Abstract
Introduction
The Ras proteins (K-Ras, N-Ras, H-Ras) are GTPases that function as molecular switches for a variety of critical cellular activities and their function is tightly and temporally regulated in normal cells. Oncogenic mutations in the RAS genes, which create constitutively-active Ras proteins, can result in uncontrolled proliferation or survival in tumor cells.
Areas covered
The paper discusses three therapeutic approaches targeting the Ras pathway in cancer: 1) Ras itself, 2) Ras downstream pathways, and 3) synthetic lethality. The most adopted approach is targeting Ras downstream signaling, and specifically the PI3K-AKT-mTOR and Raf-MEK pathways, as they are frequently major oncogenic drivers in cancers with high Ras signaling. Although direct targeting of Ras has not been successful clinically, newer approaches being investigated in preclinical studies, such as RNA interference-based and synthetic lethal approaches, promise great potential for clinical application.
Expert opinion
The challenges of current and emerging therapeutics include the lack of “tumor specificity” and their limitation to those cancers which are “dependent” upon aberrant Ras signaling for survival. While the newer approaches have the potential to overcome these limitations, they also highlight the importance of robust preclinical studies and bidirectional translational research for successful clinical development of Ras-related targeted therapies.
1. Introduction
The Ras proteins, H-Ras, K-Ras and N-Ras, are GTPases which regulate signal transduction underlying diverse cellular activities, including proliferation, survival, growth, migration, differentiation or cytoskeletal dynamism. GTP-bound (“on-state”) Ras proteins convert extracellular stimuli into intracellular signaling cascades, which eventually evoke changes in cellular activities; this signaling ceases when Ras-bound GTP is hydrolyzed to GDP as the result of another signaling cascade. Thus, in normal cells, Ras proteins function as molecular switches for critical changes in cellular activities, such as cell proliferation and survival, and their proper and tight regulation is indispensable to maintain the homeostasis of cells and, ultimately, the entire organism.
Conversely, uncontrolled activity of the Ras proteins, or the molecular components of their downstream pathways, can result in serious consequences, including cancers and other diseases. Indeed, approximately 30% of human tumors are estimated to harbor activating mutations in one of the three Ras isoforms: KRAS, NRAS and HRAS (1). KRAS is most frequently mutated among three isoforms in malignancies; its mutation rate in all tumors is estimated to be 25–30% (1). KRAS mutation is especially prominent in colorectal carcinoma (40–45% mutation rate), non-small cell lung cancer (NSCLC) (16–40%) and pancreatic ductal carcinoma (69–95%) (1). In contrast, activating mutations of NRAS and HRAS are less common (8% and 3% mutation rate, respectively). Malignant melanomas predominantly harbor NRAS mutations (20–30% prevalence) (1). The activating oncogenic mutations most commonly occur in codons 12, 13 and 61, in the GTPase catalytic domains, identically among the three isoforms. 80% of KRAS mutations are observed in codon 12, whereas NRAS mutations preferentially involve codon 61 (60%) compared to codon 12 (35%) (2). HRAS mutations are divided almost equally among codon 12 (50%) and codon 61 (40%) (2). Regardless of isoform type or codon location, all these activating mutations render Ras proteins resistant to GTP hydrolysis (and consequent Ras inactivation) stimulated by GTPase-activating proteins (GAPs). These constitutively-activated oncogenic Ras mutant proteins, therefore, initiate intracellular signaling cascades without the input of extracellular stimuli, resulting in uncontrolled cell proliferation and abnormal cell survival.
2. Ras proteins
Due to the space limitations, this section is focused on the basic background of Ras protein biology and biochemistry, particularly related to the therapeutic interventions to be discussed later. For further details on the biology and biochemistry of the Ras proteins, their activation by upstream signaling pathways, and their downstream signaling pathways, readers should refer to the excellent reviews listed in references (2–7).
2.1 Structure
The two major structural components in Ras proteins are the catalytic domain, called the G domain, and the C-terminal hypervariable region (HVR). The catalytic G domain, which is highly homologous among the three isoforms, contains the phosphate-binding loop (P-loop) and two parts of the nucleotide-binding switch regions (Switch I and Switch II) (2). All of the frequently mutated amino acid residues (Gly12, Gly13 and Gln61) are located within these motifs, which are critical for Ras catalytic activity. The HVR is the site of post-translational modifications that are required for Ras proteins to be translocated to the plasma membrane. The HVRs of the three isoforms share only 15% homology, and this divergence is proposed to contribute to the functional differences among the isoforms, although has not yet been definitively linked to function (8). Each Ras isoform undergoes a slightly different post-translational modification process due to the sequence variation in the HVRs, which thereby defines what set of mediator enzymes are allowed to access to the HVR.
To become functionally active, newly-synthesized Ras proteins are subjected to a series of post-translational modifications (9). After translation in the cytosol, Ras proteins are farnesylated on the cysteine within the “CAAX box” motif, the C-terminal region in the HVRs. This brings immature Ras proteins to the ER, where the CAAX box is truncated by proteolysis and methylated. The final modification, palmitoylation, matures Ras proteins for translocation to the plasma membrane. As Ras cannot be activated without membrane translocation, farnesylation is essential for Ras function and has been intensively studied as a target for potential pharmacological interventions. Studies using farnesyltransferase (FTase) inhibitors (FTIs), however, revealed that K-Ras and N-Ras alternatively can be geranylgeranylated, which is equally capable of facilitating translocation of Ras proteins to the membrane when farnesylation is inhibited by FTIs (10).
Ras proteins anchor in the cytoplasmic membrane via the HVR once they reach the membrane. In some cases, the Ras proteins are bound by Ras-escort proteins in the HVR. These proteins include galactin-1 and galactin-3, which have strong binding affinity to GTP-H-Ras and GTP-K-Ras, respectively (11). Ras-escort proteins stabilize the Ras proteins in the GTP-bound (active) state (6). Disruption of the interaction between these escort proteins and Ras has been exploited as a strategy to modulate aberrant Ras signaling.
2.2 Function
The importance of K-Ras expression during development is illustrated by the embryonic lethality of K-Ras knockout mice, as a result of liver defects and anemia (3). In contrast, mice with HRAS or NRAS knockouts are completely viable without any obvious phenotypes (3). Although mouse models do not entirely mimic human tumorigenesis, transgenic and knock-in mouse models provide proof of the physiological contribution of oncogenic Ras proteins to tumorigenesis. Expression of oncogenic H-Ras or K-Ras under tissue-specific promoters induces various types of malignancies in multiple transgenic mouse models (4). For example, one conditional K-Ras G12D knock-in model produced lung tumors after activation of the oncogenic KRAS gene.
2.3 Proteins controlling Ras
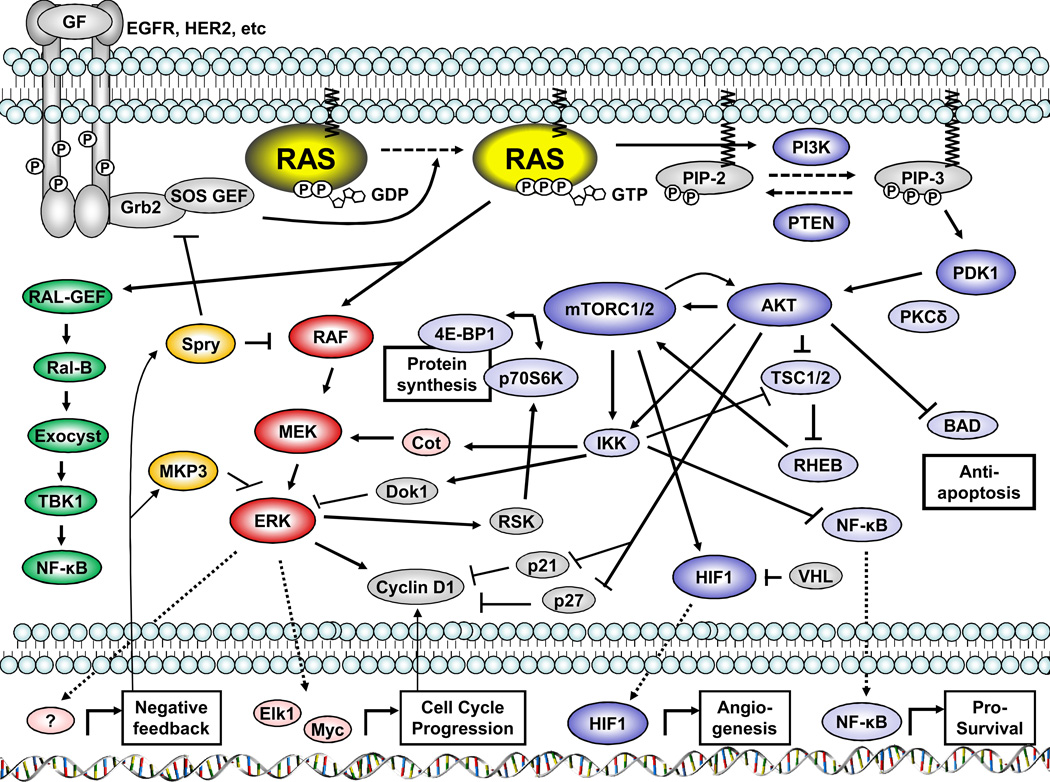
As cell proliferation signaling should be initiated only when it is required for growth, development, or tissue repair, predominantly via an extracellular stimulus (eg., receptor tyrosine kinases (RTKs) or G-protein-coupled receptors), the activity of the Ras proteins is normally tightly and temporally controlled in normal cells. For example, upon the arrival of ligands/growth factors to RTKs, the receptors homo- or hetero-dimerize, autophosphorylate each other on specific tyrosine residues and recruit adaptor proteins (e.g., Grb2 or Shc) to their SH2 domain(s), which leads to recruitment of guanine nucleotide exchange factors (GEFs) to the plasma membrane (Figure 1). Ras becomes activated when a GEF stimulates dissociation of GDP, allowing rapid replacement by the more abundant GTP. Conformational changes caused by binding of GTP increase the binding affinity of the Ras proteins to their downstream effectors, such as the Raf family proteins or the phosphatidylinositol 3 kinases (PI3K), which in turn activate a series of kinase chain-reactions. Activated Ras is eventually inactivated by hydrolysis of the bound GTP, which is accelerated by GAPs. Because the exchange of GDP and GTP is an extremely slow process in both directions under physiological conditions without catalysis by GAPs and GEFs, the balance between GAP and GEF activities is a crucial regulatory mechanism for Ras activation status (for review of GAPs and GEFs, see (12)).
Figure 1.
The aberrant activity of any of the molecules involved in Ras activation can be oncogenic. RTK family members, including epidermal growth factor receptors (EGFRs), HER2/ERBB2 or insulin-like growth factor 1 receptor (IGF1R) are frequently hyperactivated due to overexpression, genetic mutation and/or gene amplification in many types of cancers including lung, colon, breast, ovarian and stomach carcinomas (13).
2.4 Downstream effectors of Ras
The proximal downstream Ras effectors are defined as proteins which have a strong affinity to GTP-Ras, are thereby activated, and initiate a subsequent cascade of signaling (5). Ras effectors share a characteristic Ras-binding domain (the Ras core effector domain). Among more than 10 reported Ras effectors, Raf and PI3K and their downstream pathways have been most extensively studied, because of their importance both in the normal physiological setting and in tumorigenesis. Thus, these pathways have been the primary targets of cancer drug discovery and development.
The Raf-MEK-ERK pathway comprises the major part of the mitogen-activated protein kinase (MAPK) pathway system, and the Raf kinases are on the MAPK-kinase-kinase (MAPKKK) tier. The Raf family consists of three isoforms: A-Raf, B-Raf and C-Raf/Raf-1. B-Raf is the strongest MEK kinase and A-Raf is the weakest MEK activator. A-Raf preferentially activates MEK1, while B-Raf and C-Raf activate both MEK1/2 with equal efficiency (6). Activation of MEK1/2 by Raf family leads to the activation of the MAPK, ERK. The BRAF gene is mutated in 66% of melanomas and 12% of colorectal cancers, whereas mutations of C-Raf, A-Raf or MEK1/2 are rarely found in any cancer (6, 14). Regardless of the location of the mutation or aberrant activation in cascade, abnormalities in this pathway lead to elevation of phospho (activated) -ERK1/2, as observed in numerous human cancers. Activated ERKs are translocated to the nucleus and activate transcription factors whose target genes include regulators of cell proliferation or cell cycle regulation, or, in some cases, negative feedback regulators of the Raf-MEK-ERK pathway (Figure 1).
PI3Ks convert phosphatidylinositol (4,5)-bisphosphate (PIP-2) to phosphatidylinositol (3, 4,5)-triphosphate (PIP-3) by phosphorylation. Although there are three classes of PI3Ks, class I PI3Ks have been most studied and are almost exclusively the target of pharmacological PI3K inhibitors of all classes. PI3Ks are heterodimeric proteins consisting of one catalytic subunit (isoforms: p110α/PIK3CA, p110β/PIK3CB, p110δ/PIK3CD) and one regulatory subunit. PDK1 is recruited to the membrane by PIP-3, is activated, and phosphorylates AKT at Thr308. There are three AKT isoforms (AKT1/2/3). As AKTs exert either survival or apoptotic signaling, depending upon the cellular context, the downstream substrates of AKTs include a wide range of proteins, such as apoptotic regulators (e.g., BAD), transcription factors (e.g., FOXO), and other kinases (e.g., glycogen synthase kinase-3β (GSK3β), tuberous sclerosis 2 (TSC2))(7). Mammalian target of rapamycin [mTOR] is a serine/threonine kinase comprised of two types of multi-kinase complexes. mTOR complex 1 (mTORC1), regulated by TSC2, phosphorylates ribosomal S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1). Consequently, mTORC1 plays an important role in translational initiation. mTOR complex 2 (mTORC2) not only lies downstream of AKTs, but also contributes to the activation of AKTs by phosphorylating AKTs on Ser473 following initial Thr308 phosphorylation by PDK1 (15)(Figure 1).
2.5 The components of Ras signaling provide potential therapeutic targets
Because of its central role in intracellular signal transduction, malignant transformation and progression (including proliferation, migration, morphological changes and epithelial-mesenchymal transition [EMT]), Ras proteins have been a focus of research in cancer drug discovery and development. “Oncogene addiction” describes a model in which cancer cells are highly dependent upon the activity of a single oncogene (despite many other genetic abnormalities) for continued tumor cell proliferation and survival. KRAS “addiction” is among the best known examples (16). However, it is clear that the presence of a mutated Ras allele in a given tumor does not predict “oncogene addiction.” Indeed, tumor types which are uniformly addicted to a single, specific oncogene (i.e., BCR-ABL in chronic myelogenous leukemia), appear to be the exceptions rather than the rule. While targeting Ras proteins or mutant forms of Ras proteins directly became the early strategy, a number of issues have confounded this approach, and the Ras proteins themselves are no longer considered feasible pharmaceutical targets, as will be discussed later. The current most widely-adopted strategy is to target instead the components of Ras downstream signaling pathways, such as the Raf-MEK-ERK or PI3K-AKT-mTOR pathways. There have already been some notable clinical successes stemming from this approach, and many other drug candidates with better drug properties and target specificity are under clinical investigation. A newer approach, sometimes termed “synthetic lethality,” is to selectively attack cancer cells by targeting another protein, which is independent of the Ras signaling pathway, but upon which cells with mutant Ras expression (tumor cells) are dependent. This state is also sometimes termed “non-oncogene addiction.” In this approach, the activated, mutated Ras signaling is utilized as a cancer cell marker rather than drug target.
3. Targeting Ras directly
Unlike the case for many kinase inhibitors, targeting the catalytic domain of the Ras proteins is technically challenging, due to the structural characteristics of GTPases (8). This limitation redirected efforts to directly target Ras proteins into two alternative strategies; 1) preventing the expression of Ras proteins; or, 2) blocking the localization of Ras proteins to the plasma membrane where Ras proteins are activated and then function as a molecular switch.
3.1 Inhibiting Ras expression
The first approach utilizes the gene silencing techniques that prevent mRNAs of Ras proteins from being translated. Gene silencing technology utilizes two different methodologies: antisense oligodeoxynucleotides (ODNs), or RNA interference (RNAi). ISIS2503, an antisense ODN against H-Ras, produced selective suppression of H-Ras mRNA and protein in cell culture systems, and showed antitumor activities in mouse xenograft models including a pancreatic carcinoma system (17, 18). In a phase I trial, ISIS2503 was well tolerated with relatively minor adverse events, although no consistent reduction in H-Ras mRNA levels were observed in patients’ peripheral blood lymphocytes (18). Single-agent phase II trials in the patients with advanced colorectal cancer, pancreatic cancer and NSCLC did not address clinical activities (19–21). Phase II trials of ISIS2503 in combination with gemcitabine in advanced pancreatic cancer, with docetaxel in previously treated advanced NSCLC, and with paclitaxel in metastatic breast cancer, failed to demonstrate a significant improvement in response rate and survival rate, or tumor regression, compared to conventional treatment alone (22–24). The failure of ISIS2503 in human trials can be explained by insufficient recognition of the importance of the genetic background in the diseases targeted. The development of ISIS2503 was based on in vitro studies in which ISIS2503, but not a K-Ras-specific ODN, exhibited anti-tumor activity (17). However, H-Ras was infrequently mutated in the cancer types that were targeted in these clinical trials. The much more frequently mutated K-Ras has also been targeted for potential clinical application; however, the effect of K-Ras antisense ODN on tumor cell growth inhibition appears to be more variable, and unpredictably dependent upon cell or ODN types (17, 25, 26).
An advantage of the more recent RNAi technology is the extraordinary specificity against the target sequence, enabling selective silencing of an oncogenic Ras with a single point mutation, so that treatment could spare normal cells expressing a wild-type Ras (Figure 2). Several groups reported that selective knockdown of mutant K-Ras or H-Ras via small interfering RNA (siRNA) induced significant growth inhibition in cell lines of pancreatic cancers, lung cancers, colorectal cancers, and ovarian cancers, and, more encouragingly, in animal models (27–32). Although RNAi-based therapy has not progressed to human testing in malignant conditions, it appears to have better clinical potential in comparison to antisense ODNs, based on the predicted in vivo knockdown efficacy and applicability for K-Ras targeting. Nonetheless, there are very significant challenges in terms of delivery of the RNAi to the local tumor environment. High molecular-weight molecules/drugs like nucleic acids are generally more difficult to deliver effectively, and exogenous RNA could become the target of neutralization by the immune system. Furthermore, some studies have demonstrated that silencing a Ras gene/protein alone may not be sufficient to kill all tumors containing activated Ras, but rather only those tumors in which the activated Ras is critical for the survival of the tumor (“Ras-dependent” tumors). This consideration has led to the alternative concept of exploiting the finding that tumor cells harboring oncogenic RAS mutations may become dependent upon other non-oncogenic proteins for survival (“non-oncogene dependency”). Inhibition or knockdown of this non-oncogenic protein can then efficiently induce selective cytotoxicity in the Ras-mutant tumor cells, while sparing normal cells (“synthetic lethality,” to be discussed later).
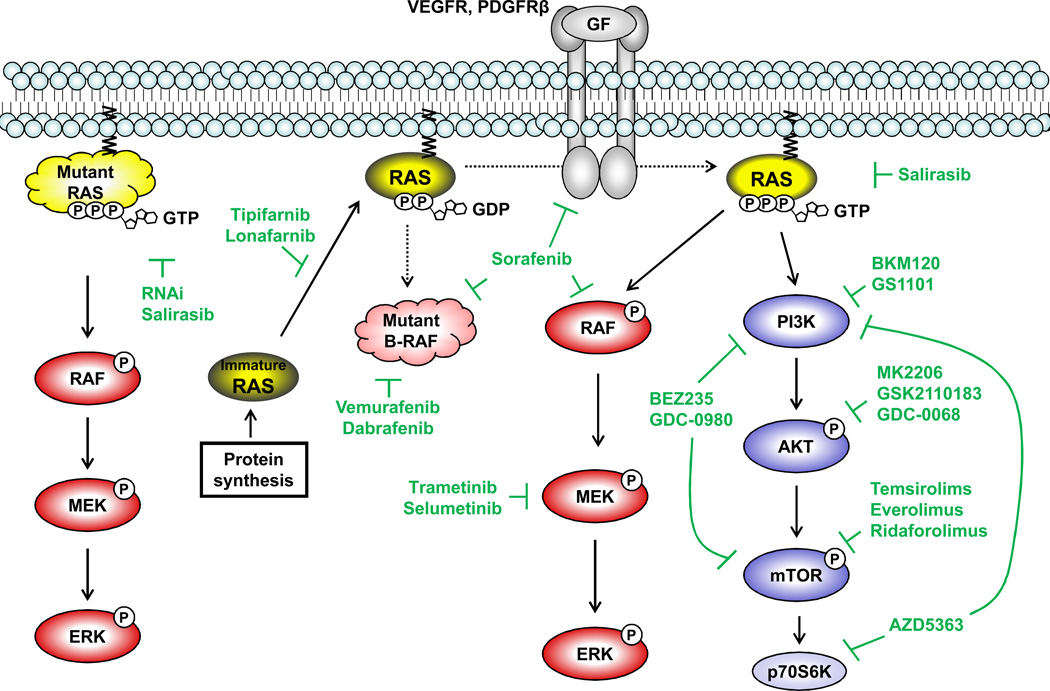
Figure 2.
3.2 Inhibiting Ras protein processing
Aside from preventing the translation of Ras proteins by RNAi, an alternative approach to targeting Ras involves the prevention of newly-translated Ras proteins from being activated, by inhibiting the post-translational modification that is necessary to translocate Ras to the plasma membrane, using FTIs. Preclinical studies demonstrated the potency of FTIs, showing efficacy against H-Ras and K-Ras substrates, and tumor growth inhibition in vitro and in vivo in a number of cancer cell line models (33–35). To date, two FTIs, tipifarnib and lonafarnib, advanced to Phase III trials; but with little success so far. Phase II trials with tipifarnib produced no responses in the most of the cancers evaluated including metastatic pancreatic cancer, NSCLC, and advanced colon cancer, but some activity in advanced breast cancer and myelodysplastic syndrome (MDS) (36–41). Multiple phase III trials of tipifarnib monotherapy in acute myeloid leukemia (AML) and in refractory advanced colorectal cancer, and in combination with gemcitabine in advanced pancreatic cancer, did not produce improvements in overall survival (42–44). A phase II/III trial of the combination of tipifarnib and gemtuzumab ozogamicin has been initiated in AML and high-risk MDS; however, the current status of the trial is unknown due to withdrawal of gemtuzumab ozogamicin from the market (45). A phase III trial of lonafarnib in combination therapy with carboplatin/paclitaxel failed to improve overall survival in advanced or metastatic NSCLC (46).
The disappointing clinical outcomes in most clinical trials testing FTIs were hinted at in some preclinical studies. For example, cell lines with no RAS mutations were also susceptible to FTIs both in vitro and in the in vivo xenograft models (33, 47), suggesting that the drugs did not selectively target oncogenic Ras. Additionally, while H-Ras is an exclusive target of FTases, K-Ras and N-Ras become geranylgeranylated alternatively in the presence of FTIs, so that they are still translocated to the plasma membrane for full activation (10). Accordingly, it was then proposed that the combinational use of FTIs and geranylgeranyltransferase (GGTase) inhibitors (GGTIs) would be required to suppress K-Ras activity (48). However, such combinations might produce undesired toxicity to normal cells, by inhibition of processing of critical molecules other than Ras that require these modifications for activation. In addition, the lack of validated biomarkers to measure any inhibitory effect of the drugs on FTase activity in clinical trials made it more challenging to assess whether pharmacodynamic goals were being achieved with the dosing regimens utilized. Finally, although FTIs were initially developed as Ras-specific inhibitors in the preclinical setting, FTIs and GGTIs appear to also act via unidentified “off-target” pathways and can no longer be considered as Ras-specific inhibitors.
In contrast to FTIs and GGTIs that are intended to inhibit the membrane recruitment of Ras proteins, the intended action of salirasib (s-trans, trans-farnesylthiosalicylic acid), a Ras farnesylcysteine mimetic, is to dislodge oncogenic Ras proteins, or physiologically-activated Ras proteins, from the plasma membrane by competing with Ras for binding to the Ras-escort proteins galectin 1 and galectin 3 (Figure 2) (11). Salirasib was shown to inhibit activation of the Raf-MEK-ERK pathway, and inhibit tumor growth, in both in vitro and in vivo in models of pancreatic, lung, colorectal, and hepatocellular carcinomas (HCCs) and brain tumors (49–52). Salirasib was well tolerated as both a single agent or in combination with gemcitabine: 79% of patients showed no drug-related toxicity greater than grade 1 (53, 54). Approximately 40% down-regulation of K-Ras expression was observed in paired biopsies from accessible tumors in two subjects (53). So far, phase I and II trials of salirasib as a single agent, or in combination with gemcitabine, in metastatic pancreatic adenocarcinoma and lung adenocarcinoma have been completed. Although have been reported (e.g., stable disease, 1-year survival rate) (53, 54), extensive further clinical testing will be required to determine if there is a significant impact on tumor response and survival, as well as reliable proof of target modulation.
4. Targeting Ras Effectors
While many investigational drugs targeting Ras effectors remain in early phase trials (Table 1), four such drugs have reached the market to date: mTOR inhibitors (temsirolimus and everolimus) and RAF inhibitors (sorafenib and vemurafenib). In general, there appears to be two approaches in the current Ras-effector drug developmental strategies; 1) focusing on particular types of disease areas by targeting one or a few isozyme(s) in the same kinase class; and 2) extending the target disease areas by expanding into diseases which share a similar genetic background or activation of similar signaling pathways, using a multi-kinase inhibitor.
Table 1.
Investigational drugs targeting Ras effectors
| Name | Targets | Currently most advanced trial phase (Combination) |
Cancer types | Clinicaltrials.gov identification | References |
|---|---|---|---|---|---|
| BKM120 | Class I PI3K | III (single), III (+ Fulvestrant) | Previously-treated ER+/HER2- breast cancer | NCT01610284, NCT01633060 | (57, 58) |
| GS1101, CAL101 |
PI3Kδ | III (single), III (+ Rituximab), III (+ Bendamustine/Rituximab), III (+ Ofatumumab) |
CLL |
NCT01539291, NCT01539512, NCT01569295, NCT01659021 |
(59, 65- 68) |
| GDC-0941 | PI3Kα/ δ | II (+ Fulvestant), II (+ Carboplatin/Paclitaxel, + Carboplatin/Paclitaxel/Bevacizumab ) |
AI-refractory advanced or metastatic breast cancer, Advanced or recurrent NSCLC |
NCT01437566, NCT01493843 | (145- 147) |
| PX-866 | PI3Kα/γ/δ | II (single), II (single) | Recurrent or metastatic castration resistant prostate cancer, Glioblastoma multiforme |
NCT01331083, NCT01259869 | (148, 149) |
| BAY80–6946 | Class I PI3K | II (single) | Relapsed, indolent or aggressive non- Hodgkin's lymphomas |
NCT01660451 | (150) |
| XL147 | Class I PI3K | II (single), I/II (+ Letrozole) | Advanced or recurrent endometrial cancer, Breast cancer |
NCT01013324, NCT01082068 | (151, 152) |
| BYL719 | PI3Kα | I/II (+Cetuximab), Ib (+MEK162) | Recurrent or metastatic head and neck squamous cell carcinoma, Advanced solid tumors |
NCT01602315, NCT01449058 | (153) |
| INK1117 | PI3Kα | I (single) | Metastatic solid tumors | NCT01449370 | |
| IPI-145 | PI3Kγ/δ | I (single) | Advanced hematologic malignancies | NCT01476657 | |
| ZSTK474 | Class I PI3K | I (single) | Advanced solid malignancies | NCT01280487 | (154) |
| AMG319 | PI3Kδ | I (single) | Relapsed or refractory lymphoid malignancies | NCT01300026 | |
| MK2206 | AKT1/2 | II (single), II (single), II (single), II (single), II (single), II (single), II (+Erlotinib Hydrochloride), II (single), II (single), II (+AZD6244), II (single) |
Refractory RCC, Refractory HCC, Refractory advanced gastric or gastroesophageal junction cancer, Platinum-refractory ovarian/fallopian tube/peritoneal cancer, Advanced PIK3CA(mu)/PTEN loss breast cancer, Relapsed refractory advanced NSCLC, Relapsed refractory acute myelogenous leukemia, Recurrent or advanced PIK3CA(mu) endometrial carcinoma, Recurrent or metastatic nasopharyngeal carcinoma, Advanced colorectal carcinoma, Relapsed or refractory DLBCL |
NCT01239342, NCT01239355, NCT01260701, NCT01283035, NCT01277757, NCT01294306, NCT01253447, NCT01307631, NCT01349933, NCT01333475, NCT01481129 |
(71–74, 155) |
| GSK2110183 | AKT1/2/3 | II (single), II (+ Ofatumumab) | Hematologic malignancies, CLL | NCT01531894, NCT01532700 | (75) |
| GDC-0068 | AKT1/2/3 | I (+ GDC-0973), I (+ GSK1120212) | Advanced solid tumors, Locally advanced or metastatic solid tumors |
NCT01562275, NCT01138085 | (76) |
| AZD5363 | AKT1/2/3, p70S6K, PKA, 14 other AGCs |
I (single), I (single), I (+paclitaxel) | Advanced solid malignancy/advanced or metastatic PIK3CA(mu) breast cancer, Advanced solid malignancy, Breast cancer |
NCT01226316, NCT01353781, NCT01625286 |
(77, 78) |
| ARQ092 | AKT1/2/3 | I (single) | Advanced solid tumors | NCT01473095 | (156) |
| GSK2141795 | AKT1/2/3 | I (+ GSK1120212) | Pancreatic cancer/endometrial cancer/colorectal cancer |
NCT01138085 | (157) |
| Ridaforolimus | mTORC1 | III (single), II (+ Exemestane) | Advanced bone and soft tissue sarcomas, Post-menopausal breast cancer |
NCT00538239, NCT01605396 | (83, 84) |
| CC-223 | mTORC1/2 | I/II (single), I (+ Erlotinib or oral Azacitidine) |
Refractory advanced tumors, NSCLC | NCT01177397, NCT01545947 | (158) |
| ME-344 | mTORC1/2 | I (single) | Refractory solid tumors | NCT01544322 | |
| AZD2014 | mTORC1/2 | I (single), I (+ Fulvestrant) | Advanced solid malignancies, Advanced metastatic breast cancer |
NCT01026402, NCT01597388 | (159) |
| OSI-027 | mTORC1/2 | I (single) | Solid tumors or lymphoma | NCT00698243 | (160) |
| BEZ235 | Class I PI3K , mTORC1/2 |
II (single), II (single) | Advanced pNET, pNET after failure of mTOR Inhibitor therapy |
NCT01628913, NCT01658436 | (86–92) |
| GDC-0980 | Class I PI3K , mTORC1/2 |
II (+ Fulvestrant), II (single), II (+ Abiraterone Acetate) |
AI-refractory advanced or metastatic breast cancer, Recurrent or persistent endometrial carcinoma, Castration-resistant prostate cancer previously- treated with docetaxel |
NCT01437566, NCT01455493, NCT01485861 |
(93, 94) |
| PF-04691502 | Class I PI3K , mTORC1/2 |
II (+ PF-05212384 ), II (+ Letrozole), II (+ Exemestane) |
Recurrent endometrial cancer, Early breast cancer, Advanced breast cancer |
NCT01420081, NCT01430585, NCT01658176 |
(161) |
| PKI-587 , PF-05212384 |
Class I PI3K , mTORC1/2 |
II (single) | Recurrent endometrial cancer | NCT01420081 | (162) |
| XL765, SAR245409 |
Class I PI3K , mTORC1/2 |
II (single), I/II (+ Letrozole) | Relapsed or refractory lymphoma or leukemia subtypes, Breast cancer |
NCT01403636, NCT01082068 | (163 164) |
| GSK2126458 | Class I PI3K , mTORC1/2 |
I (+ GSK1120212) | Advanced solid tumor | NCT01248858 | (165) |
| DS-7423 | Class I PI3K , mTORC1/2 |
I (single) | Advanced solid malignant tumors | NCT01364844 | |
|
PWT33597- 101 |
PI3Kα , mTORC1/2 |
I (single) | Advanced malignancies | NCT01407380 | (166) |
| SF1126 (LY294002 pro-drug) |
Class I PI3K , mTORC1/2 |
I (single) | Advanced or metastatic solid tumors cancer | NCT00907205 | (167) |
| Dabrafenib | B-Raf(mu) | III (+ Trametinib), III (+ Trametinib) |
BRAF(mu) melanoma, Unresectable or metastatic BRAFV600E/K(mu) melanoma |
NCT01584648, NCT01597908 | (106) |
| Regorafenib | B-Raf(mu), KIT(mu), RET(mu), VEGFR1/2/3, PDGFRβ, FGFR |
III (single), II (+ FOLFIRI) | Refractory metastatic colorectal cancer, Metastatic colorectal cancer |
NCT01584830, NCT01298570 |
(168, 169) |
| LGX818 | B-Raf(mu) | I/II (single), I (single) | BRAF-dependent advanced solid tumors, Advanced or metastatic BRAF(mu) melanoma |
NCT01543698, NCT01436656 | (170) |
| RAF265 | B-Raf, VEGFR2 |
I (single), I (+ MEK162) | Locally advanced or metastatic melanoma, Advanced solid tumors with RAS(mu) or +BRAFV600E(mu) |
NCT00304525, NCT01352273 | (171, 172) |
| RO5212054 | B-Raf(mu) | I (single) | Advanced solid tumors with BRAFV600(mu) | NCT01143753 | |
| ARQ736 | B-Raf(mu) | I (single) | Advanced solid tumors with NRAS(mu) or BRAFV600E(mu) |
NCT01225536 | |
| Trametinib | MEK1/2 | III (+ Dabrafenib), III (+ Dabrafenib) |
Unresectable or metastatic BRAF V600E/K(mu) melanoma |
NCT01597908, NCT01584648 | (116- 119) |
| Selumetinib | MEK1/2 | I/II (+ Sorafenib), II (single), II (+ Temsirolimus), II (+ Erlotinib Hydrochloride), II (+ Erlotinib Hydrochloride), II (single), II (+ MK2206), II (+ MK2206), II (+ MK2206), |
Advanced HCC, Metastatic uveal melanoma, Metastatic, recurrent, or locally advanced unresectable soft tissue sarcomas, Locally advanced or metastatic pancreatic adenocarcinoma, KRAS(WT/mu) advanced NSCLC, MCT-1 Related relapsed or refractory DLBCL, Advanced colorectal carcinoma, Relapsed BRAF(mu) melanoma, previously treated metastatic pancreatic cancer |
NCT01029418, NCT01143402, NCT01206140, NCT01222689, NCT01239290, NCT01278615, NCT01333475, NCT01519427, NCT01658943 |
(14, 120- 123) |
| MEK162 | MEK1/2 | II (single), Ib (+ BYL719), Ib/II (+ LGX818), I (single), I (+ Paclitaxel) |
Advanced melanoma, Advanced solid tumors, BRAF dependent advanced solid tumors, Advanced solid tumors, Epithelial ovarian, fallopian Tube or peritoneal cancer |
NCT01320085, NCT01449058, NCT01543698, NCT01469130, NCT01649336 |
(173) |
| Pimasertib, AS703026 |
MEK1/2 | I/II (+ Gemcitabine), I (+ Temsirolimus), I (single) |
Hematological malignancies/acute myeloid leukemia, Pancreatic cancer, Locally advanced or metastatic solid tumors |
NCT00957580, NCT01378377, NCT01390818 |
(174, 175) |
| Refametinib | MEK1/2 | I/II (+ Gemcitabine), Ib (+ BAY80- 6946) |
Advanced pancreatic cancer, Advanced cancer | NCT01251640, NCT01392521 | (176, 177) |
| WX-554 | MEK1/2 | I/II (single) | Solid tumors | NCT01581060 | (178) |
| AS703988 | MEK1/2 | I (single) | Solid tumors | NCT01453387 | |
| GDC-0973 | MEK1/2 | I (+ GDC-0941), I (+ Vemurafenib), I (+ GDC-0068) |
Locally advanced or metastatic solid tumors, Metastatic BRAF(mu) melanoma, Locally advanced or metastatic solid tumors |
NCT00996892, NCT01271803, NCT01562275 |
(146) |
| GDC-0623 | MEK | I (single) | Locally advanced or metastatic solid tumors | NCT01106599 | |
| RO4987655 | MEK1 | I (single) | Advanced solid tumors, | NCT00817518 | (179) |
| TAK-733 | MEK1/2 | I (single), I (+ Alisertib) | Advanced nonhematologic malignancies | NCT00948467, NCT01613261 | (180) |
| E6201 | MEK1, MEKK1 |
I (single) | Solid tumors | NCT00794781 | (181) |
Abbreviations
ER: estrogen receptor, AI: aromatase inhibitor, CLL: chronic lymphocytic leukemia, NSCLC: non-small cell lung cancer, RCC: renal cell carcinomas, HCC: hepatocellular carcinomas, PTEN: phosphatase and tensin homolog DLBCL: diffuse large B-cell lymphoma, pNET: pancreatic neuroendocrine tumors, mu: mutant, MCT-1: multiple copies in T-cell lymphoma-1
4.1 Targeting the PI3K-AKT-mTOR Pathway
The PI3K-AKT-mTOR pathway is well characterized for its role in cellular survival signal transduction. Physiologically, the AKT pathway promotes cell survival by inhibiting pro-apoptotic regulators, facilitating p53 degradation, modulating the activity of cell-cycle regulators and regulating cell mass (55). The involvement of the PI3K-AKT-mTOR pathway in cancer is indicated by the frequency of aberrantly high activity of the pathway in various types of cancers, in addition to the very common findings of genetic alterations in pathway components, such as oncogenic mutations of PI3KCA and AKT1, or loss of function of PETN (55). The PI3K-AKT-mTOR pathway also plays an important role in promoting tumor angiogenesis via transcriptional activation of vascular endothelial growth factor (VEGF) through mTOR, leading to the stimulation of endothelial cell survival, growth and proliferation (56).
4.1.1 PI3K Inhibitors
There are many investigational drugs in this class currently undergoing early clinical trials, and two drugs have advanced into Phase III trials to date. BKM120 is an oral pan-Class I PI3K inhibitor that also inhibits the constitutively-activated mutant PIK3CA (57). Interestingly, PIK3CA-mutant cell lines were more sensitive to BKM120 than PIK3CA wild-type lines, which might support the potential of this drug in malignancies, considering that alteration or aberrant activation of the PI3K pathway is seen many types of cancers (57). Preclinical in vivo studies demonstrated strong antitumor and antiangiogenic activities (57). In the first-in-human phase I trial, BKM120 was well tolerated (58). Consistent with other PI3K pathway inhibitors, dose-limiting toxicity included hyperglycemia (which would be expected given the established involvement of the PI3K pathway in insulin signaling), mood alteration (likely due to the effects of PI3K inhibition in the CNS) and skin rash (58). Early antitumor activity was demonstrated: one patient with triple-negative breast cancer and a KRAS mutation achieved a partial response and seven patients remained on-study for more than 8 months (58). Ongoing phase III trials are being conducted with BKM120 as a single agent, or in combination with fulvestrant, in patients with previously-treated locally-advanced or metastatic breast cancer (estrogen receptor (ER)-positive, HER2-negative). In the phase I trial, pharmacodynamic analysis demonstrated dose-dependent inhibition of the PI3K pathway by BKM120, and a possible correlation with outcome was suggested (58). Currently, several Phase II trials are being conducted to test this correlation. Combination of BKM120 with letrozole was also well tolerated in ER+/HER2- metastatic breast cancers, and combination therapies with many other chemotherapeutic agents in various types of cancers are now being tested, predominantly in phase I trials.
GS1101 (formerly CAL-101) was strategically developed as an isoform-specific inhibitor of PI3Kδ, which is exclusively expressed in leukocytes. The preclinical studies verified: 1) expression of PI3Kδ in B cells collected from chronic lymphocytic leukemia (CLL) patients; 2) elevated activation of PI3K in peripheral B cells from CLL patients, compared to B cells from healthy volunteers; and, 3) great sensitivity to GS1101 in peripheral leukemia cells from CLL patients compared to normal peripheral blood mononuclear cells (59, 60). In vitro activity of GS1101 was also demonstrated against Hodgkin lymphoma, multiple myeloma (MM) and mantle cell lymphoma cells (61–63). Phase I trials of single-agent or combinatorial use with other agents showed acceptable toxicity, reduction of AKT phosphorylation, and some clinical activity, such as reduction in lymphadenopathy and high rates of tumor regression in the majority of participating patients (64–68). Based on these results, four phase III trials of GS1101 are ongoing, either as a single-agent or in combination with rituximab, ofatumumab or bendamustine in CLL patients. Additional phase I or II studies in different types of hematological malignancies are also underway. Interestingly, recent reports of in vitro studies in glioblastoma suggested the potential application of GS1101 beyond hematological cancers (69, 70).
4.1.2 AKT inhibitors
Compared to PI3K inhibitors, there are fewer AKT inhibitors in human testing. The most advanced is MK2206, which is currently being investigated in phase II trials. MK2206 is an oral allosteric AKT inhibitor that prevents translocation of AKT proteins to the plasma membrane and subsequent activation, by binding AKT proteins and inducing a conformational change. The inhibitory action of MK2206 is highly-specific for AKT1 and AKT2 (71). In vitro studies indicated anti-proliferative activity in tumor cells with activation of HER2, with mutations of PTEN or PI3KCA, or with AKT2 amplification, the types of genetic alterations that could provoke constitutive activation of the AKT signaling pathway (72). Consistent with our understanding that aberrant AKT activation commonly serves as one mechanism of cancer drug resistance, in vivo models showed improved responses to chemotherapeutic agents when MK2206 was added to the regimen (erlotinib, carboplatin and gemcitabine in a NSCLC model, lapatinib in breast and ovarian cancer models, docetaxel in a prostate cancer model) (73). MK2206 was well-tolerated in a phase I trial, and dose-limiting toxicities included skin rash, nausea, pruritus, diarrhea and hyperglycemia (71). Reduction in phosphorylation of AKT (Ser473) in all tumor biopsies validated the pharmacodynamic endpoint (71). One patient with advanced pancreatic adenocarcinoma previously resistant to four regimens of chemotherapy experienced 23% reduction in tumor size, while two other patients with advanced pancreatic neuroendocrine tumors displayed minor reduction in tumor size (71). Stable disease was observed in three patients for four months or longer, and in another three patients for six months or longer (71). Concurrent treatment with MK2206 and trastuzumab in HER2-positive tumors produced one complete remission in a breast cancer patient and 16% of patients experienced stable disease for at least 4 months (74). Currently, additional phase II trials are underway. Trial regimens include MK2206 as a single agent, in combination therapy, in previously-treated patients, or in patients with PI3KCA mutations or PTEN loss.
GSK2110183, an oral ATP-competitive inhibitor of all three isoforms of AKT, has also advanced to phase II trials. Preliminary results from the first-in-human phase I study, focusing primarily on MM, in which the PI3K/AKT pathway is constitutively activated, exhibited good tolerability and clinical activity as monotherapy in heavily-pretreated MM patients (75). Another ATP-competitive inhibitor of AKT1/2/3, GDC-0068, was shown to effectively block phosphorylation of downstream targets of AKT in cell culture systems, and this was confirmed in in vivo xenograft models in a dose-dependent manner (76). Antitumor activity was reported in the same in vivo model, which had aberrantly activated PI3K-AKT-mTOR signaling (76). GDC-0068 recently completed a single agent safety and dose-determination phase I trial and is now undergoing phase I combination trials.
In contrast to the high target specificity of the ATP-competitive inhibitors described above, the AKT inhibitor AZD5363 was found to possess inhibitory activity against AKT isoforms, p70S6K and PKA, as well as 14 other AGC family kinases in in vitro kinase assays (Figure 2). Cell lines carrying wild-type RAS together with either an activating mutation of PI3KCA or PTEN mutation/loss were particularly sensitive to AZD5363(77). In an in vivo HER2-positive breast cancer model with trastuzumab resistance, AZD5363 displayed antitumor activity as a monotherapy and this antitumor activity was enhanced by combination with docetaxel, lapatinib, or trastuzumab (77). Furthermore, addition of AZD5363 to trastuzumab resensitized HER2-positive tumors with PI3CA mutations to the treatment (78). These preclinical studies suggested that the activity of AZD5363 can be maximized when it is used against tumors with a particular genetic profile. AZD5363 is now undergoing several phase I trials as monotherapy or combinatorial therapy.
4.1.3 mTOR inhibitors
Renal cell carcinomas (RCC) typically express high level of the transcription factor hypoxia-inducible factor 1 (HIF1), which is one substrate of mTOR. Uncontrolled transcription of pro-angiogenic factors regulated by HIF1, including VEGF, contributes to tumor angiogenesis in RCC (56). RCC, therefore, presents a potential therapeutic opportunity for the early mTOR inhibitors temsirolimus and everolimus, which bind to a component of mTORC1 and prevent initiation of the mTOR signaling cascade. Preclinical studies demonstrated these inhibitors repressed the growth of a wide range of cancer cell lines, accompanied by decreased activities of downstream markers of mTOR signaling (79, 80). Interestingly, antitumor activity was observed in some tumor models in vivo even when the cell lines themselves were insensitive to the drug in vitro, suggesting that indirect effects may have contributed to the in vivo antitumor activity, such as attenuation of tumor angiogenesis by antiangiogenic factors downstream of mTOR signaling (79). In the registration phase III trial of single-agent temsirolimus compared to interferon α, temsirolimus improved overall survival in patients with advanced RCC, and most adverse events were manageable (81). Similarly, everolimus prolonged progression-free survival (PFS) over the placebo group (4.9 months versus 1.9 months) in a phase III trial of patients of advanced RCC previously treated with sunitinib or sorafenib, leading to its approval in this disease, although overall survival was not different between everolimus-treated patients and placebo group (82). Serious adverse events included infections (10%), dyspnea (7%), and fatigue (5%) (82). Everolimus was later approved for three more indications: subependymal giant cell astrocytoma, metastatic pancreatic neuroendocrine tumors (14% of these cancers have a genetic mutation in the mTOR pathway), and ER-positive/HER2-negative advanced breast cancer.
Ridaforolimus is an investigational oral agent under development for maintenance therapy for patients with metastatic soft tissue or bone sarcoma who have stable disease or better after four or more cycles of chemotherapy. The rationale for the application of ridaforolimus to sarcoma is two-fold: 1) the mTOR pathway is involved in the development of mesenchymal cells, from which sarcomas arise; 2) mTOR inhibition decreases the expression level of EWS fusion proteins, the product of gene fusion between EWS and transcription factor genes, which is a key event in the development of Ewing sarcoma (83). Clinical trials were conducted in breast cancer, endometrial cancer, hematological malignancies, sarcoma and solid tumors in phases I or II. Generally, ridaforolimus showed good tolerability, predictable and manageable adverse events and an indication of mTOR pathway inhibition in patient samples (84). Ridaforolimus demonstrated more promising clinical activity in sarcomas in phase I and II studies compared to the phase II trials with everolimus and temsirolimus (83). Based on the phase II observation of prolonged PFS in advanced sarcoma patients, the application to a maintenance regimen was pursued in a phase III trial in patients with advanced bone and soft tissue sarcomas who had at least stable disease following prior chemotherapy. Median PFS and 6-month PFS rates were 17.7 weeks and 34% in the ridaforolimus group and 14.6 weeks and 23% in the placebo group (83). No statistical improvement in overall survival was reported. In June 2012, the US Food and Drug Administration (FDA) rejected the approval of New Drug Application for ridaforolimus in its present form and required additional clinical trial(s) for further assessment of safety and efficacy (85). A currently ongoing phase II trial is evaluating effects of the combination therapy of ridaforolimus and exemestane in comparison to single-agent treatment with ridaforolimus, dalotuzumab or exemestane on PFS in post-menopausal, ER-positive breast cancer patients. Multiple phase I trials of ridaforolimus in combination with other agents in various types of cancers are underway.
Unlike these first generation mTOR inhibitors, which are collectively called rapalogs [rapamycin analogs], new generation inhibitors currently in early phase trials are predominantly mTORC1/2 dual inhibitors. As dual inhibition of mTORC1 and mTORC2 presumably leads to the complete inhibition of the mTOR pathway, better antitumor clinical activity is expected.
4.1.4 PI3K-mTOR dual inhibitors
Since mTOR possesses a motif that structurally resembles the catalytic domain of PI3K, some inhibitors that were designed to target PI3K or mTOR have a dual-inhibitory effect on both kinases. BEZ235 inhibits class I PI3Ks and mTORC1/2 (Figure 2). Preclinical studies demonstrated growth-inhibitory activity in breast cancer cells with HER2 amplification, glioma cells, lung and ovarian cancer cells, all of which are characterized by aberrant activation of the PI3K-AKT-mTOR pathway (86–89). Interestingly, cell lines harboring KRAS or BRAF mutations, or EGFR amplification, all which would lead to PI3K-AKT activation, were less sensitive to BEZ235 in breast cancer models, while ovarian cancer cell lines with activating PI3K mutations or PTEN loss were more sensitive to the same drug (86, 88). The first-in-human phase I trial produced partial responses in patients with lung cancer and ER-positive breast cancers, and 24% of patients had stable disease over 4 months (90). BEZ235 is now being tested in phase I and Ib/II trials. Preliminary results reported that BEZ235 in combination with trastuzumab showed acceptable safety in patients with PI3K- or PTEN-altered, HER2-positive metastatic breast cancer and BEZ235 as a single agent given twice-daily produced some evidence of clinical activity (stable disease in 2 colorectal and 1 endometrial cancer) (91, 92).
GDC-0980 also inhibits both class I PI3Ks and mTORC1/2, as verified by inhibition of downstream components of the PI3K-mTOR pathway (Figure 2) (93). GDC-0980 inhibited proliferation of various cancer cell lines, producing G1 cell cycle arrest, with the greatest activity seen in breast, prostate and lung cancer lines (93). The observation that melanoma and pancreatic cancer cell lines were less susceptible to the inhibition of this pathway might be explained by the frequent mutation of KRAS or BRAF in these tumors, which could enhance drug resistance (93). Inhibition of tumor growth was observed in animal xenograft studies, including models developed from cell lines harboring activated PI3K or loss of PTEN (93). Phase I trial results indicated tolerability and showed antitumor activity, including tumor regression in patients with mesothelioma, gastrointestinal stromal tumor and adrenal cell carcinoma (94).
4.2 Targeting the Raf-MEK pathway
4.2.1 Raf inhibitors
The Raf-MEK pathway may be a particularly central component of Ras signaling to target for cancer therapeutics. Barbacid and others, using “Ras-less” cells, have demonstrated that the MAPK pathway is necessary and sufficient for proliferation and migration of normal cells, and that none of the other Ras effector pathways, including PI3K, could substitute in this model (95). Furthermore, in certain K-Ras-driven lung cancer models C-Raf, rather than A-Raf or BRAF, is the critical Raf kinase mediating the oncogenic effect of K-Ras (96).
In the search for potential therapeutics to block aberrant activation of the Raf-MEK-ERK pathway in cancer cells, pharmacological inhibitors of Raf kinases and MEK kinases have been most intensively pursued. Two Raf inhibitors have been approved by the FDA to date. Sorafenib was approved for the treatment of patients with advanced RCC and unresectable HCC. Although sorafenib was designed to target C-Raf, it also effectively inhibits C-Raf, wild-type B-Raf and the oncogenic B-Raf V600E mutant, as well as the VEGF receptor 1 (VEGFR1), VEGFR2, VEGFR3, and platelet-derived growth factor receptor-β (PDGFRβ) tyrosine kinases in biochemical assays in vitro (Figure 2) (97). The inhibitory effect on the VEGFRs was presumed to contribute to the observed disruption of tumor microvasculature in the in vivo models (97). Interestingly, a phase I trial in RCC demonstrated that a reduction of vascular permeability correlated with better PFS (98). A phase III study resulted in prolonged PFS in the patients treated with sorafenib (5.5 months) in comparison to the placebo group (2.8 months) (99). In the case of HCC, blockade of both Raf-MEK-ERK signal transduction and tumor angiogenesis is postulated to contribute to the anti-tumor activity. A phase II trial showed correlation between the pharmacodynamic marker of decreased levels of phospho-ERK expression and prolonged time to progression (TTP) (100). Both median survival and TPP were nearly 3 months longer for HHC patients treated with sorafenib than for those given placebo in the Phase III monotherapy study (99). Currently, more than 150 clinical trials in the different phases are being conducted with sorafenib in various cancers, in single or combination regimens.
The discovery of frequent BRAF mutations in a wide range of cancers attracted attention to B-Raf as a druggable target (101). Theoretically, specifically targeting mutant B-Raf, the expression of which is confined to cancer cells, would enable tumor-selective drug activity, while sparing normal cells that carry wild-type B-Raf. Most investigational drugs currently in clinical trials are selective for the BRAF-V600E mutant, which is particularly common in melanoma (and in colorectal cancer at a lower frequency). The recently FDA-approved agent vemurafenib preferentially inhibits the V600E mutant form of B-Raf over wild-type (Figure 2). Inhibition of ERK phosphorylation, induction of cell cycle arrest and apoptosis are exclusively observed in BRAF-V600E-positive cells (102). A phase II trial in previously-treated melanoma patients with mutant B-Raf achieved a remarkable response rate of 53% and a median duration of response of 6.7 months (103, 104). A phase III trial which compared the efficacy of vemurafenib to that of dacarbazine in the patients with previously-untreated BRAF-V600E-positive melanomas verified the higher response rate and improved rates of overall survival and PFS over the standard treatment group (105). Vemurafenib was approved by FDA in 2011 for the treatment of patients with previously untreated metastatic or unresectable melanoma with the BRAF-V600E mutation, with concurrent approval of a BRAF-V600E mutation assay (companion diagnostic). Among the investigational drugs in this class, the most advanced at this time is dabrafenib, which has higher specificity against mutant B-Raf and a similar preclinical profile to vemurafenib (Figure 2) (106). Encouraged by a phase II trial that confirmed a 59% response rate to dabrafenib in melanoma, several phase III trials are currently ongoing. Preliminary result from a randomized monotherapy trial reported improved median PFS over dacarbazine treatment (106).
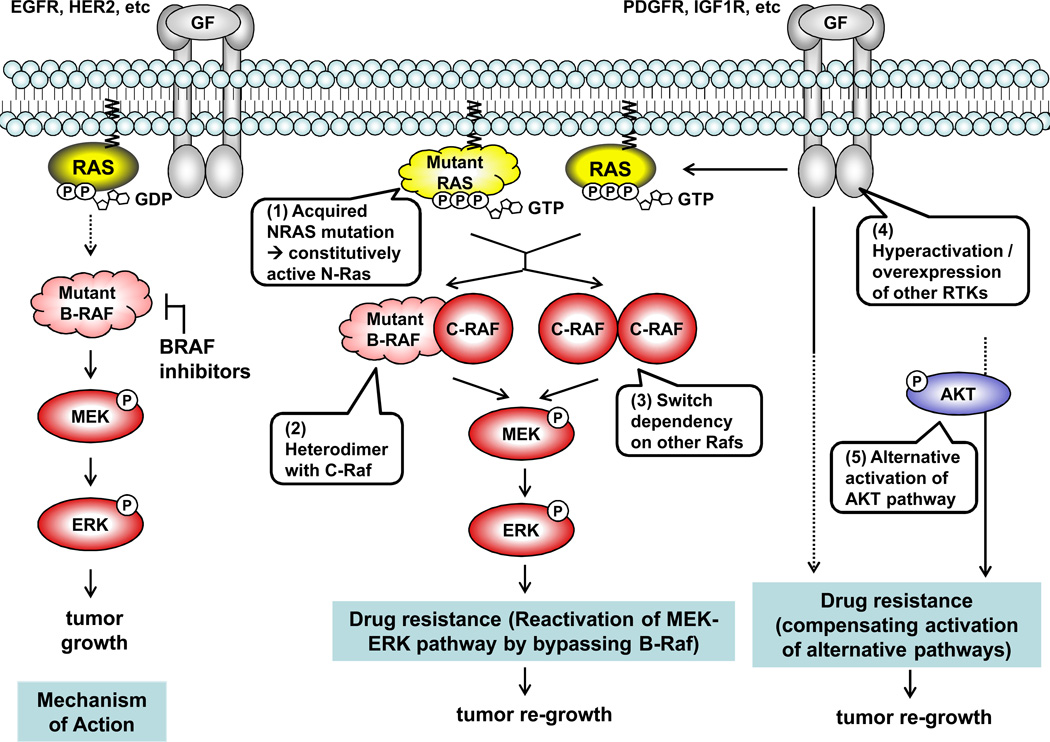
While these mutant BRAF inhibitors produce improved overall survival in the patients with BRAF mutations compared to standard treatment options, a major challenge remains: essentially all patients treated with these drugs relapse due to the development of drug resistance, with the median TTP of 7 months for vemurafenib and 5 months for dabrafenib (103, 106). Several models for resistance have been proposed: 1) reactivation of the MEK/ERK pathway bypassing BRAF (e.g., secondary mutation in NRAS, hyperactivation/overexpression of C-Raf, or activation of another MAPKK COT); or, 2) adaptive dependency upon alternative pathways (hyperactivation/overexpression of RTKs, such as PDGFRβ or IGF1R, or the AKT pathway) (Figure 3) (107–110).
Figure 3.
Interestingly, BRAF mutant-selective inhibitors, collectively called class I B-Raf inhibitors, were reported to paradoxicially activate the MEK-ERK pathway via C-Raf in a Ras activity-dependent manner in RAS-mutant cells, leading to accelerated cell proliferation (111–113). Moreover, RAS mutations (predominantly HRAS) were detected in 60% of tumor samples taken from patients who developed secondary tumors (cutaneous squamous-cell carcinomas and keratoacanthomas) after treatment with class I B-Raf inhibitors (114). In this study, HRAS mutation was demonstrated to be associated with accelerated cell proliferation due to increased MAPK pathway activity both in vitro and in vivo in response to exposure to B-Raf inhibitors. While the sequence of the event between the evolution of RAS mutations and the development of secondary tumors is still unclear, the mutational status of the RAS genes should be carefully monitored in patients who are treated with a class I B-Raf inhibitor over the course of the treatment.
4.2.2 MEK inhibitors
In contrast to the strategy underlying the development of B-Raf inhibitors to specifically target mutant oncogenic forms of the kinase, the MEK kinases are rarely mutated, and do not provide a tumor-specific target. Instead, tumor selectivity for the MEK inhibitors in development is sought by the choice of tumors to be targeted, i.e., cancer types in which Raf-MEK-ERK signaling is aberrantly activated relative to normal cells, and upon which the tumor cells are dependent. This strategy was supported by early studies using the first generation MEK inhibitor CI-1040, which showed activity in BRAF-mutant cell lines. This B-Raf mutant selectivity of CI-1040 was proposed to be MEK-dependent; mutation of BRAF was associated with enhanced and selective sensitivity to MEK inhibition, in comparison to cells harboring either a wild-type BRAF or a RAS mutation (115).
The most promising investigational MEK inhibitor is trametinib, which is now being investigated in several phase III trials. Trametinib is a highly-selective allosteric inhibitor of MEK1/2. Inhibition of ERK phosphorylation as an indicator of proof-of-concept and cell growth inhibition were confirmed in multiple cell lines with activating mutations driving the MAPK pathway (116). In vivo efficacy was also observed in models with activating mutations in BRAF or KRAS (116). A phase I study in melanoma patients indicated substantial clinical activity (the response rate) correlated with the BRAF mutational status (117). Trametinib showed tolerability with manageable adverse events and a favorable pharmacokinetics and pharmacodynamics (118). A phase III trial in metastatic melanoma patients with BRAF mutations, in comparison to dacarbazine- or paclitaxel-treatment groups, achieved improved PFS and overall survival, in the similar manner to vemurafenib but with less significance (119). 74% of patients had some degree of tumor regression and 22% had sustained tumor regression (119). Ongoing phase III trials are exploring the safety and efficacy of combination therapy of trametinib with the mutant-BRAF inhibitor dabrafenib.
Selumetinib is currently undergoing multiple trials in phases I, I/II and II. Preclinical studies demonstrated inhibition of proliferation in cell lines containing BRAF or RAS mutations of colon, pancreatic, breast cancer, and melanoma origin, while NSCLC cell lines with RAS mutations, and non-V600E-BRAF mutations, were not as sensitive as BRAF-V600E mutant cells (14, 120). In vivo activity in colorectal and pancreatic cancer models suggested the possibility of expanded indications beyond melanoma (14). While selumetinib was well tolerated, with a manageable safety profile, monotherapy phase II trials showed no clinical activity compared to conventional chemotherapies in HCC, advanced melanoma, or advanced pancreatic cancer (121–123). The pharmacodynamic marker of reduction in ERK phosphorylation in solumetinib-treated patients was achieved, despite the lack of clinical response, suggesting this agent may provide additional activity if combined with a B-Raf inhibitor (121, 122).
Interestingly, the earlier proposition that the mutational status of BRAF and RAS predicts the sensitivity of cells to MEK inhibitors was partially supported and partially refuted by a series of recent studies. A BRAF mutation was consistently an indicator of sensitivity over wild-type BRAF, whereas the correlation of RAS mutation with tumor sensitivity varied among studies using different compounds or cell lines (14, 115, 116, 120). One possible explanation for this discrepancy is that BRAF mutation could affect MEK activity with less variation among different cell systems, as it is an immediate upstream effector of MEK. In contrast, RAS mutations might produce different outcomes among different cell lines, as Ras is involved in the genesis of many signaling pathways in addition to the Raf-MEK pathway. The direct coupling of B-Raf to MEK may make these tumor cells more likely to be dependent upon MEK activity for proliferation, whereas activating Ras mutations may provide the cells with a number of proliferative signals, making them less likely to be dependent upon MEK activation alone.
While the complexity of Ras downstream signaling allows cells to have flexible and timely positive- or negative-functional regulatory options in response to changing environmental signals, it also provides for redundancy among these pathways, so that cells can develop alternative mechanisms to compensate for any failure of the original signaling pathway. This is particularly the case in the setting of the “hyper-mutator phenotype,” which characterizes malignancy. From the pharmacological point of view, this therefore presents a major challenge to targeting the Ras signaling pathways. The mechanisms underlying resistance to B-Raf inhibitors were discussed earlier. For MEK inhibitors, alternative activation of the PI3K-AKT pathway or remodeling upstream signaling (Ras or Raf) to bypass MEK has been reported (124–126). Paradoxically, the inhibitors that selectively attack single “cancer cell-specific markers” (e.g., BRAF-mutation, overexpression of PI3Kδ) or “cancer cell-specific events” (e.g., hyperactivation of the Raf-MEK or PI3K-AKT pathways) appear to provide the most facile opportunities for cancer cells to develop drug resistance despite the sometimes remarkable antitumor activities produced early in the course of the treatment. As a strategy to conquer this paradox, accumulating evidence suggests the necessity of combinational therapeutic approaches to block multiple pathways simultaneously (126–128).
5. Synthetic lethal approaches
Because activating mutations of Ras proteins are among the most frequent oncogenic events in human cancers, targeting mutated Ras should be a promising opportunity for a tumor-specific therapeutic approach. However, as described above, targeting Ras proteins themselves for anticancer therapy has been challenging for a number of reasons, and Ras proteins are now widely considered to be “undruggable” targets. Meanwhile, the recently-emerging (or rediscovered) strategies variously termed “synthetic lethality” and “non-oncogene addiction” have produced a framework for the development of indirect approaches to targeting mutant Ras in cancer cells. Two genes are in a so-called “synthetic lethal” interaction if a mutation of either gene alone is compatible with viability but simultaneous mutations of both genes lead to cell death (129). The concept of synthetic lethality is over 60 years old and has been used in yeast and drosophila, and more recently in human systems, to identify critical components of survival pathways, now including those survival pathways uniquely operative in cancers (130). Thus, inhibition of a synthetic lethal interactor of Ras by chemotherapy theoretically kills only tumorigenic cells with a mutated RAS gene without affecting normal cells. Similarly, “non-oncogene addiction” describes the situation in which transformation of a cell (whether by a known oncogene or unknown mechanisms) renders it dependent upon a normally non-essential protein for survival (131). That non-essential (non-oncogenic) protein can then become the target of a therapeutic strategy, which should be cancer-specific and spare normal cells. These concepts have provided a new approach to target oncogenic Ras indirectly: that is, to discover synthetic lethal interactors, or critical “non-oncogenes,” which are more druggable than Ras, and then develop therapeutic methods to inhibit these interactors.
Several groups employed RNAi high-throughput screening to identify synthetic lethal interactors of Ras, in which genes whose knockdown specifically killed K-Ras-dependent cancer cells were sought (132–137). One of these studies yielded TANK-binding kinase 1 (TBK1), a non-canonical IκB kinase that regulates the NFκB survival pathway, as a potential synthetic lethal partner of mutant K-Ras (132). Follow-up analyses in individual cell lines of lung cancer with mutant K-Ras or wild-type K-Ras revealed that suppression of TBK1, or its reported upstream effector Ral-B, provoked apoptosis uniquely in K-Ras-dependent cancer cell lines through activation of the NFκB signaling pathway. This approach was further supported by the observation of elevated activity of Ras and the NFκB pathway in lung adenocarcinoma clinical samples with K-Ras mutant in comparison to wild-type K-Ras samples. A simultaneous report corroborated the requirement for the NFκB pathway in cancers with KRAS mutations in a mouse model (138).
In contrast, the discovery of serine/threonine kinase 33 (STK33) as a synthetic lethal interactor with Ras now appears to be incorrect. STK33 was identified from the screening of 8 cell lines representing different types of K-Ras-dependent cancers (133). STK33 belongs to the calcium/calmodulin-dependent kinase family but its physiological function is unknown. The initial report stated that STK33 activity was required for the survival of cancer cells with K-Ras dependency. However, a more recent study questioned this conclusion (134). In this latter study, inhibition of STK33, whether by siRNA, dominant-negative mutant overexpression, or small molecule inhibitors, had no effect on the survival of KRAS mutant cells. Additionally, a synthetic lethal siRNA screening conducted in this study did not indicate STK33 as a synthetic lethal interactor.
In contrast to the above examples of RNAi-based discoveries of synthetic lethal or non-oncogene addiction targets, the earlier identification of the protein kinase C delta (PKCδ) isozyme as a Ras synthetic lethal interactor originated from a focused study of Ras signaling pathways. PKCδ is a serine/threonine kinase of the PKC family, novel class, and functions in a number of cellular activities including cell proliferation, survival or apoptosis (139). However, PKCδ is not required for the proliferation of normal cells, and PKCδ-null animals develop normally and are fertile, suggesting the potential tumor-specificity of a PKCδ-targeted approach (140). PKCδ was validated as a target in cancer cells of multiple types with activation of H-Ras or K-Ras, using both genetic (siRNA, dominant-negative PKCδ) and small molecule inhibitors (141). Inhibition of PKCδ induced apoptosis in pancreatic cancer cell lines with activating KRAS mutations at least in part through suppression of AKT signaling, and “Ras-dependency” in the tumors was not required for the cytotoxic effects (141, 142). More recently, tumors with aberrant activation of the PI3K pathway in the setting of wild-type RAS alleles have also been shown to be dependent upon PKCδ activity, potentially expanding the potential application of this approach beyond tumors with mutational activation of Ras (143). Not-yet-published studies documenting the susceptibility of melanoma cells with NRAS mutations, and melanoma lines which have become resistant to B-Raf inhibitors, to PKCδ suppression or inhibition have stimulated the development of novel, more specific, and more potent, small molecule PKCδ inhibitors as potential therapeutics in tumors with aberrant Ras signaling (144).
Although none of the Ras synthetic lethal approaches have progressed to human trials, this concept proposes a potential and unique approach to cancer types with high RAS mutational frequencies: that it, it utilizes the mutant Ras proteins as markers to identify potentially susceptible tumors, rather than as pharmacological targets. Hypothetically this approach allows a synthetic-lethal-partner-targeted therapy to confine its anti-proliferative activity only to tumorigenic cells with RAS mutations. The controversy that has been raised in the recent preclinical studies presented by different groups, however, represents a current obstacle in this research area. While these studies carefully screened a number of cell lines representing different types of cancers, context-dependent issues, such as variations in cell lines or RNAi libraries, or the complexities that arise from the combinations of these parameters, can complicate such open-ended screens, and tumor cell viability is not a molecularly-specific endpoint. Furthermore, it is noteworthy that the biological consequences of down-regulating a protein target by RNAi do not necessarily reflect the effects of a small molecule inhibitor bound to the target. An in vitro screen based primarily on RNAi should therefore be interpreted with caution if its goal is as proof-of-concept for the development of an inhibitor against the activity of the target molecule (and this is indeed generally the ultimate therapeutic plan of such RNAi screening programs. Comprehensive follow-up studies to understand signaling pathways in which the target is involved, its interaction with other proteins, and the fitness of an inhibitor of the target in the entire gamut of normal cellular activities or in vivo efficacy/toxicity are needed.
6. Conclusion
The Ras GTPases (K-Ras, N-Ras and H-Ras) function as molecular switches for critical cellular activities, such as cell proliferation or growth, differentiation, and survival in normal cells and are tightly and temporally regulated by multiple signaling pathways. Pharmacological interventions in situations of uncontrolled Ras activity or downstream signaling, which is often prominent among the deadliest types of cancers, has been sought since the discovery of H-Ras as an oncogene in bladder cancers.
The currently most widely-employed approach to inhibiting Ras signaling is to target one or more components of the Ras downstream pathways, such as the two major Ras downstream signaling pathways: PI3K-AKT-mTOR and Raf-MEK-ERK. Two Raf inhibitors and two mTOR inhibitors are currently approved and utilized in the clinic, and many investigational drugs with higher target specificity, better drug property and promising clinical activity are being investigated in clinical trials. Drug resistance has been a major issue in this category of drugs, however, suggesting the necessity of combination therapy to avoid the development of resistance and maximize clinical outcome in the use of these inhibitors.
The recently re-emerging concept “synthetic lethality” has provided a new therapeutic framework for targeted cancer therapy, which redefines the role of oncogenic Ras proteins as cancer cell “markers” rather than targets. This approach seeks to discover synthetic lethal interactors of Ras for pharmacological intervention, which should then selectively kill tumor cells harboring RAS mutations. Although therapies based on this strategy have not reached human testing, several synthetic lethal interactors have been proposed as targets, inhibitors identified, and their clinical potential is being investigated in preclinical settings.
7. Expert opinion
Since the discovery of the Ras proteins nearly half century ago, Ras has been intensively studied and has become one of the most well-understood oncoproteins. Oncogenic mutations of Ras proteins are found in up to 30% of all human tumors, and are particularly frequent in those types of cancers with the highest mortality rates, such as lung, colorectal and pancreatic cancers and melanomas. This makes the Ras proteins attractive pharmacological targets for cancer therapeutics. As effective direct inhibition of Ras activity was discovered to be unexpectedly challenging, the components of the Ras downstream signaling pathways have instead been exploited for inhibition by pharmacological agents. There has already been some notable successes employing this approach and additional promising investigational drugs are in clinical trials, some of which may emerge into the market over the next few years. It is important to note, however, that many of the approaches described above are not truly “tumor-specific.” Except for those agents which target only a mutated, oncogenic form of Ras or Ras effector (such as mutant Ras-specific siRNA, or the V600E mutant B-Raf inhibitors), all of these agents block those critical physiological Ras signaling pathways which are required for the viability of all cells, both normal and malignant. This crucial factor limits our ability to utilize them in the clinic at doses which would be more effective against the tumor, as normal cell function becomes increasingly compromised.
Other remaining challenges of Ras-effector inhibitors include drug resistance and unaddressed disease areas. The complexity and redundancy of Ras signaling pathways provide the tumor cells many opportunities for drug resistance and confine the target disease areas to those cancers with high dependency upon these pathways, such as melanomas, RCC or HCC. Although the application of combination therapies in first line regimens, to establish complete blockade of multiple Ras downstream pathways, might avoid or slow the establishment of drug resistance, it would still leave cancers with high RAS mutation rates but without Ras pathway-dependency uncovered.
The ultimate goal for Ras-related targeted therapy is to establish therapeutics that can overcome the current limitations described above: tumor-specificity and limited cancer indications. In this respect, the “synthetic lethal” approach raises the hope of generating antitumor activity in cancers with high RAS mutation rates regardless of Ras pathway dependency or independency. Because synthetic lethality utilizes aberrant Ras signaling as a “marker” for sensitivity rather than as a direct drug target, it is to be expected to be applicable to those types of cancers cannot be effectively targeted by Ras-effector-inhibitor drugs (i.e., Ras-signaling-pathway-independent cancers) while sparing normal cells unaffected. This new research framework will be accelerated in the coming years aiming clinical application.
The earlier failure of strategies to develop FTIs as Ras-specific therapeutics teaches a crucial lesson in the development of targeted therapies. Thorough preclinical studies are essential for the efficient and successful clinical development of a targeted therapeutic. While it is difficult to fully verify and validate the mechanism of action and predict proof-of-concept prior to moving into the complex and confounding variables of a clinical study, good preclinical studies enable the establishment of methodologies to create multiple validated pharmacodynamic markers which inform clinical studies, whether successful or unsuccessful. Robust preclincal data also provides a framework for improving developmental strategies for later-phase trials, such as selection of target diseases areas/patient populations, clinical endpoints and regimens.
Because Ras and its downstream signaling evokes various types of cellular responses, depending on signaling, cellular, and tissue context, the history of Ras therapeutic development highlights the importance of “bidirectional translational research” in the development of Ras-related targeted therapies. Translational research is defined as exploiting the effective transition of knowledge from the bench to the clinic to seek a better clinical outcome. Yet, the fitful progress and unexpected complexities in the clinical application of these new targeted agents also demands a return back to the bench with clinical data and samples, to develop new solutions or applications. As the recent clinical successes of Ras-effector inhibitors with high target specificity demonstrates, strong reciprocal interactions between the lab and the clinic, as well as between academia and industry, lead to greater and more rapid benefits for patients.
The future holds great promise for “Ras-targeted” therapeutic approaches. Some of the drugs targeting specific or multiple Ras-effectors in the late clinical phases show impressive activity in certain malignancies, and will likely reach the market after accelerated FDA approval. RNAi-based approaches targeted mutated RAS will be tested in the clinical, although many technical hurdles remain to be addressed. As Ras synthetic lethal interactor proteins are identified, and drugs to target them are developed, we will see a completely new type of anticancer agent/approach reach clinical testing, ideally one without toxicity to normal cells and tissues.
Article highlights box.
The Ras GTPase family proteins regulate critical cellular activities including cell proliferation, differentiation and survival. Oncogenic mutations of RAS are prominent in many types of cancers with particularly high prevalence and mortality rates. The Ras proteins or the components of their downstream signaling pathways have been studied for pharmacological intervention of aberrant Ras signaling in cancer cells as an anti-cancer therapy.
Direct targeting of the Ras proteins has been challenging. For example, the FTIs/GGTIs have failed in part due to their lack of target (Ras protein) specificity, and antisense oligonucleotides to Ras have lacked sufficient clinical activity. Newer approaches utilizing RNA interference technology, currently in preclinical studies, have the potential for future clinical application.
Among the multiple Ras downstream pathways, the Raf-MEK and PI3K-AKT-mTOR pathways have been the major focus of drug discovery/development for inhibition of Ras signaling. There are four FDA-approved drugs (Raf inhibitors and mTOR inhibitors), and some promising investigational drugs which are in the late clinical trial phases in this category. The inhibitors in these classes utilized in those cancer types which are characterized by the existence of aberrantly high Ras signaling.
One major obstacle to the application of Ras-effector inhibitors is the emergence of drug resistance. Some drugs demonstrate remarkable clinical activity initially in treatment, but tumors eventually and inevitably relapse due to the development of resistance to these drugs. Accumulating evidence suggests that employing combination therapy in the first line of treatment for a simultaneous inhibition of multiple Ras downstream pathways may prevent cancer cells from switching to alternative survival pathways and escaping.
The synthetic lethal approach identifies synthetic lethal interactors of Ras proteins, whose inhibition is toxic only to those tumor cells with aberrant Ras pathway activity. Although this approach remains in the preclinical phase, it presents the potential to provide treatment options for the cancer types with activating mutations of RAS or high Ras activity which are not addressed by current Ras-targeted therapies.
References
- 1.Fernandez-Medarde A, Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011 Mar;2(3):344–358. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prior IA, Lewis PD, Mattos C. A comprehensive survey of ras mutations in cancer. Cancer Res. 2012 May 15;72(10):2457–2466. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malumbres M, Barbacid M. RAS oncogenes: The first 30 years. Nat Rev Cancer. 2003 Jun;3(6):459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 4.Karreth FA, Tuveson DA. Modelling oncogenic ras/raf signalling in the mouse. Curr Opin Genet Dev. 2009 Feb;19(1):4–11. doi: 10.1016/j.gde.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajalingam K, Schreck R, Rapp UR, Albert S. Ras oncogenes and their downstream targets. Biochim Biophys Acta. 2007 Aug;1773(8):1177–1195. doi: 10.1016/j.bbamcr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007 May 14;26(22):3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 7.Franke TF. PI3K/akt: Getting it right matters. Oncogene. 2008 Oct 27;27(50):6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 8.Gysin S, Salt M, Young A, McCormick F. Therapeutic strategies for targeting ras proteins. Genes Cancer. 2011 Mar;2(3):359–72. doi: 10.1177/1947601911412376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appels NM, Beijnen JH, Schellens JH. Development of farnesyl transferase inhibitors: A review. Oncologist. 2005 Sep;10(8):565–578. doi: 10.1634/theoncologist.10-8-565. [DOI] [PubMed] [Google Scholar]
- 10.Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, et al. K- and N-ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem. 1997 May 30;272(22):14459–14464. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- 11.Rotblat B, Ehrlich M, Haklai R, Kloog Y. The ras inhibitor farnesylthiosalicylic acid (salirasib) disrupts the spatiotemporal localization of active ras: A potential treatment for cancer. Methods Enzymol. 2008;439:467–489. doi: 10.1016/S0076-6879(07)00432-6. [DOI] [PubMed] [Google Scholar]
- 12.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: Validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010 Dec;10(12):842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003 Jan;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 14.Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007 Mar 1;13(5):1576–1583. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Manning BD. A complex interplay between akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009 Feb;37(Pt 1):217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinstein IB. Cancer. addiction to oncogenes--the achilles heal of cancer. Science. 2002 Jul 5;297(5578):63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 17.Chen G, Oh S, Monia BP, Stacey DW. Antisense oligonucleotides demonstrate a dominant role of c-ki-RAS proteins in regulating the proliferation of diploid human fibroblasts. J Biol Chem. 1996 Nov 8;271(45):28259–28265. doi: 10.1074/jbc.271.45.28259. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham CC, Holmlund JT, Geary RS, Kwoh TJ, Dorr A, Johnston JF, et al. A phase I trial of H-ras antisense oligonucleotide ISIS 2503 administered as a continuous intravenous infusion in patients with advanced carcinoma. Cancer. 2001 Sep 1;92(5):1265–1271. doi: 10.1002/1097-0142(20010901)92:5<1265::aid-cncr1447>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Perez RP, Smith JW, III, Alberts SR, Kaufman H, Posey J, Ritch P, et al. Phase II trial of ISIS 2503, an antisense inhibitor of H-ras, in patients (pts) with advanced pancreatic carcinoma (CA) ASCO Meeting Abstracts. 2001;20:628. [Google Scholar]
- 20.Saleh M, Posey J, Pleasant L, Lobuglio AI, Moore M, Soltan AI, et al. A phase II trial of ISIS 2503, an antisense inhibitor of H-ras, as first line therapy for advanced colorectal carcinoma. ASCO Meeting Abstracts. 2000;19:1258. [Google Scholar]
- 21.Dang T, Johnson DH, Kelly K, Rizvi N, Holmlund J, Dorr A. Multicenter phase II trial of an antisense inhibitor of H-ras (ISIS-2503) in advanced non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2001;20:1325. [Google Scholar]
- 22.Goldsmith G, Larocca RV, Glisson S, Hargis JB, Ballard C, Kuross S, et al. Phase 2 trial of ISIS 2503, an antisense inhibitor of h-ras, in combination with docetaxel for patients with previously treated advanced non-small cell lung cancer. ASCO Meeting Abstracts. 2003;22:941. [Google Scholar]
- 23.Alberts SR, Schroeder M, Erlichman C, Steen PD, Foster NR, Moore DF, Jr, et al. Gemcitabine and ISIS-2503 for patients with locally advanced or metastatic pancreatic adenocarcinoma: A north central cancer treatment group phase II trial. J Clin Oncol. 2004 Dec 15;22(24):4944–4950. doi: 10.1200/JCO.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Saleh MN, Irwin D, Burton G, Hargis JB, Chitambar C, Jones CM, et al. Phase 2 trial of ISIS 2503, an antisense inhibitor of h-ras, in combination with weekly paclitaxel in the treatment of patients with metastatic breast cancer. ASCO Meeting Abstracts. 2003;22:829. [Google Scholar]
- 25.Lledo S, Alfonso R, Alino SF. Antisense gene therapy using anti-k-ras and antitelomerase oligonucleotides in colorectal cancer. Rev Esp Enferm Dig. 2005 Jul;97(7):472–480. doi: 10.4321/s1130-01082005000700002. [DOI] [PubMed] [Google Scholar]
- 26.Andreyev HJ, Ross PJ, Cunningham D, Clarke PA. Antisense treatment directed against mutated ki-ras in human colorectal adenocarcinoma. Gut. 2001 Feb;48(2):230–237. doi: 10.1136/gut.48.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002 Sep;2(3):243–247. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 28.Fleming JB, Shen GL, Holloway SE, Davis M, Brekken RA. Molecular consequences of silencing mutant K-ras in pancreatic cancer cells: Justification for K-ras-directed therapy. Mol Cancer Res. 2005 Jul;3(7):413–422. doi: 10.1158/1541-7786.MCR-04-0206. [DOI] [PubMed] [Google Scholar]
- 29.Smakman N, Veenendaal LM, van Diest P, Bos R, Offringa R, Borel Rinkes IH, et al. Dual effect of kras(D12) knockdown on tumorigenesis: Increased immune-mediated tumor clearance and abrogation of tumor malignancy. Oncogene. 2005 Dec 15;24(56):8338–8342. doi: 10.1038/sj.onc.1208995. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Thompson JA, Fang B, Liu J. Silencing of H-ras gene expression by retrovirus-mediated siRNA decreases transformation efficiency and tumorgrowth in a model of human ovarian cancer. Oncogene. 2003 Aug 28;22(36):5694–5701. doi: 10.1038/sj.onc.1206858. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Jiang G, Yang F, Wang J. Knockdown of mutant K-ras expression by adenovirus-mediated siRNA inhibits the in vitro and in vivo growth of lung cancer cells. Cancer Biol Ther. 2006 Nov;5(11):1481–1486. doi: 10.4161/cbt.5.11.3297. [DOI] [PubMed] [Google Scholar]
- 32.Zhu H, Liang ZY, Ren XY, Liu TH. Small interfering RNAs targeting mutant K-ras inhibit human pancreatic carcinoma cells growth in vitro and in vivo. Cancer Biol Ther. 2006 Dec;5(12):1693–1698. doi: 10.4161/cbt.5.12.3466. [DOI] [PubMed] [Google Scholar]
- 33.End DW, Smets G, Todd AV, Applegate TL, Fuery CJ, Angibaud P, et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001 Jan 1;61(1):131–137. [PubMed] [Google Scholar]
- 34.Liu M, Bryant MS, Chen J, Lee S, Yaremko B, Lipari P, et al. Antitumor activity of SCH 66336, an orally bioavailable tricyclic inhibitor of farnesyl protein transferase, in human tumor xenograft models and wap-ras transgenic mice. Cancer Res. 1998 Nov 1;58(21):4947–4956. [PubMed] [Google Scholar]
- 35.Rose WC, Lee FY, Fairchild CR, Lynch M, Monticello T, Kramer RA, et al. Preclinical antitumor activity of BMS-214662, a highly apoptotic and novel farnesyltransferase inhibitor. Cancer Res. 2001 Oct 15;61(20):7507–7601. [PubMed] [Google Scholar]
- 36.Adjei AA, Mauer A, Bruzek L, Marks RS, Hillman S, Geyer S, et al. Phase II study of the farnesyl transferase inhibitor R115777 in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2003 May 1;21(9):1760–1766. doi: 10.1200/JCO.2003.09.075. [DOI] [PubMed] [Google Scholar]
- 37.Cohen SJ, Ho L, Ranganathan S, Abbruzzese JL, Alpaugh RK, Beard M, et al. Phase II and pharmacodynamic study of the farnesyltransferase inhibitor R115777 as initial therapy in patients with metastatic pancreatic adenocarcinoma. J Clin Oncol. 2003 Apr 1;21(7):1301–1306. doi: 10.1200/JCO.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 38.Johnston SR, Hickish T, Ellis P, Houston S, Kelland L, Dowsett M, et al. Phase II study of the efficacy and tolerability of two dosing regimens of the farnesyl transferase inhibitor, R115777, in advanced breast cancer. J Clin Oncol. 2003 Jul 1;21(13):2492–2499. doi: 10.1200/JCO.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 39.Sparano JA, Moulder S, Kazi A, Coppola D, Negassa A, Vahdat L, et al. Phase II trial of tipifarnib plus neoadjuvant doxorubicin-cyclophosphamide in patients with clinical stage IIB-IIIC breast cancer. Clin Cancer Res. 2009 Apr 15;15(8):2942–2948. doi: 10.1158/1078-0432.CCR-08-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fenaux P, Raza A, Mufti GJ, Aul C, Germing U, Kantarjian H, et al. A multicenter phase 2 study of the farnesyltransferase inhibitor tipifarnib in intermediate- to high-risk myelodysplastic syndrome. Blood. 2007 May 15;109(10):4158–4163. doi: 10.1182/blood-2006-07-035725. [DOI] [PubMed] [Google Scholar]
- 41.Jabbour E, Kantarjian H, Ravandi F, Garcia-Manero G, Estrov Z, Verstovsek S, et al. A phase 1–2 study of a farnesyltransferase inhibitor, tipifarnib, combined with idarubicin and cytarabine for patients with newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndrome. Cancer. 2011 Mar 15;117(6):1236–1244. doi: 10.1002/cncr.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harousseau JL, Martinelli G, Jedrzejczak WW, Brandwein JM, Bordessoule D, Masszi T, et al. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009 Aug 6;114(6):1166–1173. doi: 10.1182/blood-2009-01-198093. [DOI] [PubMed] [Google Scholar]
- 43.Rao S, Cunningham D, de Gramont A, Scheithauer W, Smakal M, Humblet Y, et al. Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J Clin Oncol. 2004 Oct 1;22(19):3950–3957. doi: 10.1200/JCO.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 44.Van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004 Apr 15;22(8):1430–1438. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 45.Safety alerts for human medical products > mylotarg (gemtuzumab ozogamicin): Market withdrawal [Internet].: U.S. Food and Drug Administration; 06/21/2010; cited 9/15/2012] Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm216458.htm.
- 46.Blumenschein G, Ludwig C, Thomas G, Tan E, Fanucchi M, Santoro A, et al. A randomized phase III trial comparing lonafarnib/carboplatin/paclitaxel versus carboplatin/paclitaxel (CP) in chemotherapy-naive patients with advanced or metastatic non-small cell lung cancer (NSCLC) Lung Cancer. 2005 Jul;49(Supplement 2):S30. [Google Scholar]
- 47.Sepp-Lorenzino L, Ma Z, Rands E, Kohl NE, Gibbs JB, Oliff A, et al. A peptidomimetic inhibitor of farnesyl:Protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res. 1995 Nov 15;55(22):5302–5309. [PubMed] [Google Scholar]
- 48.Sun J, Qian Y, Hamilton AD, Sebti SM. Both farnesyltransferase and geranylgeranyltransferase I inhibitors are required for inhibition of oncogenic K-ras prenylation but each alone is sufficient to suppress human tumor growth in nude mouse xenografts. Oncogene. 1998 Mar;16(11):1467–1473. doi: 10.1038/sj.onc.1201656. [DOI] [PubMed] [Google Scholar]
- 49.Charette N, De Saeger C, Lannoy V, Horsmans Y, Leclercq I, Starkel P. Salirasib inhibits the growth of hepatocarcinoma cell lines in vitro and tumor growth in vivo through ras and mTOR inhibition. Mol Cancer. 2010 Sep 22;9:256. doi: 10.1186/1476-4598-9-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldberg L, Ocherashvilli A, Daniels D, Last D, Cohen ZR, Tamar G, et al. Salirasib (farnesyl thiosalicylic acid) for brain tumor treatment: A convection-enhanced drug delivery study in rats. Mol Cancer Ther. 2008 Nov;7(11):3609–3616. doi: 10.1158/1535-7163.MCT-08-0488. [DOI] [PubMed] [Google Scholar]
- 51.Haklai R, Elad-Sfadia G, Egozi Y, Kloog Y. Orally administered FTS (salirasib) inhibits human pancreatic tumor growth in nude mice. Cancer Chemother Pharmacol. 2008 Jan;61(1):89–96. doi: 10.1007/s00280-007-0451-6. [DOI] [PubMed] [Google Scholar]
- 52.Zundelevich A, Elad-Sfadia G, Haklai R, Kloog Y. Suppression of lung cancer tumor growth in a nude mouse model by the ras inhibitor salirasib (farnesylthiosalicylic acid) Mol Cancer Ther. 2007 Jun;6(6):1765–1773. doi: 10.1158/1535-7163.MCT-06-0706. [DOI] [PubMed] [Google Scholar]
- 53.Laheru D, Shah P, Rajeshkumar NV, McAllister F, Taylor G, Goldsweig H, et al. Integrated preclinical and clinical development of S-trans, trans-farnesylthiosalicylic acid (FTS, salirasib) in pancreatic cancer. Invest New Drugs. 2012 May 1; doi: 10.1007/s10637-012-9818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsimberidou AM, Rudek MA, Hong D, Ng CS, Blair J, Goldsweig H, et al. Phase 1 first-in-human clinical study of S-trans,trans-farnesylthiosalicylic acid (salirasib) in patients with solid tumors. Cancer Chemother Pharmacol. 2010 Jan;65(2):235–241. doi: 10.1007/s00280-009-1027-4. [DOI] [PubMed] [Google Scholar]
- 55.Carnero A. The PKB/AKT pathway in cancer. Curr Pharm Des. 2010 Jan;16(1):34–44. doi: 10.2174/138161210789941865. [DOI] [PubMed] [Google Scholar]
- 56.Brugarolas J. Renal-cell carcinoma--molecular pathways and therapies. N Engl J Med. 2007 Jan 11;356(2):185–187. doi: 10.1056/NEJMe068263. [DOI] [PubMed] [Google Scholar]
- 57.Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012 Feb;11(2):317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 58.Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, et al. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012 Jan 20;30(3):282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 59.Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010 Sep 23;116(12):2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011 Jan 13;117(2):591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ikeda H, Hideshima T, Fulciniti M, Perrone G, Miura N, Yasui H, et al. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood. 2010 Sep 2;116(9):1460–1468. doi: 10.1182/blood-2009-06-222943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meadows SA, Vega F, Kashishian A, Johnson D, Diehl V, Miller LL, et al. PI3Kdelta inhibitor, GS-1101 (CAL-101), attenuates pathway signaling, induces apoptosis, and overcomes signals from the microenvironment in cellular models of hodgkin lymphoma. Blood. 2012 Feb 23;119(8):1897–1900. doi: 10.1182/blood-2011-10-386763. [DOI] [PubMed] [Google Scholar]
- 63.Meadows SA, Kashishian A, Johnson D, Ulrich RG, Miller LL, Lannutti BJ. CAL-101 (GS-1101), a specific inhibitor of phosphatidylinositol-3-kinase-delta (PI3K{delta}), disrupts signals from the microenvironment, induces apoptosis, and enhances the antitumor activity of everolimus (RAD001), an inhibitor of mammalian target of rapamycin (mTOR), in mantle cell lymphoma (MCL) ASH Annual Meeting Abstracts. 2011 Dec 09;118(21):3730. [Google Scholar]
- 64.Kahl B, Byrd JC, Flinn IW, Wagner-Johnston N, Spurgeon S, Benson DM, et al. Clinical safety and activity in a phase 1 study of CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3-kinase P110{delta}, in patients with relapsed or refractory non-hodgkin lymphoma. ASH Annual Meeting Abstracts. 2010 Dec 03;116(21):1777. [Google Scholar]
- 65.Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM, Wagner-Johnston N, et al. CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3-kinase P110{delta}, demonstrates clinical activity and pharmacodynamic effects in patients with relapsed or refractory chronic lymphocytic leukemia. ASH Annual Meeting Abstracts. 2010 Dec 03;116(21):55. [Google Scholar]
- 66.Sharman J, de Vos S, Leonard JP, Furman RR, Coutre SE, Flinn IW, et al. A phase 1 study of the selective phosphatidylinositol 3-kinase-delta (PI3K{delta}) inhibitor, CAL-101 (GS-1101), in combination with rituximab and/or bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia (CLL) ASH Annual Meeting Abstracts. 2011 Dec 09;118(21):1787. [Google Scholar]
- 67.de Vos S, Schreeder MT, Flinn IW, Coutre SE, Leonard JP, Wagner-Johnston N, et al. A phase 1 study of the selective phosphatidylinositol 3-kinase-delta (PI3K{delta}) inhibitor, cal-101 (GS-1101), in combination with rituximab and/or bendamustine in patients with previously treated, indolent non-hodgkin lymphoma (iNHL) ASH Annual Meeting Abstracts. 2011 Dec 09;118(21):2699. [Google Scholar]
- 68.Furman RR, Barrientos JC, Sharman JP, De Vos S, Leonard J, Coutre SE, et al. A phase I/II study of the selective phosphatidylinositol 3-kinase-delta (PI3K{delta}) inhibitor, GS-1101 (CAL-101), with ofatumumab in patients with previously treated chronic lymphocytic leukemia (CLL) ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):6518. [Google Scholar]
- 69.Luk SK, Piekorz RP, Nurnberg B, Tony To SS. The catalytic phosphoinositol 3-kinase isoform p110delta is required for glioma cell migration and invasion. Eur J Cancer. 2012 Jan;48(1):149–157. doi: 10.1016/j.ejca.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 70.Kashishian A, Meadows S, Steiner B, Lannutti B. Abstract 3555: Anti-tumor activity of CAL-101, a potent selective inhibitor of the p110{delta} isoform of PI3K, in models of human glioblastoma. Cancer Research. 2011 Jul 12;71(8 Supplement):3555. [Google Scholar]
- 71.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011 Dec 10;29(35):4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 72.Lu W, Defeo-Jones D, Davis L, Hang G, Tammam J, Hatch H, et al. Abstract #3714: In vitro and in vivo antitumor activities of MK-2206, a new allosteric akt inhibitor. AACR Meeting Abstracts. 2009 Apr 18;:3714. 2009(2_Annual_Meeting) [Google Scholar]
- 73.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010 Jul;9(7):1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 74.Han HS, Swanton C, Janjigian YY, Sutherland SC, Chandarlapaty S, Lehman R, et al. A phase I study of the AKT inhibitor (MK-2206) with concurrent trastuzumab and lapatinib in patients with HER2-positive solid tumors. ASCO Meeting Abstracts. 2011 Jun 09;29(15_suppl):3028. [Google Scholar]
- 75.Spencer A, Yoon S, Harrison SJ, Morris S, Smith D, Freedman SJ, et al. Novel AKT inhibitor GSK2110183 shows favorable safety, pharmacokinetics, clinical activity in multiple myeloma. preliminary results from a phase I first-time-in-human study. ASH Annual Meeting Abstracts. 2011 Nov 18;118(21):1856. [Google Scholar]
- 76.Blake JF, Xu R, Bencsik JR, Xiao D, Kallan NC, Schlachter S, et al. Discovery and preclinical pharmacology of a selective ATP-competitive akt inhibitor (GDC-0068) for the treatment of human tumors. J Med Chem. 2012 Sep 27;55(18):8110–8127. doi: 10.1021/jm301024w. [DOI] [PubMed] [Google Scholar]
- 77.Davies BR, Greenwood H, Dudley P, Crafter C, Yu DH, Zhang J, et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: Pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012 Apr;11(4):873–887. doi: 10.1158/1535-7163.MCT-11-0824-T. [DOI] [PubMed] [Google Scholar]
- 78.Wu X, Zhang J, Zhen R, Lv J, Zheng L, Su X, et al. Trastuzumab anti-tumor efficacy in patient-derived esophageal squamous cell carcinoma xenograft (PDECX) mouse models. J Transl Med. 2012 Aug 30;10(1):180. doi: 10.1186/1479-5876-10-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Houghton PJ. Everolimus. Clin Cancer Res. 2010 Mar 1;16(5):1368–1372. doi: 10.1158/1078-0432.CCR-09-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rini BI. Temsirolimus, an inhibitor of mammalian target of rapamycin. Clin Cancer Res. 2008 Mar ;14(5):1286–1290. doi: 10.1158/1078-0432.CCR-07-4719. [DOI] [PubMed] [Google Scholar]
- 81.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007 May 31;356(22):2271–2278. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 82.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008 Aug 9;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 83.Keedy VL. Treating metastatic soft-tissue or bone sarcomas - potential role of ridaforolimus. Onco Targets Ther. 2012;5:153–160. doi: 10.2147/OTT.S19055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drugs R. 2010;10(3) Ridaforolimus. [Google Scholar]
- 85.Merck receives complete response letter from U.S. food and drug administration for investigational medicine ridaforolimus [Internet].: Merck & Co., Inc. [updated June 5, 2012; cited 9/3/2012] Available from: http://www.merck.com/newsroom/news-release-archive/research-and-development/2012_0605.html.
- 86.Liu TJ, Koul D, LaFortune T, Tiao N, Shen RJ, Maira SM, et al. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009 Aug;8(8):2204–2210. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santiskulvong C, Konecny GE, Fekete M, Chen KY, Karam A, Mulholland D, et al. Dual targeting of phosphoinositide 3-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach in human ovarian carcinoma. Clin Cancer Res. 2011 Apr 15;17(8):2373–2384. doi: 10.1158/1078-0432.CCR-10-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res. 2008 Oct 1;68(19):8022–8030. doi: 10.1158/0008-5472.CAN-08-1385. [DOI] [PubMed] [Google Scholar]
- 89.Herrera VA, Zeindl-Eberhart E, Jung A, Huber RM, Bergner A. The dual PI3K/mTOR inhibitor BEZ235 is effective in lung cancer cell lines. Anticancer Res. 2011 Mar;31(3):849–854. [PubMed] [Google Scholar]
- 90.Burris H, Rodon J, Sharma S, Herbst RS, Tabernero J, Infante JR, et al. First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors. ASCO Meeting Abstracts. 2010 Jun 14;28(15_suppl):3005. [Google Scholar]
- 91.Arkenau H, Jones SF, Kurkjian C, Infante JR, Pant S, Burris HA, et al. The PI3K/mTOR inhibitor BEZ235 given twice daily for the treatment of patients (pts) with advanced solid tumors. ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):3097. [Google Scholar]
- 92.Krop IE, Saura C, Rodon Ahnert J, Becerra C, Britten CD, Isakoff SJ, et al. A phase I/IB dose-escalation study of BEZ235 in combination with trastuzumab in patients with PI3-kinase or PTEN altered HER2+ metastatic breast cancer. ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):508. [Google Scholar]
- 93.Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, et al. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011 Dec;10(12):2426–2433. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 94.Wagner AJ, Bendell JC, Dolly S, Morgan JA, Ware JA, Fredrickson J, et al. A first-in-human phase I study to evaluate GDC-0980, an oral PI3K/mTOR inhibitor, administered QD in patients with advanced solid tumors. ASCO Meeting Abstracts. 2011 Jun 09;29(15_suppl):3020. [Google Scholar]
- 95.Drosten M, Dhawahir A, Sum EY, Urosevic J, Lechuga CG, Esteban LM, et al. Genetic analysis of ras signalling pathways in cell proliferation, migration and survival. EMBO J. 2010 Mar 17;29(6):1091–1104. doi: 10.1038/emboj.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Karreth FA, Frese KK, DeNicola GM, Baccarini M, Tuveson DA. C-raf is required for the initiation of lung cancer by K-ras(G12D) Cancer Discov. 2011 Jul;1(2):128–136. doi: 10.1158/2159-8290.CD-10-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008 Oct;7(10):3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 98.Lamuraglia M, Escudier B, Chami L, Schwartz B, Leclere J, Roche A, et al. To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: Pilot study using dynamic contrast-enhanced doppler ultrasound. Eur J Cancer. 2006 Oct;42(15):2472–2479. doi: 10.1016/j.ejca.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 99.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul 24;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 100.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006 Sep 10;24(26):4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 101.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002 Jun 27;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 102.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008 Feb 26;105(8):3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010 Aug 26;363(9):809–901. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ribas A, Kim KB, Schuchter LM, Gonzalez R, Pavlick AC, Weber JS, et al. BRIM-2: An open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAF V600E mutation-positive metastatic melanoma. ASCO Meeting Abstracts. 2011 Jun 09;29(15_suppl):8509. [Google Scholar]
- 105.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011 Jun 30;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012 Jul 28;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 107.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010 Dec 16;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010 Dec 14;18(6):683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Montagut C, Sharma SV, Shioda T, McDermott U, Ulman M, Ulkus LE, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res. 2008 Jun 15;68(12):4853–4861. doi: 10.1158/0008-5472.CAN-07-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010 Dec 16;468(7326):968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010 Mar 18;464(7287):427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010 Mar 18;464(7287):431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 113.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010 Jan 22;140(2):209–221. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Su F, Viros A, Milagre C, Trunzer K, Bollag G, Spleiss O, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012 Jan 19;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006 Jan 19;439(7074):358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gilmartin AG, Bleam MR, Groy A, Moss KG, Minthorn EA, Kulkarni SG, et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin Cancer Res. 2011 Mar 1;17(5):989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 117.Falchook GS, Lewis KD, Infante JR, Gordon MS, Vogelzang NJ, Demarini DJ, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: A phase 1 dose-escalation trial. Lancet Oncol. 2012 Aug;13(8):782–789. doi: 10.1016/S1470-2045(12)70269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Infante JR, Fecher LA, Falchook GS, Nallapareddy S, Gordon MS, Becerra C, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: A phase 1 dose-escalation trial. Lancet Oncol. 2012 Aug;13(8):773–781. doi: 10.1016/S1470-2045(12)70270-X. [DOI] [PubMed] [Google Scholar]
- 119.Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012 Jul 12;367(2):107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 120.Friday BB, Yu C, Dy GK, Smith PD, Wang L, Thibodeau SN, et al. BRAF V600E disrupts AZD6244-induced abrogation of negative feedback pathways between extracellular signal-regulated kinase and raf proteins. Cancer Res. 2008 Aug 1;68(15):6145–6153. doi: 10.1158/0008-5472.CAN-08-1430. [DOI] [PubMed] [Google Scholar]
- 121.O'Neil BH, Goff LW, Kauh JS, Strosberg JR, Bekaii-Saab TS, Lee RM, et al. Phase II study of the mitogen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2011 Jun 10;29(17):2350–2356. doi: 10.1200/JCO.2010.33.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kirkwood JM, Bastholt L, Robert C, Sosman J, Larkin J, Hersey P, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res. 2012 Jan 15;18(2):555–567. doi: 10.1158/1078-0432.CCR-11-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Pover G, et al. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2012 Jun;30(3):1216–1223. doi: 10.1007/s10637-011-9687-4. [DOI] [PubMed] [Google Scholar]
- 124.Balmanno K, Chell SD, Gillings AS, Hayat S, Cook SJ. Intrinsic resistance to the MEK1/2 inhibitor AZD6244 (ARRY-142886) is associated with weak ERK1/2 signalling and/or strong PI3K signalling in colorectal cancer cell lines. Int J Cancer. 2009 Nov 15;125(10):2332–2341. doi: 10.1002/ijc.24604. [DOI] [PubMed] [Google Scholar]
- 125.Gopal YN, Deng W, Woodman SE, Komurov K, Ram P, Smith PD, et al. Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in braf-mutant human cutaneous melanoma cells. Cancer Res. 2010 Nov 1;70(21):8736–8747. doi: 10.1158/0008-5472.CAN-10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Little AS, Balmanno K, Sale MJ, Newman S, Dry JR, Hampson M, et al. Amplification of the driving oncogene, KRAS or BRAF, underpins acquired resistance to MEK1/2 inhibitors in colorectal cancer cells. Sci Signal. 2011 Mar 29;4(166) doi: 10.1126/scisignal.2001752. ra17. [DOI] [PubMed] [Google Scholar]
- 127.Sos ML, Fischer S, Ullrich R, Peifer M, Heuckmann JM, Koker M, et al. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci U S A. 2009 Oct 27;106(43):18351–18356. doi: 10.1073/pnas.0907325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Greger JG, Eastman SD, Zhang V, Bleam MR, Hughes AM, Smitheman KN, et al. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther. 2012 Apr;11(4):909–920. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- 129.Kaelin WG., Jr Synthetic lethality: A framework for the development of wiser cancer therapeutics. Genome Med. 2009 Oct 27;1(10):99. doi: 10.1186/gm99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dobzhansky T, Krimbas C, Krimbas MG. Genetics of natural populations. xxix. is the genetic load in drosophila pseudoobscura a mutational or a balanced load? Genetics. 1960 Jun;45(6):741–753. doi: 10.1093/genetics/45.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Cell. 2007 Sep 21;130(6):986–988. doi: 10.1016/j.cell.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 132.Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009 Nov 5;462(7269):108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scholl C, Frohling S, Dunn IF, Schinzel AC, Barbie DA, Kim SY, et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell. 2009 May 29;137(5):821–834. doi: 10.1016/j.cell.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 134.Babij C, Zhang Y, Kurzeja RJ, Munzli A, Shehabeldin A, Fernando M, et al. STK33 kinase activity is nonessential in KRAS-dependent cancer cells. Cancer Res. 2011 Sep 1;71(17):5818–5826. doi: 10.1158/0008-5472.CAN-11-0778. [DOI] [PubMed] [Google Scholar]
- 135.Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF, et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the ras oncogene. Cell. 2009 May 29;137(5):835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, et al. A gene expression signature associated with “K-ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009 Jun 2;15(6):489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sarthy AV, Morgan-Lappe SE, Zakula D, Vernetti L, Schurdak M, Packer JC, et al. Survivin depletion preferentially reduces the survival of activated K-ras-transformed cells. Mol Cancer Ther. 2007 Jan;6(1):269–276. doi: 10.1158/1535-7163.MCT-06-0560. [DOI] [PubMed] [Google Scholar]
- 138.Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009 Nov 5;462(7269):104–107. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Basu A, Pal D. Two faces of protein kinase cdelta: The contrasting roles of PKCdelta in cell survival and cell death. Scientific World Journal. 2010 Nov 16;10:2272–2284. doi: 10.1100/tsw.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, et al. Exacerbated vein graft arteriosclerosis in protein kinase cdelta-null mice. J Clin Invest. 2001 Nov;108(10):1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xia S, Forman LW, Faller DV. Protein kinase C delta is required for survival of cells expressing activated p21RAS. J Biol Chem. 2007 May 4;282(18):13199–13210. doi: 10.1074/jbc.M610225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xia S, Chen Z, Forman LW, Faller DV. PKCdelta survival signaling in cells containing an activated p21Ras protein requires PDK1. Cell Signal. 2009 Apr;21(4):502–508. doi: 10.1016/j.cellsig.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen Z, Forman LW, Miller KA, English B, Takashima A, Bohacek RA, et al. Protein kinase cdelta inactivation inhibits cellular proliferation and decreases survival in human neuroendocrine tumors. Endocr Relat Cancer. 2011 Dec 1;18(6):759–771. doi: 10.1530/ERC-10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Takashima A, Faller DV. Inhibition of protein kinase C delta induces apoptosis through JNK-H2AX pathway in melanoma. AACR Annual Meeting 2013 Abstract submitted [Google Scholar]
- 145.Besse B, Soria J, Gomez-Roca C, Ware JA, Adjei AA, Dy GK, et al. A phase ib study to evaluate the PI3-kinase inhibitor GDC-0941 with paclitaxel (P) and carboplatin (C), with and without bevacizumab (BEV), in patients with advanced non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2011 Jun 09;29(15):3044. [Google Scholar]
- 146.LoRusso P, Shapiro G, Pandya SS, Kwak EL, Jones C, Belvin M, et al. A first-in-human phase ib study to evaluate the MEK inhibitor GDC-0973, combined with the pan-PI3K inhibitor GDC-0941, in patients with advanced solid tumors. ASCO Meeting Abstracts. 2012 May 30;30(15):2566. [Google Scholar]
- 147.Moreno Garcia V, Baird RD, Shah KJ, Basu B, Tunariu N, Blanco M, et al. A phase I study evaluating GDC-0941, an oral phosphoinositide-3 kinase (PI3K) inhibitor, in patients with advanced solid tumors or multiple myeloma. ASCO Meeting Abstracts. 2011 Jun 09;29(15):3021. [Google Scholar]
- 148.Jimeno A, Herbst RS, Falchook GS, Messersmith WA, Hecker S, Peterson S, et al. Final results from a phase I, dose-escalation study of PX-866, an irreversible, pan-isoform inhibitor of PI3 kinase. ASCO Meeting Abstracts. 2010 Jun 14;28(15_suppl):3089. [Google Scholar]
- 149.Jimeno A, Senzer NN, Rudin CM, Ma WW, Halmos B, Schnadig ID, et al. PX-866 and docetaxel in patients with advanced solid tumors. ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):3024. [Google Scholar]
- 150.Patnaik A, Appleman LJ, Mountz JM, Ramanathan RK, Beeram M, Tolcher AW, et al. A first-in-human phase I study of intravenous PI3K inhibitor BAY 80–6946 in patients with advanced solid tumors: Results of dose-escalation phase. ASCO Meeting Abstracts. 2011 Jun 09;29(15_suppl):3035. [Google Scholar]
- 151.Moldovan C, Soria J, LoRusso P, Guthrie T, Song C, Nguyen LT, et al. A phase I safety and pharmacokinetic (PK) study of the PI3K inhibitor XL147 (SAR245408) in combination with erlotinib in patients (pts) with advanced solid tumors. ASCO Meeting Abstracts. 2010 Jun 14;28(15_suppl):3070. [Google Scholar]
- 152.Traynor AM, Kurzrock R, Bailey HH, Attia S, Scheffold C, van Leeuwen B, et al. A phase I safety and pharmacokinetic (PK) study of the PI3K inhibitor XL147 (SAR245408) in combination with paclitaxel (P) and carboplatin (C) in patients (pts) with advanced solid tumors. ASCO Meeting Abstracts. 2010 Jun 14;28(15_suppl):3078. [Google Scholar]
- 153.Juric D, Rodon J, Gonzalez-Angulo AM, Burris HA, Bendell J, Berlin JD, et al. Abstract CT-01: BYL719, a next generation PI3K alpha specific inhibitor: Preliminary safety, PK, and efficacy results from the first-in-human study. Cancer Research. 2012 Jun 04;72(8 Supplement) CT-01. [Google Scholar]
- 154.Yaguchi S, Fukui Y, Koshimizu I, Yoshimi H, Matsuno T, Gouda H, et al. Antitumor activity of ZSTK474, a new phosphatidylinositol 3-kinase inhibitor. J Natl Cancer Inst. 2006 Apr 19;98(8):545–56. doi: 10.1093/jnci/djj133. [DOI] [PubMed] [Google Scholar]
- 155.Jonasch E, Lara P, Tannir NM. A randomized phase II study of MK-2206 in comparison with everolimus in refractory renal cell carcinoma.(NCI 8727) ASCO Meeting Abstracts. 2011 Jun 09;29(15_suppl) TPS192. [Google Scholar]
- 156.Chan TCK, Lapierre J, Ashwell MA, France DS, Chen C, Cornell-Kennon S, et al. Abstract A230: Discovery and characterization of ARQ 092, an ATP-independent, potent and selective inhibitor of AKT kinases. Molecular Cancer Therapeutics. 2011 Nov 12;10(Supplement 1):A230. [Google Scholar]
- 157.Burris HA, Siu LL, Infante JR, Wheler JJ, Kurkjian C, Opalinska J, et al. Safety, pharmacokinetics (PK), pharmacodynamics (PD), and clinical activity of the oral AKT inhibitor GSK2141795 (GSK795) in a phase I first-in-human study. ASCO Meeting Abstracts. 2011 Jun 09;29(15_suppl):3003. [Google Scholar]
- 158.Shih KC, Bendell JC, Reinert A, Jones S, Kelley RK, Infante JR, et al. Phase I trial of an oral TORC1/TORC2 inhibitor (CC-223) in advanced solid and hematologic cancers. ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):3006. [Google Scholar]
- 159.Banerji U, Dean EJ, Gonzalez M, Greystoke AP, Basu B, Krebs M, et al. First-in-human phase I trial of the dual mTORC1 and mTORC2 inhibitor AZD2014 in solid tumors. ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):3004. [Google Scholar]
- 160.Bhagwat SV, Gokhale PC, Crew AP, Cooke A, Yao Y, Mantis C, et al. Preclinical characterization of OSI-027, a potent and selective inhibitor of mTORC1 and mTORC2: Distinct from rapamycin. Mol Cancer Ther. 2011 Aug;10(8):1394–1406. doi: 10.1158/1535-7163.MCT-10-1099. [DOI] [PubMed] [Google Scholar]
- 161.Yuan J, Mehta PP, Yin MJ, Sun S, Zou A, Chen J, et al. PF-04691502, a potent and selective oral inhibitor of PI3K and mTOR kinases with antitumor activity. Mol Cancer Ther. 2011 Nov;10(11):2189–2199. doi: 10.1158/1535-7163.MCT-11-0185. [DOI] [PubMed] [Google Scholar]
- 162.Mallon R, Feldberg LR, Lucas J, Chaudhary I, Dehnhardt C, Santos ED, et al. Antitumor efficacy of PKI-587, a highly potent dual PI3K/mTOR kinase inhibitor. Clin Cancer Res. 2011 May 15;17(10):3193–3220. doi: 10.1158/1078-0432.CCR-10-1694. [DOI] [PubMed] [Google Scholar]
- 163.Cohen RB, Janne PA, Engelman JA, Martinez P, Nishida Y, Gendreau S, et al. A phase I safety and pharmacokinetic (PK) study of PI3K/TORC1/TORC2 inhibitor XL765 (SAR245409) in combination with erlotinib (E) in patients (pts) with advanced solid tumors. ASCO Meeting Abstracts. 2010 Jun 14;28(15_suppl):3015. [Google Scholar]
- 164.Nghiemphu PL, Omuro AM, Cloughesy T, Mellinghoff IK, Norden AD, Nguyen LT, et al. A phase I safety and pharmacokinetic study of XL765 (SAR245409), a novel PI3K/TORC1/TORC2 inhibitor, in combination with temozolomide (TMZ) in patients (pts) with newly diagnosed malignant glioma. ASCO Meeting Abstracts. 2010 Jun 14;28(15_suppl):3085. [Google Scholar]
- 165.Munster PN, van der Noll R, Voest EE, Dees EC, Tan AR, Specht JM, et al. Phase I first-in-human study of the PI3 kinase inhibitor GSK2126458 (GSK458) in patients with advanced solid tumors (study P3K112826) ASCO Meeting Abstracts. 2011 Jun 09;29(15_suppl):3018. [Google Scholar]
- 166.O'Farrell M, Ventura R, Tai A, Matthews DJ. Abstract 3737: The PI3K/mTOR inhibitor PWT33597 regresses 786-0 renal xenografts. Cancer Research. 2012 Jun 04;72(8 Supplement):3737. [Google Scholar]
- 167.Mahadevan D, Chiorean EG, Harris WB, Von Hoff DD, Stejskal-Barnett A, Qi W, et al. Phase I pharmacokinetic and pharmacodynamic study of the pan-PI3K/mTORC vascular targeted pro-drug SF1126 in patients with advanced solid tumours and B-cell malignancies. Eur J Cancer. 2012 Aug 23; doi: 10.1016/j.ejca.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Grothey A, Sobrero AF, Siena S, Falcone A, Ychou M, Lenz H, et al. Results of a phase III randomized, double-blind, placebo-controlled, multicenter trial (CORRECT) of regorafenib plus best supportive care (BSC) versus placebo plus BSC in patients (pts) with metastatic colorectal cancer (mCRC) who have progressed after standard therapies. ASCO Meeting Abstracts. 2012 Jan 30;30(4_suppl) LBA385. [Google Scholar]
- 169.Eisen T, Joensuu H, Nathan PD, Harper PG, Wojtukiewicz MZ, Nicholson S, et al. Regorafenib for patients with previously untreated metastatic or unresectable renal-cell carcinoma: A single-group phase 2 trial. Lancet Oncol. 2012 Oct;13(10):1055–1062. doi: 10.1016/S1470-2045(12)70364-9. [DOI] [PubMed] [Google Scholar]
- 170.Stuart DD, Li N, Poon DJ, Aardalen K, Kaufman S, Merritt H, et al. Abstract 3790: Preclinical profile of LGX818: A potent selective RAF kinase inhibitor. Cancer Research. 2012 Jun 04;72(8 Supplement):3790. [Google Scholar]
- 171.Sharfman WH, Hodi FS, Lawrence DP, Flaherty KT, Amaravadi RK, Kim KB, et al. Results from the first-in-human (FIH) phase I study of the oral RAF inhibitor RAF265 administered daily to patients with advanced cutaneous melanoma. ASCO Meeting Abstracts. 2011 Jun 09;29(15_suppl):8508. [Google Scholar]
- 172.Su Y, Vilgelm AE, Kelley MC, Hawkins OE, Liu Y, Boyd KL, et al. RAF265 inhibits the growth of advanced human melanoma tumors. Clin Cancer Res. 2012 Apr 15;18(8):2184–2198. doi: 10.1158/1078-0432.CCR-11-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Finn RS, Javle MM, Tan BR, Weekes CD, Bendell JC, Patnaik A, et al. A phase I study of MEK inhibitor MEK162 (ARRY-438162) in patients with biliary tract cancer. ASCO Meeting Abstracts. 2012 Jan 30;30(4_suppl):220. [Google Scholar]
- 174.Kim K, Kong SY, Fulciniti M, Li X, Song W, Nahar S, et al. Blockade of the MEK/ERK signalling cascade by AS703026, a novel selective MEK1/2 inhibitor, induces pleiotropic anti-myeloma activity in vitro and in vivo. Br J Haematol. 2010 May;149(4):537–549. doi: 10.1111/j.1365-2141.2010.08127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Infante JR, Gandhi L, Shapiro G, Burris HA, Bendell JC, Baselga J, et al. Phase lb combination trial of a MEK inhibitor, pimasertib (MSC1936369B), and a PI3K/mTOR inhibitor, SAR245409, in patients with locally advanced or metastatic solid tumors. ASCO Meeting Abstracts. 2012 May 30;30(15_suppl) TPS3118. [Google Scholar]
- 176.Lim HY, Yen C, Tak W, Heo J, Choi HJ, Lin C, et al. A phase II trial of MEK inhibitor BAY 86–9766 in combination with sorafenib as first-line systemic treatment for patients with unresectable hepatocellular carcinoma (HCC) ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):4103. [Google Scholar]
- 177.Van Laethem J, Heinemann V, Martens UM, Jassem J, Michl P, Peeters M, et al. A phase I/II study of the MEK inhibitor BAY 86–9766 (BAY) in combination with gemcitabine (GEM) in patients with nonresectable, locally advanced or metastatic pancreatic cancer (PC): Phase I dose-escalation results. ASCO Meeting Abstracts. 2012 May 30;30(15_suppl):4050. [Google Scholar]
- 178.Mala C, Neville NG, Haindl E, Buergle M, Schmalix W, Bevan P. A phase I, first-in-human single ascending dose study of the MEK inhibitor WX-554 given to healthy male subjects. ASCO Meeting Abstracts. 2010 Jun 14;28(15_suppl):e13666. [Google Scholar]
- 179.Leijen S, Middleton MR, Tresca P, Kraeber-Bodere F, Dieras V, Scheulen ME, et al. Phase I (ph) safety, pharmacodynamic (PD), and pharmacokinetic (PK) trial of a pure MEK inhibitor (i), RO4987655, in patients with advanced /metastatic solid tumor. ASCO Meeting Abstracts. 2011 Jun 09;29(15_suppl):3017. [Google Scholar]
- 180.Dong Q, Dougan DR, Gong X, Halkowycz P, Jin B, Kanouni T, et al. Discovery of TAK-733, a potent and selective MEK allosteric site inhibitor for the treatment of cancer. Bioorg Med Chem Lett. 2011 Mar 1;21(5):1315–1319. doi: 10.1016/j.bmcl.2011.01.071. [DOI] [PubMed] [Google Scholar]
- 181.Kumar V, Schuck EL, Pelletier RD, Farah N, Condon KB, Ye M, et al. Pharmacokinetic characterization of a natural product-inspired novel MEK1 inhibitor E6201 in preclinical species. Cancer Chemother Pharmacol. 2012 Jan;69(1):229–237. doi: 10.1007/s00280-011-1687-8. [DOI] [PubMed] [Google Scholar]