Abstract
To modulate isoflavone aglycone composition within a soy functional food, soy ingredients were processed and evaluated in a soy bread system intended for clinical trials. A soy flour/soy milk mixture (SM) was boiled, fermented, steamed, or roasted prior to dough preparation. The isoflavone compositions of five processed SM and their corresponding breads combined with and without β-glucosidase-rich almonds were examined using HPLC. Isoflavone malonyl-glucosides (>80%) were converted into acetyl and simple glucoside forms (substrates more favorable for β-glucosidase) in steamed and roasted SM. Their corresponding breads had isoflavones predominately as aglycones (∼75%) with soy–almond bread with steamed SM being more consumer acceptable than roasted. Isoflavone composition in soy bread was stable during frozen storage and toasting. A suitable glycoside-rich soy bread (31.6 ± 2.1 mg aglycone equiv/slice) using unprocessed SM and an aglycone-rich soy–almond bread (31.1 ± 1.9 mg aglycone equiv/slice) using steamed SM were developed to evaluate fundamental questions of isoflavone bioavailability in clinical trials.
Keywords: soy bread, almonds, isoflavones, aglycone, glycoside
Introduction
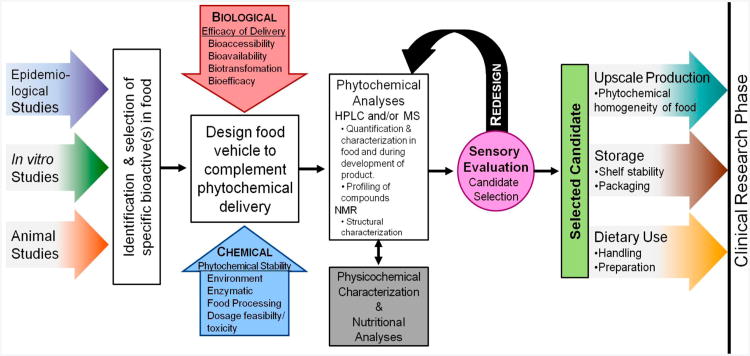
Designing and selecting an appropriate food vehicle to complement delivery of food bioactive ingredients to specific sites within the human body (e.g., oral cavity, esophagus, and small intestines) are fundamental in the development of a functional food appropriate for clinical trials. Functional foods contain bioactive ingredients that have the potential to promote health and prevent disease. Therefore, identification and selection of ingredients abundant in bioactive compounds of interest needs to be at the forefront of design. Additionally, ensuring the delivery of these bioactive compounds to biological targets is critical in directing the choice of the food vehicle (Figure 1). For this study, soy isoflavones in a soy bread model were the example.
Figure 1.
Preclinical phase of functional food development beginning with identification of target food bioactive proceeding to design of food vehicle and then to selection of final candidate(s) for clinical trials.
Of the several dietary variables of interest, emerging evidence suggests that diets rich in soy foods are associated with a lower risk of heart disease,1 hormone-dependent cancer,2 and other age-related diseases.3 Among the soy phytonutrients, isoflavones and their associated soy protein have demonstrated biological activity.4–10 Soy has functional properties that, when properly manipulated, can be utilized to manufacture soy baked goods or snack foods which can be conveniently integrated into a Westernized diet. Developing food vehicles appropriate for clinical trials requires substantial amounts of soy, typically ranging from 25 to 50% (∼50 mg of isoflavones/day) of the soy food formulation.11,12 Greater quantities of active ingredients contained in a food vehicle inversely affect dosing frequency. Reducing dosing frequency aids in compliance during clinical trials. However, >20% soy in baked goods has detrimental effects on bread quality such as loss of texture (dense and grainy),13–15 formation of off-flavors (bitter and beany),14,16,17 and loss of dough durability.18 These challenges reiterate that design and development of food vehicles inevitably involve the balance of maintaining food quality and optimizing delivery of active ingredients to biologic targets.
Specifically with soy isoflavones, ongoing discussion remains regarding their bioavailability, metabolism, and bioactivity.19–24 Isoflavones are naturally occurring heterocyclic compounds existing in three types (daidzein, genistein, and glycitein) and four chemical forms (aglycone, β-glycoside, acetyl-glycoside, and malonyl-glycoside). The aglycones compared to their glycoside forms of isoflavones are hypothesized to be more bioavailable because they are assumed to be more readily absorbed through passive absorption by the intestinal enterocytes.19,20 After ingestion, isoflavone glycosides are suggested to be hydrolyzed by β-glucosidase present in the human intestinal mucosa25 and intestinal microbiota,26 thereby yielding isoflavone aglycones (daidzein, genistein, and glycitein). Studies have suggested higher,24 lower,19,23 or no difference in absorption20,21 of isoflavone glycoside forms compared to their aglycones. These mixed results might be explained by the different forms and sources in which isoflavones have been administered, either as pure27 or within a food.19,28 Differences in processing methods, soy food matrix, isoflavone chemical composition, and other soy phytonutrients are considered to affect the bioaccessibility and bioavailability of isoflavones in soy products.29–31 For instance, meta-analysis investigating relative risk for prostate cancer between consumption of nonfermented (RR, 0.70) versus fermented (RR, 1.01) soy foods underscores the importance of understanding the role of isoflavone chemical composition and soy food type.32
The various processing methods in manufacturing different soy foods have provided evidence that significant chemical modifications of isoflavones occur during cooking and fermentation of soybeans.29,33 Isoflavones exist predominately in their malonyl-glycoside forms in native soybeans; however, they are sensitive to chemical and enzymatic transformation during soy food processing. Malonyl- and acetyl-glycoside forms when heated are susceptible to chemical conversion.34,35 Soy products such as soy milk powder undergo heated dehydration, and the isoflavone composition, which begins as predominately malonyl-glycosides, becomes abundant in acetyl-glycosides,29 whereas in fermented soy products, such as miso, the isoflavone glycosides have been converted to their aglycone forms by bacterial enzymes.29,33 The principal enzyme responsible for the conversion of glycosides to the aglycone forms is β-glucosidase, which facilitates the hydrolysis of the glycoside.36–38 This ubiquitous enzyme is found abundantly in almonds (Prunus dulcis),37 yeast (Saccharomyces cerevisiae),39 wheat flour (Triticum aestivum L.),40 and soybeans (Glycine max).36
The objectives of this study were to develop and select an acceptable aglycone-rich soy bread that will be a suitable delivery vehicle of isoflavones in clinical trials and to investigate factors (proofing, toasting, and storage of soy bread) that may alter the successful delivery of isoflavones in soy breads used for clinical trials.
Materials and Methods
Processing Soy Ingredient Mix
All analyses were performed using a patented mix of soy flour and soy milk41 (3:1, soy flour/soy milk (SM)) from a single 50 lb lot of soy flour and soy milk (Table 1). This mixture of soy ingredients will be collectively referred to as soy mix (SM). Five different processing methods were examined. SM was boiled using 80% formula water (30 min in water bath at 100 °C), microwave heated (1500 W, model R995J Sharp) for 10 min on 50% power, or incubated with 80% formula water (12 h in water bath at 40 °C). SM was steamed using a no. 30 sieve in which soy mixture was sandwiched between two filter papers (no.1, Whatman Inc., Piscataway, NJ, USA), sealed with aluminum foil, and placed over a boiling water bath. The internal temperature of the steamed SM was measured and maintained at 98 ± 2 °C for 2 h. SM was dry roasted by spreading soy mixture on an aluminum baking sheet (5 mm depth) and heated for 60 min at 175 °C. SM was fermented with 80% formula water, covered with polyethylene wrap (Fisherbrand, Fisher Scientific Inc., Fair Lawn, NJ, USA), and stored at ambient conditions (25 ± 5 °C) for 96 h. Many other variations in fermentation (3–10 days) and thermal processing such as boiling with 1% acetic acid solution and frying were examined but produced bread with poor quality and taste; therefore, these variables were not included in the results or discussion.
Table 1. Ingredients and Formulation of Soy Bread and Soy–Almond Bread.
| ingredient | soy bread (% wet basis) | soy–almond bread (% wet basis) |
|---|---|---|
| water | 36.4 | 36.4 |
| high gluten enriched bromated wheat flour (Bouncer, Bay State Milling, Quincy, MA, USA) | 38.7 | 35.7 |
| vital gluten (Hodgson Mill, Effingham, IL, USA) | 0.9 | 0.9 |
| defatted soy flour, Baker's Nutrisoy (ADM Protein Specialties Division, Decatur, IL, USA) | 14.2 | 14.2 |
| soy milk powder, Benesoy (Devansoy, Carroll, IA, USA) | 4.7 | 4.7 |
| Crisco vegetable shortening (Proctor & Gamble, Cincinnati, OH, USA) | 1.9 | 1.8 |
| sugar (Domino Foods Corp., Yonkers, NY, USA) | 1.9 | 1.8 |
| instant active dry yeast, SAF instant (Lesaffre Group, Marcq-en-Baroeul, France) | 0.3 | 0.3 |
| kosher salt (Morton International Inc., Chicago, IL, USA) | 0.8 | 0.8 |
| dough conditioner (Caravan Products Co., Totowa, NJ, USA) | 0.1 | 0.1 |
| whole raw almond meal (Whole Foods, Austin, TX, USA) | 0 | 3.2 |
Preparation of Soy and Soy–Almond Bread
All ingredients were measured to produce a finished dough weighing 1200 g using the proportions described in Table 1. Whole raw almonds (Whole Foods, Austin, TX, USA) were frozen and ground into meal no more than 30 min prior to dough formation using a commercial blender (model 50200MP, Hamilton Beach Brands Inc., Glen Allen, VA, USA) and passed through a no. 20 stainless steel sieve (USA Standard Testing Sieve) prior to inclusion. The soy (SB) and soy–almond breads (SAB) were produced using a sponge-dough process. Two sponge mixtures were prepared. Sponge A (flour, gluten, yeast, and water) was stored under ambient conditions for 2 h. Sponge B (untreated or processed soy mixture and water with or without almond meal) rested in an electric proofing cabinet (CM2000 combination module FlavorView InterMetro Industries Corp., Wilkes-Barre, PA, USA) set at maximum humidity (90–100% RH) at 40 °C for 2 h. The two sponge mixtures were combined with the addition of remaining dry ingredients and water in a 5 qt bowl KitchenAid planetary mixer (model KV25GO, KitchenAid, St. Joseph, MI, USA) affixed with a dough hook attachment. The dough was developed for 15 min. Loaves were formed, panned, and proofed in a high-humidity proofing cabinet at 40 °C for 60 min. Proofed loaves were baked for 50 min at 150 °C in a convection oven (jet air oven, model JA14, Doyon, Liniere, Quebec, Canada). Breads were determined to be done once an internal temperature of 95 ± 2 °C was achieved. Finished loaves were cooled for 4 h and stored in sealed polyethylene bags. Because the water content among the many processed soy ingredients varied and these differences in moisture were suspected to affect final isoflavone concentration, the formula water used during dough production was adjusted to balance for differences in moisture content between the treated and untreated soy ingredients. Formula water was adjusted to target the SM moisture content to 13%.
Analysis of Isoflavones
Chemicals
HPLC grade acetonitrile (ACN), acetic acid, methanol (MeOH), and water were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Dimethyl sulfoxide (ACS spectrophotometric grade) was from Sigma-Aldrich (St. Louis, MO, USA). Daidzein, genistein, glycitein, daidzin, genistin, glycitin, acetyl-daidzin, malonyl-genistin, and acetyl-genistin were from LC Laboratories Division (PKC Pharmaceuticals, Inc., Woburn, MA, USA) and acetyl-glycitin, malonyl-daidzin, and malonyl-glycitin from Wako Chemicals USA, Inc. (Richmond, VA, USA).
Sample Preparation
(a) Soy Ingredients and Soy Breads
Processed SM was sampled from each processing method, and three replicates were sampled from eight different batches. In finished soy breads with or without almond, three replicate samples were collected using a ground center slice of eight loaves baked during eight baking days. Bread samples were freeze-dried, ground to a fine powder using a mortar and pestle, and stored at −25 °C. Using methods described by Ahn-Jarvis and colleagues, isoflavones were extracted from soy and bread samples (500 mg) using 60% aqueous ACN solution (10 mL).42 Extracts were dried under nitrogen and stored at −80 °C until HPLC analysis. Extracts were redissolved in 80% MeOH for HPLC analysis.
(b) Soy and Soy–Almond Dough Proofing
Three replicate dough samples (1.0 g) were collected from three batches of SB with untreated SM and of SAB with steamed SM immediately after development of the sponge (10 min) and at 60, 120, and 180 min in a humidified proofing cabinet (40 °C). The samples were quickly weighed and immediately extracted for isoflavones first with 100% ACN followed by 60% ACN, as described earlier.
(c) Soy Bread and Soy–Almond Bread Toasting Studies
Two candidate soy breads (SB with untreated SM and SAB with steamed SM) that were to be used for clinical trials were toasted using a vertical pop-up toaster (Hamilton Beach, type T71, 780 W). Toast samples were collected prior to toasting (0 min) and after 1, 2, 3, and 4 min of toasting. Replicate samples were from three loaves of SB or SAB baked on three different baking days. Samples were freeze-dried, aliquoted (500 mg), and extracted for isoflavones as described previously.
HPLC Analysis
A Waters model 2690 HPLC equipped with a Waters 2996 photodiode array detector (PDA), autosampler (10 °C), and column heater at 30 °C (Waters Associates, Milford, MA, USA) was used for the isoflavone analysis of the soy and bread extracts. Reversed phase HPLC using a 3.0 × 100 mm, 3 μm particle, Symmetry PS C18 column (Waters Associates) affixed to a Waters guard column was used. A binary mobile phase (1% aqueous acetic acid/ACN) gradient began at 95:5 for 2 min, progressed linearly to 65:35 in 23 min and to 25:75 in 2 min, and returned to 95:5 in 2 min for a total run time of 29 min. Injection volume was 10 μL.
Authenticated standards were prepared as described by Ahn-Jarvis and colleagues. In brief, authenticated standards were solubilized in MeOH, or small amounts of dimethyl sulfoxide were added to help crystals into solution. Calibration curves with correlation coefficients (R2 = 0.996 ± 0.002) were generated from a working mixture. HPLC peak areas of each IF were analyzed and quantified using Empower Pro (Empower 2.0, Waters Associates) software. Glycitein and malonyl- and acetyl-glycoside standards were always prepared just prior to use because these standards are known to be unstable in dilute aqueous solutions.
Sensory Analysis
The Ohio State University human subjects Institutional Review Board approved exemption (NIH, category 6) for this study (Exempted Research OSUIRB#2009E0656). Participants were prescreened and discouraged from participating if they had allergies to wheat, rye, soy, and almonds; were pregnant or nursing women; or had color blindness or difficulty with discerning brown colors. Completed consumer acceptance evaluations were received from 60 participants. Sample preparation and experimental conditions for consumer acceptance and descriptive analyses are described in detail by Ahn-Jarvis and colleagues.43 A nine-point hedonic scale was used to assess overall acceptability and acceptability of flavor, texture, and color of six soy breads (one SB and five SAB variables).44
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics, version 19.0 (SPSS, IBM, Somers, NY, USA). For SM, soy, or soy–almond bread data the mean ± standard deviation (SD) was derived from 24 samples (4 replicates from 6 different batches of each processing variable of SM, SB, or SAB). Isoflavone content was reported as miligrams of AE per gram of dry mass (DM) or as micrograms of AE per gram of soy mix, soy, or soy–almond bread dry mass. For the proofing and toasting studies the reported mean ± SD reflects nine replicates (triplicate analyses from three different batches) for each time point. Comparisons of SM, soy bread, and soy–almond bread were done using a two-factor repeated-measures analysis of variance with interaction terms for comparison of processing treatment and individual isoflavones or chemical forms (aglycones, β-glucosides, acetyl-glucosides, or malonyl-glucosides). Similarly, significant changes in isoflavones during proofing were evaluated using a two-factor repeated-measures analysis of variance with interaction terms for comparison of proofing duration (time) and chemical forms. When a factor effect or an interaction was considered to be significant (F test, p < 0.05), a Tukey–Kramer multiple-means comparison test was used to identify different groups. Significant differences were considered with comparisons with p < 0.05. Differences in consumer acceptance among the six soy breads evaluated were reported as the mean ± SD, and significant differences among groups were discerned using ANOVA with Tukey's post hoc tests.
Results
Isoflavone Composition in Processed Soy Mix
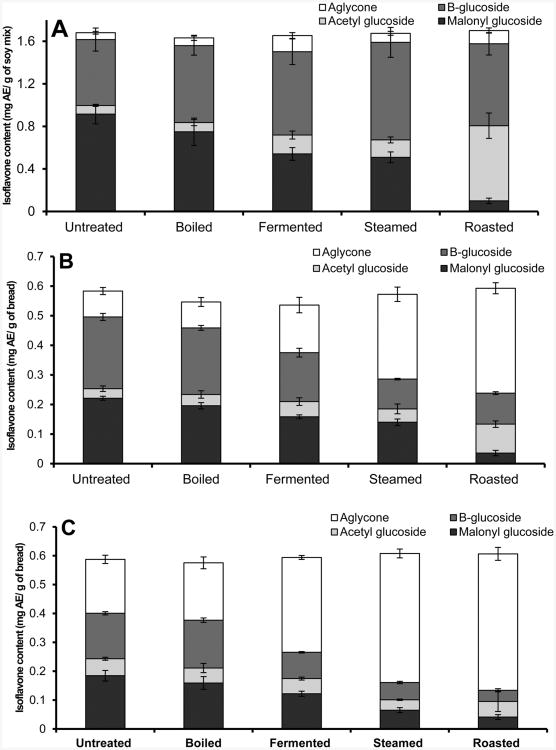
A mixture of soy milk powder (protein, 49%; fat, 25%; and carbohydrates, 31%) and defatted soy flour (protein, 53%; fat, 3%; and carbohydrates, 44%) was used to investigate the effects of processing on modulating the isoflavone composition of finished soy breads with and without almonds. The 12 chemical forms of soy isoflavones observed in the processed and unprocessed soy mixture (SM) are reported in Table 2 and were used to derive the isoflavone composition (aglycones, β-glucosides, malonyl-glucosides, and acetyl-glucosides) of the SM variables reported in Figure 2A. The total isoflavone content in SM did not significantly change with processing (p = 0.880). Although changes in aglycone content (4–9%) from processing were not distinctively different, the composition of glycoside forms had significant differences. In particular, malonyl-glycosides clustered into three different groups with roasted having 6% of it is total isoflavones as malonyl-glycosides followed by steamed (30%), fermented (32%), boiled (46%), and then untreated (54%) having had the most (Figure 2A). Roasted SM had the highest composition of acetyl-glycosides (41%) compared to all other treatment variables. Notably, no significant differences in the composition and content of isoflavones were observed between boiled, microwave heated, and incubated SM or their corresponding soy and soy–almond breads; hence, these experimental variables were collectively regarded as boiled.
Table 2. Isoflavone Distributiona in Soy Mixtureb.
| aglycone (μg AE/g of dry mass soy mix) | β-glycoside (μg AE/g of dry mass soy mix) | acetyl-glycoside (μg AE/g of dry mass soy mix) | malonyl-lycoside (μg AE/g of dry mass soy mix) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| total IF | Dai | Gen | Gly | β-Dai | β-Gen | β-Gly | Ace-Dai | Ace-Gen | Ace-Gly | Mal-Dai | Mal-Gen | Mal-Gly | |
| untreated | 1680 ± 217 | 32.4 ± 6.7A | 22.3 ± 7.9A | 9.6 ± 3.5A | 271.9 ± 55.4DEF | 319.8 ± 47.9DEF | 28.3 ± 6.1A | 38.1 ± 3.4A | 41.9 ± 5.6A | 0.7 ± 0.6A | 290.3 ± 24.0DEF | 513.9 ± 56.6FG | 111.8 ± 12.6ABC |
| boiled | 1630 ± 239 | 31.4 ± 9.7A | 30.3 ± 9.9A | 10.3 ± 6.2A | 308.5 ± 38.1DEF | 380.3 ± 53.3EFG | 34.7 ± 9.0A | 42.3 ± 14.6A | 43.4 ± 14.8A | 0.9 ± 0.8A | 233.8 ± 49.7CD | 420.1 ± 79.6FG | 96.0 ± 8.0AB |
| fermented | 1655 ± 196 | 41.9 ± 11.8A | 56.5 ± 13.3A | 20.8 ± 6.9A | 316.8 ± 58.1DEF | 437.5 ± 72.5EFG | 28.9 ± 11.9A | 47.1 ± 12.0A | 87.5 ± 39.8A | 18.9 ± 7.5A | 183.0 ± 48.8BC | 301.7 ± 33.9DEF | 76.6 ± 10.5AB |
| steamed | 1680 ± 200 | 38.9 ± 7.1A | 59.7 ± 6.2A | 16.4 ± 8.6A | 364.4 ± 47.8EF | 531.6 ± 94.9G | 28.9 ± 11.9A | 71.6 ± 3.8AB | 48.9 ± 3.6A | 9.1 ± 3.9A | 163.0 ± 39.8ABC | 302.0 ± 44.5DEF | 74.0 ± 13.2 AB |
| roasted | 1700 ± 251 | 41.4 ± 10.6A | 64.0 ± 11.5A | 17.6 ± 3.9A | 301.5 ± 32.0DEF | 431.1 ± 74.7EFG | 37.4 ± 4.9 A | 315.3 ± 51.0DEF | 347.5 ± 68.1EF | 43.7 ± 9.1A | 8.3 ± 4.6A | 39.3 ± 14.3A | 52.5 ± 10.9A |
Mean ± SD (n = 24).
Two-factor ANOVA among various processing treatments and compound. Significant difference (p ≤ 0.05) denoted by different superscripted letters. IF, isoflavone; Dai, daidzein; Gen, genistein; Gly, glycitein; β-, simple β-glycoside; ace-, acetyl; mal-, malonyl
Figure 2.
Distribution of aglycones, β-glycosides (B-glycoside), acetyl-glycosides, and malonyl-glycosides among the various processing treatments: (A) soy ingredient mix; (B) soy bread; (C) soy–almond bread.
Isoflavone Composition in Soy Bread
Soy breads (6% fat, 26% protein, and 68% carbohydrates) with untreated or processed SM were used to investigate the changes in isoflavone content during the breadmaking process (sponge development, sponge fermentation, dough proofing, and bread baking). Similar to SM, quantities of individual isoflavones in soy and soy–almond bread are reported in Table 3 and were used to derive the isoflavone profile in Figure 2B,C. No significant differences in total isoflavone content were observed in finished soy breads (p = 0.998) (Table 3). However, aglycone content increased significantly in breads with roasted and steamed SM having the greatest aglycone content (∼50% aglycones of total isoflavones), followed by fermented (31%), and untreated and boiled SM having the least (16%) (Figure 2B). Proportionate decreases in glycoside forms were observed in SB. Specifically, SB with fermented, steamed, and roasted SM had a 25 ± 8% decrease in β-glycosides, and SB with untreated and boiled SM had a 9% decrease in malonyl-glycosides compared to their SM.
Table 3. Distributiona of Isoflavones in Soy and Soy–Almond Bread Made from Processed Soy Mixb.
| aglycone (μg AE/g of dry mass soy bread) | β-glycoside (μg AE/g of dry mass soy bread) | acetyl-glycoside (μg AE/g of dry mass soy bread) | malonyl-glycoside (μg AE/g of dry mass soy bread) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| total IF | Dai | Gen | Gly | β-Dai | β-Gen | β-Gly | Ace-Dai | Ace-Gen | Ace-Gly | Mal-Dai | Mal-Gen | Mal-Gly | |
| Soy Bread | |||||||||||||
| untreated | 591.1 ± 35.8 | 37.8 ± 4.2CD | 41.5 ± 5.3CDE | 11.2 ± 2.3A | 84.6 ± 6.0FG | 124.7 ± 6.5GH | 11.8 ± 3.7A | 13.2 ± 2.1AB | 17.0 ± 3.2A | 1.5 ± 0.6A | 82.2 ± 46EFG | 134.3 ± 14.3H | 31.2 ± 7.1BC |
| boiled | 571.4 ± 59.0 | 40.5 ± 5.9BCD | 46.2 ± 6.5CDE | 8.3 ± 3.8A | 86.8 ± 10.8EFG | 117.5 ± 18.3GH | 10.1 ± 4.2A | 13.3 ± 3.7AB | 16.7 ± 5.4A | 5.8 ± 1.6A | 73.4 ± 15.0FG | 120.6 ± 25.0GH | 32.4 ± 3.6CD |
| fermented | . 568.0 ± 44.4 | 73.8 ± 24.1FG | 90.5 ± 22.2FGH | 10.2 ± 2.8A | 69.6 ± 17.6EF | 96.7 ± 15.1FGH | 6.6 ± 1.9A | 11.3 ± 8.9A | 11.7 ± 2.8A | 10.5 ± 4.7A | 58.0 ± 14.3DEF | 103.4 ± 12.1FGH | 25.7 ± 4.6 |
| steamed | 594.2 ± 69.5 | 126.2 ± 30.7GH | 162.2 ± 37.2™ | 16.6 ± 5.7AB | 47.3 ± 10.6DEF | 76.4 ± 15.0FG | 12.9 ± 9.9A | 7.3 ± 6.4A | 12.6 ± 10.3A | 7.4 ± 2.0A | 42.9 ± 8.7CDE | 63.0 ± 12.3 DEF | 19.5 ± 6.2AB |
| roasted | 597.0 ± 52.4 | 134.8 ± 26.2H | 202 ± 13.91 | 12.2 ± 3.6A | 38.5 ± 12.9CD | 54.7 ± 6.1DEF | 25.0 ± 4.2AB | 37.3 ± 6.6CD | 49.1 ± 5.8CDE | 8.0 ± 2.3A | 9.0 ± 2.1A | 15.1 ± 3.3AB | 11.3 ± 2.9A |
| Soy Almond Bread | |||||||||||||
| untreated | 561.1 ± 55.9 | 73.8 ± 10.7DE | 70.5 ± 8.8CD | 13.0 ± 5.4AB | 51.5 ± 10.9BCD | 88.5 ± 19.5E | 97.4 ± 10.7E | 15.9 ± 2.5AB | 97.6 ± 17.4E | 9.7 ± 5.5A | 8.9 ± 3.4A | 7.9 ± 1.7A | 26.5 ± 6.5AB |
| boiled | 546.8 ± 39.8 | 77.2 ± 12.5DE | 66.2 ± 2.1CD | 8.4 ± 2.1A | 53.0 ± 10.8BCD | 87.0 ± 144E | 88.3 ± 20.2E | 36.6 ± 13.2ABC | 79.1 ± 9.9E | 11.2 ± 3.1A | 6.7 ± 4.3A | 6.4 ± 8.3A | 28.8 ± 7.5AB |
| fermented | . 557.4 ± 46.1 | 114.0 ± 12.8E | 41.4 ± 10.5ABC | 16.1 ± 11.5AB | 35.5 ± 11.6ABC | 165.6 ± 22.4F | 55.3 ± 12.5BCD | 9.6 ± 3.8A | 70.5 ± 19.2CD | 17.0 ± 4.2AB | 4.9 ± 1.8A | 5.1 ± 1.7A | 25.9 ± 3.7AB |
| steamed | 589.4 ± 40.6 | 159.3 ± 18.7F | 28.9 ± 4.3AB | 10.0 ± 6.8A | 20.8 ± 13.1AB | 246.2 ± 146G | 31.2 ± 5.6AB | 10.8 ± 10.2A | 30.6 ± 15.1AB | 29.4 ± 4.7AB | 8.5 ± 6.2A | 5.4 ± 2.3A | 9.2 ± 4.6A |
| roasted | 592.1 ± 48.8 | 160.9 ± 29.5F | 13.6 ± 2.2AB | 29.7 ± 6.0AB | 16.7 ± 3.0AB | 260.9 ± 20.2G | 20.7 ± 8.2AB | 18.9 ± 13.8AB | 18.1 ± 11.5AB | 37.3 ± 8.7ABC | 5.5 ± 3.8A | 8.7 ± 3.9A | 1.2 ± 0.6A |
Mean ± SD (n = 24).
Two-factor ANOVA among various processing treatments and compounds within each bread type. Significant difference (p ≤ 0.0S) denoted by different superscripted letters. IF, isoflavone; Dai, daidzein; Gen, genistein; Gly, glycitein; β-, simple β-glycoside; ace-, acetyl; mal-, malonyl.
Isoflavone Composition in Soy–Almond Bread
Soy–almond breads (8% fat, 26% protein, and 66% total carbohydrates) with untreated or processed SM were used to investigate the effect of almond meal (rich in β-glucosidase) in enhancing isoflavone conversion into aglycones during the breadmaking process. No isoflavones were detected in almond meal (limits of quantification, S/N 10, 0.26–13.4 μmol/g). The total isoflavones of the various SAB did not significantly vary (p = 0.551) (Table 3). In SAB aglycone content was greater than that of the corresponding SB. The highest content of aglycones was in SAB with roasted and steamed SM (∼75%) followed by fermented (53%), then boiled, and untreated (∼31%) SM having the least (Figure 2C). Corresponding decreases in glycoside forms were observed in the various SAB. Specifically, β-glycosides and malonyl-glycosides were the lowest in SAB with steamed (10%) and roasted (7%) SM, followed by fermented (∼20%), and greatest in untreated (31%) and boiled (29%) SM.
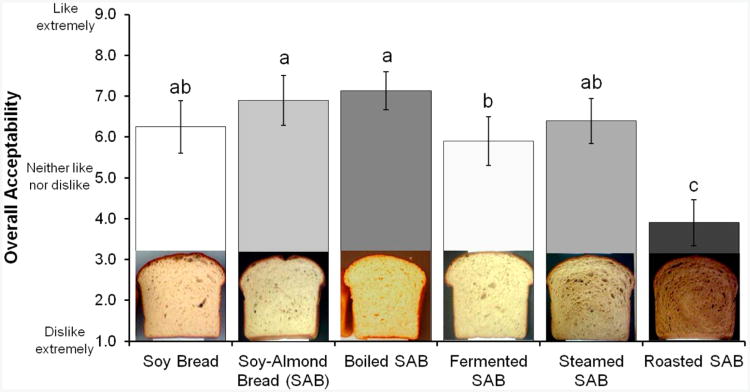
Sensory
Sensory evaluations were conducted to select for an acceptable soy bread with high glycoside content and another with high aglycone content for use in clinical trials evaluating the bioavailability of isoflavones. For consumer testing, SB with untreated SM and SAB with five types (untreated, boiled, fermented, steamed, and roasted) of SM were evaluated (Figure 3). Although SAB with roasted SM had the greatest aglycone content, consumer acceptability indicated that roasted was not an acceptable SAB. However, overall acceptability of SAB with steamed SM (6.4 ± 0.6) was comparable to that of SB (6.2 ± 0.8). SAB with roasted SM had significantly the lowest acceptability scores for flavor (3.8 ± 0.7), aroma (4.0 ± 0.8), texture (5.1 ± 0.9), crumb color (5.2 ± 1.0), and crust color (5.8 ± 1.0). The acceptability scores of SAB with steamed SM for flavor (6.4 ± 0.8), aroma (6.5 ± 0.8), texture (6.5 ± 0.9), crumb color (6.8 ± 0.8), and crust color (7.1 ± 0.8) did not significantly differ from those of soy bread.
Figure 3.
Consumer acceptance (n = 60) of soy with untreated SIM and soy–almond bread (SAB) with untreated, fermented, steamed, and roasted SIM. One-way ANOVA comparison of nine-point hedonic scores among the five soy breads. Significant difference (p ≤ 0.05) is indicated by different letters.
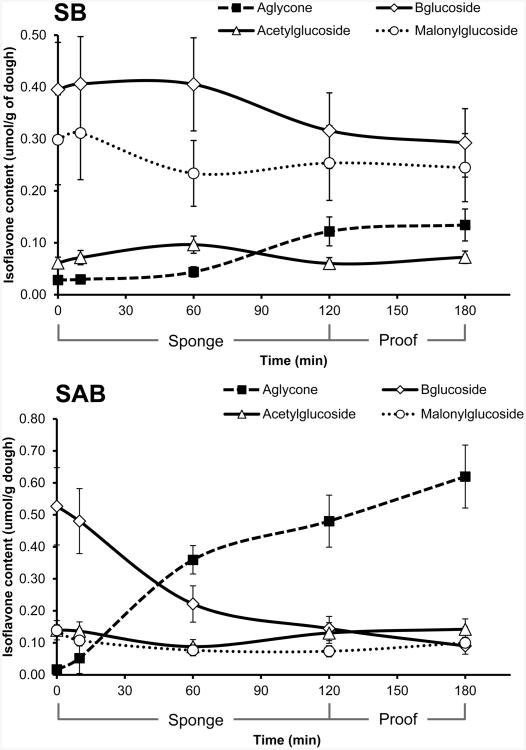
Isoflavone Composition in Soy and Soy–Almond Bread during Proofing
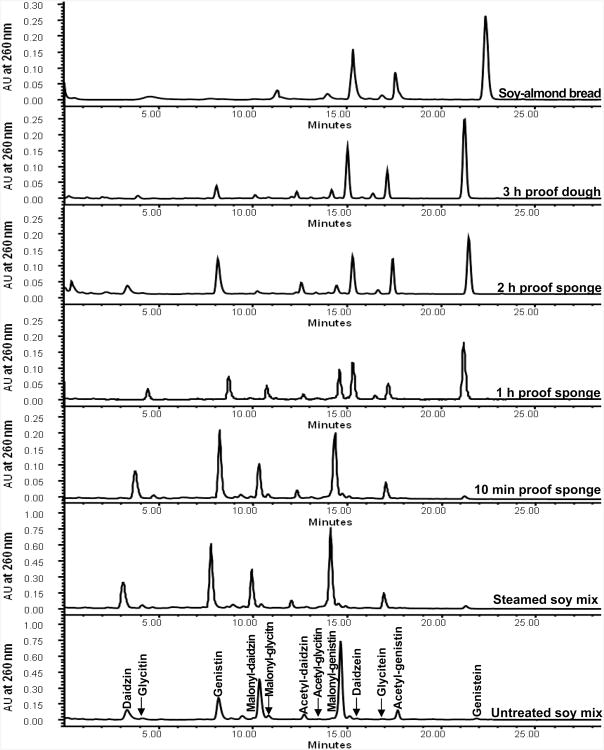
Changes in isoflavone composition during the breadmaking process were observed in soy bread with untreated SM and in SAB with steamed SM (two soy breads selected for use in clinical trials) (Figure 4). Mass spectroscopy was used to verify the identity of all isoflavones analyzed (data not shown). The total isoflavone content in the two soy breads did not differ between the two breads and was not altered by the breadmaking process (p = 0.221). Although starting glycoside compositiona significantly differed (p = 0.014) between the two soy bread types, starting aglycone contenta in SB (0.028 ± 0.007 μmol/g of dough) and SAB (0.017 ± 0.003 μmol/g of dough) did not significantly differ (p = 0.434). However, significant changes in aglycone content were observed in SAB (p = 0.001) 10 min after sponge assembly. The aglycone content increased to 7% (0.053 ± 0.008 μmol/g of dough) of total isoflavones at 10 min, to 49% (0.394 ± 0.044 μmol/g of dough) at 1 h, to 65% (0.529 ± 0.081 μmol/g of dough) at 2 h, and to 75% (0.620 ± 0.098 μmol/g of dough) at 3 h. In SB dough significant change in aglycone content were delayed to 1 h of the sponge period, when the aglycone content increased to 6% (0.047 ± 0.009 μmol/g of dough) and then to 15% (0.125 ± 0.027 μmol/g of dough) at 2 h and to 17% (0.142 ± 0.031 μmol/g of dough) of total isoflavones at 3 h. The increase in aglycones during the bread processing period was associated with a concomitant decrease in glycoside forms within SB and SAB (Figure 5).
Figure 4.
Obtained HPLC chromatograms (260 nm) of steamed soy–almond bread starting with untreated soy mix (bottom) to steamed soy mix to finished steamed soy–almond bread (top).
Figure 5.
Changes in isoflavone composition (n = 9/time point) during sponge fermentation, dough proofing of soy bread (SB) and soy–almond bread (SAB) with steamed SM over a 3 h period (40 °C at 95 ± 5% RH) prior to baking.
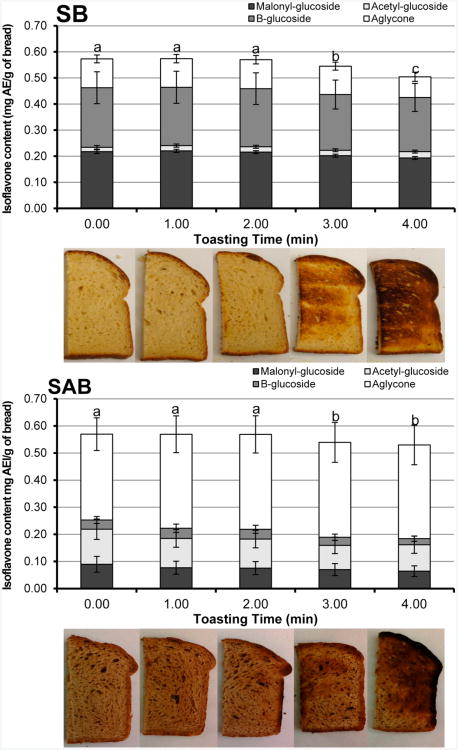
Isoflavone Composition in Soy and Soy–Almond Bread with Toasting
The composition and total isoflavone concentration (μg AE/g of DM) of the SB and SAB did not significantly change (p > 0.05) during storage (−25 °C) and were as follows: 597.2 ± 42.5 (day 0), 594.4 ± 32.8 (day 30), 589.6 ± 40.9 (day 90), and 593.9 ± 38.2 (day 180); however, bread texture had changed. Toasting improved the texture. The effects of toasting on isoflavone composition were examined in SB and SAB because significant differences in the isoflavone composition in soy bread crumb and crust had been observed in earlier studies.10 A significant decrease in total isoflavones was observed after 3 min of toasting (p = 0.045) (Figure 6). Although the aglycone contents between SB (0.108 ± 0.012 mg AE/g of dm) and SAB (0.342 ± 0.014 mg AE/g of dm) were significantly different (p = 0.003), the aglycone content from toasting was not significantly affected within SB (p = 0.998) or within SAB (p = 0.168). However, a significant decrease in glycoside forms was observed. In particular, β-glycosides (p = 0.001) and malonyl-glycosides (p = 0.023) in soy breads decreased by 7 and 8%, respectively, in breads toasted for >3 min. Similarly, SAB when toasted for >3 min had a significant decrease in β-glycosides (p = 0.005) by 22%, acetyl-glycosides (p = 0.006) by 26%, and malonyl-glycosides (p = 0.038) by 25% compared to untoasted SAB.
Figure 6.
Changes in isoflavone composition in SB (top) and SAB (bottom) during toasting (150 °C). One-way ANOVA (n = 9) was used for comparison of total isoflavone content among various time points. Significant difference (p ≤ 0.05) is indicated by different letters. Aglycone, combined daidzein, genistein, and glycitein; B-glycoside, β-glycoside.
Discussion
Functional foods intended for clinical use not only need to maintain their identity as a food but need to perform as dietary food vehicles capable of delivering food-bioactive compounds to the target site, at a concentration sufficient to induce a biological response. Drawing from insights gained in drug development, a functional food was developed using a systematic approach (Figure 1). Once soy isoflavones were identified as the compound of interest, they were characterized in soy ingredients, chemically modulated to potentially improve their bioavailability, and monitored for stability and homogeneity during production, storage, and administration.
A sponge-dough breadmaking process was used to make significant changes in isoflavone composition. Relative levels were expressed as percent aglycones (100 × total aglycones/ total isoflavones), or the three glycoside forms (100 × total β-glycoside, acetyl-glycosides, or malonyl-glycoside/total isoflavones) of the total isoflavones were used to compare the changes in isoflavone composition because soy concentrations differed between SM and soy breads. Although the percent aglycones in SM were not significantly different, the percent aglycones significantly increased in the finished soy breads with and without almond meal. In the SM significant changes were found in the glycoside composition. Most notably, roasted SM had the greatest percentage of acetyl-glycosides, steamed SM had the greatest percentage of β-glycosides, and untreated and boiled SM had the greatest percentage of malonyl-glycoside. Toda and colleagues reported isoflavone compositions in cooked, steamed, and roasted soybeans that were similar to those found in this study.45 Moreover, Murphy and colleagues had similar findings in toasted soy flour (150 °C for 4 h) with a significant increase in acetyl-glycosides with a corresponding decrease in malonyl-glycosides and no significant change in aglycones or degradation of isoflavones.34
In soy breads the percent aglycone increased with untreated/ boiled SM by 15%, with fermented SM by 22%, and with steamed and roasted SM by 50%. This increase in aglycone content in soy breads may be attributed to the endogenous β-glucosidase activity shown to be present in the various soy bread ingredients with the soy mix lending perhaps the greatest activity and specificity.46 Sponge fermentation at 40 °C and the neutral pH of bread system (pH 5.5–6.5) provide the optimal conditions for β-glucosidase activity.37,46 Isoflavone changes observed during our proofing studies mirror those reported by earlier studies; β-glucosidase activity was found to be highest during sponge fermentation, and the resultant conversion of soy isoflavone glycosides to aglycones can be associated with the β-glucosidase endogenous in untreated soy.38,47
Conversion of glycosides to aglycone was enhanced when almond meal, a natural source of β-glucosidase, was added to the formulation (5% w/w) using the same fermentation period. The aglycone content doubled in SAB with untreated and boiled SM compared to their soy bread counterpart from 15% to 32%. Similarly, SAB with fermented SM had a 22% increase in aglycone content over their soy bread analogue. SAB with steamed and roasted SM had >74% aglycone content, which was the highest of all variations studied. Almond β-glucosidase has been observed from previous studies to hydrolyze the glucose moiety from the simple β-glycoside of isoflavones but not the malonyl- or acetyl-glycoside forms.36,37,48 However, endogenous β-glucosidase isolated from soybean roots36,49 was capable of cleaving malonyl forms of isoflavone glycosides in early studies. Chuankhayan and colleagues reported that endogenous β-glucosidase in soy flour hydrolyzed 29% of the acetyl-glycosides.50 The elevated levels of aglycone in SAB variants compared to SB suggest that almond β-glucosidase provides the critical increase in β-glucosidase activity over soy β-glucosidase alone.
Processing the SM prior to their addition into the soy bread system changed the proportion of the various isoflavone species. Specifically, fermented, steamed, and roasted SM had the lowest quantities of malonyl-glycosides. Early studies have reported that under heat (>100 °C) and neutral pH isoflavone standards convert into glycoside forms that are more energetically favorable.35,51 Chien and colleagues reported that isoflavones (genistein and their glycoside forms) when heated (100 °C) under moist and dry conditions result in a rate of conversion from malonyl-genistin to genistin (k = 1.80/h) that is greater than conversion to the acetyl-genistin (k = 0.773/h),51 whereas Mathias and colleagues observed the same demalonylation of isoflavone standards under moist conditions but the conversion of malonyl-glycosides to acetyl-glycosides under dry conditions.35 These same changes in malonyl-glycosides under moist and dry heating were observed in soy flour and soy milk processing.52 Changes made to the glycoside composition of SM in combination with almond meal and dough fermentation conditions could explain the higher levels of aglycones in the SAB with steamed and roasted SM.
Once isoflavone composition was successfully manipulated, sensory evaluation was used to select for glycoside-rich and aglycone-rich soy breads. To ensure that the therapeutic intent of the soy breads is being achieved and not being biased by inconsistencies in dietary compliance, the overall liking of the two interventional agents needs to be similar.53,54 In earlier studies, aglycones have been associated with producing bitter flavors in soy foods.17,55 Therefore, several translations of SAB with various percentages of aglycone were screened. SB with untreated SM containing 15% aglycone and SAB with steamed SM containing 74% aglycone were selected as dietary intervention tools for clinical trials evaluating isoflavone bioavailability.
Controlling and maintaining isoflavone composition during large-scale production is equally as critical as selecting the proper study agents. Proofing duration affects bread quality15,16 and isoflavone composition.38,47 In this study, changes in aglycone content were observed as early as 10 min after sponge formation in SAB but after 60 min in SB. Although no changes in total isoflavone content were observed, proofing studies showed that aglycone content increased with longer proofing times in SAB but not in SB.38,56 Therefore, proofing time was carefully monitored during commercial production of soy breads to minimize differences in aglycone content between batches.
Before the selected soy breads can enter the clinical phase of functional food development, isoflavone stability during storage, distribution, and administration (dietary use) was evaluated. Storage of soy breads in residential freezers (−25 °C) for 6 months showed no change in isoflavone content or composition, although texture was compromised. Toasting improved the palatability of these soy breads. Alterations in isoflavone composition from study participants heating soy breads during a clinical trial may introduce added variability, especially because previous studies have shown that heating soy foods can cause changes in isoflavone composition.29,52,57,58 Findings from this study reveal that soy breads could be warmed for no more than a couple of minutes, and no browning can occur to maintain isoflavone composition.
The process of preclinical functional food development was applied using soy bread as the model for isoflavone delivery. Chemical modulation of the isoflavones to potentially affect their bioavailability in soy breads was achieved. Conventional breadmaking methods using a sponge-dough technique enhanced the conversion of isoflavone glycosides to their respective aglycones. The combination of processed soy mix and almond meal enhanced the conversion of glycosides to their respective aglycones. Two acceptable soy breads, one glycoside-rich (15% aglycones) and the other aglycone-rich (74% aglycones), were developed and selected for use in future clinical trials. This functional food innovation will be used as a tool to address questions of isoflavone absorption, metabolism, and biological impact in clinical trials.
Acknowledgments
Funding: This study was supported by Ohio Agricultural Research and Development Center (OARDC) support of a SEED grant and by The Center for Advanced Functional Foods Research and Entrepreneurship, OSU Comprehensive Cancer Center, The Nutrient and Phytochemical Analytic Shared Resource (NPASR), and a Pelotonia graduate fellowship, and by The National Cancer Institute support of R21 CA125909.
Abbreviations Used
- ACN
acetonitrile
- ace
acetyl
- ANOVA
analysis of variance
- dai
daidzein
- DM
dry mass
- gen
genistein
- gly
glycitein
- HPLC
high-performance liquid chromatography
- IF
isoflavones
- mal
malonyl
- MeOH
methanol
- SAB
soy–almond bread
- SB
soy bread
- SM
soy ingredient mix
- RH
relative humidity
Footnotes
Notes: The authors declare no competing financial interest.
References
- 1.Erdman JW., Jr AHA Science Advisory: soy protein and cardiovascular disease: a statement for healthcare professionals from the Nutrition Committee of the AHA. Circulation. 2000;102:2555–2559. doi: 10.1161/01.cir.102.20.2555. [DOI] [PubMed] [Google Scholar]
- 2.Kranse R, Dagnelie PC, van Kemenade MC, de Jong FH, Blom JH, Tijburg LB, Weststrate JA, Schroder FH. Dietary intervention in prostate cancer patients: PSA response in a randomized double-blind placebo-controlled study. Int J Cancer. 2005;113:835–840. doi: 10.1002/ijc.20653. [DOI] [PubMed] [Google Scholar]
- 3.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113:71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 4.Zhou JR, Mukherjee P, Gugger ET, Tanaka T, Blackburn GL, Clinton SK. Inhibition of murine bladder tumorigenesis by soy isoflavones via alterations in the cell cycle, apoptosis, and angiogenesis. Cancer Res. 1998;58:5231–5238. [PubMed] [Google Scholar]
- 5.Zhou JR, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. 1999;129:1628–1635. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 6.Shen JC, Klein RD, Wei Q, Guan Y, Contois JH, Wang TT, Chang S, Hursting SD. Low-dose genistein induces cyclin-dependent kinase inhibitors and G(1) cell-cycle arrest in human prostate cancer cells. Mol Carcinog. 2000;29:92–102. doi: 10.1002/1098-2744(200010)29:2<92::aid-mc6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Pollard M, Wolter W, Sun L. Diet and the duration of testosterone-dependent prostate cancer in Lobund-Wistar rats. Cancer Lett. 2001;173:127–131. doi: 10.1016/s0304-3835(01)00673-5. [DOI] [PubMed] [Google Scholar]
- 8.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP) Cancer Res. 2001;61:6777–6782. [PubMed] [Google Scholar]
- 9.Zhou J, Yu L, Zhong Y, Blackburn G. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J Nutr. 2003;133:516. doi: 10.1093/jn/133.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YC, Albrecht D, Bomser J, Schwartz SJ, Vodovotz Y. Isoflavone profile and biological activity of soy bread. J Agric Food Chem. 2003;51:7611–7616. doi: 10.1021/jf034679c. [DOI] [PubMed] [Google Scholar]
- 11.Liu K, Limpert WF. Soy flour: varieties, processing, properties, and applications. In: Liu K, editor. Soybeans as Functional Foods and Ingredients. AOCS Press; Champaign, IL: 2005. pp. 101–121. [Google Scholar]
- 12.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 13.Buck JS, Walker CE, Watson KS. Incorporation of corn gluten meal and soy into various cereal-based foods and resulting product functional, sensory, and protein quality. Cereal Chem. 1987;64:264–269. [Google Scholar]
- 14.Fleming SE, Sosulski FW. Breadmaking properties of four concentrated plant proteins. Cereal Chem. 1977;54:1124–1140. [Google Scholar]
- 15.Erdman JW, Jr, O'Connor MP, Weingartner KE, Solomon LW, Nelson AI. Production, nutritional value and baking quality of soy-egg flours. J Food Sci. 1977;42:964–968. [Google Scholar]
- 16.Brewer MS, Potter SM, Sprouls G, Reinhard M. Effect of soy protein isolate and soy fiber on color, physical and sensory characteristics of baked products. J Food Qual. 1992;15:245–262. [Google Scholar]
- 17.Matsuura M, Obata A, Fukushima D. Objectionable flavor of soy milk developed during the soaking of soybeans and its control. J Food Sci. 1989;54:602–605. [Google Scholar]
- 18.Endres JG. Soy Protein Products: Characteristics, Nutritional Aspects, And Utilization. AOCS Press; Champaign, IL: 2001. American Oil Chemists' Society, Soy Protein Council. [Google Scholar]
- 19.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 20.Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr. 2003;77:1459–1465. doi: 10.1093/ajcn/77.6.1459. [DOI] [PubMed] [Google Scholar]
- 21.Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, Offord EA. Hydrolysis of isoflavone glycosides to aglycones by beta-glycosidase does not alter plasma and urine isoflavone pharmacoki-netics in postmenopausal women. J Nutr. 2002;132:2587–2592. doi: 10.1093/jn/132.9.2587. [DOI] [PubMed] [Google Scholar]
- 22.Setchell KD, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, V C, Heubi JE. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr. 2003;133:1027–1035. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 23.Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006;136:2291–2296. doi: 10.1093/jn/136.9.2291. [DOI] [PubMed] [Google Scholar]
- 24.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 25.Setchell KD, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr. 2002;76:447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- 26.Turner NJ, Thomson BM, Shaw IC. Bioactive isoflavones in functional foods: the importance of gut microflora on bioavailability. Nutr Rev. 2003;61:204–213. doi: 10.1301/nr.2003.jun.204-213. [DOI] [PubMed] [Google Scholar]
- 27.Setchell KDR. Absorption and metabolism of soy isoflavones – from food to dietary supplements and adults to infants. J Nutr. 2000;130:654s–655s. doi: 10.1093/jn/130.3.654S. [DOI] [PubMed] [Google Scholar]
- 28.Faughnan M, Hawdon A, Ah-Singh E, Brown J, Millward DJ, Cassidy A. Urinary isoflavone kinetics: the effect of age, gender, food matrix and chemical composition. Br J Nutr. 2004;91:567–574. doi: 10.1079/BJN20041087. [DOI] [PubMed] [Google Scholar]
- 29.Coward L, Smith M, Kirk M, Barnes S. Chemical modification of isoflavones in soy foods during cooking and processing. Am J Clin Nutr. 1998;68:1486S–1491S. doi: 10.1093/ajcn/68.6.1486S. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer. 2007;57:1–10. doi: 10.1080/01635580701267677. [DOI] [PubMed] [Google Scholar]
- 31.Reinwald S, Akabas SR, Weaver CM. Whole versus the piecemeal approach to evaluating soy. J Nutr. 2010;140:2335S–2343S. doi: 10.3945/jn.110.124925. [DOI] [PubMed] [Google Scholar]
- 32.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr. 2009;89:1155–1163. doi: 10.3945/ajcn.2008.27029. [DOI] [PubMed] [Google Scholar]
- 33.Okabe Y, Shimazu T, Tanimoto H. Higher bioavailability of isoflavones after a single ingestion of aglycone-rich fermented soybeans compared with glucoside-rich non-fermented soybeans in Japanese postmenopausal women. J Sci Food Agric. 2011;91:658–663. doi: 10.1002/jsfa.4228. [DOI] [PubMed] [Google Scholar]
- 34.Murphy PA, Barua K, Hauck CC. Solvent extraction selection in the determination of isoflavones in soy foods. J Chromatogr, B: Anal Technol Biomed Life Sci. 2002;777:129–138. doi: 10.1016/s1570-0232(02)00342-2. [DOI] [PubMed] [Google Scholar]
- 35.Mathias K, Ismail B, Corvalan CM, Hayes KD. Heat and pH effects on the conjugated forms of genistin and daidzin isoflavones. J Agric Food Chem. 2006;54:7495–7502. doi: 10.1021/jf061322a. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh M, Graham TL. Partial purification and characterization of a soybean β-glucosidase with high specific activity towards isoflavone conjugates. Phytochemistry. 2001;58:995–1005. doi: 10.1016/s0031-9422(01)00380-6. [DOI] [PubMed] [Google Scholar]
- 37.Ismail B, Hayes K. β-Glycosidase activity toward different glycosidic forms of isoflavones. J Agric Food Chem. 2005;53:4918–4924. doi: 10.1021/jf0404694. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YC. Changes in distribution of isoflavones and beta-glucosidase activity during soy bread proofing and baking. Cereal Chem. 2004;81:741–745. [Google Scholar]
- 39.Inamdar AN, Kaplan JG. Purification and properties of an inducible β-glucosidase of bakers' yeast. Can J Biochem. 1966;44:1099–1108. doi: 10.1139/o66-127. [DOI] [PubMed] [Google Scholar]
- 40.Sue M, Ishihara A, Iwamura H. Purification and characterization of a hydroxamic acid glucoside β-glucosidase from wheat (Triticum aestivum L.) seedlings. Planta. 2000;210:432–438. doi: 10.1007/s004250050029. [DOI] [PubMed] [Google Scholar]
- 41.Vodovotz Y, Ballard C. Formula and process for making soy-based bakery products. U S Patent. 2009;7:592–028. [Google Scholar]
- 42.Ahn-Jarvis JH, Clinton SK, Riedl KM, Vodovotz Y, Schwartz SJ. Impact of food matrix on isoflavone metabolism and cardiovascular biomarkers in adults with hypercholesterolemia. Food Funct. 2012;3:1051–1058. doi: 10.1039/c2fo10284f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn-Jarvis JH, Schwartz SJ, Vodovotz Y. Optimizing isoflavone-rich food delivery systems for human clinical trials. In. In: Preedy V, editor. Food and Nutritional Components in Focus Isoflavones: Chemistry, Analysis, Function and Effects. Royal Society of Chemistry Publishing; Cambridge, UK: 2013. pp. 399–422. [Google Scholar]
- 44.Lawless HT, Heymann H. Sensory Evaluation of Food: Principles and Practices. Chapman and Hall; New York: 1998. [Google Scholar]
- 45.Toda T, Sakamoto A, Takayanagi T, Yokotsuka K. Changes in isoflavone compositions of soybean foods during cooking process. Food Sci Technol Res. 2000;6:314–323. [Google Scholar]
- 46.Graham TL. Flavonoid and isoflavonoid distribution in developing soybean seedling tissues and in seed and root exudates. Plant Physiol. 1991;95:594–603. doi: 10.1104/pp.95.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riedl KM, Zhang YC, Schwartz SJ, Vodovotz Y. Optimizing dough proofing conditions to enhance isoflavone aglycones in soy bread. J Agric Food Chem. 2005;53:8253–8258. doi: 10.1021/jf0508549. [DOI] [PubMed] [Google Scholar]
- 48.Song X, Xue Y, Wang Q, Wu X. Comparison of three thermostable β-glucosidases for application in the hydrolysis of soybean isoflavone glycosides. J Agric Food Chem. 2011;59:1954–1961. doi: 10.1021/jf1046915. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki H, Takahashi S, Watanabe R, Fukushima Y, Fujitat N, Noguchi A, Yokoyama R, Nishitani K, Nishino T, Nakayama T. An isoflavone conjugate-hydrolyzing β-glucosidase from the roots of soybean (Glycine max) seedlings. Purification, gene cloning, phylogenetics, and cellular localization. J Biol Chem. 2006;281:30251–30259. doi: 10.1074/jbc.M605726200. [DOI] [PubMed] [Google Scholar]
- 50.Chuankhayan P, Rimlumduan T, Svasti J, Cairns JR. Hydrolysis of soybean isoflavonoid glycosides by Dalbergia β-glucosidases. J Agric Food Chem. 2007;55:2407–2412. doi: 10.1021/jf062885p. [DOI] [PubMed] [Google Scholar]
- 51.Chien JT, Hsieh HC, Kao TH, Chen B. Kinetic model for studying the conversion and degradation of isoflavones during heating. Food Chem. 2005;91:425–434. [Google Scholar]
- 52.Chiarello MD, Le Guerroue J, Chagas CMS, Franco OL, Bianchini E, Joao MJ. Influence of heat treatment and grain germination on the isoflavone profile of soy milk. J Food Biochem. 2006;30:234–247. [Google Scholar]
- 53.Schwartz JI, Yeh KC, Berger ML, Tomasko L, Hoover ME, Ebel DL, Stauffer LA, Han R, Bjornsson TD. Novel oral medication delivery system for famotidine. J Clin Pharmacol. 1995;35:362–367. doi: 10.1002/j.1552-4604.1995.tb04074.x. [DOI] [PubMed] [Google Scholar]
- 54.Dove WF. Developing food acceptance research. Science. 1946;103:187. doi: 10.1126/science.103.2668.187. [DOI] [PubMed] [Google Scholar]
- 55.Huang H, Liang H, Kwok KC. Effect of thermal processing on genistein, daidzein and glycitein content in soymilk. J Sci Food Agric. 2006;86:1110–1114. [Google Scholar]
- 56.Shao S, Yang R, Tsao R, Duncan AM, Marcone MF, Rajcan I. Tracking isoflavones: from soybean to soy flour, soy protein isolates to functional soy bread. J Funct Foods. 2009;1:119–127. [Google Scholar]
- 57.Shimoni E. Stability and shelf life of bioactive compounds during food processing and storage: soy isoflavones. J Food Sci. 2004;69:160–166. [Google Scholar]
- 58.Lee SW, Lee JH. Effects of oven-drying, roasting, and explosive puffing process on isoflavone distributions in soybeans. Food Chem. 2009;112:316–320. [Google Scholar]