner objects of the list (Objects 2–3) was confirmed by subsequent group comparisons for each phase. Thus, Group Neo-H-ibo performed similarly to Group Neo-C in Phase 1 (1 vs 4;U = 21, p > .05), Phase 2 (1 vs 3; U = 24, p > .05), Phase 3 (1 vs 2; U =23, p > .05), Phase 4 (2 vs 4; U = 19, p > .05), and Phase 5 (3 vs 4; U = 12.5, p > .05). However, for temporal order discrimination between Object 2 versus Object 3 (Phase 6), Group Neo-H-ibo took significantly more sessions (average: 5) as compared to Group Neo-C (average: 1 session; U = 0, p = 0.002). Thus, animals with neonatal hippocampal lesions were selectively impaired in making temporal order judgments between objects positioned in the middle of the list (i.e. 2 vs 3), but not on those including either the first object or the last object of the list (i.e. 1–2, 1–3, 1–4, 2–4, 3–4).
3.2.3 4-SOMT Probe Trials
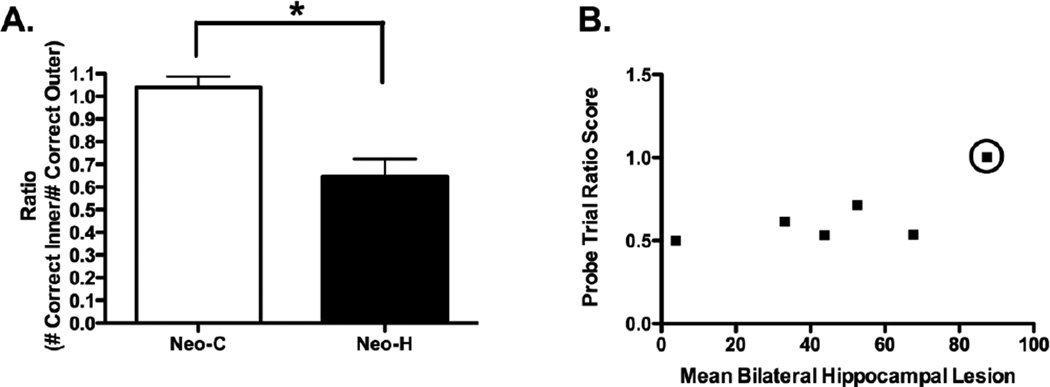
The results obtained from probe trials testing are shown in Figure 3A and confirmed the specificity of the temporal order memory impairment of animals with neonatal hippocampal lesions. Ratio scores for Group Neo-C averaged 1.04, indicating that control animals made as many correct choices on temporal order judgments between Objects 1 and 4 of the list than between Objects 2 and 3. By contrast, Group Neo-H-ibo obtained an averaged ratio score of 0.65, indicating that the animals made more correct choices on temporal order judgments between Objects 1 and 4 than between Objects 2 and 3. This group difference reached significance [U = 34, p = 0.01).
Figure 3.
(A) Averaged ratio of scores (± SEM) on the 4-SOMT probe trials for sham-operated controls (Group Neo-C, white bars) and monkeys with neonatal hippocampal lesions (Group Neo-Hibo, black bars). Scores are cumulative correct responses on the inner object discrimination (2 vs 3) divided by the number of correct responses on the outer discrimination (1 vs 4) across the three-day probe sessions. A score near one indicates equivalent performance on the two trial types, whereas a score below one indicates poor performance on the inner object discrimination. * indicates p = 0.002. (B) A graph of the correlation between the extent of hippocampal lesion and the ratio score earned on the 4-SOMT probe test (r = 0.820, p = 0.046), however it is clear that this correlation is driven by one individual (Neo-H-3 highlighted with a circle), and this correlation does not remain when this animal is removed from the analysis (r = 0.698, p = 0.199).
3.3 Correlation of Lesion and Behavior
To examine the relationship between lesion extent and memory performance a correlation analysis was performed using all behavioral measures and the extent of damage to the hippocampus as measured from the FLAIR images. The only significant correlation detected was between amount of bilateral damage to the hippocampus and the ratio score on the probe trials (r = 0.820, p = 0.046)s. However, as illustrated in Figure 3B, this correlation was mostly driven by ratio score in case Neo-H-3, which approached 1.0 as for the control animals, despite that this case had the largest hippocampal lesion of the group (Neo-H-3 is identified with a circle in Figure 3B). Thus, this positive correlation did not reach significance when this animal was removed from the analysis (r = 0.698, p = 0.199).
4. Discussion
The goal of the current investigation was to assess the effects of neonatal hippocampal lesions on serial order memory in monkeys. The data showed that for both the 3-SOMT and 4-SOMT tasks, control animals took more sessions to acquire pairings using the first two objects (1–2) of the list. Interestingly, the neonatal lesions of the hippocampus spared performance on the 3-SOMT task, indicating normal memory for unique sequential non-spatial events. This sparing was still present in the 4-SOMT task during which the animals with neonatal hippocampal lesions performed similarly to controls in all pairings except when the pairings included the two middle items of the list (2–3). This specific impairment was further confirmed when, after reaching criterion on the 4-SOMT task, animals’ performance was probed by tests that included either the two middle items of the list (2–3, inner pairings) or the first and last items of the list (1–4, outer pairings). In these probe tests, control animals showed equal performance for the inner or outer pairings, whereas animals with neonatal hippocampal lesions demonstrated better performance on the outer than on the inner pairings. The impairment in monitoring working memory in the serial order task in animals with neonatal hippocampal lesions parallels a similar impairment in a self-order memory task [1], although the magnitude of the impairment was milder after neonatal hippocampal lesions than after adult-onset dorsolateral prefrontal cortex lesions. As will be discussed below, the pattern of results indicates disruption of working memory processes known be mediated by the dorsolateral prefrontal cortex or its functional interactions with the hippocampus.
4.1 Memory for the order of elements and the hippocampus
Growing evidence supports a role of the hippocampus in the retrieval for the order of elements. Electrophysiology studies in rodents have shown that hippocampal neuronal activity reflects processing of the order of events in both spatial [28, 29] and nonspatial episodes [30] and hippocampal activation or lesion in humans has also been related to memory for the order of elements [31–34]. Further, lesion studies in rodents demonstrated impaired memory for the order of associated elements that compose an episode after selective hippocampal damage [14, 15, 17–19, 35]. The impairment in temporal order judgments observed was global affecting pairings of any two items of the list. Additionally, although no studies to date have examined the effects of adult hippocampal lesions in monkeys on the serial order memory task used in the present study, Charles and colleagues [36] showed that fornix transection in adult macaques impaired recency memory. Both of these findings contrast with the temporal order memory impairment present only for pairings with the inner items (2–3) of the list in monkeys with neonatal hippocampal lesions. The present findings also demonstrate that recency memory was intact following neonatal damage to the hippocampus and suggest that the nature of the impairment in animals with neonatal hippocampal lesions differs from the impairment found after adult-onset damage to the hippocampal system. One obvious difference between the earlier studies and this one relates to the timing of the lesions, which were done in adult animals in the previous reports but in infancy in the current study. Neural reorganization that follows neonatal brain lesions could have allowed the use of alternate strategies to solve the task that may depend on brain areas other than the hippocampus. As already alluded to by Petrides [3], good performance on the task could be accomplished by remembering the two endpoint items, i.e. Objects 1 and 3 of the 3-SOMT and Objects 1 and 4 for the 4-SOMT. Indeed, the presence of strong memories for the endpoints of serial order memory task has already been demonstrated in primates, rodents and pigeons [16, 37]. Thus, using this strategy, animals with neonatal hippocampal lesions could maintain normal performance on all pairings of the 3-SOMT and all pairings but one (2–3) of the 4-SOMT. However, performance on pairings 2–3 of the 4-SOMT can be accomplished only if animals remember the temporal order of each item in the list. Thus, the findings provide additional evidence that the hippocampus is involved in memory for temporal order. This conclusion, however, will need to be confirmed by a direct investigation of the effects of adult-onset hippocampal lesions on the serial order memory task.
It is worth noting here that the unimpaired performance of case Neo-H-3 is particularly puzzling as this animal was not impaired on any component of serial order memory despite having the larger hippocampal lesion of the group and had already shown no impairment on monitoring working memory processes, also in contrast to the impairment found in the remaining animals of the lesion group [1]. A thorough examination of the animals’ lesion and behavioral data provided no potential explanation for why this single animal was so different than the rest of the lesion group. Interestingly, this animal had no impairment on two independent measures of monitoring working memory processes despite showing similar deficits as the rest of the group for incidental recognition memory [22] and memory for object-place associations [38, 39]. One possible explanation for the temporal order memory deficit found in five animals with incomplete hippocampal lesions is that an abnormal functioning of the spared hippocampal tissue may have had the potential of altering functions of other brain regions [40]. Another possibility may relate to the use of alternative strategies that this animal may have developed to solve the task, as alluded to in earlier reports [41, 42].
4.2 Memory for the order of elements and the lateral prefrontal cortex
Substantial evidence also exists for a critical role of the lateral prefrontal cortex in memory for the temporal order of events [3, 18, 43–45] and neurons in this prefrontal area fired selectively to items within a specific sequence that predicts behavioral judgments of temporal order [46, 47]. In the original serial order memory study by Petrides [3], the author demonstrated that focal mid-dlPFC lesions did not impair performance on the 3-SOMT, but did impair performance on the 4-SOMT. As found in monkeys with neonatal hippocampal lesions, the temporal order judgment deficit was specifically restricted to comparisons between the middle items of the list (e.g. 2 and 3) and not for all comparisons that included endpoint items of the list (e.g. 1–2, 1–3, 1–4, 2–4, 3–4). Despite similar pattern of sparing and impairment in the 3-SOMT and 4-SOMT tasks after the neonatal hippocampal and mid-dlPFC lesions, it is worth noting that the magnitude of impairment found in temporal order judgments for items 2 and 3 of the list differed between the two types of lesions. Specifically, unlike monkeys with adult lesions of the mid-dlPFC that never met criterion in the 2–3 pairings, those with neonatal hippocampal lesions took longer to learn, but eventually met criterion.
One potential explanation for this difference may lie in the ability of animals with neonatal lesions to develop an alternative strategy to solve the 4-SOMT task. Because the testing in the 4-SOMT required the animals to meet criterion successively for each pairings (i.e. learn first pairings 1–4 and then 1–3 and so on), animal could develop the strategy to pay attention to and remember only one object of the list for each discrimination. This strategy would allow the subject to successfully complete any discrimination, including the most difficult discrimination containing only inner (2 vs 3) objects. To test this possibility, we employed probe tests in which animals had to attend to all objects in the series as they did not know which recency discrimination they would face on any particular trial. As a result, while normal animals completed outer (1 vs 4) and inner (2 vs 3) object discriminations with equal accuracy, Neo-Hibo fell to chance on inner object discriminations, indicating an impairment in temporal order memory similar to that following the dorsolateral prefrontal lesions. Thus, the similarity in the pattern of sparing and impairment in temporal order memory may further suggest that the temporal order memory deficit found in monkeys with neonatal hippocampal lesions could also have resulted from a dysfunction of the dorsolateral prefrontal cortex and its interactions with the hippocampus. Interestingly, preliminary findings from a recent resting state fMRI study performed on some animals of the present study [48] have indicated that functional connectivity between the hippocampus and the DLPFC was significantly affected by neonatal hippocampal lesions.
4.3 Conclusions
The present results demonstrate that neonatal hippocampal lesions in monkeys yield significant deficits in memory for the temporal order of elements that required the monitoring of information in working memory. The results further suggest that the deficits may relate to an alteration of hippocampal-prefrontal interactions; a proposal that is currently being tested with neuroimaging studies, and ultimately by direct postmortem anatomical investigation of the brain of these animals. Thus, the impairment following neonatal hippocampal lesions in monkeys in incidental recognition memory [22], spatial memory [38, 39, 49], pre-pulse inhibition, as well as working memory processes [1] parallel similar impairments reported in rodents with neonatal hippocampal lesions. Given that the constellation of behavioral and cellular changes described in the rodent model mimics many aspects of schizophrenia, the data in monkeys provide a potential nonhuman primate model for translational research in schizophrenia.
Highlights.
Serial order memory was assessed in adult monkeys with neonatal hippocampal lesions
Early hippocampal lesions were found to impair serial order memory
This data suggests a role for the hippocampus in dlPFC function and/or development
Acknowledgements
This work was supported by grants from NIMH (MH-58846), NICHD (HD-35471), the Yerkes Base Grant NIH RR00165 (currently supported by the Office of Research Infrastructure Programs/OD P51OD11132), and the Center for Behavioral Neuroscience grant NSF IBN-9876754 to JB, as well as from a NIMH (T32-MH0732505) predoctoral fellowship to EH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heuer E, Bachevalier J. Neonatal hippocampal lesions in rhesus macaques alter the monitoring, but not maintenance, of information in working memory. Behav Neurosci. 2011;125:859–870. doi: 10.1037/a0025541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrides M. Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. J Neurosci. 1995;15:359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrides M. Functional specialization within the dorsolateral frontal cortex for serial order memory. Proc Biol Sci. 1991;246:299–306. doi: 10.1098/rspb.1991.0158. [DOI] [PubMed] [Google Scholar]
- 4.Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- 5.Bertolino A, Roffman JL, Lipska BK, van Gelderen P, Olson A, Weinberger DR. Reduced N-acetylaspartate in prefrontal cortex of adult rats with neonatal hippocampal damage. Cereb Cortex. 2002;12:983–990. doi: 10.1093/cercor/12.9.983. [DOI] [PubMed] [Google Scholar]
- 6.Lipska BK, Aultman JM, Verma A, Weinberger DR, Moghaddam B. Neonatal damage of the ventral hippocampus impairs working memory in the rat. Neuropsychopharmacology. 2002;27:47–54. doi: 10.1016/S0893-133X(02)00282-8. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- 8.Endo K, Hori T, Abe S, Asada T. Alterations in GABA(A) receptor expression in neonatal ventral hippocampal lesioned rats: comparison of prepubertal and postpubertal periods. Synapse. 2007;61:357–366. doi: 10.1002/syn.20393. [DOI] [PubMed] [Google Scholar]
- 9.Friedman HR, Goldman-Rakic PS. Activation of the hippocampus and dentate gyrus by working-memory: a 2-deoxyglucose study of behaving rhesus monkeys. J Neurosci. 1988;8:4693–4706. doi: 10.1523/JNEUROSCI.08-12-04693.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 11.Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci. 2006;26:4596–4601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunsaker MR, Fieldsted PM, Rosenberg JS, Kesner RP. Dissociating the roles of dorsal and ventral CA1 for the temporal processing of spatial locations, visual objects, and odors. Behav Neurosci. 2008;122:643–650. doi: 10.1037/0735-7044.122.3.643. [DOI] [PubMed] [Google Scholar]
- 14.Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116:286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- 15.Kesner RP, Hunsaker MR, Ziegler W. The role of the dorsal CA1 and ventral CA1 in memory for the temporal order of a sequence of odors. Neurobiol Learn Mem. 2010;93:111–116. doi: 10.1016/j.nlm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Kesner RP, Novak JM. Serial position curve in rats: role of the dorsal hippocampus. Science. 1982;218:173–175. doi: 10.1126/science.7123228. [DOI] [PubMed] [Google Scholar]
- 17.Lee I, Jerman TS, Kesner RP. Disruption of delayed memory for a sequence of spatial locations following CA1- or CA3-lesions of the dorsal hippocampus. Neurobiol Learn Mem. 2005;84:138–147. doi: 10.1016/j.nlm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Devito LM, Eichenbaum H. Memory for the order of events in specific sequences: contributions of the hippocampus and medial prefrontal cortex. J Neurosci. 2011;31:3169–3175. doi: 10.1523/JNEUROSCI.4202-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barker GR, Warburton EC. Evaluating the neural basis of temporal order memory for visual stimuli in the rat. Eur J Neurosci. 2011;33:705–716. doi: 10.1111/j.1460-9568.2010.07555.x. [DOI] [PubMed] [Google Scholar]
- 20.Heuer E, Bachevalier J. Modulation of serial order memory following neonatal lesions of the hippocampus in the rhesus macaque. Society for Neuroscience Abstracts Online Washington DC. 2008;791:2. On Line. [Google Scholar]
- 21.Goursaud AP, Bachevalier J. Social attachment in juvenile monkeys wth neonatal lesion of the hippocampus, amygdala and orbitofrontal cortex. Behav Brain Res. 2007;176:75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J Neurosci. 2010;30:9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heuer E, Bachevalier J. Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys. Behav Neurosci. 2011;125:137–149. doi: 10.1037/a0022539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. J Neurosci Methods. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- 25.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 26.Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: A volumetric magnetic resonance imaging study. Hippocampus. 2009 doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeamer AE, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. J Neurosci. doi: 10.1523/JNEUROSCI.0022-10.2010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 29.Foster DJ, Wilson MA. Hippocampal theta sequences. Hippocampus. 2007;17:1093–1099. doi: 10.1002/hipo.20345. [DOI] [PubMed] [Google Scholar]
- 30.Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumaran D, Maguire EA. An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol. 2006;4:e424. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehn H, Steffenach HA, van Strien NM, Veltman DJ, Witter MP, Haberg AK. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci. 2009;29:3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross RS, Brown TI, Stern CE. The retrieval of learned sequences engages the hippocampus: Evidence from fMRI. Hippocampus. 2009;19:790–799. doi: 10.1002/hipo.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayes AR, Isaac CL, Holdstock JS, Hunkin NM, Montaldi D, Downes JJ, et al. Memory for single items, word pairs, and temporal order of different kinds in a patient with selective hippocampal lesions. Cogn Neuropsychol. 2001;18:97–123. doi: 10.1080/02643290125897. [DOI] [PubMed] [Google Scholar]
- 35.Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charles DP, Gaffan D, Buckley MJ. Impaired recency judgments and intact novelty judgments after fornix transection in monkeys. J Neurosci. 2004;24:2037–2044. doi: 10.1523/JNEUROSCI.3796-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sands SF, Wright AA. Primate memory: retention of serial list items by a rhesus monkey. Science. 1980;209:938–940. doi: 10.1126/science.6773143. [DOI] [PubMed] [Google Scholar]
- 38.Blue SN, Kazama AM, Bachevalier J. Neuroscience Meeting Planner. Vol. 98. Washington DC: Society for Neuroscience Online; 2009. The normal development of object-place association memory is altered by neonatal hippocampal lesions in rhesus monkeys; p. 7. [Google Scholar]
- 39.Glavis-Bloom C, Alvarado MC, Bachevalier J. Neonatal hippocampal damage impairs specific food/place associations in adult macaques. Behav Neurosci. doi: 10.1037/a0031498. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bachevalier J, Meunier M. Cerebral ischemia: are the memory deficits associated with hippocampal cell loss? Hippocampus. 1996;6:553–560. doi: 10.1002/(SICI)1098-1063(1996)6:5<553::AID-HIPO8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 41.Kimble DP, Pribram KH. Hippocampectomy and behavior sequences. Science. 1963;139:824–825. doi: 10.1126/science.139.3557.824. [DOI] [PubMed] [Google Scholar]
- 42.Waxler M, Rosvold HE. Delayed alternation in monkeys after removal of the hippocampus. Neuropsychologia. 1970;8:137–146. doi: 10.1016/0028-3932(70)90001-1. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell JB, Laiacona J. The medial frontal cortex and temporal memory: tests using spontaneous exploratory behaviour in the rat. Behav Brain Res. 1998;97:107–113. doi: 10.1016/s0166-4328(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 44.Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 45.Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ninokura Y, Mushiake H, Tanji J. Representation of the temporal order of visual objects in the primate lateral prefrontal cortex. J Neurophysiol. 2003;89:2868–2873. doi: 10.1152/jn.00647.2002. [DOI] [PubMed] [Google Scholar]
- 47.Averbeck BB, Lee D. Prefrontal neural correlates of memory for sequences. J Neurosci. 2007;27:2204–2211. doi: 10.1523/JNEUROSCI.4483-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li C, Li Z, Hu X, Bachevalier J, Zhang X. Quantitative functional connectivity between the hippocampus and dorsolateral prefrontal cortex is altered in a neonatal hippocampal lesion macaque model; ISRM 20th Annual Meeting; Melbourne, Australia. 2012. [Google Scholar]
- 49.Glavis-Bloom C, Alvarado MC, Bachevalier J. Neuroscience Meeting Planner. Vol. 574. Washington DC: Society for Neuroscience Online; 2006. Neonatal hippocampal damage impairs specific place/food associations in adult macaques; p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hodos W, Bobko P. A weighted index of bilateral brain lesions. J Neurosci Methods. 1984;12:43–47. doi: 10.1016/0165-0270(84)90046-3. [DOI] [PubMed] [Google Scholar]