SUMMARY
The immediate early gene NARP is an AMPAR binding protein that is specifically enriched at excitatory synapses onto fast-spiking parvalbumin-positive interneurons (FS (PV) INs). Here we show that transgenic deletion of NARP decreases the number of excitatory synaptic inputs onto FS (PV) INs, and reduces net excitatory synaptic drive onto FS (PV) INs. Accordingly, the visual cortex NARP −/− mice is hyper-excitable, and unable to express ocular dominance plasticity, although many aspects of visual function are unimpaired. Importantly, the number and strength of inhibitory synaptic contacts from FS (PV) INs onto principle neurons in the visual cortex is normal in NARP −/− mice, and enhancement of this output recovers the expression of experience-dependent synaptic plasticity. Thus the recruitment of inhibition from FS (PV) INs plays a central role in enabling the critical period for ocular dominance plasticity.
INTRODUCTION
Understanding the processes that initiate and terminate critical periods for receptive field plasticity is a subject of intense investigation. The initiation of the critical period for ocular dominance plasticity is widely believed to be triggered by the maturation of inhibitory synapses targeting the somata of principal neurons in the visual cortex (Hensch et al., 1998; Huang et al., 1999; Di Cristo et al., 2007). Increased perisomatic inhibition would reduce excitability in principal neurons, enabling mechanisms of activity-dependent synaptic plasticity to discriminate between inputs from the two eyes (Jiang et al., 2007; Toyoizumi and Miller, 2009; Kuhlman et al., 2010). The activation of inhibitory GABA receptors would also limit activity at NMDA receptors and restrict subsequent induction of synaptic plasticity at excitatory synapses onto principal neurons (Kirkwood and Bear, 1994; Rozas et al., 2001; Artola and Singer, 1987; Jang et al., 2009).
The evidence supporting the idea that maturation of inhibition determines the timing of the critical period is based on experimental manipulations of inhibitory output. For example, promotion of the early maturation of inhibitory synapses onto principle neurons induces a precocious initiation of the critical period (Huang et al., 1999; Di Cristo et al., 2007; Sugiyama et al., 2008). Similarly, premature expression of ocular dominance plasticity is enabled by enhancement of inhibitory output with diazepam, a positive allosteric modulator of ligand-bound GABAA receptors (Seighart, 1995; Fagiolini and Hensch, 2000). Conversely, direct or indirect reduction of the strength of inhibitory output restores ocular dominance plasticity in post-critical period adults (He et al., 2006; Sale et al., 2007; Harazouv et al., 2010). However, recent evidence suggests a disconnection between the maturation of inhibitory output and the termination of the critical period for ocular dominance plasticity (Huang et al., 2010). The maturation of perisomatic inhibition, characterized by a plateau in inhibitory synaptic density, IPSC amplitudes and the loss of endocannabinoid-dependent iLTD, reaches adult levels ~ postnatal day 35 (P35) in the rodent visual cortex (Morales et al., 2002; Huang et al., 1999; Di Cristo et al., 2007; Jiang et al., 2010). Nonetheless, robust juvenile-like ocular dominance plasticity persists beyond P35 (Sawtell et al., 2003; Fischer et al., 2007; Heimel et al., 2007; Lehmann and Lowel, 2008; Sato and Stryker, 2008). Importantly, enhancing inhibitory output with diazepam blocks ocular dominance plasticity in late postnatal development (Huang et al., 2010). This suggests that inhibitory synapses are functional at this age, but are not efficiently recruited by visual experience.
The possibility that the recruitment of inhibitory circuitry might control the timing of the critical period for ocular dominance plasticity prompted us to examine the regulation of excitatory inputs onto interneurons in the visual cortex. We focused specifically on the recruitment of inhibition mediated by FS (PV) INs, which mediate the majority of perisomatic inhibition, and therefore exert powerful control of neuronal spiking output. We studied mice lacking the gene for NARP (neuronal activity-regulated pentraxin a.k.a. NP2) an immediate early gene that is rapidly expressed in the visual cortex in response to light exposure following dark adaptation (Tsui et al., 1996). NARP is a calcium-dependent lectin that is secreted by pyramidal neurons, and accumulates at excitatory synapses onto FS (PV) INs where it forms an AMPAR-binding complex with NP1 and NPR (O’Brien et al., 1999; Xu et al., 2003; Chang et al., 2010). NARP accumulation onto FS (PV) INs is inhibited by degradation of the proteoglycans of the perineuronal net (Chang et al., 2010), a manipulation previously shown to enhance ocular dominance plasticity in adults (Pizzorusso et al., 2002; 2006). Importantly, NARP −/− mice are unable to scale EPSCs onto FS (PV) INs in response to changes in synaptic activity (Chang et al., 2010), demonstrating the importance of NARP in activity-dependent plasticity at these synapses.
NARP −/− mice therefore provide a unique opportunity to examine how excitatory drive onto FS (PV) INs contributes to the timing of the critical period for ocular dominance plasticity. We found that NARP −/− mice have a reduction in the number of excitatory synaptic inputs onto FS (PV) INs, while inhibitory synapses onto pyramidal neurons are unchanged. The reduction in excitatory drive onto FS (PV) INs renders the visual cortex of NARP −/− mice hyper-excitable, and unable to express ocular dominance plasticity. Nonetheless, other forms of synaptic plasticity, which are prominent in the pre-critical stage of development, are normal in NARP −/− mice. Importantly, ocular dominance plasticity can be triggered at any age in NARP −/− mice by enhancing inhibitory output with diazepam. Thus the ability to recruit inhibition, rather than the strength of inhibitory synapses, plays a central role in the initiation of the critical period for ocular dominance plasticity.
RESULTS
Reduced excitatory drive onto FS (PV) INs in NARP −/− mice
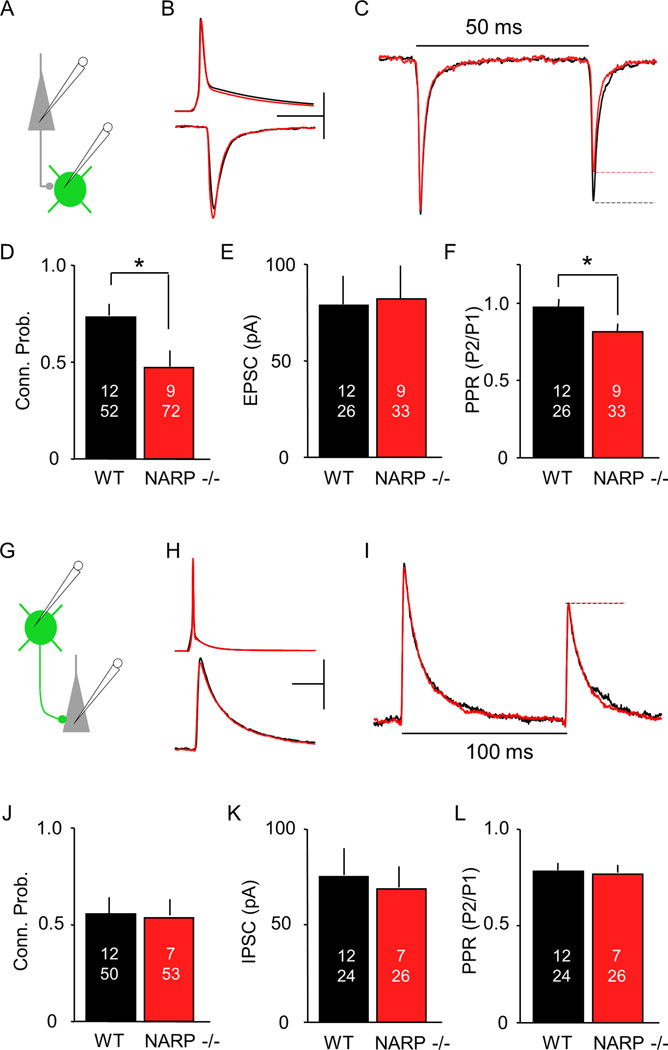
To ask how the absence of NARP impacted excitatory synaptic drive onto inhibitory interneurons, we crossed NARP −/− mice with G42 mice, which express GFP in fast spiking, parvalbumin positive interneurons ((FS (PV) INs); Jiang et al., 2010). Unitary excitatory postsynaptic currents (uEPSCs) were recorded pairs of pyramidal (Pyr) and FS (PV) interneurons from layer II/III in slices of visual cortex prepared from three week old (postnatal day 21 – 25) NARP −/− and age-matched wild type mice (Fig 1A, B). In the absence of NARP, the probability of connectivity between any Pyr->FS (PV) IN pair was significantly reduced (connection probability average ± SEM: NARP−/− 0.47±0.06, n = 9 mice, 72 pairs; WT 0.73±0.06, n=12, 52; p= 0.0007, Fisher exact test; Fig 1D). However, in connected pairs, the uEPSC amplitude was normal (NARP−/− 82.2±16.3 pA, n = 9, 33; WT 72.0±13.0%, n=10, 35; p=0.62, t-test; Fig 1B, E). Importantly, the absence of NARP did not affect connectivity from FS (PV) INs onto pyramidal cells (Fig 1G–L). No differences were detected between wild type and NARP−/− mice in either the probably of connectivity (p=0.20; Fig 1J), the amplitude of the unitary IPSC evoked by direct depolarization of the FS (PV) IN (p=0.69; Fig 1K) or the paired pulse response ratio (p=0.83; Fig 1L). Thus, the absence of NARP specifically reduced the connectivity from pyramidal neurons onto FS (PV) INs, while the connectivity from FS (PV) IN onto pyramidal neurons was unimpaired.
Fig 1. Altered connectivity between layer II/III pyramidal neurons and FS (PV) INs in the visual cortex of NARP −/− mice.
A–F. uEPSCs in FS (PV) IN (green) evoked by action potentials in a nearby pyramidal neuron (grey). A. Experimental schematics B. Average of all responses recorded in connected Pyr->FS (PV) IN pairs (20 responses per pair) from P21-P25 NARP−/− (red) and age-matched wild type mice (black). Top: Action potentials in pyramidal neurons: bottom: uEPSCs in FS (PV) INs. Calibration Bar: 50 mV, 50 pA, 10 msec. C. Average uEPSC evoked with paired pulse stimulation, normalized to 1st uEPSC (NARP−/−: red, WT: black). D-F. Effects of NARP deletion on the probability of finding a connected pair (D), the uEPSC amplitude (E), and the paired pulse response ratio (F). G-L. uIPSCs in pyramidal neuron (grey) evoked by action potentials in a nearby FS (PV) IN (green). G. Experimental schematics H. Average of all responses recorded in connected FS (PV) IN -> Pyr neuron pairs (20 responses per pair). Top: Action potentials in FS (PV) INs; bottom: uIPSCs in pyramidal neurons. Calibration Bar: 40 mV, 40 pA, 10 msec. I. Average uIPSC evoked with paired pulse stimulation, normalized to 1st uIPSC (NARP−/−: red, WT: black). J-L. Effects of NARP deletion on the probability of finding a connected pair (J), the uIPSC amplitude (K), and the paired pulse response ratio (L). The number of mice and cell pairs is presented in each bar. *=p<0.01, t-test
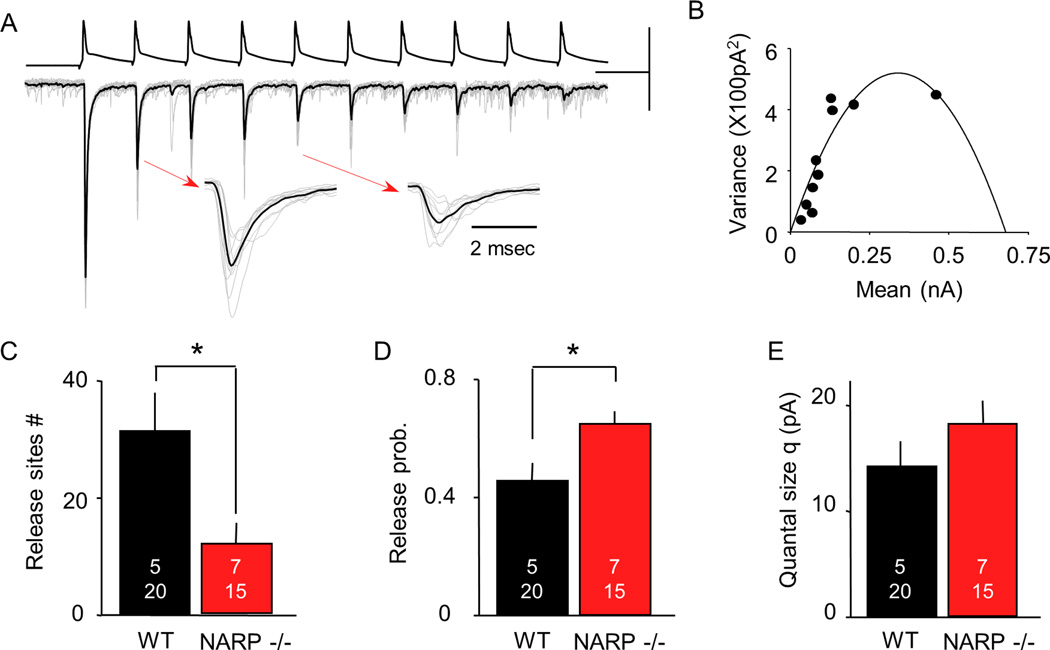
As a first estimation of neurotransmitter release probability, we examined the paired-pulse response ratio (PPR) of the uEPSCs in Pyr->FS (PV) IN pairs. We found that the PPR was decreased in NARP −/− mice (NARP−/− 0.80±0.04, n = 4, 17; WT 0.99±0.05, n=10, 35; p= 0.007, t-test; Fig 1C, F), suggesting that the excitatory synapses that persist may have enhanced presynaptic function. This prompted us to ask if the absence of NARP affects quantal parameters such as quantal size (Q), the number of presynaptic release sites (N) and the presynaptic release probability (P) at the remaining Pyr ->FS (PV) IN synapses. To obtain these parameters, we performed a mean- variance analysis of the uEPSC evoked by 50 Hz trains of 5 or 10 action potentials in the pyramidal neuron, as described (Fig 2A; Scheuss et al 2001; Huang et al 2010). This analysis allows quantal parameters (N, P, Q) to be estimated from the parabola fit to the relationship between mean and variance of the uEPSCs within the train (Fig 2B, see methods). We first tested the validity of this approach by increasing extracellular [Ca2+] from 2 mM to 4 mM. As expected, this resulted in an increase in the magnitude of the uEPSC (paired t-test: p=0.008, n=6 pairs) that was associated with an increase in release probability (p<0.001), but no change in quantal size (p=0.307) or the number of release sites (p=0.426). Alternatively, the addition of a low dose of the glutamate receptor antagonist kynurenic acid (200 mM) resulted in a decrease the magnitude of the uEPSC (paired t-test: p=0.039; n=6 pairs) that was associated with a decrease quantal size (p=0.008), but no change in release probability (p=0.807) or the number of release sites (p=0.722; Supp Fig 1). Application of the mean-variance approach to Pyr->FS (PV) IN uEPSCs in NARP −/− mice (postnatal day 21– 25) revealed a decrease in the number of presynaptic release sites (N; NARP−/− 11.8±2.0, n = 7,15; WT 31.5±7.1, n=5, 205; p=0.016, t-test; Fig 2C) associated with an increase in presynaptic release probability (P; NARP−/− 0.66±0.05, n = 7,15; WT 0.46±0.06, n=5, 20; p=0.010, t-test; Fig 2D), but no change in quantal size (Q: NARP−/− 18.2±2.4, n = 7.15; WT 14.2±2.3, n=5, 20; p=0.231, t-test; Fig 2E). Together, this demonstrates a net reduction in the excitatory drive onto FS (PV) INs in the visual cortex of NARP−/− mice.
Fig 2. NARP deletion reduces the number of release sites and increases the release probability at Pyr to FS (PV) IN connections.
A. Representative experiment illustrating the estimation of synaptic parameters through a mean variance analysis of uEPSCs evoked by 50 Hz trains (of 10 pulses). 15 consecutive trials (grey) are superimposed, along with averaged response (black). Scale bars: 200 mV, 200 pA, 20 msec. Expanded uEPSCs, indicated by arrows, were evoked by the 2nd and 5th pulse of the trains. B. The relationship between mean uEPSC amplitude and variance for each of the 10 uEPSCs within the train was fitted with a parabola. C-E. Synaptic parameters estimated from the parabolic fit (see methods) in NARP−/− (red) and WT mice (black) include the number of release sites (C), the release probability (D), and the quantal size (E). The number of mice and cell pairs is presented in each bar. *=p<0.01, t-test.
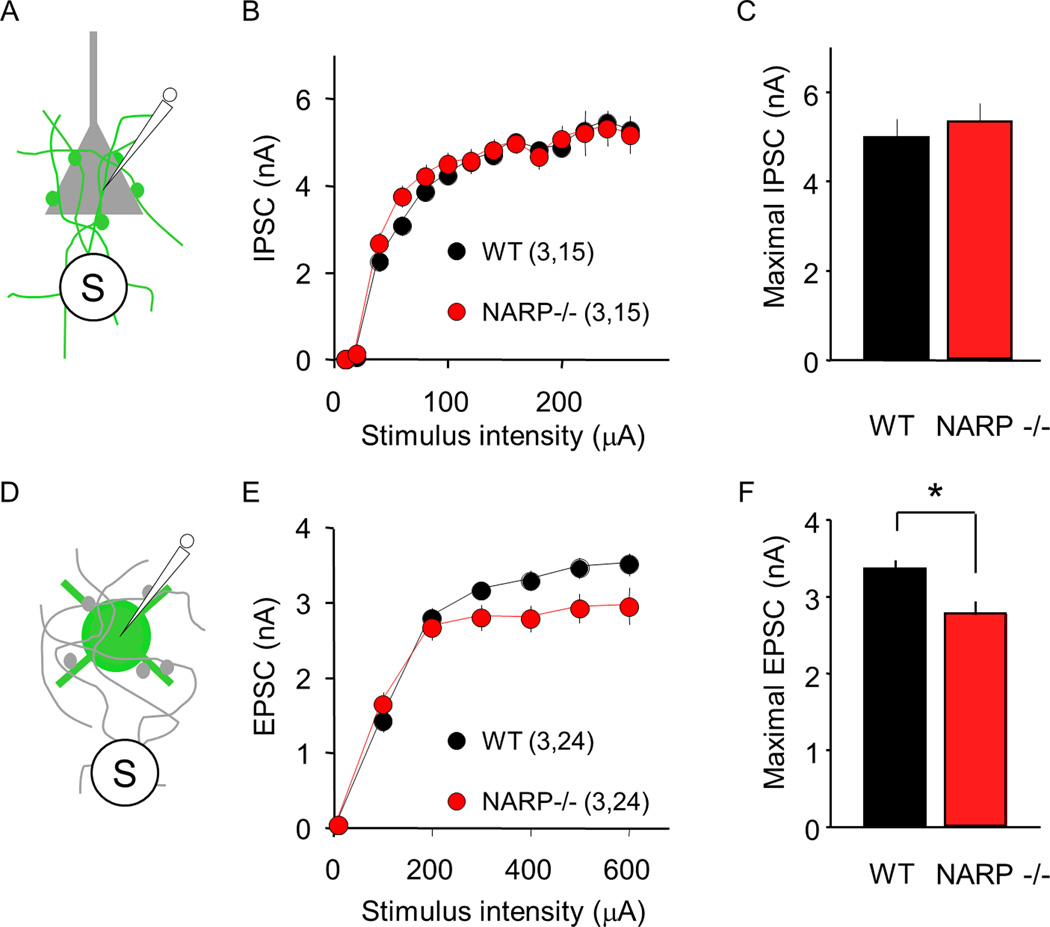
To ask how the reduction in excitatory input from proximal pyramidal neurons onto FS (PV) INs impacts total functional excitatory input or inhibitory output, we examined the maximal, extracellularly-evoked IPSC in pyramidal neurons (eIPSC; Fig 3A–C), and the maximal extracellularly-evoked EPSC in FS (PV) IN (eEPSC; Fig 3D–F). This allows an estimation of the combined strength of all available inputs, which we have previously used to characterize developmental changes in the strength of inhibition onto pyramidal neurons (Huang et al., 1999; Morales et al., 2002; Jiang et al., 2007; Huang et al., 2010). In these experiments, the stimulating electrode was placed in layer IV, which effectively recruits horizontal inputs onto layer II/III neurons (Morales et al., 2002). These experiments were performed at postnatal day 35 (± 2 days), when the maturation of inhibitory output is complete in wild types. In pyramidal neurons we observed a similar input/output relationship for the eIPSC in NARP−/− and wild type mice (one way ANOVA, F1,335= 0.16, p=0.689; Fig 3B) and similar amplitude of the maximal eIPSC (NARP−/− 5.4±0.4 pA, n = 3,15; WT 5.2±0.4, n=3, 15; p=0.5, t-test; Fig 3C). In contrast, the input/output relationship for the eEPSC was significantly different in NARP −/− and wild type mice (one way ANOVA, F1,299=10.93, p=0.0011; Fig 3E), and the amplitude of the maximal eEPSC was significantly reduced (NARP−/− 3.35±0.12 pA, n = 3, 24; WT 2.76±0.17, n=3, 24; p=0.010, t-test; Fig 3F). Thus the absence of NARP decreased the strength of total excitatory drive onto FS (PV) INs, without affecting the strength of inhibitory output evoked by depolarization of FS (PV) INs.
Fig 3. Normal inhibitory input onto pyramidal cell but reduced excitatory input onto FS (PV) INs in NARP−/− mice.
A–C. Extracellularly-evoked IPSCs (eIPSCs) recorded in pyramidal neurons are normal in P35 NARP−/− mice. A. Pharmacologically-isolated eIPSCs were recorded in layer II/III pyramidal neurons, evoked by extracellular stimulation of the underlying layer IV. B. Input-output relationship for eIPSCs in NARP−/− (red) and WT controls (black). C. Maximal IPSC computed by averaging eIPSC amplitudes evoked by the 3 largest stimulus intensities. D–F. Extracellularly- evoked EPSCs (eEPSCs) recorded in FS (PV) INs are reduced in P35 NARP−/− mice. D. Pharmacologically-isolated eEPSCs were recorded in layer II/III FS (PV) INs, evoked by extracellular stimulation of the underlying layer IV. E. Input-output relationship for eEPSCs in NARP−/− (red) and WT controls (black). F. Maximal EPSC computed by averaging eEPSC amplitudes evoked by the 3 largest stimulus intensities. Number of mice and neurons in parentheses in B and E. *=p<0.02; t-test.
Hyper-excitable visual cortex in NARP −/− mice
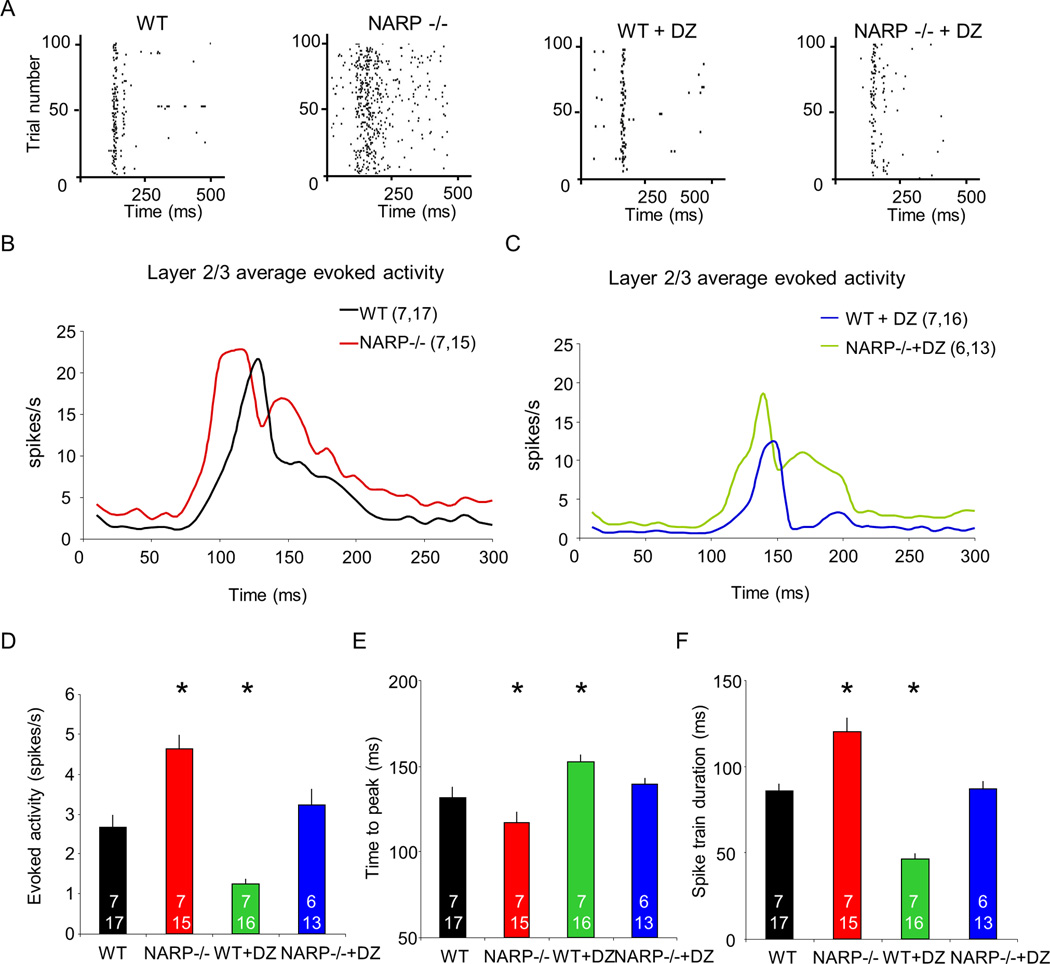
We predicted that the decrease in excitatory drive from pyramidal neurons to FS (PV) INs in NARP −/− mice would reduce the ability to recruit fast perisomatic inhibition and increase overall cortical excitability. To test this hypothesis, we examined single unit spiking output in the binocular region of the primary visual cortex of P28 mice in vivo. In NARP −/−mice, visually-evoked activity of neurons in layer II/III (response to 1 Hz reversals of 0.04 cycles/degree; 100% contrast gratings; presented at preferred orientation) had a larger average spike rate (median evoked activity ± SEM (spikes/second): WT 2.45±0.32, n=6,16; NARP −/− 4.32±0.34, n=6,21, Fig 4D) an earlier time to peak (average time to peak ± SEM (msecs): WT 132±6, n=6,16; NARP −/− 117±7, n=6,21; WT + DZ 153±4, n=6,25; NARP −/− + DZ, 139±4, n=6,17, one way ANOVA, F3,57=8.449, p<0.001; Fig 4E) and a longer duration (average msecs ± SEM: WT 76±5, n=6,16; NARP −/− 101±6, n=6,21; WT + DZ 54±3, n=6,25; NARP −/− + DZ 78±5, n=6,17; one way ANOVA, F3,57=32.370, p<0.001; Fig 4F) than wild types. To ask if enhancing inhibitory output could reverse this cortical hyper-excitability, we administered diazepam, a positive allosteric modulator of ligand-bound GABAA receptors (Sieghart, 1995). Acute diazepam (15 mg/kg, i.p.) significantly reduced the average spike rate, the time to peak and the response duration of visually-evoked activity in NARP −/− and wild type mice (evoked: WT + DZ 1.16±0.13, n=6,25; NARP −/− + DZ 2.98±0.40, n=6,17, Kruskal-Wallis test, H(3)=37.812, p<0.001, Fig 4D; spontaneous: WT + DZ 0.44±0.06, n=6,25, NARP −/− + DZ 0.87±0.09, n=6,17, Kruskal-Wallis test, H(3)=28.980, p<0.001). In all cases we observed parallel changes in spontaneous and evoked neuronal firing rates, resulting in no net change in signal to noise ratio (evoked activity / (evoked activity + spontaneous activity) average ± SEM: WT 0.74±0.03, n=6,16; NARP −/− 0.75±0.03, n=6,21; WT + DZ 0.74±0.03, n=6,25; NARP −/− + DZ 0.80±0.03, n=6,17; Kruskal-Wallis test, H(3)=2.201, p=0.532). Similar enhancement of visually-evoked and spontaneous activity was observed in neurons from layer IV of NARP −/− mice (Supp Fig 2), indicating widespread hyper-excitability in the primary visual cortex of NARP −/− mice.
Fig 4. Enhanced neuronal excitability in layer 2/3 of NARP −/− visual cortex.
A. Representative raster plots of neuronal activity acquired in layer II/III of P28 visual cortex of wild type, NARP −/−, wild type + diazepam and NARP −/− + diazepam mice. In each case, activity is shown in response to visual stimulus in preferred orientation (1 Hz reversals of 0.04 cycles/degree; 100% contrast gratings, starting a time 0). B. Post-stimulus time histograms of average evoked activity of wild type and NARP −/− mice in response to visual stimulus in preferred orientation. Kruskal-Wallis H test, H=9.366, p=0.002. C. Post-stimulus time histograms of average evoked activity of wild type +diazepam and NARP −/− + diazepam in response to visual stimulus in preferred orientation. Kruskal-Wallis H test, H=21.01, p<0.001. D. Median evoked activity from layer II/III of P28 visual cortex of wild type, NARP −/−, wild type + diazepam and NARP −/− + diazepam mice. Kruskal-Wallis test, H(3)=37.812, p<0.001, *p<0.05 Mann-Whitney post hoc versus wild type controls. E. Time to peak evoked activity from layer II/III of P28 visual cortex of wild type, NARP −/−, wild type + diazepam and NARP −/− + diazepam. One way ANOVA, F3,57=8.449, p<0.001, *p<0.05 Bonferroni's post hoc versus wild type controls. F. Spike train duration from layer II/III of P28 visual cortex of wild type, NARP −/−, wild type + diazepam and NARP −/− + diazepam mice. One way ANOVA, F3,57=32.370, p<0.001, *p<0.05 Bonferroni's post hoc versus wild type controls.
Normal vision in NARP −/− mice
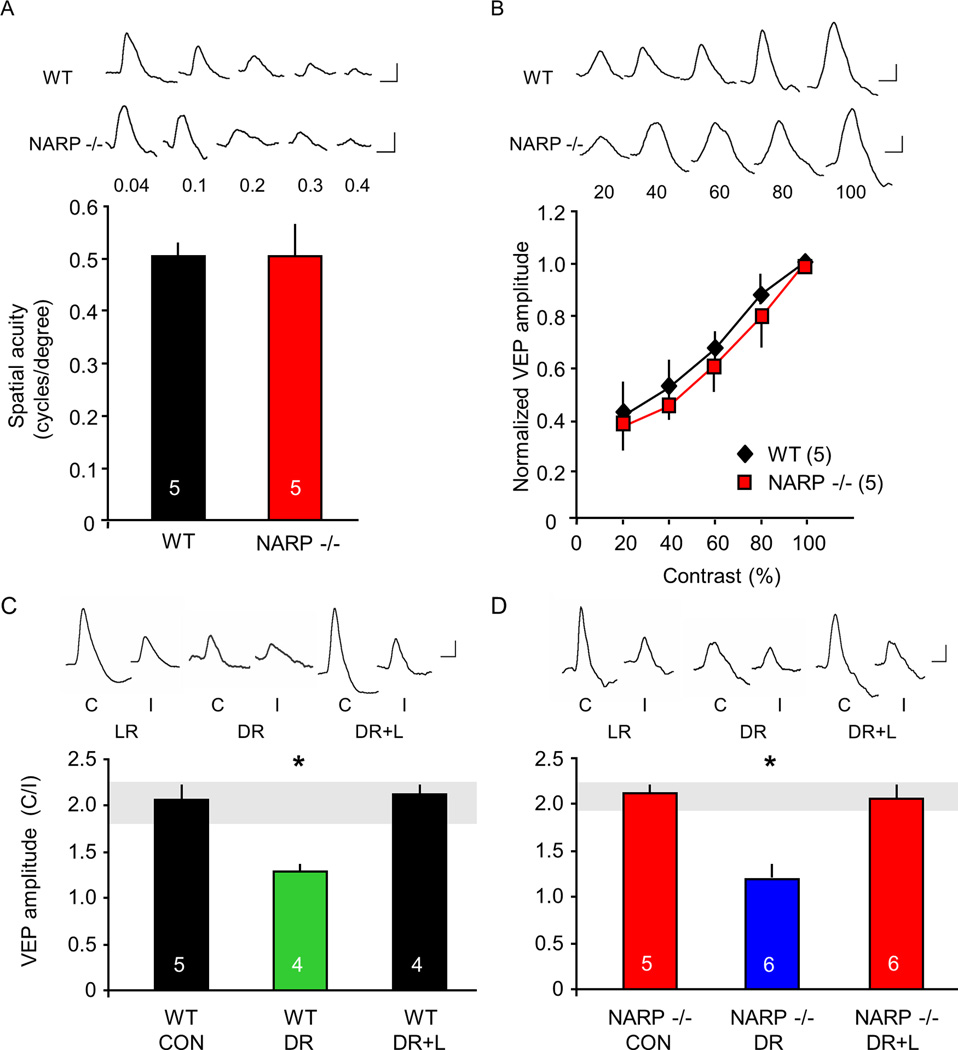
We used visually evoked potentials (VEPs) to ask if the absence of NARP, and the resulting increase in cortical excitability, impacted visual acuity or visual cortical plasticity. Visual acuity was estimated by extrapolating the linear regression of the VEP amplitude versus the spatial frequency of the visual stimulus (range from 0.04 – 0.6 cycles/degree) to 0 mV (Porciatti et al., 1999). In these experiments, we used VEPs recorded from the surface of the binocular visual cortex, to focus on synaptic potentials generated in superficial laminae (Katzner et al., 2009). We found that juvenile (P30) NARP −/− mice had an estimated spatial acuity of 0.48±0.04 cycles/degree (average ± SEM, n=5), which was indistinguishable from age-matched wild type controls (0.49±0.02 cycles/degree, n=5; p=0.86, t-test; Fig 5A). Manipulation of the visual stimulus from 20 to 100% contrast revealed similar contrast sensitivity in NARP −/− and wild type vision (Two way repeated measures ANOVA, F1,6=0.003, p=0.955; Fig 5B).
Fig 5. Normal vision in NARP −/− mice.
A. Comparable spatial acuity in NARP −/− mice and age-matched (P30) wild types. Spatial acuity is extrapolated from the linear regression of VEP amplitude versus spatial frequency of the visual stimulus. B. Comparable contrast sensitivity in NARP −/− mice and age-matched (P30) wild types. C. Two-fold VEP contralateral bias in P30 wild types (CON). Dark rearing (DR) from birth to P30 significantly inhibits the experience-dependent expression of VEP contralateral bias. Exposing dark-reared subjects to three days of normal lighted environment (DR+L) at P28 increases the VEP contralateral bias to the normal range (grey horizontal bar). One way ANOVA; F2,10=273.61, p<0.001. D. VEP contralateral bias is normal in P30 NARP −/− mice (CON). Dark rearing (DR) from birth to P30 significantly inhibits the experience-dependent expression of VEP contralateral bias. Exposing dark-reared subjects to three days of light (DR+L) at P28 increases VEP contralateral bias to the normal range (grey horizontal bar). One way ANOVA; F2,14=72.947, p<0.001; *p<0.01 Bonferroni’s post-hoc versus control. Inset: representative VEP waveforms. Scale bars: 50 ms, 50 µV.
To ask if the absence of NARP disrupts the organization of the visual cortex, we quantified ocular preference and retinotopy over the medio-lateral extension of V1. To examine ocular preference, we calculated the ratio of VEP amplitudes in response to separate stimulation of the contralateral and ipsilateral eye (Supp Fig 3). In both wild type and NARP −/− mice, recordings medial to the binocular region of the primary visual cortex revealed responses to contralateral eye stimulation only, as expected of monocular visual cortex. Recordings from a narrow area, ranging from ~ 3.0 – 3.5 mm lateral to the intersection of lambda and bregma, revealed responses to visual stimulation of both eyes, as expected of binocular visual cortex. Recordings lateral to the binocular region of the primary visual cortex revealed a loss of contralateral preference, as expected for the lateral medial region of secondary visual cortex (Rossi et al., 2001). Retinotopy was also similar in wild type and NARP −/− mice. The area of visual space resulting in the largest VEP amplitude moved along the visual field azimuth, from contralateral visual field to the meridian as the recording site was moved laterally across the binocular region of the primary visual cortex, and reversed toward the contralateral visual cortex as the recording site moved laterally from the binocular region of the primary visual cortex into LM (Supp Fig 3D). The orientation selectivity and orientation tuning of NARP −/− mice was also similar to wild types (Supp Fig 4). Thus many aspects of visual system organization and function are normal in NARP −/− mice.
The binocular primary visual cortex of rodent has a contralateral bias that depends on early binocular visual experience (McCurry et al., 2010). To ask if NARP −/− mice retained normal experience-dependent regulation of VEP contralateral bias, we examined VEP contralateral bias at the site in binocular visual cortex that yielded the largest ipsilateral eye VEP (typically 3.3 mm lateral to the intersection of lambda and bregma). Dark-rearing from birth to postnatal day 30 (P30) prevented the expression of the VEP contralateral bias in both genotypes. Similarly, bringing dark-reared subjects (at P30) into a normal lighted environment (3 days) increased the contralateral bias to the normal range (VEP amplitude contralateral eye/ipsilateral eye, average ± SEM: wild type DR 1.26±0.03, n=4; DR+L 2.05±0.03, n=4; one way ANOVA, F2,10=273.61, p<0.001, Fig 5C; NARP −/− DR 1.29±0.02, n=6; DR+L 2.12±0.04, n=6; one way ANOVA, F2,14=72.947, p<0.001, Fig 5D). In both NARP −/− and wild type mice, the experience-dependent regulation of VEP contralateral bias was mediated by changes in the amplitude of the contralateral eye VEP (Supp Fig 5). Thus the expression of a form of synaptic plasticity that is dependent on early visual experience is intact in NARP −/− mice.
Absence of ocular dominance plasticity in NARP −/− mice
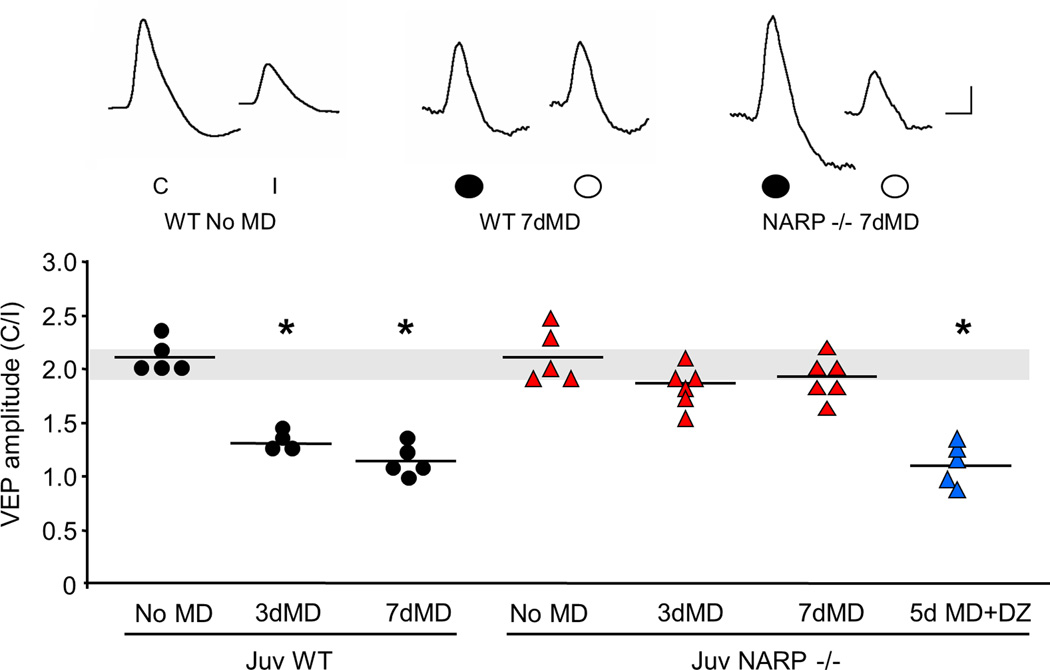
To ask how the absence of NARP affects ocular dominance plasticity, we examined the response to brief (3 days) and prolonged (7 days) monocular deprivation (MD) on the VEP contralateral bias initiated at P25, the peak of the critical period (Fagiolini et al., 1994; Gordon and Stryker, 1996; Fagiolini and Hensch, 2000). As expected, both brief and prolonged monocular deprivation of the dominant contralateral eye significantly decreased the VEP contralateral bias in juvenile wild type mice (VEP amplitude contralateral eye/ipsilateral eye average ± SEM: no MD 2.19±0.03, n=5; 3d MD 1.32±0.05, n=4; 7d MD 1.18±0.04, n=5; Fig 6). In contrast, no shift in ocular dominance was observed in juvenile NARP −/− mice following either brief or prolonged monocular deprivation (no MD 2.16±0.10, n=5; 3d MD 1.91±0.07, n=6; 7d MD 1.92±0.07, n=6). Importantly, enhancing inhibitory output with diazepam (15 mg/kg, 1x/day) enabled ocular dominance plasticity in juvenile NARP −/− mice (5d MD+DZ 1.09±0.08, n=5). No shift in ocular dominance was observed following diazepam alone (VEP amplitude contralateral eye/ipsilateral eye, average ± SEM: NARP −/− + DZ no MD, 2.08±0.11, n=3, t-test versus NARP −/− no MD, p=0.61).
Fig 6. Absence of ocular dominance plasticity in juvenile NARP −/− mice.
Brief (3 days) and prolonged (7 days) monocular deprivation of the dominant, contralateral eye, induced a significant decrease in the VEP contralateral bias in juvenile (P25) wild type, but not NARP −/− mice. Diazepam (DZ, for 5 days initiated at P25) enabled ocular dominance plasticity in NARP −/− mice. One way ANOVA (F6,29=51.187, p<0.001); *p<0.05 Bonferroni's post hoc versus WT no MD control. Normal VEP contralateral bias is depicted by grey horizontal bar. Inset: representative VEP waveforms. C=contralateral eye, I=ipsilateral eye. Scale bars: horizontal 50 ms, vertical 50 µV.
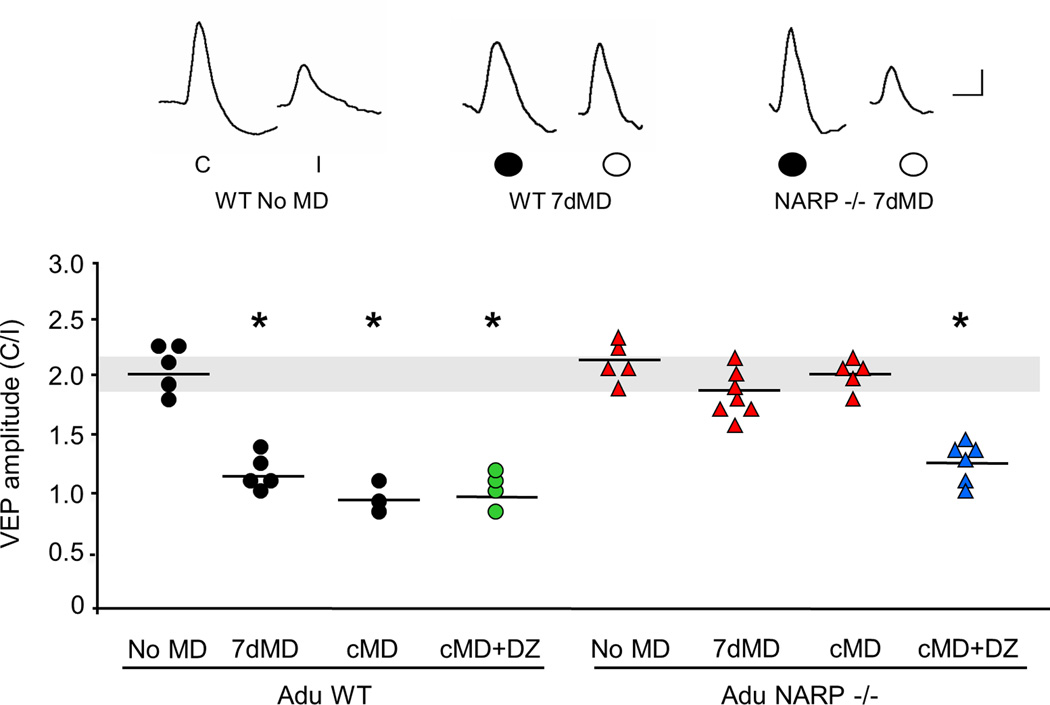
Ocular dominance plasticity persists into adulthood in wild type mice (Sawtell et al., 2003; Sato and Styker, 2008) and may utilize mechanisms distinct from those recruited by monocular deprivation earlier in development (Pham et al., 2004; Fischer et al., 2007; Ranson et al., 2012). To ask if adult NARP −/− mice could express ocular dominance plasticity, we examined the response to monocular deprivation for 7 days beginning at P90 (Fig 7). However this manipulation did not induce a shift in ocular dominance in NARP −/− mice (VEP amplitude contralateral eye/ipsilateral eye average ± SEM: adult NARP −/− no MD 2.15±0.13, n=5; 7d MD 1.93±0.09, n=7). To confirm the absence of ocular dominance plasticity in NARP −/− mice, we examined the VEP contralateral bias after chronic monocular deprivation (80 days beginning at P21). Surprisingly, the normal ocular dominance of NARP −/− mice persisted following chronic monocular deprivation (VEP amplitude contralateral eye/ipsilateral eye average ± SEM: cMD 2.00±0.11, n=5). Increasing inhibitory output with diazepam for the last 5 days of chronic monocular deprivation enabled an ocular dominance shift in adult NARP −/− mice (15 mg/kg, i.p.; cMD+DZ 1.17±0.10, n=6; Fig 7). As expected, adult wild type mice expressed a significant shift in contralateral bias in response to prolonged (7 days) and chronic (80 days) monocular deprivation (VEP amplitude contralateral eye/ipsilateral eye average ± SEM: adult WT no MD 2.04±0.20, n=5; 7d MD 1.14±0.13, n=5; cMD 0.99±0.17, n=3), which was unaffected by diazepam in adulthood (cMD+DZ 0.98±0.09, n=4). Thus, in the absence of NARP, the visual system is unable to respond to monocular deprivation, despite functional inhibitory output.
Fig 7. Absence of ocular dominance plasticity in adult NARP −/− mice.
Prolonged monocular deprivation (7 days) of the dominant, contralateral eye, initiated in adulthood (P90) and chronic monocular deprivation (from P21 – P100) induced a significant decrease in the VEP contralateral bias in adult wild type, but not NARP −/− mice. Diazepam (DZ, during the last 5 days of chronic MD) enabled ocular dominance plasticity in chronically- deprived NARP −/− mice, but does not change the ocular dominance shift in wild type mice. One way ANOVA (F7,32=18.706, p<0.001); *p<0.05 Bonferroni's post hoc versus WT no MD. Normal VEP contralateral bias is depicted by grey horizontal bar. Inset: representative VEP waveforms. C=contralateral eye, I=ipsilateral eye. Scale bars: 50 ms, 50 µV.
Differential response of NARP −/− mice to low frequency versus high frequency visual stimulation
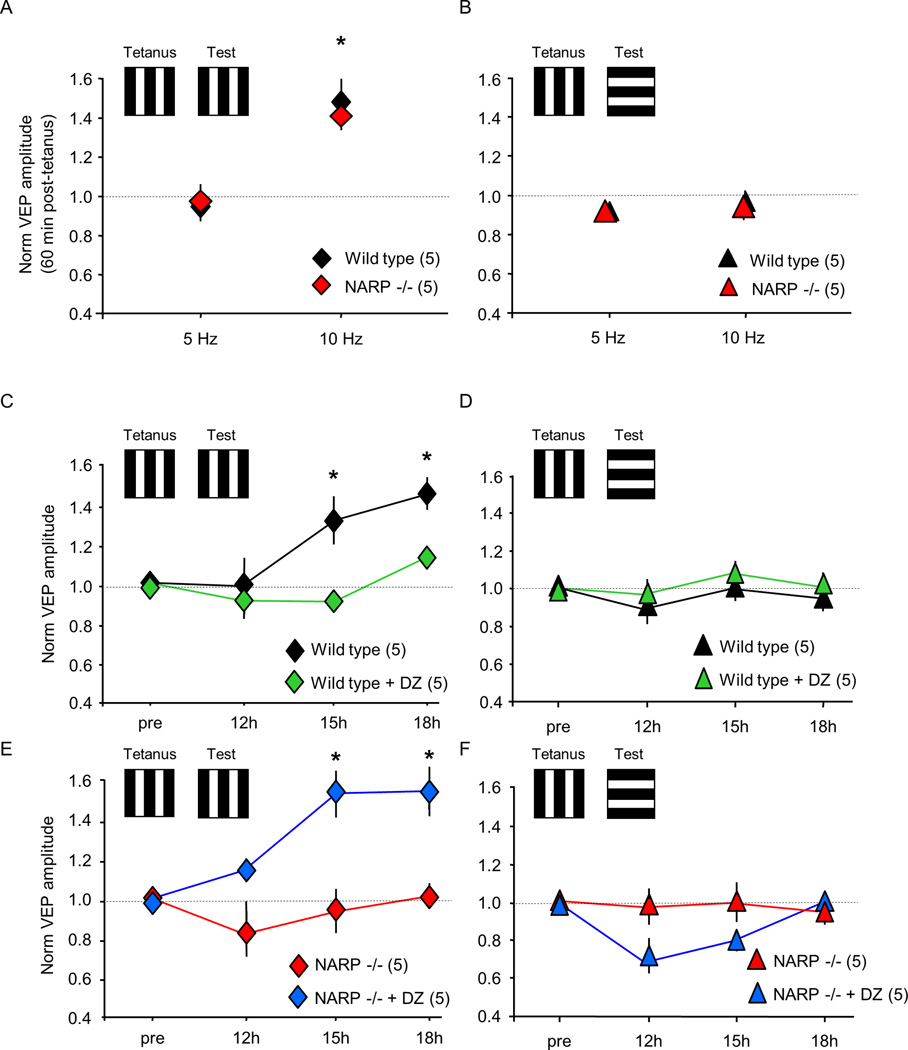
Although NARP −/− mice do not express ocular dominance plasticity, other forms of experience-dependent synaptic plasticity, such as the plasticity of the VEP contralateral bias, remain intact (Fig 5). To further explore the range of deficits in synaptic plasticity in NARP −/− mice, we examined the response to repetitive visual stimulation, previously shown to induce robust changes in VEP amplitudes in vivo (Sawtell et al., 2003; Frenkel et al., 2006; Ross et al., 2008; Cooke and Bear, 2010; Beste et al., 2011). High frequency visual stimulation (10 Hz reversals of 0.04 cycles/degree, 100% contrast, vertical gratings) induced a rapid enhancement of the VEP amplitude in P30 NARP −/− and wild type mice (VEP amplitude 60 mins post-stimulation normalized to pre-stimulation: WT 1.48±0.12, n=5; NARP −/− 1.41±0.06, n=5; two way ANOVA, F1,1=0.316, p=0.584; Fig 8A). The enhancement in VEP amplitude was dependent on the temporal frequency of the visual stimulation, as visual stimulation at an intermediate temporal frequency (5 Hz) did not affect VEP amplitudes in either genotype (VEP amplitude 60 mins post-stimulation normalized to pre-stimulation: WT 1.00±0.03, n=3; NARP −/− 0.97±0.10, n=3). The increase in VEP amplitude induced by 10 Hz visual stimulation was specific for the orientation of the visual stimulus, as no VEP enhancement was observed in response to a rotated grating (10 Hz: WT 0.96±0.05, n=5; NARP −/− 0.94±0.06, n=5; 5 Hz: WT 0.94±0.03, n=3; NARP −/− 0.97±0.08, n=3; two way ANOVA, F1,1=0.002, p=0.968; Fig 8B). In contrast,, low frequency visual stimulation (1 Hz reversals of 0.04 cycles/degree, 100% contrast vertical gratings) induced a slowly emerging increase in VEP amplitude in wild type mice (VEP amplitude post/pre stimulation: 12h 0.98±0.14; 15h 1.32±0.12; 18h 1.45±0.08; n=5), that was inhibited by diazepam (12h 0.91±0.01; 15h 0.91±0.04; 18h 1.13±0.01; n=5, two way ANOVA with repeated measures, F1,8=18.288, p=0.003; *p<0.01 versus wild type control; Fig 8C). As previously reported, the enhancement of VEP amplitude was selective for the orientation of the visual stimulus, as no increase in VEP amplitude was observed in response to a rotated grating (12h 0.88±0.12; 15h 0.99±0.04; 18h 0.94±0.06, n=5; Fig 8D; Cooke and Bear, 2010). However, the slow, stimulusselective response plasticity was absent in NARP −/− mice (12h 0.82±0.12; 15h 0.93±0.11; 18h 1.01±0.06; n=5; Fig 8E), but could be enabled by diazepam (12h 1.14±0.06; 15h 1.53±0.12; 18h 1.55±0.13; n=5, two way ANOVA with repeated measures, F1,8=12.247, p=0.008; *p<0.01 versus NARP −/− control; Fig 8E). The response enhancement evoked in the presence of diazepam was selective for the orientation of the familiar visual stimulus (12h 0.68±0.06; 15h 0.79±0.05; 18h 0.99±0.02; n=5; Fig 8F). Thus, the absence of NARP completely eliminates the expression of several essential form of experience-dependent synaptic plasticity, while other aspects of circuit function and plasticity remain unchanged.
Fig 8. Differential response of NARP −/− mice to low versus high frequency visual stimulation.
A. High frequency visual stimulation (10 Hz reversals of 0.04 cycle/degree 100% contrast vertical gratings) induced a rapid increase in VEP amplitude in P30 wild type and NARP −/− mice, but 5 Hz reversals were ineffective *=p<0.05 t-test versus pre-stimulation baseline. B. Enhancement of VEP amplitude following high frequency visual stimulation did not transfer to a visual stimulus of a novel orientation. C. Low frequency visual stimulation (1 Hz reversals of 0.04 cycle/degree 100% contrast vertical gratings) induced a slow increase in VEP amplitude in P30 wild type mice (black symbols), which was inhibited by diazepam (15 mg/kg, i.p; 30 mins prior to stimulation; green symbols). Two way repeated measures ANOVA: F1,8=18.288, p=0.003; *p<0.01 Bonferroni’s post hoc versus pre-stimulation. D. Enhancement of VEP amplitudes following low frequency visual stimulation did not transfer to a visual stimulus of a novel orientation. E. Low frequency visual stimulation (1 Hz reversals of 0.04 cycle/degree 100% contrast vertical gratings) did not change VEP amplitudes in P30 NARP −/− mice (red symbols). Administration of diazepam (30 mins prior to stimulation), enabled the enhancement of VEP amplitudes by low frequency visual stimulation (blue symbols; 15 mg/kg, i.p.). Two way repeated measures ANOVA: F1,8=12.247, p=0.008; *p<0.01 Bonferroni’s post hoc versus pre-stimulation. F. The enhancement of VEP amplitudes in NARP −/− mice by low frequency visual stimulation enabled by diazepam, did not transfer to a visual stimulus of a novel orientation.
DISCUSSION
Transgenic deletion of NARP allowed us to demonstrate that the strength of excitatory drive onto FS (PV) INs plays a central role in the initiation of the critical period for ocular dominance plasticity. Transgenic deletion of the immediate early gene NARP decreases the number of excitatory synaptic connections onto FS (PV) INs, thereby decreasing net excitatory drive onto neurons that mediate the majority of perisomatic inhibition. Importantly, net inhibitory drive from FS (PV) INs is unchanged in NARP−/−mice. Nonetheless, the visual cortex of NARP −/− mice is hyper-excitable, and unable to express several cardinal forms of synaptic plasticity, including ocular dominance plasticity, which are typically robust during an early postnatal critical period. Pharmacological reduction of the hyper-excitability in NARP −/− mice compensates for the deficit in the recruitment of inhibition, and allows the expression of ocular dominance plasticity. We propose that NARP-dependent recruitment of inhibition from FS (PV) INs is necessary to ensure the precision of pyramidal cell activity necessary to engage these forms of synaptic plasticity (Jiang et al., 2007; Toyoizumi and Miller, 2009; Kuhlman et al., 2010). The NARP-dependent enhancement of excitatory drive onto FS (PV) INs is therefore an important, novel locus for the regulation of the critical period for ocular dominance plasticity.
Role of NARP in initiation of the critical period
NARP is selectively enriched at excitatory synapses onto FS (PV) INs (Chang et al., 2010), the fast-spiking basket cells which mediate rapid feed-forward and feed-back inhibition onto neuronal somata (Kawaguchi and Kubota, 1997; Ascoli et al., 2008). Perisomatic inhibition from FS (PV) INs is therefore ideally located to exert powerful temporal and spatial control of the spiking output of principle neurons (Pouille and Scanziani, 2001; Goldberg et al., 2008; Kulman et al., 2010). Indeed, it has been proposed that a suprathreshold level of perisomatic inhibition is necessary to promote the synaptic plasticity between principle neurons that initiates the critical period for ocular dominance plasticity (Huang et al., 1999; Di Cristo et al., 2007). Alternatively, plasticity at synapses that mediate feedback inhibition onto principle neurons in the visual cortex has been proposed to mediate the shift in ocular dominance induced by monocular deprivation (Maffei et al., 2006). Changes in interneuron excitability and ocularity have been reported in response to monocular deprivation (Yazaki-Sugiyama et al., 2009; Gandhi et al., 2008; Kameyama et al., 2010). Our work suggests for the first time that a critical step in the initiation of the critical period is the recruitment of inhibition through NARP-dependent enhancement of excitatory drive onto FS (PV) INs. The deficit in the ability to recruit inhibition prevents the induction of ocular dominance plasticity in NARP −/− mice, despite the presence of normal perisomatic inhibition. Importantly, sensory experience has been shown to strengthen excitation from thalamic afferents onto feed-forward inhibitory interneurons in layer IV of rodent barrel cortex (Chittajallu and Isaac, 2010), and in the visual cortex, these inputs are remodeled by monocular deprivation (Kuhlman et al., 2011).
Absence of critical period plasticity in NARP −/− mice
Monocular deprivation prior to the initiation of the critical period (~ P18 in rodents) is ineffective, demonstrating that a developmental change in visual cortical circuitry is necessary to initiate ocular dominance plasticity. In the absence of NARP, the visual system is retained in a hyper-excitable state that is reminecsent of this pre-critical period. The method that we used to assess ocular dominance plasticity, examination of the contralateral bias of VEPs evoked by simple visual stimuli, has a lower threshold for the detection of changes induced by MD than other methods, such as change in visual acuity (Prusky and Douglas, 2003; Heimel et al., 2007). In addition, our VEP recordings were performed in superficial layers of the visual cortex, where ocular dominance plasticity is expressed long into postnatal development in wild types (Fischer et al., 2007; Heimel et al., 2007; Lehmann and Lowel, 2008; Sato and Stryker, 2008). Despite this, we saw no evidence for a shift in ocular dominance in NARP −/− mice, including in response to monocular deprivation of unusually long duration (> ten weeks). This suggests that the visual system cannot compensate for the absence of NARP, and is unable to recruit the inhibition necessary to enable ocular dominance plasticity. We cannot rule out the possibility that monocular deprivation in NARP −/− mice induces changes in the strength of synapses outside the recording radius of our electrode.
Visual function and pre-critical period plasticity are normal in NARP −/− mice
Previous work has identified an important role for neuronal pentraxins in the refinement of retinogeniculate synapses in the dLGN (Bjartmar et al., 2006). The ipsilateral eye input to the dLGN of NARP −/− (a.k.a. NP2), was slightly expanded at P10 compared to age matched WTs, but this expansion was mild compared to NP1/2 double KO mice. Despite the initial deficit in the refinement of retinogeniculate synapses, the binocular inputs to the dLGN of P30 NP1/2 KO mice become more segregated by P30. In our experiments, the single deletion of NARP (NP2) did not disrupt the macroorganziation of V1. Indeed, the anatomical boundaries between V1b and V1m and LM were similar in wild type and NARP −/− mice, and no differences were observed in retinotopy within V1b or the distribution of ocular preference along the mediolateral aspect of the primary visual cortex. Although other aspects of visual system organization not tested here may be disrupted in NARP −/− mice, our results clearly demonstrate that many aspects of visual cortex organization are unimpaired despite the deficit in the recruitment of inhibition. In addition, many aspects of visual function that mature before or during the critical period, including contralateral bias, spatial acuity and contrast sensitivity, were normal in NARP−/− mice (Huang et al., 1999; Prusky and Douglas, 2004; Heimel et al., 2007). The absence of a change in visual acuity was not unexpected, as the parallel increase in evoked and spontaneous single unit activity in NARP −/− visual cortex mice predicts that visual detection thresholds would remain unchanged. Similarly, other transgenic manipulations that induce hyper-excitability in the visual cortex (i.e. GAD 65 −/−; Hensch et al., 1998) have normal retinotopy and orientation selectivity, while manipulations that decrease inhibition in the visual cortex (i.e., dark-exposure, environmental enrichment) are not accompanied by a loss of spatial acuity (He et al., 2007; Sale et al., 2007).
Interestingly, not all forms of experience-dependent synaptic plasticity are absent in NARP −/− mice. NARP −/− mice retain the ability to express experience-dependent enhancement of the VEP contralateral bias, which is dependent on early binocular visual experience and reflects the complement of thalamocortical projections serving each eye (McCurry et al., 2010; Coleman et al., 2009). In addition, NARP −/− mice retain the ability to express experience-dependent enhancement of VEP amplitudes in response to high frequency (10 Hz) visual stimulation. Normal long term potentiation (in response to 100 Hz stimulation) and long term depression (in response to 3 Hz stimulation) of the hippocampal Schaffer collateral pathway also persists in hippocampus of double (NP1 and NP2) knock out mice (Bjartmar et al., 2006). This suggests that these forms of synaptic plasticity do not require gating by fast inhibition or can be engaged by a lower level of inhibitory output. Brief monocular deprivation during the critical period induces a rapid depression of synapses serving the deprived eye and a slow strengthening of synapses serving the non-deprived eye (Sawtell et al., 2003; Frenkel and Bear, 2004; Tagawa et al., 2005; Sato and Stryker, 2008). Importantly, despite the persistence of some forms of experience-dependent synaptic potentiation, we did not observe a strengthening of non-deprived eye synapses, even following unusually long durations of monocular deprivation. The potentiation of the inputs serving the non-deprived eye may be constrained in NARP −/− mice by the absence of deprived eye depression, which has been proposed to be required to first lower the threshold for strengthening of synapses serving the nondeprived eye (Smith et al., 2010).
Unique phenotype of NARP −/− mouse
There are important differences in the phenotype of the NARP −/− mouse from other transgenic models with deficits in ocular dominance plasticity. Transgenic manipulations that impair synaptic plasticity at excitatory synapses onto pyramidal neurons, such as deletion of the activity-regulated cytoskeletal protein arc, block the expression of ocular dominance plasticity along with a wide range of other forms of homeostatic and hebbian plasticity (McCurry et al., 2010). On the other hand, transgenic manipulations that result in hyper-excitability of the visual cortex, such as deletion of the synaptic isoform of the GABA synthetic enzyme GAD65, impair both GABAergic synaptic transmission (Choi et al., 2002) and the response to brief monocular deprivation (Hensch et al., 1998). Nonetheless, slightly longer durations of monocular deprivation can reliably induce ocular dominance shift in the visual system of GAD 65 −/− mice (Fagiolini and Hensch, 2000). Ocular dominance plasticity is absent in NARP −/− mice, even in response to prolonged duration of monocular deprivation, suggesting that the visual cortex is unable to compensate for the absence of NARP. Nonetheless, several forms of experience-dependent synaptic plasticity, such as plasticity of the VEP contralateral bias and the response to high frequency visual stimulation, are retained. The unique phenotype of the NARP −/− mouse underscores the importance of the recruitment of fast inhibition, via regulation of excitatory drive onto FS (PV) INs, in the induction of fundamental forms of experience-dependent synaptic plasticity in the mammalian visual cortex.
EXPERIMENTAL PROCEDURES
Wild type and NARP −/− mice (Bjartmar et al., 2006) were of C57BL/6, 129/SVJII mixed genetic background. All subjects were raised on a 12 h light/dark cycle, with food and water available ad libitum. All procedures conform to the guidelines of the U.S. Department of Health and Human Services and the Institutional Animal Care and Use Committees of the University of Maryland and Johns Hopkins University. Monocular deprivation was performed under ketamine/xylazine anesthesia (50 mg/10 mg/kg, i.p.). The margins of the upper and lower lids of one eye were trimmed and sutured together. The animals were returned to their home cages and disqualified in the event of suture opening or infection.
Visual cortical slices (300 µm) were prepared as described (Huang et al., 2010) in ice-cold dissection buffer containing (in mM): 212.7 sucrose, 5 KCl, 1.25 NaH2PO4, 10 MgCl2, 0.5 CaCl2, 26 NaHCO3, 10 dextrose, bubbled with 95% O2/ 5% CO2 (pH 7.4). Slices were transferred to normal artificial cerebrospinal fluid (ACSF) for at least one hour prior to recording. Normal ACSF was similar to the dissection buffer except that sucrose was replaced by 124 mM NaCl, MgCl2 was lowered to 1 mM, and CaCl2 was raised to 2 mM.
Visualized dual whole-cell voltage-clamp recordings were made from pairs of FS (PV) INs and pyramidal neurons with glass pipettes filled with (in mM): KGluconate 130, CaCl2 0.2, NaCl 8, EGTA 2, NaGTP 0.5, MgATP 4, and HEPES 10, pH 7.2. Only cells with membrane potentials < −65 mV, series resistance < 20 MW, input resistance > 100 MW (with < 15% variation over the experiment) were studied. Data were filtered at 5 kHz and digitized at 10 kHz using Igor Pro (Wave Metrics Inc., Lake Oswego, Oregon). uEPSCs were recorded in voltage clamp in the FS (PV) INs at −70 mV, and evoked by suprathreshold somatic current injection (2 msec) in presynaptic pyramidal neurons. uIPSCs were recorded in voltage clamp in pyramidal neurons at 0 mV, and evoked by suprathreshold somatic current injection (2 msec) in presynaptic FS (PV) INs (Jiang et al., 2010). At least 20 responses evoked at 0.1 Hz with paired pulse stimulation (interstimulus interval: 50 ms for Pyr ->FS (PV) IN pairs; 100 ms for FS (PV) IN -> Pyr pairs) were used to confirm a synaptic connection, and to compute the amplitudes of the unitary responses.
Mean variance analysis was performed on responses evoked by 15 stimulus trains (5 or 10 stimuli at 50 Hz) delivered at 20 sec intervals. The uEPSC amplitude was measured for each stimulus, and the mean (I) and variance (Var) were plotted against each other. Synaptic parameters including number of release sites (N), and quantal size (q) were obtained by fitting the data to the parabola: Var = qI-I2/N as previously described (Scheuss et al., 2001). We considered only those cases in which the R2 value of the fit was > 0.5.
In vivo electrophysiology was performed under isoflurane anesthesia (~1.5% in 100% O2 via modified nose cone). The dura covering binocular visual cortex was exposed through a hole (~ 3 mm diameter) in the skull. The exposed brain was kept moist with artificial cerebral spinal fluid (ACSF), and the room humidity was supplemented (ZD300Y0, Zenith). Subjects were retained in a stereotax in a darkened room (without visual stimulation) between measurements. Body temperature was maintained at 37 degrees C via circulating water heating pad (T/PUMP, Gaymar Industries Inc.), monitored with a rectal probe (BAT-12, Sensortek Inc.). A broad-band signal was collected from the lateral aspect (binocular region) of the primary visual cortex (site of largest ipsilateral eye VEP, typically 3.3 mm lateral to the intersection of lambda and the midline), with a tungsten microelectrode (0.5 MΩ) relative to a ground screw in the frontal bone (Supp Fig 3). Laminar placement of the electrode was confirmed by time to VEP peak and the shape of the VEP waveform: layer II/III, ~250 µm below the pia + primary positive peak, time to peak ~ 130 msec (average +/− SEM 131.76+/−5.88 ms, n=7); layer IV, ~450 µm below the pia + primary negative peak, time to peak ~ 105 msec (107.38+/−3.17 ms, n=6). 50 Hz low pass filter was used to isolate VEPs in response to 1 Hz reversals of full screen 100% contrast gratings (0.04 cycles/degree and 40 cd/m2 luminosity) presented on a computer monitor 25 cm from eyes. To estimate spatial acuity, the VEP amplitude was plotted against the spatial frequency of the visual stimulus (0.04 – 0.6 cycles/degree), and the linear regression was extrapolated to zero VEP amplitude. To estimate contrast sensitivity, the VEP amplitude was plotted against the contrast of the visual stimulus (20– 100%). VEPs were averaged in synchrony with the visual stimulus using OpenEX software (TDT).
700-7k Hz band pass filter was used to isolate multi-unit activity, which was sorted into single- units based on waveform shape and principal component analysis (OpenEx software; TDT). Spontaneous firing rates were measured over 100 seconds in response to blank screen. Evoked spiking rates were measured in response to visual stimulus in preferred orientation (from 9 orientations ranging from 0 degrees (vertical) to 180 degrees). Duration of evoked single unit activity was determined by comparison with 50 msec pre-stimulus baseline. Orientation selectivity index = (response evoked by preferred – orthogonal orientation)/(pref+ortho). Orientation tuning was determined by plotting spiking activity against stimulus orientation from −90 to 90 degrees from preferred orientation. Single unit activity was assigned to cortical lamina based on shape of VEP waveform.
Plasticity of VEP amplitude induced by repetitive visual stimulation was assessed under continuous isoflurane anesthesia (~1.5% in 100% O2). 60 minutes after recording baseline VEPs (evoked by 100 reversals of 0.04 cycles/degree; 100% contrast vertical and horizontal gratings; reversing at 1 Hz), high frequency visual stimulation (5 – 10 Hz reversals of same gratings; 1000 reversals) was delivered at a single orientation (vertical). 60 min after delivery of high frequency visual stimulation, VEPs were acquired with baseline stimulation (1 Hz) in response to vertical and horizontal gratings. Low frequency visual stimulation (1 Hz reversals of same gratings; 1000 reversals) was delivered at a single orientation (vertical). 12, 15 and 18 hours after delivery of low frequency visual stimulation, VEPs were acquired with baseline stimulation (1 Hz) in response to vertical and horizontal gratings.
Diazepam (Sigma) was dissolved in 10% Tween 80, 20% DMSO and 70% saline to a final concentration of 2 mg/ml. Neuronal spiking rates in diazepam are reported 45 minutes after administration.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, et al. Petilla terminology: Nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C, Wascher E, Güntürkün O, Dinse HR. Improvement and impairment of visually guided behavior through LTP- and LTD-like exposure-based visual learning. Curr Biol. 2011;21:876–882. doi: 10.1016/j.cub.2011.03.065. [DOI] [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Rentería RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, Cho R, Worley P, Malenka RC, Ball S, Peachey NS, Copenhagen D, Chapman B, Nakamoto M, Barres BA, Perin MS. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ, Worley PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci. 2010;13:1090–1097. doi: 10.1038/nn.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Isaac JT. Emergence of cortical inhibition by coordinated sensory-driven plasticity at distinct synaptic loci. Nat Neurosci. 2010;13:1240–1248. doi: 10.1038/nn.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Morales B, Lee HK, Kirkwood A. Absence of long-term depression in the visual cortex of glutamic Acid decarboxylase-65 knock-out mice. J Neurosci. 2002;22:5271–5276. doi: 10.1523/JNEUROSCI.22-13-05271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JE, Law K, Bear MF. Anatomical origins of ocular dominance in mouse primary visual cortex. Neuroscience. 2009;161:561–571. doi: 10.1016/j.neuroscience.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. J Neurosci. 2010;30:16304–16313. doi: 10.1523/JNEUROSCI.4333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristo G, Chattopadhyaya B, Kuhlman SJ, Fu Y, Bélanger MC, Wu CZ, Rutishauser U, Maffei L, Huang ZJ. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat Neurosci. 2007;10:1569–1577. doi: 10.1038/nn2008. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vision Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Fischer QS, Graves A, Evans S, Lickey ME, Pham TA. Monocular deprivation in adult mice alters visual acuity and single-unit activity. Learn Mem. 2007 Apr 6;14(4):277–286. doi: 10.1101/lm.392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Sawtell NB, Diogo AC, Yoon B, Neve RL, Bear MF. Instructive effect of visual experience in mouse visual cortex. Neuron. 2006;51:339–349. doi: 10.1016/j.neuron.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Yanagawa Y, Stryker MP. Delayed plasticity of inhibitory neurons in developing visual cortex. Proc Natl Acad Sci U S A. 2008;105(43):16797–16802. doi: 10.1073/pnas.0806159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, Viegi A, Berardi N, Maffei L. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci. 2010;30:361–371. doi: 10.1523/JNEUROSCI.2233-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci. 2006;26:2951–2955. doi: 10.1523/JNEUROSCI.5554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat Neurosci. 2007;10:1134–1136. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
- Heimel JA, Hartman RJ, Hermans JM, Levelt CN. Screening mouse vision with intrinsic signal optical imaging. Eur J Neurosci. 2007;25:795–804. doi: 10.1111/j.1460-9568.2007.05333.x. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;82:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Gu Y, Quinlan EM, Kirkwood A. A refractory period for rejuvenating GABAergic synaptic transmission and ocular dominance plasticity with dark exposure. J Neurosci. 2010;30:16636–16642. doi: 10.1523/JNEUROSCI.4384-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Cho KH, Kim HS, Hahn SJ, Kim MS, Rhie DJ. Age-dependent decline in supragranular long-term synaptic plasticity by increased inhibition during the critical period in the rat primary visual cortex. J Neurophysiol. 2009;101:269–275. doi: 10.1152/jn.90900.2008. [DOI] [PubMed] [Google Scholar]
- Jiang B, Huang S, de Pasquale R, Millman D, Song L, Lee HK, Tsumoto T, Kirkwood A. The maturation of GABAergic transmission in visual cortex requires endocannabinoid-mediated LTD of inhibitory inputs during a critical period. Neuron. 2010;66:248–259. doi: 10.1016/j.neuron.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Treviño M, Kirkwood A. Sequential development of long-term potentiation and depression in different layers of the mouse visual cortex. J Neurosci. 2007;27:9648–9652. doi: 10.1523/JNEUROSCI.2655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama K, Sohya K, Ebina T, Fukuda A, Yanagawa Y, Tsumoto T. Difference in binocularity and ocular dominance plasticity between GABAergic and excitatory cortical neurons. J Neurosci. 2010;30(4):1551–1559. doi: 10.1523/JNEUROSCI.5025-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994;14:1634–1645. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Lu J, Lazarus MS, Huang ZJ. Maturation of GABAergic inhibition promotes strengthening of temporally coherent inputs among convergent pathways. PLoS Comput Biol. 2010 Jun 3;6:e1000797. doi: 10.1371/journal.pcbi.1000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Tring E, Trachtenberg JT. Fast-spiking interneurons have an initial orientation bias that is lost with vision. Nat Neurosci. 2011;14:1121–1123. doi: 10.1038/nn.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann K, Löwel S. Age-dependent ocular dominance plasticity in adult mice. PLoS One. 2008 Sep 1;3(9):e3120. doi: 10.1371/journal.pone.0003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- McCurry CL, Shepherd JD, Tropea D, Wang KH, Bear MF, Sur M. Loss of Arc renders the visual cortex impervious to the effects of sensory experience or deprivation. Nat Neurosci. 2010;13:450–457. doi: 10.1038/nn.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J. Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Pham TA, Graham SJ, Suzuki S, Barco A, Kandel ER, Gordon B, Lickey ME. A semi-persistent adult ocular dominance plasticity in visual cortex is stabilized by activated CREB. Learn Mem. 2004;11:738–747. doi: 10.1101/lm.75304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002 Nov 8;298(5596):1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006 May 30;103(22):8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V, Pizzorusso T, Maffei L. The visual physiology of the wild type mouse determined with pattern VEPs. Vision Res. 1999;39:3071–3081. doi: 10.1016/s0042-6989(99)00022-x. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. Eur J Neurosci. 2003 Jan;17(1):167–173. doi: 10.1046/j.1460-9568.2003.02420.x. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Characterization of mouse cortical spatial vision. Vision Res. 2004;44:3411–3418. doi: 10.1016/j.visres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Ranson A, Cheetham CE, Fox K, Sengpiel F. Homeostatic plasticity mechanisms are required for juvenile, but not adult, ocular dominance plasticity. Proc Natl Acad Sci U S A. 2012;109:1311–1316. doi: 10.1073/pnas.1112204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RM, McNair NA, Fairhall SL, Clapp WC, Hamm JP, Teyler TJ, Kirk IJ. Induction of orientation-specific LTP-like changes in human visual evoked potentials by rapid sensory stimulation. Brain Res Bull. 2008;76:97–101. doi: 10.1016/j.brainresbull.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Rozas C, Frank H, Heynen AJ, Morales B, Bear MF, Kirkwood A. Developmental inhibitory gate controls the relay of activity to the superficial layers of the visual cortex. J Neurosci. 2001;21:6791–6801. doi: 10.1523/JNEUROSCI.21-17-06791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proc Natl Acad Sci U S A. 2001 May 22;98(11):6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- Sato M, Stryker MP. Distinctive features of adult ocular dominance plasticity. J Neurosci. 2008;28:10278–10286. doi: 10.1523/JNEUROSCI.2451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Scheuss V, Neher E. Estimating synaptic parameters from mean, variance, and covariance in trains of synaptic responses. Biophys J. 2001;81:1970–1989. doi: 10.1016/S0006-3495(01)75848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364:357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SL, Trachtenberg JT. Experience-dependent binocular competition in the visual cortex begins at eye opening. Nat Neurosci. 2007;10:370–375. doi: 10.1038/nn1844. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Tagawa Y, Kanold PO, Majdan M, Shatz CJ. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nat Neurosci. 2005;8:380–388. doi: 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- Toyoizumi T, Miller KD. Equalization of ocular dominance columns induced by an activity-dependent learning rule and the maturation of inhibition. J Neurosci. 2009;29:6514–6525. doi: 10.1523/JNEUROSCI.0492-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O'Brien RJ, Worley P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003 Jul 31;39(3):513–528. doi: 10.1016/s0896-6273(03)00463-x. [DOI] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Kang S, Câteau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature. 2009;462(7270):218–221. doi: 10.1038/nature08485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.