Abstract
Microtubules (MTs) composed of αβ-tubulin heterodimers are highly dynamic polymers, whose stability can be regulated by numerous endogenous and exogenous factors. Both the anti-mitotic drug, Taxol, and microtubule-associated proteins (MAPs) stabilize this dynamicity by binding to and altering the conformation of MTs. In the current study, amide hydrogen/deuterium exchange coupled with mass spectrometry (HDX-MS) was used to examine the structural and dynamic properties of the MT complex with the microtubule binding domain of MAP4 (MTB-MAP4) in the presence and absence of Taxol. The changes in the HDX levels indicate that MTB-MAP4 may bind to both the outside and the luminal surfaces of the MTs, and that Taxol reduces both of these interactions. The MTB-MAP4 binding induces conformational rearrangements of α- and β-tubulin that promote an overall stabilization of MTs. Paradoxically, despite Taxol’s negative effects on MAP4 interactions with the MTs, its binding to the MTB-MAP4-MT complex further reduces the overall deuterium incorporation, suggesting that a more stable complex is formed in the presence of the drug.
MTs are highly dynamic protein polymers that are crucial in many aspects of cellular function, including mitosis. Mitosis can be disrupted by arresting microtubule dynamics, which may be accomplished with drugs that interact with MTs (1). One such class of drugs is the microtubule stabilizing agents, which bind to the MT polymer and prevent its disassembly, thereby inducing loss of dynamicity of the mitotic spindle required for progression of mitosis (2). The prototype of this class of drugs is Taxol, whose activity and properties have been studied extensively (3, 4). The dynamic behavior of MTs is also regulated in vivo by microtubule associated proteins (MAPs) (5). Some of these regulatory proteins, including stathmin, sequester tubulin dimers and promote microtubule disassembly, whereas others stimulate assembly and favor stable MTs (5, 6). Tau, MAP2 and MAP4 belong to a family of MAPs that promotes microtubule assembly and stabilization. Tau and MAP2 are found predominantly in neuronal and some glial cells, whereas MAP4 is ubiquitously expressed (7, 8). All members of this family have a similar primary structure, with each including an N-terminal projection (PJ) domain and a C-terminal microtubule-binding (MTB) domain (9–11). This latter MTB domain is responsible for binding to and regulating MT assembly. It can be subdivided into three distinct regions: a proline-rich region, an assembly promoting region, and a highly hydrophobic tail region (12). The assembly promoting region, which contains three, four, or five 18-residue imperfect repeats, is homologous in all family members and can bind to MTs and promote microtubule elongation (13, 14). The proline-rich regions of tau, MAP2 and MAP4 are similar in structure, but display no significant sequence homology. While sequences of tau and MAP2 tail regions bear a close resemblance to each other, MAP4 differs significantly from the other family members (12). Clearly, despite the sequence similarities found in this family of proteins, there may be differences in their mechanisms of microtubule stabilization, not least because MAP4 has not been studied as intensely as MAP2 and tau. As the predominant MAP in normal and cancer cells, MAP4 was selected in our study to investigate the structural changes in the αβ-tubulin heterodimer upon binding of endogenous microtubule stabilizers. Since MAP4 is ubiquitously expressed and shares characteristic structures with neural MAP2 and tau, analysis of structural changes in MTs induced by MAP4 binding will further our understanding of the role MAPs play in the regulation of MT dynamics.
While we have obtained valuable information concerning the interaction of different types of microtubule-stabilizing drugs with tubulin and MTs (15, 16), our experimental system thus far has been limited by the absence of endogenous factors that further regulate MT dynamics, altering their conformation in vivo. Thus, here we expand our current HDX-MS studies to include MAP4 that is an endogenous dynamic regulator. The HDX-MS method is ideal for probing the interactions between protein-protein and protein-ligand complexes. A typical HDX-MS experiment involves the comparison of HDX rates of peptic peptides before and after interacting with its binding partners. A reduced HDX rate indicates that the peptide is either involved in the direct interactions with its partners or its dynamicity has been reduced by the binding interactions. Understanding how MAPs stabilize MTs in the presence and absence of Taxol may provide important insights into their role in modulating drug effects and MT dynamics. In the present study, we demonstrate that MTB-MAP4 significantly reduces deuterium incorporation into MTs, suggesting that it stabilizes the polymer via its effects on the multiple interfaces that comprise a stable MT polymer. This effect of MAP4 is further enhanced in the presence of Taxol, indicating cooperative action of these two stabilizers on MT dynamics.
Results
CET was chosen as it is is composed of only one α- and one β-tubulin isotype, α1 and βVI, respectively, and has limited posttranslational modifications (20), thereby significantly simplifying data analysis and eliminating any ambiguity in assignment of measured masses and conformational effects of isotype-specific peptides. Moreover, the core sequence of chicken βVI-tubulin is nearly 90% identical both to the most abundant mammalian brain tubulin βII and to human βI, the major isotype in non-neuronal tissue. This further enhances our ability to understand the conformational dynamics of MT stabilization in a broader context.
Global HDX of Microtubules
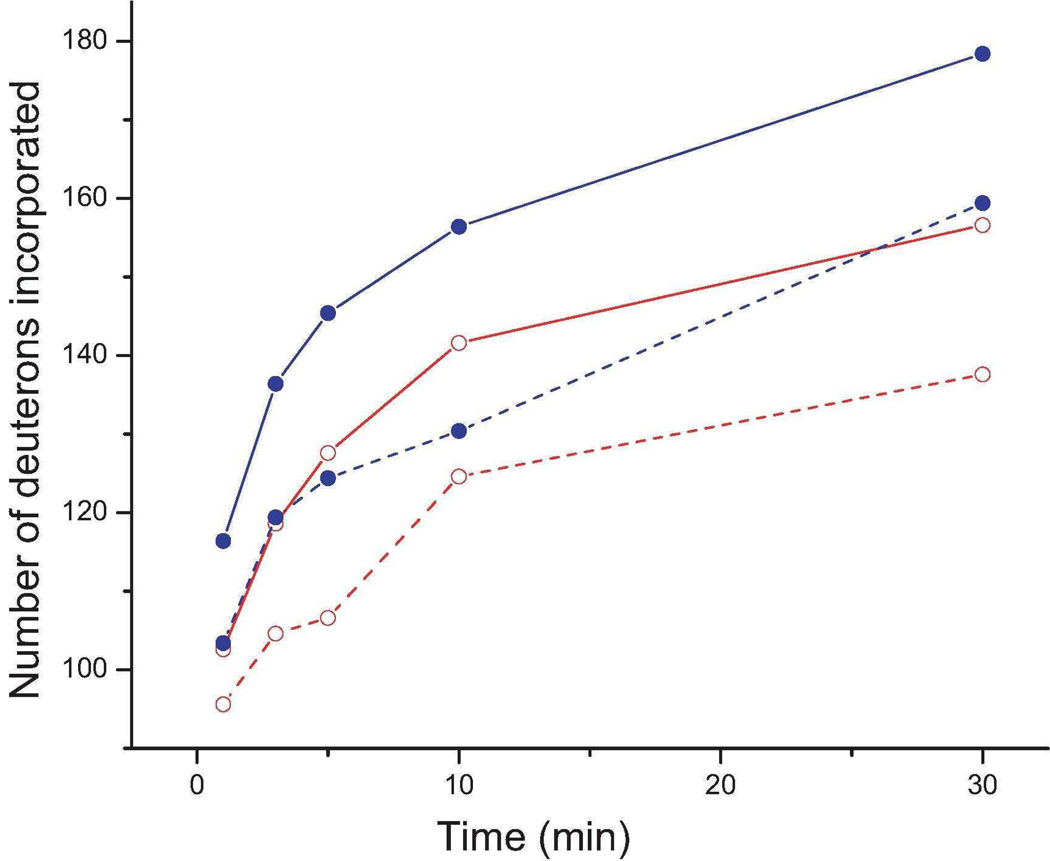
We compared the time courses of HDX in intact chicken erythrocyte tubulin complexes in the presence and absence of MTB-MAP4 (Figure 1). Binding of MTB-MAP4 to GMPCPP-MTs led to a significant reduction in deuterium incorporation into both α- and β-tubulin, confirming previously established stabilizing activity of MAP4 on the entire MT polymer (21).
Figure 1.
Global deuterium incorporation into chicken erythrocyte α- (open circle, red) and β-tubulin (filled circle, blue) in the presence of GMPCPP alone (control, solid lines), or with MAP4 added (dashed lines).
Regional HDX of Microtubules
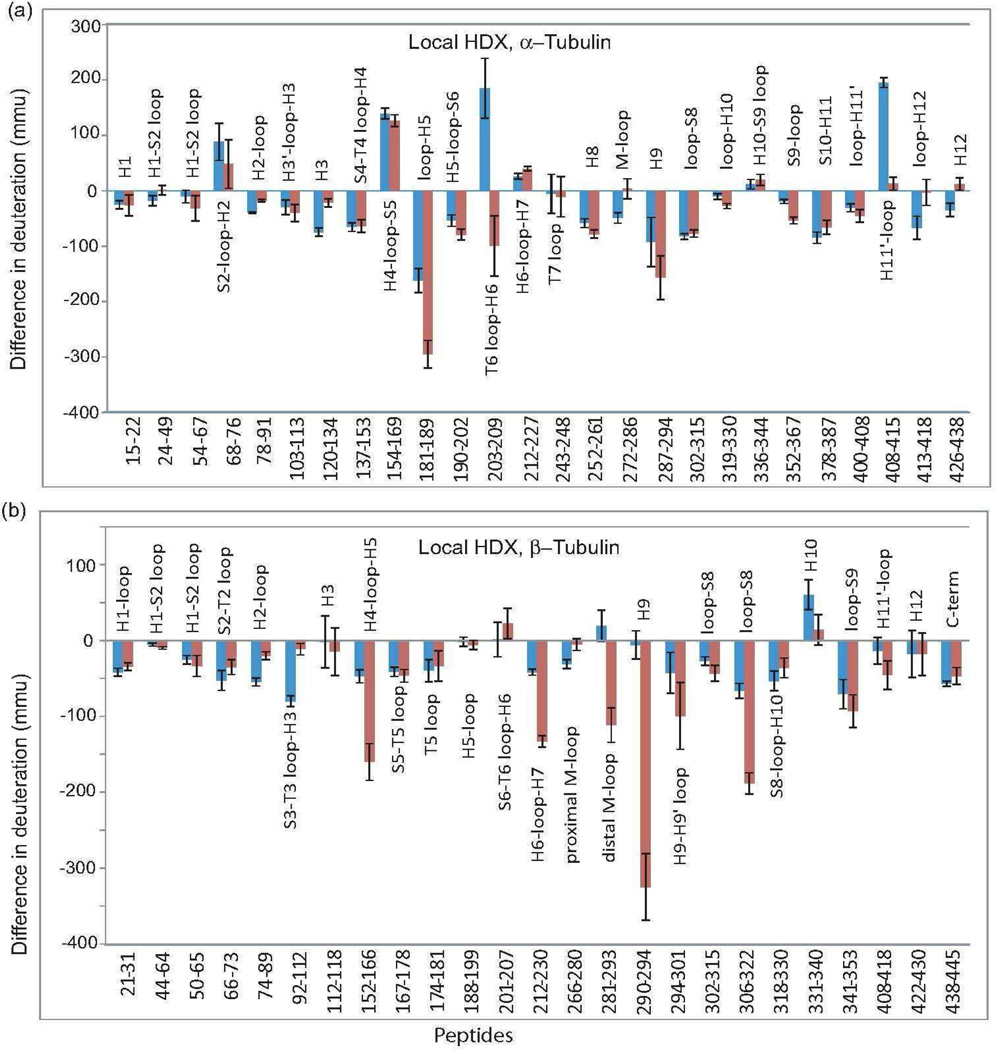
The CET peptic peptides were identified as described under “Materials and Methods.” Under all conditions utilized for HDX experiments, 32 α- and 33 β-tubulin peptides were consistently detected, corresponding to 70 and 68% sequence coverage, respectively. Figure 2 summarizes the effects of MTB-MAP4 alone (blue bars) and MTB-MAP4+Taxol (red bars) on deuterium labeling. The ΔHDX values for each peptide shown in Figure 2 correspond to the differences between the centroid values (xc) of the mass distributions of the peptides from GMPCPP-stabilized MTs in the presence and absence (control) of MTB-MAP4 or MTB-MAP4+Taxol. Figure 3 maps these changes in deuterium incorporation onto an αβ-tubulin heterodimer structure (PDB file 1JFF). More detailed deuterium exchange information at the interdimer interface, intradimer interface and interprotofilament region are illustrated in figures 4, 5 and 6, respectively.
Figure 2.
MAP4-induced alterations in deuterium incorporation referenced against GMPCPP-stabilized microtubules for (a) α-tubulin and (b) β-tubulin. Peptides resulting from the digest of the microtubule complexes with MTB-MAP4 and MTB-MAP4+Taxol are indicated in blue and red, respectively. Data denote the mean±S.D. of three separate experiments. Differences in deuteration per amino acid are expressed in mmu. Peptides are labeled with the corresponding amino acid numbers, as well as secondary structure designations based on Löwe et al. (22).
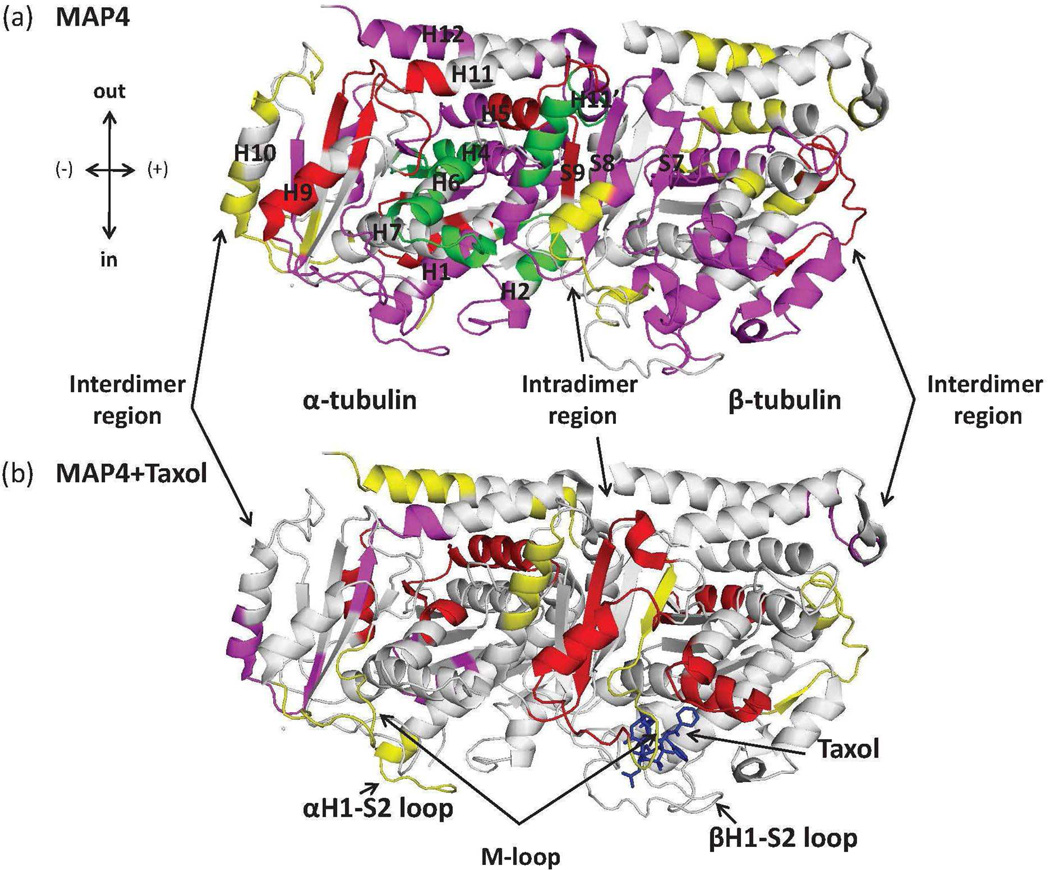
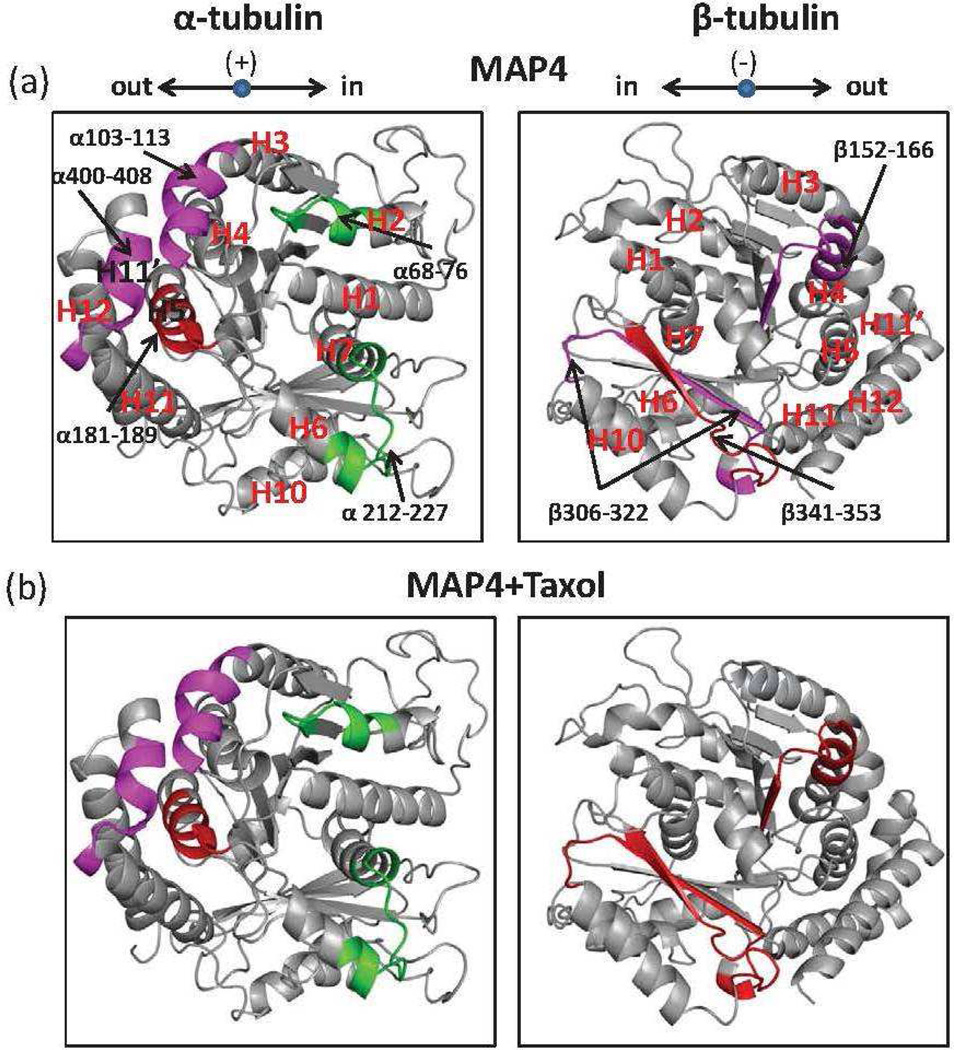
Figure 3.
Structure of tubulin heterodimer (PDB code 1JEF): map of local HDX alterations in the presence of MAP4 (a) and MAP4+Taxol (b) referenced against GMPCPP-MTs. Peptides are color-coded as follows: yellow=insignificant change in deuterium incorporation; green=significant increase in deuterium incorporation; magenta ; red=significant reduction in deuterium incorporation with weak and strong effects and grey= undetected peptides. For clarity, only those peptides that are significantly different from those shown in a are shown in color in b. Peptides shown in grey in b represent those in which no change has occurred in deuterium incorporation plus those not detected in this experiment. Orientation of the dimer is indicated in the upper left corner: “in” refers to the inside (luminal side) of the MT, “out” to the outside, (+) to the plus end (GTP-cap) and (−) to the minus end (MT organizing center). Peptides are labeled with the corresponding amino acid numbers, as well as secondary structure designations based on Löwe et al. (22).
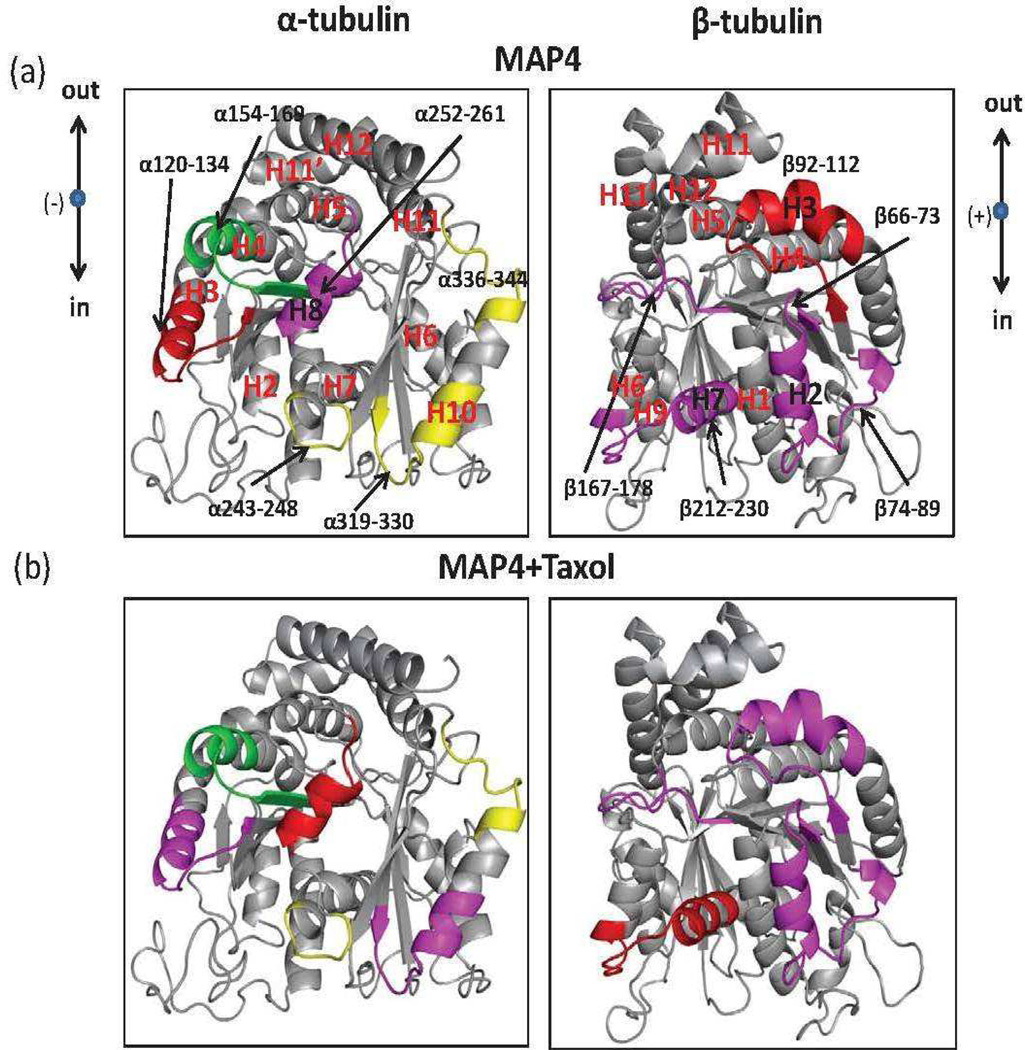
Figure 4.
Interdimer interface of the tubulin dimer (PDB code 1JFF): map of local HDX alterations in the presence of MAP4 (a) and MAP4+Taxol (b). Peptides are color-coded as follows: yellow=insignificant change in deuterium incorporation; green=significant increase in deuterium incorporation; magenta and red=significant reduction in deuterium incorporation with weak and strong effects, respectively. The directional coordinates are shown in a, adjacent to each α- and β-tubulin component of the interface, with designations as indicated in Figure 3. Peptides are labeled with the corresponding amino acid numbers, as well as secondary structure designations based on Löwe et al. (22).
Figure 5.
Intradimer interface of the tubulin dimer (PDB code 1JFF): map of local HDX alterations in the presence of MAP4 (a) and MAP4+Taxol (b). Peptides are color-coded as follows: yellow=insignificant change in deuterium incorporation; green=significant increase in deuterium incorporation; magenta and red=significant reduction in deuterium incorporation with weak and strong effects, respectively. The directional coordinates are shown in a, on top of each α- and β-tubulin component of the interface, with designations as indicated in Figure 3. Peptides are labeled with the corresponding amino acid numbers, as well as secondary structure designations based on Löwe et al. (22).
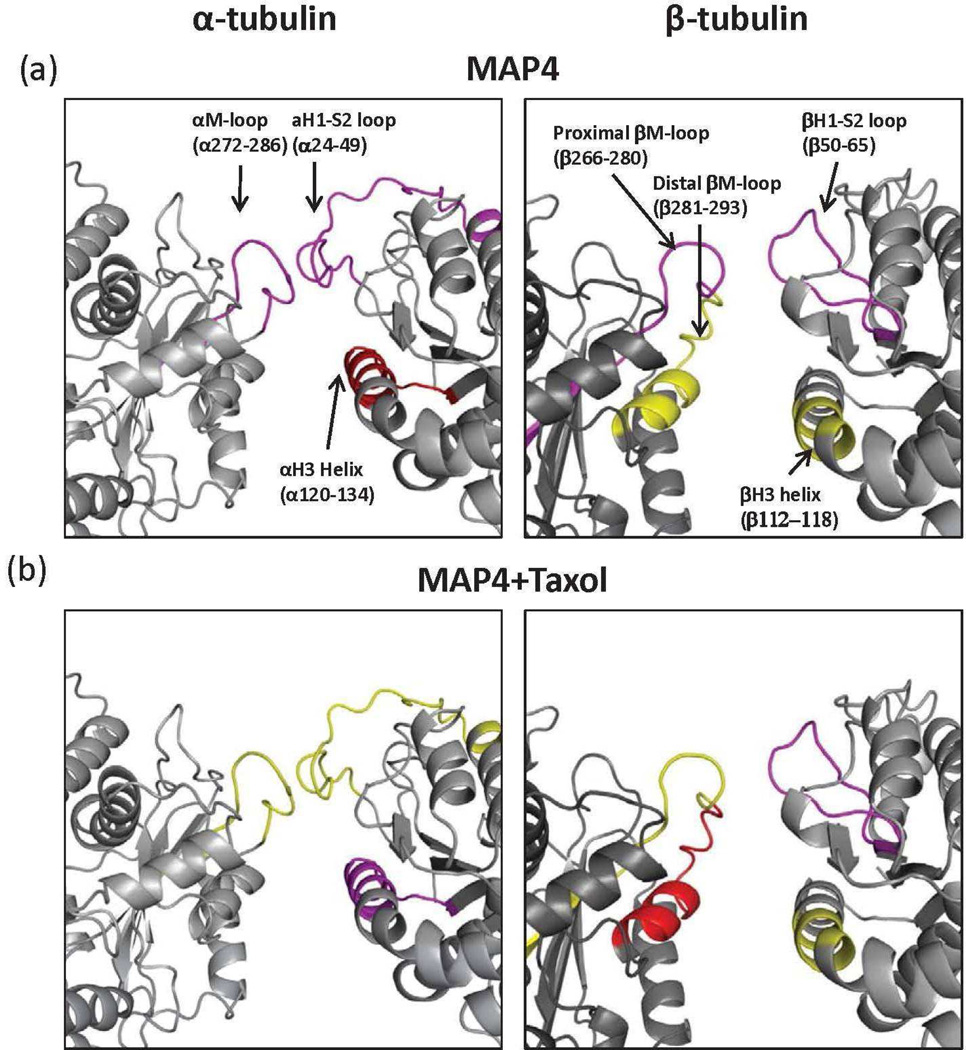
Figure 6.
Lateral interface between adjacent protofilaments (21) : map of local HDX alterations in the presence of MAP4 (a) and MAP4+Taxol (b). The interactions between adjacent protofilaments are shown as if viewed down the length of the protofilament with the upper portion corresponding to the inside and the lower portion corresponding to the outside of the microtubule. Peptides are color-coded as follows: yellow=insignificant change in deuterium incorporation; green=significant increase in deuterium incorporation; magenta and red=significant reduction in deuterium incorporation with weak and strong effects, respectively. Secondary structure designations are based on Löwe et al. (22).
Interdimer interface
Figure 4 shows the exchange map at the interdimer interface, where adjacent αβ-tubulin heterodimers interact head to tail along the length of the protofilament. Extensive reduction of deuterium incorporation induced by MTB-MAP4 in this region was observed on most peptides in both α- and β-tubulins. These include peptides α120-134 (H3-loop-S4) and α252-261 (H8) in α-tubulin, and peptides β66-73 (S2-loop-H2), β74-89(H2-loop), β92-112 (S3-T3 loop-H3), β167-178 (S5-T5 loop) and β212-230 (H6-loop-H7) in β-tubulin. No significant effects of MAP4 were observed on peptides α243-248 (T7 loop), α319-330 (loop-H10) and α336-344 (H10-S9 loop) in α-tubulin, while peptide α154-169 (H4-loop-S5) was deprotected. In our previous studies (15, 16, 19), peptide α154-169 was protected in the presence of microtubule stabilizing agents. However, in the presence of MAP4, we observed the opposite effect. Therefore, we hypothesize that MAP4 displaced α154-169 from the interdimer interface, exposing it to the deuterated solvent. Deprotection of this region in α-tubulin, in turn, allowed for strong interactions between the aforementioned peptide α120-134 and its β-tubulin partners β74-89 and β92-112. The addition of Taxol to the MTB-MAP4-MT complex significantly altered deuterium incorporation into multiple peptides at the interdimer interface. Peptide α120-134 and its partners β74-89 and β92-112 became less protected in the presence of Taxol, suggesting a loss of stabilization in this portion of the interdimer interface. Conversely interacting pair α319-330 and β212-230 gained significant protection when Taxol was added to the MTB-MAP4-MT complex. Complementary peptides α252-261 and β167-178, as well as α243-248 and β66-73 retained the same level of protection, and peptide α154-169 remained strongly deprotected. Although incorporation of deuterium along the peptide chain was altered in the presence of Taxol, the overall level of protection of this region remained the same as indicated by the P-values which are greater than 0.05 (Table 1).
Table 1.
Quantitative summary of MT-stabilizing trends for MAP4 in the presence and absence of Taxol.
| Interface | Total ΔHDX (α) | P | Total ΔHDX (β) | P | ||
|---|---|---|---|---|---|---|
| MAP4 | MAP4+Taxol | MAP4 | MAP4+Taxol | |||
| Interdimer | −136 ± 38 | −118 ± 40 | 0.6 | −270 ± 18 | −246 ± 18 | 0.1 |
| Intradimer | −108 ± 43 | −292 ± 54 | 0.003 | −184 ± 24 | −441 ± 36 | 0.000 |
| Lateral | −142 ± 15 | −17 ±21 | 0.001 | −81 ± 41 | −298 ± 43 | 0.001 |
Peptide α154-166 was not included in the total ΔHDX calculation at the interdimer interface for reasons referred to in the “Results” section.
Intradimer Interface
Figure 5 maps the changes in deuterium incorporation at the intradimer interface, the region of interaction between the α- and β-tubulin components of a heterodimer. Peptides β152-166 (H4-loop-S5), β306-322 (loop-S8), and β341-353 (loop-S9) are located on the β-tubulin side of the interface. All three peptides exhibited significantly reduced deuterium incorporation when MTB-MAP4 was bound. Peptides corresponding to the α-tubulin side of the intradimer interface were also significantly affected by MTB-MAP4 binding. While α103-113 (H3’-loop-H3), α181-191 (loop-H5) and α400-408 (loop-H11’) became more protected, peptides α68-76 (S2-loop-H2) and α 212-227 (H6-loop-H7) were deprotected in the presence of MTB-MAP4. The addition of Taxol enhanced the stabilizing activity of MTB-MAP4 on MTs, as evidenced by further reduction in the levels of deuterium incorporation into almost all of the peptides in this region, with particularly strong effects on α181-189 (loop-H5), β152-166 (H4-loop-S5), and β306-322 (loop-S8). In fact, based on total ΔHDX values(Table 1), the increase in protection in the presence of Taxol was most significant at the intradimer interface.
Interprotofilament region
The major components of the lateral interface in a microtubule are the M-loop (α and β274-288) and the N-terminal H1-S2 loop (α and β24-64). In α-tubulin, both the M-loop (α272-286) and the corresponding portion of the H1-S2 loop (α24-49) were protected from deuterium incorporation by MTB-MAP4 (Figure 6a). Similarly, the H1-S2 loop (β21-31, β44-64 and β50-65) in β-tubulin showed slightly reduced deuterium incorporation. While the proximal M-loop peptide β266-280 was significantly protected by MTB-MAP4, the distal portion of the M-loop (β281-293) remained unaffected (Figure 6a). A drastic change in deuterium incorporation into the M-loop occurred when Taxol was added to the MTB-MAP4-microtubule complex, as depicted in figure 2 and figure 6b. Specifically, Taxol enhanced protection of the distal M-loop (β281-293) by 140 mmu, while it deprotected the proximal part of the M-loop (β266-280) by 26 mmu, with respect to the MTB-MAP4-microtubule complex. The significance of these findings is further explained in the Discussion section. In contrast, peptide α272-286, corresponding to the M-loop in α-tubulin, exhibited increased deuterium incorporation upon Taxol binding (ΔΔHDX = 53 mmu). In summary, MTB-MAP4 stabilized lateral interactions between both α-tubulin and β-tubulin subunits in adjacent protofilaments (Figure 6a and Table1). In the presence of Taxol, lateral α-contacts were weakened at the expense of significantly strengthened β-tubulin contacts (Figure 6b and Table 1).
Taxol-binding site
MTB-MAP4 induced a significant reduction in deuterium incorporation into peptide β212-230 (H6-loop-H7), where Leu217, Leu219, His229 and Leu230 have been shown to establish hydrophobic contacts with Taxol (22). As would be expected, Taxol binding led to further protection of this β-tubulin peptide. However, the distal M-loop (β281-293), which also participates in Taxol binding, as noted above in the context of lateral interactions, was only protected in the presence of Taxol. MTB-MAP4 alone showed no effect on stabilizing this portion of the Taxol-binding site.
MAP binding sites
Although the exact MAP binding sites within a microtubule are still under investigation, the C-terminal portions of both α- and β-tubulin, including helices H11, H12 and the hypervariable C-terimus are involved in the interactions between MTs and MAPs (23–25). Our previous HDX results demonstrated significant protection of the α-tubulin H12 helix from deuterium incorporation by Taxol and other microtubule stabilizing agents (15, 16). In this study, the HDX data indicated that the α-tubulin helices H11’ (represented by peptide α400-408) and H12 (represented by peptides α413-418 and α426-438) were significantly protected by MTB-MAP4 binding. Interestingly, the loop connecting H11’ and H12 (represented by peptide α408-415) was deprotected under the influence of MTB-MAP4. Addition of Taxol to the MTB-MAP4-microtubule complex actively altered deuterium incorporation into the aforementioned MAP-interacting tubulin peptides. Particularly, deuterium levels in the α-tubulin H12 helix (peptides α413-418 and α426-438) were increased in the presence of Taxol. In contrast, deuterium incorporation into peptide α408-415 (H11’-H12 loop) was reduced following Taxol binding, such that the overall effect on this region of α-tubulin returned to the control level (GMPCPP alone).
In β-tubulin, the C-terminal peptide β438-445 exhibited significantly reduced deuterium incorporation. This C-terminal peptide is not shown in the figures because it has not been observed in the crystallography studies, presumably because of its flexibility. Due to the weak signal of peptides β408-418 (H11’-loop) and β422-430 (H12), a conclusive determination of whether they are protected by MTB-MAP4 or not, could not be made. No significant changes were observed at the C-terminus (β438-445) upon Taxol binding. Peptide β408-418 (H11’-loop) gained enhanced protection by Taxol as indicated by the average value of ΔHDX (Figure 2), although this change was not statistically significant (P>0.05).
Discussion
Our previous observations demonstrated that microtubule stabilizing agents induce a reduction of deuterium labeling across the entire length of the microtubule, despite having binding sites limited to a small region in β-tubulin (15, 16, 19). Our current work shows similar findings with MTB-MAP4-stabilized MTs. The longitudinal and lateral interactions between tubulin dimers are strengthened under the influence of MAP4, as suggested by reduced deuterium incorporation into most peptides at the intradimer, interdimer and lateral interfaces (Table 1). The conformational effects are apparent both in the lumen and on the outside of the microtubule, and are seen in both α- and β-tubulin (Figure 2 and Figure 3). This global MT-stabilizing activity of MTB-MAP4 is mimetic of the microtubule stabilizing agents, as measured in our previous HDX experiments (15, 16). However, the overall protective effects produced by MTB-MAP4 binding are noticeably weaker than those induced by combined MTB-MAP4 and Taxol binding. In the presence of MTB-MAP4 alone, the sum of ΔHDX values for all peptides is −365.6 and −786.8 mmu in α- and β-tubulin, respectively. Addition of Taxol further decreases these values to −927.2 mmu in α- and −1614.4 mmu in β-tubulin. This implies that the degree of stabilization induced by MTB-MAP4 binding is limited, which is consistent with the observation made by the Bulinski group that MAP4 stabilizes MTs to an extent similar to that of low concentrations of Taxol (18).
Our result, that the protective effects of MTB-MAP4 were more than doubled by addition of Taxol suggests a complementary mode of MT stabilization between these two regulators. Predictably, some of the most dramatic effects are seen in peptides involved in the binding of Taxol in its β-tubulin luminal site, including the M-loop (β281-293) and the internal H6-loop-H7 region (β212-230). While the effects of Taxol on the interdimer interface were variable, very strong enhancement of MT stabilization at the intradimer interface was apparent, as evidenced by significant decrease in deuterium incorporation into this region in the presence of Taxol (Table 1). By itself, Taxol has been shown to have no effect on stabilizing lateral interactions between adjacent α-tubulin subunits (15, 16), yet it appears to weaken the stability of lateral interactions in the presence of MAP4. This weakening, however, is accomplished at the expense of substantial stabilization of the lateral β–β contacts (Table 1). Thus, the MT stabilization by Taxol and MAP4 is via the complementary stabilization of both longitudinal (intradimer) and lateral interactions.
The MTB-MAP4 used in our studies contains all major components of the human MAP4 MT-binding domain, including the entire proline-rich region, the assembly-promoting repeated sequence region, and the hydrophobic C-terminus (18). It therefore has all the elements required for microtubule binding and assembly (18). MAPs, including tau and MAP2, as well as motor proteins, such as kinesin and dynein, have been shown to bind to the external H12 helix of the microtubule (26, 27). Consistent with these findings, our HDX results demonstrate that MTB-MAP4 induces significant protection of the C-terminal regions of both α- and β-tubulin, including helices αH11 (α378-387) and αH12 (α413-418 and α426-438), as well as the βC-terminal peptide (β438-445). This implicates α- and β-tubulin C-terminal regions in directly binding MTB-MAP4, which corroborates, via an independent approach, the binding model proposed for tau by Kar et al. from structural data (28). In their model the positively charged proline-rich region of tau interacts with the negatively charged acidic residues on the tubulin C-termini. Our results are also consistent with the observations made by cryo-electron microscopy, that tau binds longitudinally along the outer ridges of Taxol-stabilized MTs (26). Of note, when Taxol was added to the preformed MTB-MAP4-MT complex, the protection of the αH12 helix (α413-418 and α426-438) was diminished. These results suggest that Taxol may reduce the binding affinity of MTB-MAP4 for the outer surface of the MTs. Our previous HDX studies indicated a significant protection of the α-tubulin H12 helix by Taxol and other microtubule stabilizing agents(15, 16). It was hypothesized that this effect of the microtubule stabilizing agents would perturb the binding of MT-associated proteins to MTs, and this has been confirmed in the current study. Consistent with its location and high flexibility, the βC-terminal tail (β438-445) was not protected by Taxol addition.
It has also been reported that Taxol and discodermolide significantly reduce the affinity of tau for MTs by binding to an overlapping luminal site in β-tubulin (29). Based on the results of our study the same may be true for Taxol and MAP4, such that a portion of the Taxol binding pocket may also be involved in the binding of MAP4 to MTs. Critical residues for the binding of the taxane ring are located in the proximal M-loop of β-tubulin, and include Pro272, Leu273, Thr274, Ala277 and Arg276 (22). In our HDX data, this region is represented by peptide β266-280, which is strongly protected from deuterium incorporation by MTB-MAP4, with this effect reversed in the presence of Taxol (Figures 2 and 3b). This indicates that the proximal M-loop may be part of a shared luminal binding site for Taxol and MAP4. Taxol, therefore, competes with MTB-MAP4 for binding at this site, thereby reducing MAP4-MT interactions. These results are consistent with both FRET (30) and the cryo-electron microscopy (28) studies of tau bound to MTs, in which the repeating motif of tau was shown to interact with the β-tubulin taxane binding site in the absence of Taxol. The distal M-loop, represented by peptide β281-293 in our study, is one of the main participants in lateral interactions between adjacent MT protofilaments. Contacts made between this part of the M-loop on one side, and the H1-S2 loop and H3 helix on the other, hold the protofilaments together, maintaining a straight and stable MT polymer (31). Residues comprising the distal M-loop have also been shown to influence the binding of Taxol in its β-tubulin luminal site (32). The results of the present study further confirm involvement of the distal M-loop in the binding of Taxol. MTB-MAP4 alone, however, has no effect on this region, which suggests that this portion of the M-loop is not involved in binding MAP4. To summarize, MAP4 and Taxol appear to share an overlapping luminal binding site in β-tubulin involving only the proximal part of the M-loop, and not the distal part. Furthermore, addition of Taxol to the MTB-MAP4-MT complex weakens this interaction between MAP4 and tubulin.
We have previously reported that the MT polymer adopts a straight conformation once bound by Taxol, as evidenced by enhanced protection in the intradimer, interdimer and interprotofilament regions (15, 16, 19). All of these interfaces are also strongly protected by MTB-MAP4 (Figure 3 and Table 1), albeit to a lesser extent than in the presence of a drug. This strongly suggests that similar to the microtubule stabilizing agents, MAP4 induces MT stability via transforming the MT conformation from curved to straight. The driving force for this straightening could be the direct result of simultaneous binding of MTB-MAP4 to both the outside and the luminal portion of the MT. Stabilization of MTs by Taxol was suggested to follow a cooperative mechanism in which the conformational changes induced by Taxol binding to β-tubulin could stabilize α-tubulin and extend this effect to all other tubulin dimers along the protofilament (33). This was further demonstrated in our original study of Taxol-stabilized MTs (19). This global stabilizing activity of Taxol appears to further enhance the effects of MAP4, as evidenced by significantly increased protection of the intradimer and lateral β–β interprotofilament interactions, suggesting that Taxol contributes to straightening the tubulin conformation, thus maintaining a more stable microtubule structure.
The cooperative MT stabilization between Taxol and MAP4 suggests that drugs, such as Taxol, may alter the physiological activity of endogenous MT effectors by recruiting MAPs as partners in inducing mitotic arrest. In fact, results of multiple studies support this hypothesis. For example, it has been demonstrated that p53 mutant baby rat kidney (BRK) cells overexpressing MAP4 were more sensitive to Taxol and less sensitive to MT destabilizing drugs of the vinca alkaloid class (34). Additionally, vinca-resistant human leukemia cells were shown to overexpress MAP4 (35), whereas Taxol-resistant A549 cells expressed more hyperphosphorylated forms of MAP4 as the level of resistance increased (36). Phosphorylated MAP4 does not bind to MTs or promote stabilization of the polymer (37, 38).
We have shown that similar to the microtubule stabilizing agents, MAP4 stabilizes the entire MT via its activity on all the major interfaces that comprise the MT polymer. We have also demonstrated that Taxol and MAP4 may share an overlapping luminal binding site involving the proximal β-tubulin M-loop. Binding of Taxol to MTs appears to weaken both this luminal and external interactions between MAP4 and MTs. Despite this effect, enhancement of stabilization of the intradimer and lateral interfaces observed in the presence of Taxol suggests that endogenous effectors of MT dynamics may cooperate with microtubule stabilizing agents in stabilizing MTs and regulating mitotic activity of the cells.
Materials and Methods
Tubulin, MAPs and other materials
Tubulin was isolated from chicken erythrocytes as previously described (17). Phosphocellulose-purified chicken erythrocyte tubulin (CET) was stored in a nucleotide-free buffer (50 mM MES, 1 mM EGTA, 0.2 mM MgCl2, pH 6.8) at −80 °C. This tubulin contains a single α- and β-isotype, α1 and βVI. Purified tubulin was fully functional as assessed by measuring its ability to polymerize at 37°C in the presence of equimolar Taxol, and its morphology was normal, as determined by negative-staining transmission electron microscopy following assembly (not shown). MTB-MAP4 was prepared as previously described (18) and was composed of the entire proline-rich region, three assembly promoting repeats, and the C-terminus, but had its projection domain (PJ) truncated.
Taxol was obtained from the Drug Development Branch, National Cancer Institute, Bethesda, MD. Porcine stomach pepsin was purchased from Sigma-Aldrich. Taxol was stored as 5 mM solutions in DMSO at −20 °C. Deuterium oxide (99.9%) was obtained from Cambridge Isotope Laboratories. Tris(2-carboxyethyl)phosphine (TCEP, 0.5 M), guanidinium hydrochloride, formic acid (FA), and trifluoroacetic acid (TFA) were from Pierce. GMPCPP (100 mM) was purchased from Jena Bioscience, Germany. Acetonitrile was purchased from Fisher Scientific. All other reagents were of highest purity available.
HDX LC-MS system and peptide identification
Instrumentation, experimental design and peptide identification were performed as previously described (15).
HDX/MS experiments
All tubulin samples were clarified by centrifugation at 50,000 rpm, at 4 °C for 10 min prior to assembly. For each experiment, 40 µM tubulin was preincubated with 1 mM GMPCPP in MEM buffer (50 mM MES, 1 mM EGTA, and 0.2 mM MgCl2, pH 6.8) at 37 °C for 30 min. For experiments done with the MTB-MAP4-microtubule complex, tubulin was preincubated with equimolar MTB-MAP4 at 37 °C for 30 min. For the experiments done with the MTB-MAP4-Taxol-microtubule complex, three stepwise additions of increasing concentrations (10 µM, 100 µM, and 1mM) of Taxol were added to tubulin preassembled in the presence of MTB-MAP4 and GMPCPP, to a final concentration of 20 µM Taxol. The incubation time was 10 min after the first two additions and 15 min after the last one.
All HDX experiments were done in triplicate as described previously (15, 16). Briefly, assembled CET, under various conditions described above, was subjected to HDX for 5 min, quenched with chilled phosphate buffer (0.5 M, pH 2.5), followed by immediate pepsin digestion in solution for 5 min. The peptic peptides were then separated by HPLC and analyzed by FT-ICR MS. For global HDX experiments, the deuterium incorporation into intact tubulin pre-incubated with GMPCPP or GMPCPP+MTB-MAP4 was measured using LC-MS at five different time points (1, 3, 5, 10 and 30 min) after further incubation at 37°C in deuterated 0.1 M MEM buffer.
Data analysis and presentation
The MS distribution for each peptide was fitted to a Gaussian curve and the centroid value (xc) was determined using OriginPro8. Changes in deuterium incorporation (ΔHDX) were defined as the difference between the xc values of the GMPCPP-stabilized MTs in the presence and absence of MTB-MAP4 or MTB-MAP4+Taxol. Changes in deuteration due to Taxol addition were designated as ΔΔHDX and subjected to the same statistical analysis as the ΔHDX values. Briefly, all differences with a P-value <0.05 were considered significant. For a detailed description of the statistical analysis of data, refer to Khrapunovich-Baine et al. (15).
Peptides that exhibited significant changes in deuterium incorporation were mapped onto the tubulin dimer structure (1JFF) and onto a structure of a microtubule protofilament pair previously constructed in our laboratory (19). Molecular representations of tubulin in all figures were generated using Pymol.
Acknowledgement
This work was supported by NIH Grant CA124898 (NCI) and the National Foundation for Cancer Research (to S. B. H.).
References
- 1.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 2.Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999;10:947–959. doi: 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–7295. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz SB, Fojo T. Microtubule Stabilizing Agents. In: Fojo T, editor. Cancer Drug Discovery and Development: The Role of Microtubules in Cell Biology, Neurobiology, and Oncology. Totowa, NJ: Humana Press; 2008. pp. 307–336. [Google Scholar]
- 5.Kavallaris M, Don S, Verrills NM. Microtubule-Associated Proteins and Microtubule-Interacting Proteins. In: Fojo T, editor. Cancer Drug Discovery and Development: The Role of Microtubules in Cell Biology, Neurobiology, and Oncology. Totowa, NJ: Humana Press; 2008. pp. 83–104. [Google Scholar]
- 6.Cassimeris L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr Opin Cell Biol. 2002;14:18–24. doi: 10.1016/s0955-0674(01)00289-7. [DOI] [PubMed] [Google Scholar]
- 7.Bulinski JC, Borisy GG. Widespread distribution of a 210,000 mol wt microtubule-associated protein in cells and tissues of primates. J Cell Biol. 1980;87:802–808. doi: 10.1083/jcb.87.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 9.Lee G, Cowan N, Kirschner M. The primary structure and heterogeneity of tau protein from mouse brain. Science. 1988;239:285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- 10.Lewis SA, Wang DH, Cowan NJ. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science. 1988;242:936–939. doi: 10.1126/science.3142041. [DOI] [PubMed] [Google Scholar]
- 11.Chapin SJ, Bulinski JC. Non-neuronal 210 × 10(3) Mr microtubule-associated protein (MAP4) contains a domain homologous to the microtubule-binding domains of neuronal MAP2 and tau. J Cell Sci. 1991;98(Pt 1):27–36. doi: 10.1242/jcs.98.1.27. [DOI] [PubMed] [Google Scholar]
- 12.Aizawa H, Emori Y, Murofushi H, Kawasaki H, Sakai H, Suzuki K. Molecular cloning of a ubiquitously distributed microtubule-associated protein with Mr 190,000. J Biol Chem. 1990;265:13849–13855. [PubMed] [Google Scholar]
- 13.Cann JR, York EJ, Stewart JM, Vera JC, Maccioni RB. Small zone gel chromatography of interacting systems: theoretical and experimental evaluation of elution profiles for kinetically controlled macromolecule-ligand reactions. Anal Biochem. 1988;175:462–473. doi: 10.1016/0003-2697(88)90570-2. [DOI] [PubMed] [Google Scholar]
- 14.Ennulat DJ, Liem RK, Hashim GA, Shelanski ML. Two separate 18-amino acid domains of tau promote the polymerization of tubulin. J Biol Chem. 1989;264:5327–5330. [PubMed] [Google Scholar]
- 15.Khrapunovich-Baine M, Menon V, Huang Yang CP, Northcote PT, Miller JH, Hogue Angeletti R, Fiser A, Horwitz SB, Xiao H. Hallmarks of molecular action of microtubule stabilizing agents: Effects of epothilone B, ixabepilone, peloruside A, laulimalide on microtubule conformation. J Biol Chem. 2011 doi: 10.1074/jbc.M110.162214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khrapunovich-Baine M, Menon V, Verdier-Pinard P, Smith AB, 3rd, Angeletti RH, Fiser A, Horwitz SB, Xiao H. Distinct pose of discodermolide in taxol binding pocket drives a complementary mode of microtubule stabilization. Biochemistry. 2009;48:11664–11677. doi: 10.1021/bi901351q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy DB, Wallis KT. Isolation of microtubule protein from chicken erythrocytes and determination of the critical concentration for tubulin polymerization in vitro and in vivo. J Biol Chem. 1983;258:8357–8364. [PubMed] [Google Scholar]
- 18.Nguyen HL, Chari S, Gruber D, Lue CM, Chapin SJ, Bulinski JC. Overexpression of full- or partial-length MAP4 stabilizes microtubules and alters cell growth. J Cell Sci. 1997;110(Pt 2):281–294. doi: 10.1242/jcs.110.2.281. [DOI] [PubMed] [Google Scholar]
- 19.Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, Burd B, Angeletti R, Fiser A, Horwitz SB, Orr GA. Insights into the mechanism of microtubule stabilization by Taxol. Proc Natl Acad Sci U S A. 2006;103:10166–10173. doi: 10.1073/pnas.0603704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudiger M, Weber K. Characterization of the post-translational modifications in tubulin from the marginal band of avian erythrocytes. Eur J Biochem. 1993;218:107–116. doi: 10.1111/j.1432-1033.1993.tb18357.x. [DOI] [PubMed] [Google Scholar]
- 21.Olson KR, McIntosh JR, Olmsted JB. Analysis of MAP 4 function in living cells using green fluorescent protein (GFP) chimeras. J Cell Biol. 1995;130:639–650. doi: 10.1083/jcb.130.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowe J, Li H, Downing KH, Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J Mol Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 23.Cross D, Dominguez J, Maccioni RB, Avila J. MAP-1 and MAP-2 binding sites at the C-terminus of beta-tubulin. Studies with synthetic tubulin peptides. Biochemistry. 1991;30:4362–4366. doi: 10.1021/bi00231a036. [DOI] [PubMed] [Google Scholar]
- 24.Cross D, Farias G, Dominguez J, Avila J, Maccioni RB. Carboxyl terminal sequences of beta-tubulin involved in the interaction of HMW-MAPs. Studies using site-specific antibodies. Mol Cell Biochem. 1994;132:81–90. doi: 10.1007/BF00925677. [DOI] [PubMed] [Google Scholar]
- 25.Serrano L, de la Torre J, Maccioni RB, Avila J. Involvement of the carboxyl-terminal domain of tubulin in the regulation of its assembly. Proc Natl Acad Sci U S A. 1984;81:5989–5993. doi: 10.1073/pnas.81.19.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Bassam J, Ozer RS, Safer D, Halpain S, Milligan RA. MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J Cell Biol. 2002;157:1187–1196. doi: 10.1083/jcb.200201048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuno N, Toba S, Edamatsu M, Watai-Nishii J, Hirokawa N, Toyoshima YY, Kikkawa M. Dynein and kinesin share an overlapping microtubule-binding site. EMBO J. 2004;23:2459–2467. doi: 10.1038/sj.emboj.7600240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kar S, Fan J, Smith MJ, Goedert M, Amos LA. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 2003;22:70–77. doi: 10.1093/emboj/cdg001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kar S, Florence GJ, Paterson I, Amos LA. Discodermolide interferes with the binding of tau protein to microtubules. FEBS Lett. 2003;539:34–36. doi: 10.1016/s0014-5793(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 30.Makrides V, Massie MR, Feinstein SC, Lew J. Evidence for two distinct binding sites for tau on microtubules. Proc Natl Acad Sci U S A. 2004;101:6746–6751. doi: 10.1073/pnas.0400992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sui H, Downing KH. Structural basis of interprotofilament interaction and lateral deformation of microtubules. Structure. 2010;18:1022–1031. doi: 10.1016/j.str.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao S, He L, Chakravarty S, Ojima I, Orr GA, Horwitz SB. Characterization of the Taxol binding site on the microtubule. Identification of Arg(282) in beta-tubulin as the site of photoincorporation of a 7-benzophenone analogue of Taxol. J Biol Chem. 1999;274:37990–37994. doi: 10.1074/jbc.274.53.37990. [DOI] [PubMed] [Google Scholar]
- 33.Amos LA. Focusing-in on microtubules. Curr Opin Struct Biol. 2000;10:236–241. doi: 10.1016/s0959-440x(00)00070-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang CC, Yang JM, White E, Murphy M, Levine A, Hait WN. The role of MAP4 expression in the sensitivity to paclitaxel and resistance to vinca alkaloids in p53 mutant cells. Oncogene. 1998;16:1617–1624. doi: 10.1038/sj.onc.1201658. [DOI] [PubMed] [Google Scholar]
- 35.Kavallaris M, Tait AS, Walsh BJ, He L, Horwitz SB, Norris MD, Haber M. Multiple microtubule alterations are associated with Vinca alkaloid resistance in human leukemia cells. Cancer Res. 2001;61:5803–5809. [PubMed] [Google Scholar]
- 36.Martello LA, Verdier-Pinard P, Shen HJ, He L, Torres K, Orr GA, Horwitz SB. Elevated levels of microtubule destabilizing factors in a Taxol-resistant/dependent A549 cell line with an alpha-tubulin mutation. Cancer Res. 2003;63:1207–1213. [PubMed] [Google Scholar]
- 37.Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci. 1998;23:307–311. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- 38.Chang W, Gruber D, Chari S, Kitazawa H, Hamazumi Y, Hisanaga S, Bulinski JC. Phosphorylation of MAP4 affects microtubule properties and cell cycle progression. J Cell Sci. 2001;114:2879–2887. doi: 10.1242/jcs.114.15.2879. [DOI] [PubMed] [Google Scholar]