Abstract
Hydroxyurea (HU) is underutilized in sickle cell disease (SCD). Patient adherence with taking HU and with required drug monitoring is a provider perceived barrier to HU utilization.(1-4) To determine process issues that may contribute to these barriers we sought to: 1) describe how providers monitor and adjust HU dosing in children with SCD in clinical practice and 2) identify providers' methods of assessing HU adherence. A pilot-tested survey was emailed to American Society of Pediatric Hematology/Oncology (ASPHO) members. Descriptive statistics were performed. Thirty-one percent (n=350) of 1128 surveys were returned; 63% (220 of 350) of respondents provided care for children with SCD. Most providers (64.7%) follow labs monthly and almost half (41.9%) see patients monthly. The majority (61.9%) adjusted HU dosing using maximum tolerated dose commonly determined using ANC (27.9%), platelets (26.5%), and WBC count (11.2%). Adherence was primarily assessed using patient interview (84.2%), MCV (75.3%), and HbF levels (70.7%). The majority of providers perform monthly monitoring and assess HU adherence using unreliable methods. Determining optimal frequency of monitoring HU and more reliable methods of assessing adherence are essential to balancing safety and the elimination of barriers to promote HU utilization.
Keywords: Hydroxyurea, Monitoring, Adherence, Sickle Cell Disease, Children
HU is efficacious at decreasing painful events, acute chest syndrome, transfusions, and mortality in SCD.(5-8) Despite this proven efficacy, HU is underutilized (1-4) limiting its effectiveness. This underutilization has been recognized by the National Institutes of Health.(2) Key provider-related barriers contributing to HU underutilization revolve around adherence; specifically, patient adherence with laboratory monitoring and with taking HU.(1-4) Therefore, determining providers' processes for monitoring HU is the first step towards addressing these barriers because the intensity of monitoring may affect patient adherence. Ultimately, addressing these barriers may improve effectiveness of HU.
Currently, there are no evidence-based guidelines outlining HU use in SCD. Data are limited to expert opinion (9). Although expert reviews such as that completed by Ware (9) are important, evidence-based guidelines are necessary. Adult data reveal variation in providers' practices of: 1) monitoring effects of HU, 2) dose adjustment, and 3) tracking adherence.(4) Presently, there are no data describing these same providers' practices in children with SCD from a national perspective in a “real-world” setting. One prior study of HU adherence is limited to a single institution's experience and may not reflect national practices.(10) Identifying providers' practices will provide consensus for an approach to HU monitoring, lay the groundwork for future evaluation of guideline adherence, and determine if novel methods of assessing and promoting adherence are needed.
We conducted an anonymous survey of pediatric hematology/oncology providers to achieve the following objectives: 1) describe HU monitoring and dose adjustment and 2) identify methods of assessing HU adherence. These data were collected as part of a larger study evaluating HU utilization in children with SCD.(1)
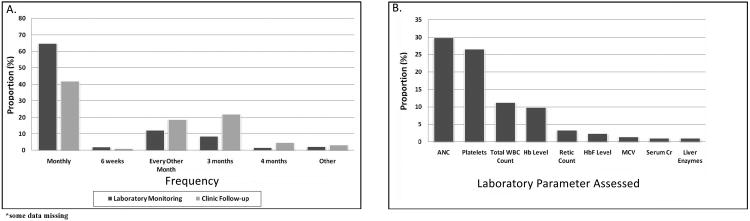
Thirty-one percent (n=350) of 1128 surveys were returned and 63% (220 of 350) of respondents report caring for children with SCD. The majority of respondents were physicians (n=213; 96.8%) and half were female (n=103; 46.8%). A diagram of the final study population and detailed provider demographics have been published.(1) Figure 1 displays the frequency of laboratory and clinical monitoring and the laboratory parameters used to determine maximum tolerated dose (MTD). To adjust HU dosing, 61.9%used MTD, 27.9% used symptom relief, and 31% made adjustments until reaching a pre-determined maximum dose with doses ranging between 20-40 mg/kg/day and 30.7% using a maximum dose of 30-35 mg/kg/day.
Figure 1.
Provider-reported Methods of Monitoring Hydroxyurea.
A) Frequency of Laboratory Monitoring and Clinic Follow-up while Patients are Taking Hydroxyurea. B) Laboratory Parameters used to Determine Maximum Tolerated Dose of Hydroxyurea. Data for Figure 1B were derived from the following question: “How do you adjust hydroxyurea dosing? Choose ALL that apply”. Potential answers were: 1) Increase until maximum tolerated dose (Please indicate criteria used below. i.e. WBC, platelets, hemoglobin), 2) Until maximum dose reached (Please indicate the maximum mg/kg/day below), 3) Symptom relief.” Data depicted reflect providers' that selected answer number 1 above and indicated the specific criteria used to determine maximum tolerated dose. ANC=absolute neutrophil count; WBC=white blood cell; MCV=mean cell volume; Cr=creatinine;
Providers discontinued HU when there was no clinical benefit (76.3%), myelotoxicity (61.4%), and no rise in HbF (17.2%) or mean cell volume (MCV) (11.2%). The majority of patients were on HU for 6 months (34%) or 1 year (36.3%) until absence of clinical benefit was determined.
Providers used variable methods to assess adherence. The majority utilized patient interview (84.2%), MCV (75.3%), or HbF (70%). Other less common methods included obtaining pharmacy records (22.8%), pill count (7.9%), and patient logs (6%).
The NHLBI recently released draft guidelines for HU use in SCD that propose monitoring every 8 weeks after patients reach a stable dose.(11) These guidelines identified optimal frequency of monitoring as a research gap. They also state future research must ensure toxicities are appropriately monitored, however, this research should also account for the fact that adherence with monitoring is a barrier to HU use. Our data reveal most providers follow labs monthly and almost half see patients monthly; however, it is unclear whether this frequency of monitoring prevents toxicity. In SCD patients, frequent monitoring may be an important component to non-adherence. In a study of HU adherence, patients identified obtaining pharmacy refills and coming to clinic as barriers to HU use.(10) Interestingly, only 28% of families felt more frequent clinic visits would improve adherence.(10)
Our study suggests the most common reason HU was permanently discontinued was the absence of clinical benefit, not toxicity. Our data do not reveal how often providers discontinue HU for this reason; they only reveal providers identified absent clinical benefit as the most common reason HU was discontinued. This finding was interesting in light of the potential for HU to prevent end-organ damage. Our prior work found acute chest syndrome and pain were the most common reasons for starting HU, not improved end-organ function (1). Therefore, if these clinical symptoms did not improve, patients were likely perceived to have not had clinical benefit. Furthermore, since non-adherence is a barrier to HU utilization, it is possible that the lack of clinical benefit may be due, in part, to non-adherence. In a prior efficacy trial of HU in children with SCD, treatment failures were likely due to non-adherence instead of lack of efficacy.(12)
Existing data reveal adjusting HU using MTD is associated with higher Hb, MCV and HbF.(9) MTD is also associated with improved and sustained clinical and hematological benefit.(9, 13) Although MTD was the most common method used to adjust HU dosing, our data reveal 40% of providers do not use this proven method. Guidelines outlining use of MTD to adjust HU dosing may guide providers in using HU to its maximum benefit.
We found HU adherence was assessed by the majority of providers using patient interview or a laboratory parameter (MCV, HbF). Patient interview is a proven unreliable method of assessing adherence in children with asthma.(14, 15) We have no reason to believe this method will be more reliable in SCD; however, this has not been specifically studied. Future research should evaluate the reliability of patient interview for assessing HU adherence. In addition, using laboratory parameters to assess HU adherence can be misleading since data reveal adherence explained only 23% of HbF variability, suggesting patients could be adherent and benefit clinically from HU without a good HbF response.(16) Although HU can increase MCV and HbF, this response is not universal thus alternative methods of assessing adherence may be more appropriate for these patients. A minimal number of providers used methods likely more reliable for assessing adherence such as following pharmacy records, pill count, or patient logs.(17) A combination of methods (i.e. pill count, patient logs, pharmacy refills, laboratory parameters) is likely most optimal for assessing adherence. Novel methods of promoting adherence, such as using text messaging that is proven effective in diabetes (18), may also be needed. Optimizing adherence is necessary as opposed to not prescribing HU because of non-adherence.
Our study includes some limitations. Our targeted population included only ASPHO members and practice patterns of non-ASHPO members may differ. The data were self-reported. Since the survey was anonymous, we have no data about non-responders and we could not analyze practice variability within the same institution. The use of blood smear examination or ANC to assess adherence was not asked. Future work should assess these as markers of adherence. The response rate was 31%. A definitive response rate deemed acceptable or unacceptable in survey research is not clear from the literature. Other published survey research reveals similar response rates.(19-21) Moreover, the denominator of ASPHO members practicing hematology and caring for children with SCD is unknown by ASPHO.
In summary, the majority of providers report monthly monitoring of patients on HU, almost half do not adjust dosing using the proven method of MTD, and the majority assess adherence with an unreliable method. Research into the optimal frequency of monitoring, promoting use of MTD to adjust dosing, and alternative methods of promoting and assessing adherence are necessary to encourage safe utilization of HU. Addressing these issues becomes vital as indications for HU in SCD are expanded.
Methods
These survey data were collected as part of a larger study evaluating the utilization of HU in children with SCD, thus details of the survey methodology have been previously published.(1) Briefly, an anonymous web-based cross-sectional survey was emailed to 1316 pediatric hematology/oncology providers identified through the published 2008 ASPHO membership directory. Of those emailed, 247 returned undeliverable, 59 of these emails were subsequently successfully delivered, and thus the final number of emails successfully delivered was 1128. A flow diagram documenting the final study population has been previously published.(1) ASPHO is an international professional society of pediatric hematology/oncology providers who are in some way involved in the care and/or research of children with cancer and other blood diseases. Details of the survey questions that address the main objectives of the manuscript are included as supplemental material. To increase honesty in responding, the survey was administered anonymously. The anonymity of the survey also allowed for individuals to report on their own practice even if this practice differed from other providers within the same institution. Initial and reminder emails were sent from February through July 2009. Reminder emails were sent only to non-responders and the survey program allowed for a single survey completion from each email address. Providers that take care of patients with SCD were identified by the survey, thus the response rate includes all respondents; however, the remainder of the data include only those individuals providing care to children with SCD.
The study was approved by the Institutional Review Board of the Children's Hospital of Wisconsin, which allowed for completion of the survey to serve as implied consent.
Statistical Analysis
All statistical analyses were conducted with SPSS version 14.0 for Windows (SPSS, Chicago, IL). Respondent survey data were extracted, inputted by hand into SPSS, and descriptive statistics were calculated. We report proportions, medians, and interquartile ranges (IQRs) as appropriate.
Acknowledgments
The authors would like to thank Danielle Jirovec for her assistance with data collection and Matthew Myrvik, PhD for his critical review of the manuscript. This work was supported in part by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute U54 HL090503 (AB), K23 HL80092 (JP), and the Midwest Athletes Against Childhood Cancer Fund.
Funding: This work was supported in part by a grant from the National Institutes of Health National Heart, Lung, and Blood Institute U54 HL090503 (AB) and K23 HL80092 (JP), and the Midwest Athletes Against Childhood Cancer Fund
Footnotes
Conflicts of Interest: None for any of the authors
Author Contributions: A.M. Brandow designed research, performed research, analyzed data, and wrote manuscript. J. A. Panepin to designed research and revised manuscript.
References
- 1.Brandow AM, Jirovec DL, Panepinto JA. Hydroxyurea in children with sickle cell disease: practice patterns and barriers to utilization. Am J Hematol. 2010;85:611–613. doi: 10.1002/ajh.21749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brawley OW, Cornelius LJ, Edwards LR, Gamble VN, Green BL, Inturrisi C, James AH, Laraque D, Mendez M, Montoya CJ, Pollock BH, Robinson L, Scholnik AP, Schori M. National Institutes of Health Consensus Development Conference statement: hydroxyurea treatment for sickle cell disease. Annals of Internal Medicine. 2008;148:932–938. doi: 10.7326/0003-4819-148-12-200806170-00220. [DOI] [PubMed] [Google Scholar]
- 3.Lanzkron S, Haywood C, Jr, Hassell KL, Rand C. Provider barriers to hydroxyurea use in adults with sickle cell disease: a survey of the Sickle Cell Disease Adult Provider Network. J Natl Med Assoc. 2008;100:968–973. [PubMed] [Google Scholar]
- 4.Zumberg MS, Reddy S, Boyette RL, Schwartz RJ, Konrad TR, Lottenberg R. Hydroxyurea therapy for sickle cell disease in community-based practices: a survey of Florida and North Carolina hematologists/oncologists. Am J Hematol. 2005;79:107–113. doi: 10.1002/ajh.20353. [DOI] [PubMed] [Google Scholar]
- 5.Voskaridou E, Christoulas D, Bilalis A, Plata E, Varvagiannis K, Stamatopoulos G, Sinopoulou K, Balassopoulou A, Loukopoulos D, Terpos E. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS) Blood. 2010;115:2354–2363. doi: 10.1182/blood-2009-05-221333. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg MH, Barton F, Castro O, Pegelow CH, Ballas SK, Kutlar A, Orringer E, Bellevue R, Olivieri N, Eckman J, Varma M, Ramirez G, Adler B, Smith W, Carlos T, Ataga K, DeCastro L, Bigelow C, Saunthararajah Y, Telfer M, Vichinsky E, Claster S, Shurin S, Bridges K, Waclawiw M, Bonds D, Terrin M. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289:1645–1651. doi: 10.1001/jama.289.13.1645. [DOI] [PubMed] [Google Scholar]
- 7.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 8.Ferster A, Vermylen C, Cornu G, Buyse M, Corazza F, Devalck C, Fondu P, Toppet M, Sariban E. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood. 1996;88:1960–1964. [PubMed] [Google Scholar]
- 9.Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood. 2010;115:5300–5311. doi: 10.1182/blood-2009-04-146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornburg CD, Calatroni A, Telen M, Kemper AR. Adherence to hydroxyurea therapy in children with sickle cell anemia. J Pediatr. 2010;156:415–419. doi: 10.1016/j.jpeds.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.rover.nhlbi.nih.gov/guidelines/scd/about.htm.
- 12.de Montalembert M, Brousse V, Elie C, Bernaudin F, Shi J, Landais P. Long-term hydroxyurea treatment in children with sickle cell disease: tolerance and clinical outcomes. Haematologica. 2006;91:125–128. [PubMed] [Google Scholar]
- 13.Zimmerman SA, Schultz WH, Davis JS, Pickens CV, Mortier NA, Howard TA, Ware RE. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103:2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 14.Burgess SW, S P, Morawska A, Devadason SG. Assessing adherence and factors associated with adherence in young children with asthma. Respirology. 2008;13:559–563. doi: 10.1111/j.1440-1843.2008.01292.x. [DOI] [PubMed] [Google Scholar]
- 15.Jentzsch NS, Camargos PA, Colosimo EA, Bousquet J. Monitoring adherence to beclomethasone in asthmatic children and adolescents through four different methods. Allergy. 2009;64:1458–1462. doi: 10.1111/j.1398-9995.2009.02037.x. [DOI] [PubMed] [Google Scholar]
- 16.Thornburg CD, Calatroni A, Telen M, Kemper AR. Adherence to hydroxyurea therapy in children with sickle cell anemia. J Pediatr. 2010;156:415–419. doi: 10.1016/j.jpeds.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 18.Franklin VL, Waller A, Pagliari C, Greene SA. A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabet Med. 2006;23:1332–1338. doi: 10.1111/j.1464-5491.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Joffe S, Kim HT, Socie G, Gilman AL, Wingard JR, Horowitz MM, Cella D, Syrjala KL. Physicians' attitudes about quality-of-life issues in hematopoietic stem cell transplantation. Blood. 2004;104:2194–2200. doi: 10.1182/blood-2003-07-2430. [DOI] [PubMed] [Google Scholar]
- 20.Wong EC, Perez-Albuerne E, Moscow JA, Luban NL. Transfusion management strategies: a survey of practicing pediatric hematology/oncology specialists. Pediatr Blood Cancer. 2005;44:119–127. doi: 10.1002/pbc.20159. [DOI] [PubMed] [Google Scholar]
- 21.Streiff MB, Smith B, Spivak JL. The diagnosis and management of polycythemia vera in the era since the Polycythemia Vera Study Group: a survey of American Society of Hematology members' practice patterns. Blood. 2002;99:1144–1149. doi: 10.1182/blood.v99.4.1144. [DOI] [PubMed] [Google Scholar]