Abstract
Background
The emerging attention on in-home care in Canada assumes that chronic disease management will be optimized if it takes place in the community as opposed to the health care setting. Both the patient and the health care system will benefit, the latter in terms of cost savings.
Objectives
To compare the effectiveness of care delivered in the home (i.e., in-home care) with no home care or with usual care/care received outside of the home (e.g., health care setting).
Data Sources
A literature search was performed on January 25, 2012, using OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Wiley Cochrane Library, and the Centre for Reviews and Dissemination database, for studies published from January 1, 2006, until January 25, 2012.
Review Methods
An evidence-based analysis examined whether there is a difference in mortality, hospital utilization, health-related quality of life (HRQOL), functional status, and disease-specific clinical measures for in-home care compared with no home care for heart failure, atrial fibrillation, coronary artery disease, stroke, chronic obstructive pulmonary disease, diabetes, chronic wounds, and chronic disease / multimorbidity. Data was abstracted and analyzed in a pooled analysis using Review Manager. When needed, subgroup analysis was performed to address heterogeneity. The quality of evidence was assessed by GRADE.
Results
The systematic literature search identified 1,277 citations from which 12 randomized controlled trials met the study criteria. Based on these, a 12% reduced risk for in-home care was shown for the outcome measure of combined events including all-cause mortality and hospitalizations (relative risk [RR]: 0.88; 95% CI: 0.80–0.97). Patients receiving in-home care had an average of 1 less unplanned hospitalization (mean difference [MD]: –1.03; 95% CI: –1.53 to –0.53) and an average of 1 less emergency department (ED) visit (MD: –1.32; 95% CI: –1.87 to –0.77). A beneficial effect of in-home care was also shown on activities of daily living (MD: –0.14; 95% CI: –0.27 to –0.01), including less difficulty dressing above the waist or below the waist, grooming, bathing/showering, toileting, and feeding. These results were based on moderate quality of evidence. Additional beneficial effects of in-home care were shown for HRQOL although this was based on low quality of evidence.
Limitations
Different characterization of outcome measures across studies prevented the inclusion of all eligible studies for analysis.
Conclusions
In summary, education-based in-home care is effective at improving outcomes of patients with a range of heart disease severity when delivered by nurses during a single home visit or on an ongoing basis. In-home visits by occupational therapists and physical therapists targeting modification of tasks and the home environment improved functional activities for community-living adults with chronic disease.
Plain Language Summary
It is assumed that patients with chronic disease will benefit if they are living at home and being looked after at home or in the community. In addition, there may be cost savings to the health care system when care is provided in the community or in the home instead of in hospitals and other health care settings.
This evidence-based analysis examined whether in-home care given by different health care professionals improved patient and health system outcomes. Patients included those with heart failure, atrial fibrillation, coronary artery disease, stroke, chronic obstructive pulmonary disease, diabetes, chronic wounds, and with more than one chronic disease. The results show that in-home care delivered by nurses has a beneficial effect on patients’ health outcomes. Patient mortality and/or patient hospitalization were reduced. In-home care also improved patients’ activities of daily living when delivered by occupational therapists and physical therapists. In addition, the results showed that in-home care delivered by nurses has a beneficial effect on health system outcomes, reducing the number of unplanned hospitalizations and emergency department visits.
Background
In July 2011, the Evidence Development and Standards (EDS) branch of Health Quality Ontario (HQO) began developing an evidentiary framework for avoidable hospitalizations. The focus was on adults with at least 1 of the following high-burden chronic conditions: chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), atrial fibrillation, heart failure, stroke, diabetes, and chronic wounds. This project emerged from a request by the Ministry of Health and Long-Term Care for an evidentiary platform on strategies to reduce avoidable hospitalizations.
After an initial review of research on chronic disease management and hospitalization rates, consultation with experts, and presentation to the Ontario Health Technology Advisory Committee (OHTAC), the review was refocused on optimizing chronic disease management in the outpatient (community) setting to reflect the reality that much of chronic disease management occurs in the community. Inadequate or ineffective care in the outpatient setting is an important factor in adverse outcomes (including hospitalizations) for these populations. While this did not substantially alter the scope or topics for the review, it did focus the reviews on outpatient care. HQO identified the following topics for analysis: discharge planning, in-home care, continuity of care, advanced access scheduling, screening for depression/anxiety, self-management support interventions, specialized nursing practice, and electronic tools for health information exchange. Evidence-based analyses were prepared for each of these topics. In addition, this synthesis incorporates previous EDS work, including Aging in the Community (2008) and a review of recent (within the previous 5 years) EDS health technology assessments, to identify technologies that can improve chronic disease management.
HQO partnered with the Programs for Assessment of Technology in Health (PATH) Research Institute and the Toronto Health Economics and Technology Assessment (THETA) Collaborative to evaluate the cost-effectiveness of the selected interventions in Ontario populations with at least 1 of the identified chronic conditions. The economic models used administrative data to identify disease cohorts, incorporate the effect of each intervention, and estimate costs and savings where costing data were available and estimates of effect were significant. For more information on the economic analysis, please contact either Murray Krahn at murray.krahn@theta.utoronto.ca or Ron Goeree at goereer@mcmaster.ca.
HQO also partnered with the Centre for Health Economics and Policy Analysis (CHEPA) to conduct a series of reviews of the qualitative literature on “patient centredness” and “vulnerability” as these concepts relate to the included chronic conditions and interventions under review. For more information on the qualitative reviews, please contact Mita Giacomini at giacomin@mcmaster.ca.
The Optimizing Chronic Disease Management in the Outpatient (Community) Setting mega-analysis series is made up of the following reports, which can be publicly accessed at http://www.hqontario.ca/evidence/publications-and-ohtac-recommendations/ohtas-reports-and-ohtac-recommendations.
Optimizing Chronic Disease Management in the Outpatient (Community) Setting: An Evidentiary Framework
Discharge Planning in Chronic Conditions: An Evidence-Based Analysis
In-Home Care for Optimizing Chronic Disease Management in the Community: An Evidence-Based Analysis
Continuity of Care: An Evidence-Based Analysis
Advanced (Open) Access Scheduling for Patients With Chronic Diseases: An Evidence-Based Analysis
Screening and Management of Depression for Adults With Chronic Diseases: An Evidence-Based Analysis
Self-Management Support Interventions for Persons With Chronic Diseases: An Evidence-Based Analysis
Specialized Nursing Practice for Chronic Disease Management in the Primary Care Setting: An Evidence-Based Analysis
Electronic Tools for Health Information Exchange: An Evidence-Based Analysis
Health Technologies for the Improvement of Chronic Disease Management: A Review of the Medical Advisory Secretariat Evidence-Based Analyses Between 2006 and 2011
Optimizing Chronic Disease Management Mega-Analysis: Economic Evaluation
How Diet Modification Challenges Are Magnified in Vulnerable or Marginalized People With Diabetes and Heart Disease: A Systematic Review and Qualitative Meta-Synthesis
Chronic Disease Patients’ Experiences With Accessing Health Care in Rural and Remote Areas: A Systematic Review and Qualitative Meta-Synthesis
Patient Experiences of Depression and Anxiety With Chronic Disease: A Systematic Review and Qualitative Meta-Synthesis
Experiences of Patient-Centredness With Specialized Community-Based Care: A Systematic Review and Qualitative Meta-Synthesis
Objective of Analysis
The objective of this evidence-based health technology assessment was to determine the effectiveness of in-home care in optimizing chronic disease management in the community. The assumption is that there will be cost savings to the health care system when patient moves from the health care setting to the community or the home. (1)
Clinical Need and Target Population
Based on the 1994/95 National Population Health Survey (NPHS), 522,900 Canadians aged 18 years or older were receiving formal home care. (2) This number grew to 545,000 in 1996/97. (2) The largest group of individuals receiving home care were the elderly and the chronically ill. However, people with a range of health conditions may receive home care. (2)
In 1995, use of home care services in Ontario increased dramatically with age, from about 50 per 1,000 population in women 65 years and older to more than 250 per 1,000 population in women 85 years and older. Men displayed a similar age-related increase in the use of home care services. (1)
In 2010, 125,724 Ontario seniors aged 65 years or more who had been assessed by the Resident Assessment Instrument Home Care were receiving publicly funded home care on an ongoing basis (i.e., expecting to receive or receiving services for at least 60 days). The majority were female (66.9%), and about 40% were aged 75 years or more. Overall, 38% were married, indicating that about one-third may have the advantage of a spouse as a caregiver. Less than 5% of the clients who received home care were without a family caregiver. Multimorbidity was common, with diabetes (26.4%), Alzheimer disease/dementia (22.7%), stroke (18.4%), chronic obstructive pulmonary disease (COPD) (17.2%), cancer (13.7%), heart failure (12.9%), and psychiatric diseases (12.7%) the most prevalent. (3)
Canadian Context
Publicly funded home care in Canada is administered by the provincial or territorial government or by regional health authorities. The way home care works in Canada is as follows: a client is referred to receive home care services, at which point a case manager is assigned to the client. The case manager meets with the client and any potential caregiver to conduct an assessment, and then coordinates care, authorizes services, and provides ongoing monitoring and evaluation. Home care service providers typically are a personal support worker and/or a nurse, either public employees and/or agency employees. A personal support worker assists with basic daily living needs whereas a nurse provides clinical care. The home care team may also include occupational therapists, physiotherapists, pharmacists, nurse practitioners, social workers, dietitians, and physicians. A majority of clients (50%-69%) across Canada are receiving home care services provided by personal support workers. (3)
In Ontario, home care services may begin at the time of hospital discharge, with a care coordinator assessing patient need. Alternately, a rapid response nurse may provide an in-home visit within 24 hours of discharge and provide medication reviews and education on symptom and lifestyle management. (Personal communication, Community Expert, December 3, 2012).
Home care services are publicly funded in Ontario, Manitoba, Quebec, Prince Edward Island, and the 3 territories. Provincial plans in British Columbia, Alberta, Saskatchewan, New Brunswick, Nova Scotia, and Newfoundland and Labrador cover most services. However, additional fees may be required for some personal and community support services. Community support services include general house cleaning, meal preparation or delivery, or help with running errands. (3)
Ontario Context
In Ontario, formal home care services are either government-funded or privately paid for. The Community Care Access Centres (CCACs) administers the former, and the case manager determines the type and amount of service delivered. Among Ontarian adults aged 65 years and older, 8% of women and 6% of men received government-funded services. (4) In total, there are 14 CCACs in communities across Ontario that are funded by Local Health Integration Networks through the Ministry of Health and Long-Term Care. CCAC advice and services are covered by the Ontario Health Insurance Plan (OHIP). (5)
The top 5 ranked type of home care services delivered to Ontario residents in fiscal year 2011/2012 by the CCAC were, by number of services delivered
Combined personal support and homemaking services (n = 17,557,390)
Nursing visits (n = 6,058,730)
Case management (n = 2,100,812)
Personal services (n = 1,862,877)
Occupational therapy (512,784 sessions) (6)
The rank of the remaining type of home care services were as follows:
Physiotherapy (443,289 sessions)
Nursing shifts (n = 376,905)
Speech language therapy (252,038 sessions)
Respite (n = 112,596)
Homemaking services (n = 72,790)
Social work (n = 55,494)
Nutrition/dietetic (47,865 sessions)
Other services (n = 37,304)
Placement services (n = 2,376)
Psychology (n = 340)
Respiratory services (n=216) (6)
In-Home Care
The aim of in-home and continuing care is to provide care for acute or chronically ill individuals in the home, in the community, in supportive housing, or in long-term care facilities. In-home and continuing care, delivered to recovering, disabled, or chronically or terminally ill individuals, maintains or improves the health status of individuals in need. (2) Offered are a variety of health services including nursing, personal care, physiotherapy, occupational therapy, speech therapy, social work, dietician services, homemaking, respite care, day programs for Alzheimer disease, Meals on Wheels, and friendly visitor programs, which can maintain or improve the health status of individuals in need. (2)
For the purposes of this evidence-based analysis, in-home care is defined as care predominately in the patient’s home. This includes ongoing in-home assessment, case management, and coordination of a range of services provided in the home or in the community that are curative, preventive, or supportive in nature and that aim to enable clients to live at home, thus preventing or delaying the need for long-term care or acute care. Palliative care and rehabilitation are not considered in this analysis. Supportive care includes personal care, meal preparation, and homemaking tasks. (2)
In-Home Care as a Component of Multidisciplinary Care
Multidisciplinary care may constitute an in-home care component. For example, a number of systematic reviews/meta-analyses have examined multidisciplinary care in relation to heart failure. (7-9) Multidisciplinary care was examined as a complex intervention, (8) as part of a disease management program, (9) or in subgroups based on the setting in which the intervention was delivered including the home. (7)
In a systematic review/meta-analysis that examined multidisciplinary care in heart failure by intervention setting including home visits, (7) 12 of the 30 included studies had a home visit component. The search strategy was current as of 2004. Included studies were published between 1993 and 2005. Multidisciplinary interventions were nurse-led programs, medication reviews, medication adherence interventions, patient education, or enhanced monitoring. Home visits were defined as one or more planned visits by a health care professional to educate or improve patient self-management, but excluded visits to take blood samples, set up physiological monitoring, or deliver wound care. Results showed a 20% reduction in all-cause admissions (relative risk [RR]: 0.80; 95% CI: 0.71–0.89), a 38% reduction in heart failure admissions (RR: 0.62; 95% CI: 0.51–0.74), and a nonsignificant 13% reduction in all-cause mortality (RR: 0.87; 95% CI: 0.72–1.06). (7)
Since multidisciplinary care tends to be used synonymously with disease management programs that focus on the continuum of care across health delivery systems, the systematic reviews / meta-analyses that examined multidisciplinary care were not considered for this evidence-based analysis.
Alternate In-Home Care Strategies
A number of health care strategies involve an in-home care component. However, many are out-of-scope and therefore are not part of this evidence-based analysis. They include the following:
Early supported discharge. Patients after stroke conventionally receive much of their rehabilitation in hospital. Services have been developed that offer patients an early discharge from hospital with more rehabilitation at home. (10)
Transitional care. Also known as integrated care or disease management programs, transitional care focuses on improving the experience of patients when they are discharged from acute hospital care to other types of care. Transitional care may include home visits as part of the coordinated service. It aims to address the needs of the 20% of patients who experience an adverse clinical event within 30 days of the discharge from hospital. (11)
Hospital-at-home. Hospitalizations result in a high demand on hospital resources and high health care costs. Hospital-at-home is a safe alternative to hospitalization in, for example, acute exacerbation of COPD where patients admitted to hospital may be discharged on the fourth day of admission to receive care at home provided by specialized respiratory nurses. (12)
Home-based rehabilitation as an alternative to hospital-based programs for pulmonary rehabilitation in patients with COPD, for example, expands the recognition, application, and accessibility of pulmonary rehabilitation for these patients. (13) Similar considerations exist for patients undergoing cardiac rehabilitation. Hospital-based cardiac rehabilitation attracts those who prefer supervision during exercise, need the camaraderie of a group, are willing to make travel arrangements, and believe they lack self-discipline. Home-based cardiac rehabilitation attracts the more self-disciplined patients who believe that rehabilitation should fit in with their lives rather than their lives fitting in with the rehabilitation. The patients who prefer home-based care also dislike group therapy and express practical concerns such as travel or transportation to group hospital therapy. (14)
Evidence-Based Analysis
Research Question
To compare the effectiveness of care delivered in the home (i.e., in-home care) with no home care or with usual care / care received outside of the home (e.g., a health care setting).
Literature Search
Search Strategy
A literature search was performed on January 25, 2012, using OVID MEDLINE, OVID MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, EBSCO Cumulative Index to Nursing & Allied Health Literature (CINAHL), the Wiley Cochrane Library, and the Centre for Reviews and Dissemination database for studies published from January 1, 2006, until January 25, 2012. The start date for the literature search was selected based on scoping of the literature and identification of a number of systematic reviews that had already been completed at that time (see Results). Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search.
Inclusion Criteria
English language full-text reports
published between January 1, 2006, and January 25, 2012
randomized controlled trials (RCTs), systematic reviews, meta-analyses, health technology assessments
adults aged ≥ 18 years
at least one in-home care visit had to have occurred
in-home care provided by any type of health or medical professional or social assistance provider
studies on multidisciplinary care when findings for home visits were presented separately
Exclusion Criteria
studies using telemonitoring or telemedicine to deliver in-home care
telephone-based follow-up service or patients using self-management strategies alone
studies on hospice care, end-of-life care, or palliative care delivered in the home
studies comparing different delivery models of in-home care
studies on the effectiveness of transitional care, early supportive discharge, hospital-at-home, or rehabilitation
Outcomes of Interest
hospital utilization (admissions, readmissions, length of stay [LOS], emergency department [ED] utilization, admissions to long-term care facilities)
survival/mortality
health-related quality of life (HRQOL) / functional status
disease-specific clinical measures / physiological measures
patient satisfaction
Statistical Analysis
A meta-analysis was performed using Review Manager Version 5. (15) For continuous data a mean difference was calculated, and for dichotomous data a risk ratio was calculated for RCTs. A fixed effect model was used unless significant heterogeneity was observed (e.g., P ≤ 0.10), and then a random effects model was used to address significant heterogeneity. When heterogeneity was not accounted for using a random effects model, a post-hoc subgroup analysis was considered. For continuous variables with mean baseline and mean follow-up data, a change value was calculated (if not presented in the original paper) as the difference between the 2 mean values (e.g., follow-up minus baseline). To allow for analysis and account for the change value, a corresponding standard deviation (SD) was calculated using 3 parameters: baseline SD, follow-up SD, and a correlation coefficient. The correlation coefficient represents the strength of the relationship between the 2 SDs. A correlation coefficient of 0.5 was used for this analysis. For all other continuous variables, a mean difference was calculated based on values at follow-up. Graphical display of the forest plots was also examined. A P value of less than 0.05 was considered statistically significant. P values in the text have been rounded to 3 decimal places. When the data were available, a subgroup analysis by disease category was performed.
Quality of Evidence
The quality of the body of evidence for each outcome was examined according to the GRADE Working Group criteria. (16) The overall quality was determined to be very low, low, moderate, or high using a step-wise, structural methodology.
Study design was the first consideration; the starting assumption was that RCTs are high quality, whereas observational studies are low quality. Five additional factors—risk of bias, inconsistency, indirectness, imprecision, and publication bias—are then taken into account. Limitations or serious limitations in these areas result in downgrading the quality of evidence. Finally, 3 main factors are considered that may raise the quality of evidence: large magnitude of effect, dose response gradient, and accounting for all residual confounding. (16) For more detailed information, please refer to the latest series of GRADE articles. (16)
As stated by the GRADE Working Group, the final quality score can be interpreted using the following definitions:
| High | Very confident that the true effect lies close to that of the estimate of the effect |
| Moderate | Moderately confident in the effect estimate—the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | Confidence in the effect estimate is limited—the true effect may be substantially different from the estimate of the effect |
| Very Low | Very little confidence in the effect estimate—the true effect is likely to be substantially different from the estimate of effect |
Results of Evidence-Based Analysis
The database search yielded 1,277 citations published between January 1, 2006, and January 25, 2012 (with duplicates removed). Articles were excluded based on information in the title and abstract. The full texts of potentially relevant articles were obtained for further assessment. Figure 1 shows the breakdown of when and for what reason citations were excluded from the analysis.
Figure 1: Citation Flow Chart.
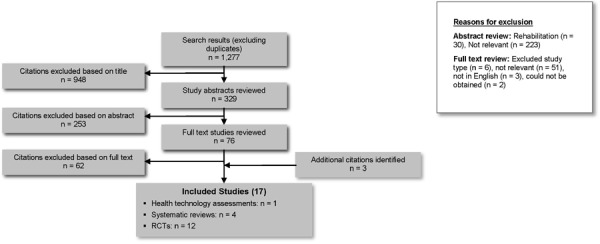
Seventeen studies (1 health technology assessment, 4 systematic reviews, 12 RCTs) met the inclusion criteria. The reference lists of the included studies were manually searched to identify any other potentially relevant studies, and 2 other RCTs were identified. One additional systematic review was identified from a review of MEDLINE. These were also included in this analysis.
Aside from the 17 studies analyzed in this evidence-based analysis, a clinical RCT conducted in Ontario, Canada, was also assessed for inclusion in this analysis. This RCT compared the effectiveness of community leg ulcer clinics with home care for treating patients with leg ulcers. (17) In-home care was considered usual care and care in community leg ulcer clinics was considered the intervention. Because of the reverse comparison, this study was excluded from this evidence-based analysis.
In addition, an RCT that used home-based care for heart failure patients was brought to the attention of the researcher; however, its date of publication was outside of the literature search dates. There was some agreement between our results and those of this study. (18)
For each included study, the study design was identified and is summarized below in Table 1, which is a modified version of a hierarchy of study design by Goodman. (19)
Table 1: Body of Evidence Examined According to Study Design.
| Study Design | Number of Eligible Studies | |
|---|---|---|
| RCT Studies | ||
| Systematic review of RCTs | 5a | |
| Large RCTb | 9 | |
| Small RCT | 3 | |
| Observational Studies | ||
| Systematic review of non-RCTs with contemporaneous controls | - | |
| Non-RCT with non-contemporaneous controls | - | |
| Systematic review of non-RCTs with historical controls | - | |
| Non-RCT with historical controls | - | |
| Database, registry, or cross-sectional study | - | |
| Case series | - | |
| Retrospective review, modelling | - | |
| Studies presented at an international conference | - | |
| Expert opinion | - | |
| Total | 17 | |
Abbreviation: RCT, randomized controlled trial.
Two systematic reviews included only RCTs; (20;21) 2 systematic reviews included RCTs in addition to other study designs (22;23) with only the information on RCTs used for this evidence-based analysis; one health technology assessment of RCTs. (24)
Large RCTs ≥ 150 subjects.
Health Technology Assessments
Heart Failure
A health technology assessment conducted by the Tufts-New England Medical Centre Evidence-Based Practice Centre under contract to the Agency for Healthcare Research and Quality in the United States compared the effectiveness of interventions that support postdischarge care with that of usual care in heart failure patients to prevent hospital readmission. (24) The magnitude of all-cause hospital readmissions was the primary outcome, whereas all-cause mortality, length of hospital stay, cost, quality of life, and a combined endpoint of mortality and readmissions were examined as secondary outcomes. The articles searched were published from 1990 to 2007. The 1990 search date was chosen as a starting point because that was the year when the medical management of heart failure started to advance rapidly, bringing about changes in practice patterns. RCTs were included if the population of interest was made up of heart failure patients and if the mean age of the population was 50 years or older. A number of interventions were examined, including home visits. These were defined as being done by “a member of the multidisciplinary heart failure team who visited the patient at home to assess clinical stability and provide care to optimize health.” The comparison group was defined as usual care, routine care, or standard care, which included non-structured care (e.g., discharge instructions, information on next appointment). A meta-analysis was performed based on the intervention of home visit (e.g., the setting where the intervention was initiated after an index hospitalization). Included were 37 studies that provided information on hospital readmissions and 30 studies that provided quantitative data for the intervention and control group. Among these were 4 studies on home visits. The meta-analysis of these 4 studies showed a statistically significant reduced risk of hospital readmission in the intervention group receiving home visits compared with the usual care group (RR: 0.82; 95% CI: 0.69–0.97). The remaining outcomes were not analyzed by intervention setting. The results were based on good to poor quality of evidence according to a 3-level customized grading scheme (i.e., good as the highest quality). The studies included in the meta-analysis were published from 1998 to 2002. The home visits were nurse-led, and in 2 of the 4 studies, there was mention of home services provided in the control group. The authors concluded that interventions that used home visits reduced the risk of hospital readmissions.
There were no health technology assessments identified for the remaining chronic conditions of interest: stroke, coronary artery disease, atrial fibrillation, COPD, diabetes, or chronic wound care.
Systematic Reviews
COPD
A systematic review examined the effectiveness of in-home care provided for COPD patients by respiratory health care worker programs. Outcomes were mortality, hospitalizations, HRQOL, lung function, and exercise tolerance. (20) Inclusion criteria allowed for RCTs with at least 3 months of follow-up, a home visit as intervention, and COPD defined according to standard criteria. Home visits were defined as a visit to the patient’s home by a respiratory nurse or respiratory health worker to facilitate health care, educate, provide social support, identify deteriorations, and reinforce correct use of inhaler therapy. The control group received routine care without access to a respiratory nurse / health care worker. The search was current as of 2009. The results of the meta-analysis of the 9 RCTs identified showed a beneficial effect of home visits by a respiratory nurse on HRQOL assessed using St George’s Respiratory Questionnaire (SGRQ; mean difference [MD]: −2.60; 95% CI: −4.81 to −0.39; 4 studies). There was no effect of home visits on mortality (5 studies), hospitalizations (5 studies), or exercise tolerance (2 studies). Data for a meta-analysis of lung function, ED visits, and general practitioner or family doctor visits were insufficient. The evidence was based on heterogeneous quality of evidence ranging from low (e.g., not possible to implement blinding) to high. The authors concluded that in-home care provided by respiratory health care worker programs for COPD improved HRQOL though heterogeneous data precluded conclusions about the other outcomes.
An integrative systematic review examined nursing care provided by nurse clinics in the chronic phase of COPD. (22) A nurse clinic was defined as a respiratory nurse with advanced respiratory competence and a primary role in delivering formalized service within a multidisciplinary team. The search included RCTs and other study designs published from 1996 and 2006. Studies on acute services were excluded. No meta-analysis was performed. From the 20 articles identified (reporting on 16 studies in total), 4 themes emerged, 1 of which was home-based respiratory care. This theme was covered in 9 articles, of which 6 were RCTs. The authors found no difference in hospitalizations except in 2 studies that showed a significant reduction in hospital admissions and readmissions and ED use. There was no difference for HRQOL and mortality. There was some suggestion of improved disease-related knowledge and patient satisfaction. For these studies, the service provided included health assessment, teaching disease facts, disease management, breathing technique and medications, advice on activities of daily living (ADL), healthy lifestyle, symptom awareness, the management of exacerbations, information on service referrals and telephone contact with health professionals. A majority of studies examining home-based respiratory care used an RCT design; however, 3 of the 9 studies were a non-RCT design. For the RCTs included, the control groups were described as usual care or standard protocols, booklets about COPD, following recommendations by physicians; a control group of 1 RCT included home visits by physicians. Because the authors summarized their data for heterogeneous study designs, it is difficult to interpret their results on health care resources, HRQOL, and mortality. Therefore, the contribution of RCT findings to the outcome measures is not clear. The authors concluded that the chronic management of COPD has been mainly conceptualized as home-based respiratory care; they could not conclude whether advanced nursing is more effective than usual care.
Multimorbidity
A systematic review examined comprehensive geriatric assessment interventions and the effect on ED use. (23) The interventions were defined based on the setting where they were implemented, including the outpatient setting of home care. The interventions were grouped into 5 general categories. The search strategy was current as of 2004 and included RCTs as well as other types of study designs. Inclusion criteria allowed for studies including the frail elderly, with their potential for multiple comorbidities, and patients 60 years of age or older. No meta-analysis was performed due to the heterogeneity of the studies. Identified were 26 studies, including 16 RCTs, that used a variety of intervention settings; 4 studies used in-home care as the intervention setting. Of these 4 studies, only 1 was considered eligible based on criteria established for this evidence-based analysis (e.g., RCT study, appropriate intervention type). This RCT, which was conducted in Italy, showed a reduced time to first ED use (hazard ratio: 0.64; P < 0.025). (25) The nature of the intervention in this study was case management—a case manager such as a nurse or social worker coordinated community services including home support, nursing care, and meals on wheels—with the control group described as usual care. (25) However, closer examination showed that both the intervention and the comparison groups included elements of home care. (25) The authors stated that the main difference between the intervention and the comparison groups was the element of case management and care planning present in the intervention group. Although the control group were able to receive the in-home care established in the community, it was considered fragmented. Overall, the authors of this systematic review concluded that interventions initiated in the outpatient setting reduced ED use whereas hospital-based interventions had less of an effect on ED use. (23)
A qualitative systematic review examined the effectiveness of home-based health promotion provided by professional nurses on patient outcomes. (21) Patient outcomes included mortality, admissions, health status, functional status, use of health and social services, and cost. The search strategy was current as of 2003, and inclusion criteria allowed for studies that used an RCT design and for community-living adults aged 65 years and older. The home-based care component included ongoing home visits or telephone contacts. Excluded studies were therapeutic or rehabilitative, involved hospital-at-home care or patients who had been discharged from the hospital. Identified were 12 RCTs. Only 2 studies included individuals in the control group receiving usual in-home care services. The intervention group received a diverse range of in-home care services including education on nutrition, exercise, stress management, substance abuse, emotional and social functions, instrumental activities of daily living (IADL), accessing health care, supportive physical and psychosocial nursing care, functional assessment, and integrated and interdisciplinary case management, to name a few. The nurses’ role included preventive care (e.g., early identification and management of health problems) and health promotion strategies (e.g., health education, goal setting). There were between 1.9 and 14.1 visits, and they lasted from 0.5 to 2 hours. The results showed favourable and significant effects for the intervention group of home-based nursing care for mortality (4 of 11 studies), functional status (4 of 8 studies), level of depression (1 of 4 studies), hospital admissions (5 of 9 studies), nursing home use (5 of 10 studies), and use of other health and social services (6 of 9 studies). Methodological limitations of included studies were randomization, blinding of outcome assessors, and incomplete follow-up. Other limitations were lack of detailed information on the content of the intervention (e.g., frequency of visits for some studies, and duration of visits) and control group (e.g., primary care, usual home care, or geriatric clinic), which specific subgroups of older individuals would most likely benefit from the intervention, and lack of information on depression and social support. The authors concluded that, despite overall positive results, it is not clear how the nursing role makes a difference in patient outcomes.
No eligible systematic reviews were identified for the remaining chronic conditions of interest: heart failure, stroke, coronary artery disease, atrial fibrillation, diabetes, or chronic wound care.
Randomized Controlled Trials
The systematic literature search found 12 RCTs eligible for this evidence-based analysis (Tables A2-A5).
Description of Studies
Of the 12 identified RCTs, 1 study was on diabetes, (26) 6 on heart failure, (27-32) 1 on COPD, (33) 1 on stroke, (34) and 3 on multimorbid chronic disease. (35-37) The sample sizes ranged from fewer than 150 subjects (28;30;33), 150 subjects or more, (26;27;29;31;32;34-37) up to even larger RCTs with more than 300 subjects. (27;36;37) The length of follow-up ranged from 1 to 3 months in 1 study (33) to 10 years in another. (32) There were 4 studies with outcome data at 6 months of follow-up (26;27;34;37) and 4 studies lasting between 1 and 2 years. (28;29;31;35) For the 6 studies on heart failure, the majority of patients were classified at study entry as New York Heart Association (NYHA) functional status class II in 2 studies, (28;30) class II/III in 1 study, (32) class III/IV in 1 study, (27) and class IV in 1 study. (29) The information was unknown for 1 study. (31) The in-home care intervention was delivered by nursing professionals in 5 studies, (28-31;34) by nursing professionals plus a pharmacist in 2 studies, (32;35) by community health workers in 1 study, (26) and allied health professionals including community pharmacists in 4 studies. (27;33;36;37) Half of the studies (6 of (12) were designed with 1 or a few scheduled in-home care visits. (27;28;30-33) Four studies scheduled ongoing in-home care visits, (26;29;36;37) and 2 provided in-home care visits as needed. (34;35) The contact time during the in-home care visit ranged from a minimum of 20 to 30 minutes (33) to a maximum of 2 hours. (28;30;34) A majority of studies (10 of 12) were designed to deliver an in-home care intervention that educated patients on disease facts, lifestyle modification, and medication use. (26-35) Two studies focused on the home environment and task performance. (36;37)
Diabetes
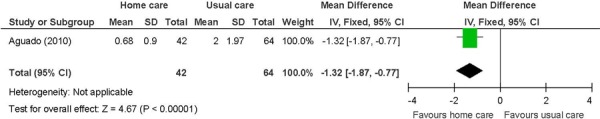
A randomized controlled clinical trial conducted in Detroit, United States, examined whether a culturally defined diabetes self-management home-based intervention administered by community health workers improved physiological measures in comparison with usual care in patients with type 2 diabetes. (26) Outcomes included hemoglobin A1c (HbA1c), systolic blood pressure (SBP), diastolic blood pressure (DBP), and low density lipoprotein (LDL) cholesterol, among others. (26) Primary or secondary outcomes were not explicitly stated but glycemic control was emphasized and therefore taken as the primary outcome. Eligible patients were identified from medical records, were at least 18 years of age with a physician-confirmed diagnosis of type 2 diabetes, and were self-identified as African American or Latino/Hispanic. Excluded were individuals with diabetes-related complications. Randomization was stratified by race/ethnicity and health care site. Allocation concealment was not stated. Interventionists were not blinded, although the data analysts were. Physiological measures were determined from medical records at baseline and at the 6-month follow-up. Analysis was described as an intent-to-treat. However, for the analysis on physiological measures, there were between 51 and 56 patients in the intervention group and between 55 and 65 patients in the control group, a reduction from the original 84 in the intervention group and 99 in the control group. There were no baseline differences, except for mean age (home care [HC]: 50; 95% CI: 47−52 vs. usual care [UC]: 55; 95% CI: 53−57 year; P = 0.02). The baseline and 6-month follow-up measures and change were presented as adjusted means.
Heart Failure
A randomized controlled clinical trial conducted in Barcelona, Spain, examined the effectiveness of a single home-based educational intervention compared with that of usual care in patients with heart failure. (28) The primary outcomes included number of unplanned hospitalizations, visits to the ED due to heart failure, and all-cause mortality. The secondary outcome relevant to this evidence-based analysis was HRQOL. Patients were eligible for inclusion if they displayed heart failure according to the Framingham criteria, had class II to IV NYHA function, and had left ventricular ejection fraction of less than 45% on echocardiography. The study did not include patients with dementia or neoplastic disease or with a previous acute coronary syndrome or who were taking dobutamine, lived out of the geographic region, were not community living, or were without a telephone. Patients were randomized using a table of random numbers before hospital discharge. Allocation concealment was not mentioned. The physicians involved in assessment and follow-up were blinded to group assignment. Relevant primary outcomes were assessed at 6 and 24 months by 1 physician reviewing medical records. Quality of life was measured using the Medical Outcomes Study Short Form 36-Item Health Survey (SF-36), a generic health questionnaire, and the Minnesota Living with Heart Failure Questionnaire (MLWHFQ). Quality of life was ascertained at baseline by personal interview and at follow-up by telephone interview. Other baseline data were ascertained before hospital discharge. The discharging physician was blinded to group assignment. The analysis did not mention intent-to-treat. There were no baseline differences. The sample size for examining the SF-36 and MLWHFQ was reduced.
A randomized controlled clinical trial conducted in Barcelona, Spain, examined the effectiveness of a home-based intensive intervention program in comparison with usual care in heart failure patients. (29) The primary outcome was combined all-cause mortality and hospitalizations due to worsening of heart failure. The secondary outcomes relevant to this evidence-based analysis were cardiovascular death, hospitalizations due to cardiovascular disease, quality of life, and patient satisfaction. Eligible individuals were hospitalized for suspected heart failure according to the Framingham criteria and had a diagnosis of heart failure at discharge in the first or second diagnostic position. Exclusion criteria included concomitant illness and a survival prognosis of less than 1 year, a cognitive deficit, not residing in the geographic region, and clinical trial involvement in the preceding 3 months. Randomization was determined from a central data management site using a random generator and stratified by hospital. Allocation concealment was not mentioned. A standardized questionnaire ascertained information on baseline data. HRQOL was determined by the MLWHFQ. Hospital admissions and discharges were ascertained from record services. Clinical outcomes were classified by a committee blinded to group assignment. Personnel ascertaining information on HRQOL measures were aware of assignment status. Follow-up was 1 year. There was a baseline difference in the number of patients with COPD as a comorbidity (HC: 34% vs. UC: 20.1%; P = 0.01), with no other baseline differences. The analysis stated an intent-to-treat analysis. There was a reduced sample size for examining MLWHFQ.
A randomized controlled clinical trial conducted in Thailand examined the effectiveness of a home-based program on symptom alleviation and well-being in comparison with usual care in heart failure patients. (30) The primary outcome was not stated. Symptom alleviation was not considered relevant to this evidence-based analysis. Eligible patients were at least 40 years of age, with functional class II NYHA criteria, stable medication use, ability to verbally communicate, living within the designated geographic area, and not living alone. Exclusion criteria were not stated, but criteria for dropping out included the presence of severe symptoms and complications from heart or comorbid diseases. Patients were randomized but other specific details were not stated, including information on allocation concealment. At baseline and follow-up at 8 and 12 weeks, a researcher measured well-being in the home for both the intervention and the control group. There was no mention of blinding or of an intent-to-treat analysis. There were no baseline differences.
A randomized controlled clinical trial conducted in the United Kingdom examined the effectiveness of a home-based intervention delivered by community pharmacists to heart failure patients. (27) The primary outcome was unplanned hospitalizations. The secondary outcomes were all-cause mortality and HRQOL (e.g., EuroQoL and MLWHFQ). Eligible patients were over 18 years of age, were admitted to emergency departments with heart failure, and were taking 2 or more drugs at the time of discharge. Patients were excluded if living in long-term care facilities, on the waiting list for surgery for heart disease, or with a terminal malignancy. Randomization was computer generated, and patients were stratified by the NYHA class and recruitment site. Allocation concealment was achieved using a third party telephone randomization process. An intent-to-treat analysis was specified. Blinding was not mentioned. Follow-up was 6 months. There were no baseline differences except for social class and use of a drug adherence aid, with the intervention group less likely to be from a non-manual labour social class (HC: 44.1% vs. UC: 54.7%; P value not specified) but more likely to use some form of drug adherence aid (HC: 26.5% vs. 15.5%; P value not specified). Post-randomization exclusions occurred in the intervention and control groups (HC: n = 20; UC: n = 26 post-randomization exclusions).
A randomized controlled clinical trial conducted in Spain compared the clinical effectiveness of a home-based education program with that of usual care in heart failure patients. (31) The primary outcome was combined unplanned hospitalizations and all-cause mortality. Secondary outcomes were unplanned hospitalizations, all-cause mortality, LOS, and ED use. Only ED visits were examined in the first 6 months of follow-up. Eligible patients did not have severe cognitive deficits, COPD, a psychiatric illness, or other terminal disease. They lived in the geographic area and had family support. Randomization was prepared by a central site and stratified by service location of recruitment. Assignment was performed by the process of closed envelopes. The randomization sequence was concealed until after assignment. Attending personnel involved outside of in-home care were unaware of patient assignment. Follow-up was up to 12 months and data were ascertained by telephone and review of clinical records. Analysis was intent-to-treat. There was no baseline differences on factors considered to be of interest.
A randomized controlled clinical trial conducted in Australia compared the clinical effectiveness of a nurse-led home-based intervention with that of usual care in heart failure patients. (32) The primary outcome was combined unplanned hospitalizations and all-cause mortality. A secondary outcome was all-cause mortality, as described in a previous publication. (38) Eligible patients were at least 55 years of age, had cardiologist-diagnosed heart failure, a history of at least 1 hospital admission for acute heart failure, functional impairment according to NYHA class II, III, or IV, and impaired left ventricular systolic function (≤ 55% ejection fraction). Exclusion criteria were a terminal malignancy or planned cardiac surgery. Randomization occurred using a blinded computerized protocol. There was no mention of allocation concealment. Baseline data were determined through patient interviews or medical record reviews before discharge. Follow-up was a minimum of 7.5 years, and data on hospital activity and mortality were ascertained from a computerized medical record system and death registry. Outcomes were ascertained in a blinded manner. Analysis was intent-to-treat. Baseline differences noted were that the intervention group were more likely to have had a prior acute myocardial infarction (HC: 55% vs. UC: 50%; P value not shown), left bundle-branch block (HC: 32% vs. UC: 21%; P value not shown), and a higher blood urea concentration (data not shown).
COPD
A randomized controlled clinical trial conducted in Louisiana, United States, compared the effectiveness of educational support either through a home visit or reading material compared with that of usual care in patients with COPD. (33) This evidence-based analysis examined only the effects of home visits. The primary outcome was HRQOL measured by SGRQ. (Secondary outcomes, for example, health knowledge, were not relevant to this evidence-based analysis.) Individuals were 18 years or older and had spirometry-confirmed, physician-diagnosed moderate to severe COPD. Having a Grade 4 reading literacy was also considered an eligibility criterion. Exclusion criteria included congestive heart failure, asthma, and severe cognitive impairment. Randomization was performed by randomly drawn letter cards. Allocation concealment was not mentioned. Personnel were not blinded to group assignment. Length of follow-up was about 30 to 90 days (Personal communication, Clinical Expert, April 24, 2012). There was no mention of an intent-to-treat analysis. There were no baseline differences between the intervention and the control group.
Stroke
A randomized controlled clinical trial conducted in Ohio, United States, compared the effectiveness of comprehensive postdischarge care management with that of organized stroke department care without postdischarge care. (34) The primary outcome was based on 5 domains including elements of neuromotor function, days spent in an institution, quality of life, management of risk, and stroke knowledge and lifestyle modification. Relevant individual outcomes for this evidence-based analysis were all-cause mortality, mean length of hospital stay, quality of life measured by the stroke-specific scale, and physiological outcomes, all secondary outcomes. Patients were eligible if they had a confirmed ischemic stroke, National Institutes of Health Stroke Scale score of 1 or more, were discharged home, lived in the geographic region, had no other dominating illness, spoke English, and did not have an endarterectomy planned. Randomization was generated by the study biostatistician, and group assignment was performed by a research assistant using the sealed envelope method. Length of follow-up was 6 months. Outcome measures relevant to this evidence-based analysis were ascertained by medical record review or at the home visit. Additional information ascertained at the home visit by a research nurse was blinded to patient assignment. Telephone interviews were also conducted. An intent-to-treat analysis was noted. There were no baseline differences except for the percentage of patients with diabetes as a comorbidity being higher in the intervention group (HC: 42% vs. UC: 29%; P value not shown) and the mean number of hospital days in the prior year being higher for the control group (HC: 0.6, standard error (SE): 0.3 vs. UC: 2.1, SE: 0.3; P value not shown).
Multimorbidity
A randomized controlled clinical trial conducted in a rural village near Ottawa, Canada, examined the effectiveness of the Anticipatory and Preventive Team Care (ATPCare) program on quality of care for chronic disease management. (35) The ATPCare program was designed as an in-home care intervention. The primary outcome was not relevant to this evidence-based analysis. Relevant outcomes included ED visits and all-cause hospitalizations. Eligible individuals were at least 50 years of age, enrolled in the Family Health Network, and at risk of functional decline, physical deterioration, and need of emergency services. Individuals were excluded if they displayed cognitive impairment, language, or cultural barriers, were expected to live less than 6 months, and were not residing in the geographical area for the study period. A central system assigned concealed random treatment allocation. Length of follow-up was up to 18 months. Health care utilization information was ascertained from an outcome questionnaire and verified by chart audit of electronic medical records by personnel blinded to group assignment. An intent-to-treat analysis was noted. There were no baseline differences except for age, with the intervention group younger than the control group (HC: 69.6 vs. UC: 72.8 years, P = 0.018). (39;40)
A randomized controlled clinical trial conducted in Philadelphia, United States, compared the effectiveness of a home-based program that reduces declining abilities in chronically ill elderly individuals with that of usual care. (36) The primary outcome for this study was mortality; however, this study was an extension of previous work by the same investigators who had examined functional difficulties as the primary outcome at the 6-month follow-up. (37) Eligible individuals for both studies were community living, ambulatory, at least 70 years of age, English speaking, cognitively intact, and reporting 1 or more functional difficulties. There was no mention of exclusions. Randomization was generated by the project statistician and prepared using double, opaque envelopes. Randomization was performed by race and living arrangement. Length of follow-up was between 2.5 and 5.25 years for the outcome of mortality, depending on when the baseline interviews were conducted. Length of follow-up was 6 months for the primary outcome of functional difficulties. The National Death Index records were used to determine mortality. Trained interviewers were blinded to group assignment. An intent-to-treat analysis was mentioned but it was not clear how this was used when examining functional difficulties. There were no baseline differences.
Meta-Analysis
An analysis was performed to address the research question on the effectiveness of care delivered in the home (i.e., in-home care) compared with no home care or usual care / care received outside of the home (e.g., health care setting). Studies with data in a format suitable for analysis are shown below for the outcomes of combined events of all-cause mortality and hospitalizations, all-cause mortality, cardiovascular-specific mortality, unplanned hospitalizations, heart failure-specific hospitalizations, LOS, ED visits, HRQOL, and functional difficulties. When data were available, the analysis was performed by disease subgroup.
The study by Gray et al (35;40) with useable information for hospitalizations and ED visits was excluded from this evidence-based analysis because the information for hospitalizations was based on all-cause hospitalizations, rather than unplanned hospitalizations as in the other 2 studies, and ED visits were based on the assumption that every deceased patient had 1 ED visit, which was different from the other included study. (35;40) One study had information on patient satisfaction but was not included in the analysis since it did not use a validated questionnaire. (29)
The interpretation of the results differs based on the outcome measure. For consistency, a beneficial effect of in-home care appears on the left-hand side of the plots. Results are presented as a risk ratio for RCTs with dichotomous data, as a mean difference at follow-up for continuous data, or as a mean difference based on change values for the HRQOL outcomes (i.e., SF-36, MLWHFQ, SGRQ). When the sample size differed between baseline and follow-up for HRQOL measures, to be conservative the smaller of the 2 sample sizes was used. (27-(29)
The outcomes were examined and are displayed in Figures 2-16 below.
Figure 2: Combined All-Cause Mortality and Readmissions/Hospitalizationsa,b,c,d,*.
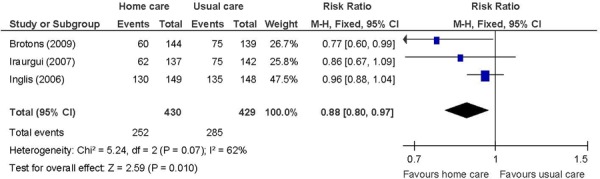
Figure 16: Instrumental Activities of Daily Livinga,b,c.
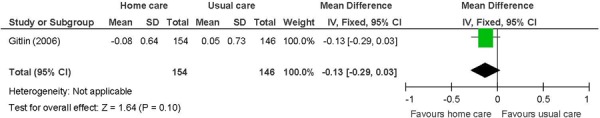
Abbreviations: CI, confidence interval; IADL, instrumental activities of daily living; SD, standard deviation.
Chronic disease multimorbid patients. (37)
Primary outcome in study. IADL include light housework, shopping, preparing meals, managing money, telephone use, and taking medications.
Change from baseline, with a negative value indicating an improvement as lower scores are favoured.
Figure 3: All-Cause Mortalitya,b.
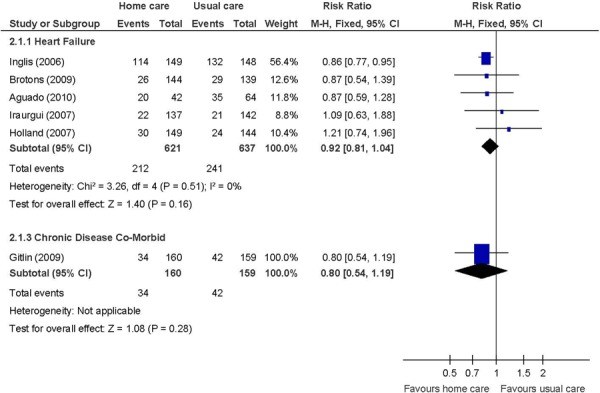
Figure 4: Cardiovascular-Specific Mortalitya,b,*.
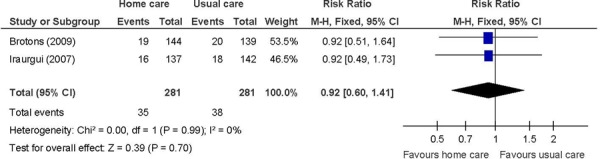
Figure 5: Unplanned Readmissions/Hospitalizationsa,b,c,d.
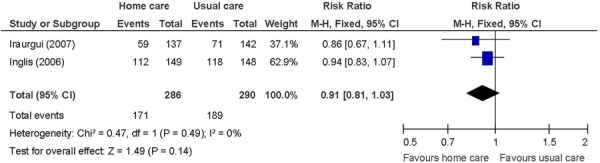
Figure 6: Heart Failure-Specific Readmissions/Hospitalizationsa,b,c.
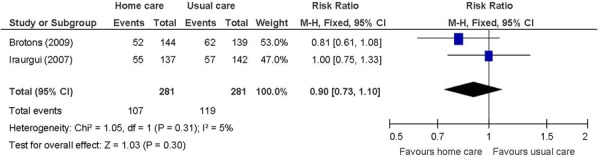
Figure 7: Mean Number of Unplanned Readmissions/Hospitalizationsa,b,c.
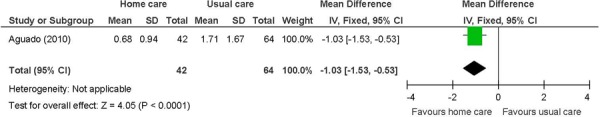
Figure 8: Mean Number of Heart Failure-Specific Readmissions/Hospitalizationsa,b,c,*.
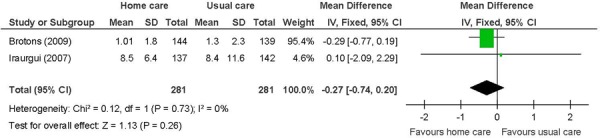
Figure 9: Mean Length of Hospital Staya,b,c.
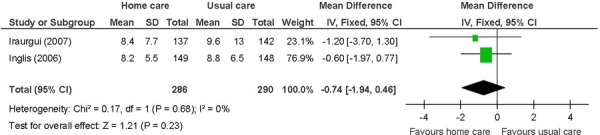
Figure 10: Mean Number of Emergency Department Visitsa,b,c.
Abbreviations: CI, confidence interval; SD, standard deviation.
Number of events.
Heart failure patients in 1 study. (28)
Not identified as a primary outcome.
Figure 11: General Well-Being (assessed using SF-36)a,b,c,d,e,f,g.
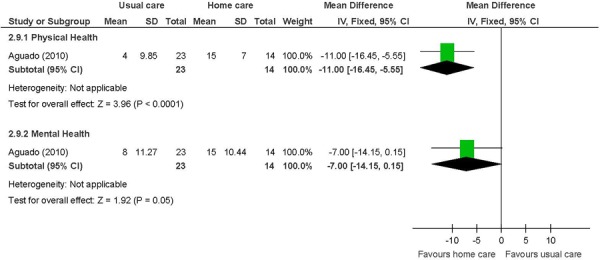
Abbreviations: CI, confidence interval; MCID, minimal clinically important difference; SD, standard deviation; SF-36, Medical Outcomes Study Short Form 36-Item Health Survey.
Heart failure patients. (28)
Not identified as a primary outcome.
Change from baseline, with a positive value indicating an improvement as higher scores are favoured.
Range for physical MCID: 10-40 points.
Range for mental MCID: 15-37.5 points.
Physical component scale includes physical functioning, role-physical, bodily pain, and general health.
Mental component scale includes vitality, social functioning, role-emotional, and mental health.
Figure 12: Heart Failure-Specific Well-Being (MLWHFQ)a,b,c,d,e.
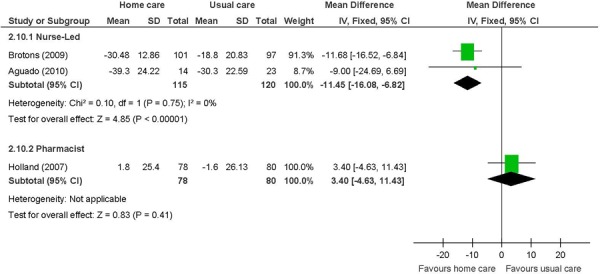
Abbreviations: CI, confidence interval; SD, standard deviation; MCID, minimal clinically important difference; MLWHFQ, Minnesota Living With Heart Failure Questionnaire.
Not identified as a primary outcome.
Change from baseline, with a negative value indicating an improvement as lower scores are favoured.
Includes questions on symptoms and signs, physical activity, social interaction, sexual activity, work, and emotions.
MCID is 5 points.
Figure 13: COPD-Specific Well-Being (SGRQ)a,b,c,d,e.
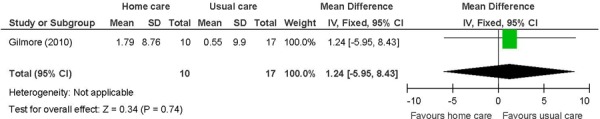
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; MCID, minimal clinically important difference; SD, standard deviation; SGRQ, St George’s Respiratory Questionnaire.
COPD patients. (33)
Primary outcome in study. (33)
Change from baseline, with a negative value indicating an improvement as lower scores are favoured.
Includes symptoms, activity, and impacts.
MCID is 4 points.
Figure 14: Activities of Daily Livinga,b,c.
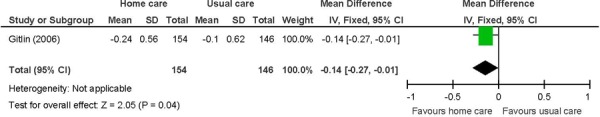
Abbreviations: CI, confidence interval; SD, standard deviation.
Chronic disease multimorbid patients. (37)
Primary outcome in study. Activities of daily living include difficulty dressing above waist or below waist, grooming, bathing/showering, toileting, and feeding.
Change from baseline, with a negative value indicating an improvement as lower scores are favoured.
Figure 15: Mobilitya,b,c.
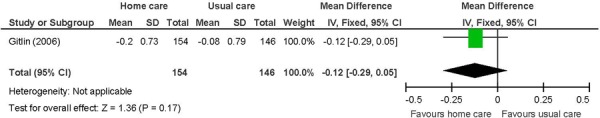
Abbreviations: CI, confidence interval; SD, standard deviation.
Chronic disease multimorbid patients. (37)
Primary outcome in study. Mobility includes getting in/out of the car, walking indoors, walking one block, climbing one flight of stairs, moving in/out of a chair, and moving in/out of bed.
Change from baseline, with a negative value indicating an improvement as lower scores are favoured.
Results of Meta-Analysis
The results of the meta-analysis show a beneficial effect of in-home care compared with usual care, without significant heterogeneity (P > 0.10) (where relevant), for the following outcomes:
Heart failure patients receiving in-home care had, on average, about one less unplanned hospitalization compared with heart failure patients receiving usual care (MD: −1.03; 95% CI: −1.53 to −0.53; P < 0.001 (I2: n/a; P = n/a)
Heart failure patients receiving in-home care had, on average, about one-and-a-half fewer ED visits compared with those receiving usual care (MD: −1.32; 95% CI: −1.87 to −0.77; P < 0.001 (I2: n/a; P = n/a)
Heart failure patients receiving in-home care were more likely to have increased HRQOL compared with those receiving usual care. A statistically significant and clinically relevant effect was shown for physical well-being (MD: −11.00, 95% CI: −16.45 to −5.55; P < 0.001), and a statistically significant and clinically relevant effect was shown for nurse-led in-home interventions on HRQOL specific to heart failure (MD: −11.45; 95% CI: −16.08 to −6.82; P < 0.001; I2: 0%, P = 0.75)
Chronic disease multimorbid patients receiving in-home care were more likely to report less difficulties in ADL compared with patients receiving usual care (MD: −0.14; 95% CI: −0.27 to −0.01; P = 0.04).
In addition,
Heart failure patients receiving in-home care were 12% less likely to experience an event of the combined of all-cause mortality and hospitalizations compared with those receiving usual care (RR: 0.88; 95% CI: 0.80−0.97; P = 0.010; I2: 62%; P = 0.07). Using a fixed effect model, heterogeneity was borderline. The point estimate remained the same and heterogeneity was not reduced when using a random effects model (RR: 0.88, 95% CI: 0.74−1.05; P = 0.15; I2: 62%; P = 0.07). The confidence interval also widened for a nonstatistically significant beneficial effect of in-home care in the latter.
The results did not show statistically significant effects of in-home care compared with results of usual care for the following outcomes:
All-cause mortality by disease category
Cardiovascular-specific mortality
Heart failure-specific hospitalizations
Length of hospital stay
Mental well-being and heart failure-specific HRQOL when in-home care was delivered by community pharmacists
HRQOL for COPD patients
Functional difficulties including mobility and IADL
These results were without significant heterogeneity (P > 0.10) (where relevant).
Qualitative Assessment
Physiological Outcomes
Two studies had information on physiological outcomes including HbA1c, SBP, DBP, and lipid levels. (26;34) One study involved diabetes patients, (26) and the other stroke patients. (34) These studies could neither be meta-analyzed together nor individually because the data in the papers were not in a useable format. For HbA1c, the study of diabetes patients showed a beneficial effect of in-home care, (26) and the study on stroke patients did not show a difference between the intervention and the control groups. (34) There were no differences between the intervention and the control groups for SBP, DBP, and lipid levels in both studies. (26;34) Overall, the benefits of in-home care were shown for lowering HbA1c in diabetes patients.
Summary of the Literature Review
In summary, education-based in-home care is effective at improving patient outcomes when it is delivered by nurses during a single home visit or on an ongoing basis to patients with a range of heart disease severity. In-home visits by occupational therapists and physical therapists targeted at modifying tasks and the home environment improved functional activities for community-living chronic disease adults.
The beneficial effect of in-home care on the combined events of all-cause mortality and hospitalizations was based on 3 studies that included heart failure patients. (29;31;32) The disease severity ranged from NYHA class II to IV in a majority of patients. The nature of the home care intervention was similar although the frequency of the home care visits differed. The length of follow-up was 1 year in 2 studies (29;31) and up to 10 years in the third. (32) Longer follow-up accounted for the higher proportion of events in the longer-term follow-up study. Overall, in-home care has a beneficial effect on the combined events of all-cause mortality and hospitalizations. The GRADE quality of evidence was moderate.
The beneficial effect of in-home care on the mean number of unplanned hospitalizations and ED visits was based on 1 study of heart failure patients. (28) The results showed unplanned hospitalizations down by 1, and ED visits down by a mean of about one-and-a-half. The standard deviations for this study were quite small. The beneficial effect of in-home care on physical well-being, assessed using the SF-36, was also based on this study. Two summary component scales, the physical and mental component scales, which are made up from the 36 questions in the 8 individual domains covered by the questionnaire, (41) were reported. A difference of 11 points is considered within the range of possible values for a minimal clinically important difference. (42) A factor contributing to the success of the in-home care intervention in this 1 study, and hence to the results, may have been the high educational level of a majority of the individuals in the intervention group (63% with a secondary school education). (28) Overall, in-home care has a beneficial effect on lowering hospital utilization and improving HRQOL. The GRADE quality of evidence was moderate quality for unplanned hospitalizations and ED visits, and low for the physical component of the SF-36.
The lack of a beneficial effect on unplanned hospitalizations, characterized as the number of events, may be due to the heterogeneity in the data provided in the 2 studies, with 1 study apparently considering the number of occasions so that each patient may contribute more than one event (32) and the other study considering only first-ever hospitalizations. (31) Imprecision may have also been a factor considering the sample size calculations. (31;32) The GRADE quality of evidence was low quality for unplanned hospitalizations when characterized as event data.
The lack of an effect on heart failure-specific hospitalizations suggests that the reasons for readmissions are due to different causes or comorbid conditions and not due to the index diagnosis. Imprecision may have also been a factor considering the sample size calculations. (29;31) The GRADE quality of evidence was low quality for heart failure-specific hospitalizations.
The beneficial effect of a nurse-led in-home care intervention on HRQOL in heart failure patients was based on 2 studies that used the MLWHFQ. (28;29) The MLWHFQ is a heart failure-specific questionnaire. It contains 21 questions that ask about symptoms and signs relevant to heart failure, physical activity, social interaction, sexual activity, work, and emotions. The maximum score is 105, with a lower score indicating better HRQOL. (41) A difference of about 12 points is considered to be beyond the specified clinically relevant change score of 5 points. (43) The result was weighted heavily on 1 study in which the nurse-led intervention was provided monthly for the duration of the 1-year study. (29) Also, the heart failure patients in this study were NYHA class IV, which may have been the population with the potential for the largest improvement in HRQOL. Overall, nurse-led in-home care has a beneficial effect on HRQOL; however, the GRADE quality of evidence was considered low quality.
The beneficial effect of in-home care on ADL was based on 1 study. (37) The ADL index is based on the mean perceived difficulty across 6 areas including dressing above the waist, dressing below the waist, grooming, bathing/showering, toileting, and feeding. Difficulty is rated on a score of 1 to 5, with higher scores indicating increased difficulty. A trend for a beneficial effect was shown for the other 2 measures of physical function including mobility and IADL; however, they did not reach statistical significance. Mobility assesses 6 areas including getting in/out of the car, walking indoors, walking one block, climbing one flight of stairs, moving in/out of a chair, and moving in/out bed. The IADL index comprises 6 areas including light housework, shopping, preparing meals, managing money, telephone use, and taking medications. The in-home care intervention of occupational therapists and physical therapists targeting task modifications and home hazards may have been more effective at improving the ADL compared with the other 2 indexes that assess challenges outside of the home and more complex activities. The clinical significance of the difference between comparison groups for ADL is not known. The GRADE quality of evidence was moderate for all 3 functional status measures.
There were no differences between the intervention and the control group for the remaining outcomes. For length of hospital stay, it was not clear whether the data in 1 study referred to the condition under study or if the duration of hospitalization was for another medical reason or referred to overall duration of hospitalization. (32) For all-cause mortality, there was no difference between the intervention and the control groups when studies were analyzed by disease category. For the mental health component of the SF-36, there was no difference between the intervention and the control groups. The mental health component is made up of vitality, social functioning, role-emotional, and mental health domains whereas the physical component is made up of physical functioning, role-physical, bodily pain, and general health domains. Therefore, the mental health component scale may be perceived as more complex, requiring as it does a more substantive intervention than nurse-led in-home care education on disease management to observe improvements.
There was no difference between the intervention and the control groups for pharmacist-led in-home care on heart failure-specific HRQOL. (27) In this 1 study, the lack of ongoing visits may have been the limiting factor although additional study design limitations including post-randomization exclusions may have had an effect. (27) There was no difference between the intervention and the control group for COPD-specific HRQOL measured by SGRQ. (33) The mean difference for the total SGRQ was 1.24 (95% CI: –5.95 to 8.43, P = 0.74) while a clinically significant change value is 4 units. (44) The confidence interval crosses the clinically significant threshold; therefore, a lack of precision may have been a limiting factor (HC, n = 10 vs. UC, n = 17 patients).
The GRADE quality of evidence for all outcomes is shown in Appendix 2.
Conclusions
Based on moderate quality of evidence, there was a beneficial effect of in-home care:
on the combined events of all-cause mortality and hospitalizations in heart failure patients;
on unplanned hospitalizations in heart failure patients;
on emergency department (ED) visits in heart failure patients;
on the functional measure of activities of daily living (ADL) in chronic ill multimorbid patients.
Based on moderate quality of evidence, there was no difference between in-home care and usual care:
for all-cause mortality in chronically ill multimorbid patients;
for the functional measure of mobility in chronically ill multimorbid patients;
for the functional measure of instrumental activities of daily living (IADL) in chronically ill multimorbid patients.
Based on low quality of evidence, there was a beneficial effect:
of in-home care on the physical component scale of the Medical Outcomes Study Short Form 36-Item Health Survey (SF-36), which assessed health-related quality of life (HRQOL) in heart failure patients;
of nurse-led in-home care on the heart failure-specific HRQOL in heart failure patients;
of in-home care on hemoglobin A1c in diabetes patients.
Based on low quality of evidence, there was no difference:
for all-cause mortality in heart failure patients;
for cardiovascular-specific mortality in heart failure patients;
for heart failure-specific hospitalizations in heart failure patients;
for length of hospital stay in heart failure patients;
between in-home care and usual care for the mental health component of the SF-36 HRQOL in heart failure patients;
between pharmacist-led in-home care and usual care for heart failure-specific HRQOL in heart failure patients;
between in-home care and usual care for the physiological measures of systolic blood pressure (SBP), diastolic blood pressure (DBP), and lipid levels in diabetes and stroke patients.
Based on indeterminate evidence, there was no difference between in-home care and usual care for chronic obstructive pulmonary disease (COPD)-specific HRQOL.
Existing Guidelines for Home Care
While there are no specific guidelines for use of in-home care in Canada, listed below are the client populations and service programs offered by the Toronto Central Community Care Access Centres that deliver home care (Personal communication, Community Expert, January 7, 2013). (5)
-
Adult*
Seniors Integrated Care
Seniors Enhanced Care (Frail Seniors*)
Community Independence Program (Seniors Independent Living*)
Adult Supportive Care
Telehomecare Program
-
Post-acute / Short-term support
Rapid Response Program*
Acute and Rehab Transitional Program
Child and Family – Long and Short Stay
End of Life
-
Urban Health (Mental Health / Homeless)
Urban Health Program
Intercity Access Program
Acquired Brain Injury Program
An asterisk indicates the programs relevant to this evidence-based analysis.
Glossary
- Advanced practice nurse
Advanced level of clinical nursing practice that includes the clinical nurse and the nurse practitioner.
- Ambulatory
Individuals who experience some difficulty with everyday living but who are not totally dependent or homebound or who are receiving services to address functional problems.
- Client
The person who is receiving home care services.
- Clinical nurse
A nurse that provides clinical guidance and nursing leadership and promotes evidence-based practice to complex care clients.
- Disease management
Coordinated multidisciplinary comprehensive care across the care continuum and specifically for chronic disease.
- Disease management program
Multidisciplinary programs that target recently hospitalized patients in an effort to optimize their longer-term management, including post-acute discharge care within the community.
- Family Health Network
A type of group practice that provides primary care services to rostered patients.
- Multidisciplinary care models
Aims to address the needs of individuals from many perspectives, e.g., medical, psychological, behavioural, and financial. Involves a team of many different health professionals who also attempt to bridge patient care from the hospital to other care delivery or the home.
- New York Heart Association Functional Classification
Ranks patients’ limitations during physical activity, e.g., class I/II: none or mild limitation; class III: moderate limitation; class IV: severe limitation.
- Nurse practitioner
Nurses who provide care in rural and remote areas that would otherwise not receive medical care and who possess the skills to diagnosis and manage disease within legislative scope.
- Rehabilitation
The physical restoration of a sick or disabled person by therapeutic measures and re-education to participation in the activities of a normal life within the limitations of the person’s physical disability.
Acknowledgements
Editorial Staff
Joanna Odrowaz, BSc (Hons)
Medical Information Services
Kaitryn Campbell, BA(H), BEd, MLIS
Kellee Kaulback, BA(H), MISt
Expert Panel for Health Quality Ontario: Optimizing Chronic Disease Management in the Community (Outpatient) Setting.
| Name | Title | Organization |
|---|---|---|
| Shirlee Sharkey (chair) | President & CEO | Saint Elizabeth Health Care |
| Theresa Agnew | Executive Director | Nurse Practitioners’ Association of Ontario |
| Onil Bhattacharrya | Clinician Scientist | Li Ka Shing Knowledge Institute, St. Michael’s Hospital, University of Toronto |
| Arlene Bierman | Ontario Women’s Health Council Chair in Women’s Health | Department of Medicine, Keenan Research Centre in the Li Ka Shing Knowledge Institute, St. Michael’s Hospital, University of Toronto |
| Susan Bronskill | Scientist | Institute for Clinical Evaluative Sciences |
| Catherine Demers | Associate Professor | Division of Cardiology, Department of Medicine, McMaster University |
| Alba Dicenso | Professor | School of Nursing, McMaster University |
| Mita Giacomini | Professor | Centre of Health Economics & Policy Analysis, Department of Clinical Epidemiology & Biostatistics |
| Ron Goeree | Director | Programs for Assessment of Technology in Health (PATH) Research Institute, St. Joseph’s Healthcare Hamilton |
| Nick Kates | Senior Medical Advisor | Health Quality Ontario – QI McMaster University Hamilton Family Health Team |
| Murray Krahn | Director | Toronto Health Economics and Technology Assessment (THETA) Collaborative, University of Toronto |
| Wendy Levinson | Sir John and Lady Eaton Professor and Chair | Department of Medicine, University of Toronto |
| Raymond Pong | Senior Research Fellow and Professor | Centre for Rural and Northern Health Research and Northern Ontario School of Medicine, Laurentian University |
| Michael Schull | Deputy CEO & Senior Scientist | Institute for Clinical Evaluative Sciences |
| Moira Stewart | Director | Centre for Studies in Family Medicine, University of Western Ontario |
| Walter Wodchis | Associate Professor | Institute of Health Management Policy and Evaluation, University of Toronto |
Appendices
Appendix 1: Literature Search Strategies
Home Care – Final Search Strategy
Database: Ovid MEDLINE(R) <1946 to January Week 3 2012>, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations <January 25, 2012>, Embase <1980 to 2012 Week 03>
Search Strategy:
_____________________________________________________________________________________
137 exp Coronary Artery Disease/ (211925)
2 exp Myocardial Infarction/ use mesz (133578)
3 exp heart infarction/ use emez (216783)
4 (coronary artery disease or cad or heart attack).ti. (44430)
5 ((myocardi* or heart or cardiac or coronary) adj2 (atheroscleros* or arterioscleros* or infarct*)).ti. (149495)
6 or/1–5 (539636)
7 exp Atrial Fibrillation/ use mesz (28093)
8 exp heart atrium fibrillation/ use emez (55436)
9 ((atrial or atrium or auricular) adj1 fibrillation*).ti,ab.(73456)
10 or/7–9 (99330)
11 exp heart failure/ (300723)
12 ((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)).ti,ab.(234410)
13 11 or 12 (381620)
14 exp Stroke/ (177913)
15 exp Ischemic Attack, Transient/ use mesz (16370)
16 exp transient ischemic attack/ use emez (19656)
17 exp stroke patient/ use emez (5632)
18 exp brain infarction/ or exp cerebrovascular accident/ use emez (100939)
19 (stroke or tia or transient ischemic attack or cerebrovascular apoplexy or cerebrovascular accident or cerebrovascular infarct* or brain infarct* or CVA).ti,ab.(281020)
20 or/14–19 (391349)
21 exp Diabetes Mellitus, Type 2/ use mesz (68223)
22 exp non insulin dependent diabetes mellitus/ use emez (101510)
23 exp diabetic patient/ use emez (12865)
24 (diabetes or diabetic* or niddm or t2dm).ti,ab.(764490)
25 or/21–24 (789402)
26 exp Skin Ulcer/ (72029)
27 ((pressure or bed or skin) adj2 (ulcer* or sore* or wound*)).ti,ab.(28663)
28 (decubitus or bedsore*).ti,ab. (8526)
29 or/26–28 (90720)
30 exp Pulmonary Disease, Chronic Obstructive/ use mesz (17049)
31 exp chronic obstructive lung disease/ use emez (54703)
32 (chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory) adj (disease* or disorder*)).ti,ab. (54430)
33 (copd or coad).ti,ab. (45643)
34 chronic airflow obstruction.ti,ab. (1063)
35 exp Emphysema/ (37422)
36 exp chronic bronchitis/ use emez (6977)
37 ((chronic adj2 bronchitis) or emphysema).ti,ab. (50825)
38 or/30–37 (159227)
39 exp Chronic Disease/ (340679)
40 ((chronic* adj2 disease*) or (chronic* adj2 ill*)).ti,ab. (219900)
41 39 or 40 (506233)
42 6 or 10 or 13 or 20 or 25 or 29 or 38 or 41 (2605524)
43 exp Home Care Services/ use mesz (36884)
44 exp home care/ use emez (46848)
45 exp home care agencies/ or exp home health aides/ use mesz (48362)
46 exp House Calls/ use mesz (2048)
47 ((home or domicil* or communit*) adj2 (visit* or care or caring or caregiver* or healthcare or assist* or aid* or agenc* or service* or rehabilitation)).ti,ab.(86989)
48 (homecare or homemaker service* or home nurs* or meals on wheels).ti,ab. (3972)
49 43 or 44 or 45 or 46 or 47 or 48 (143324)
50 42 and 49 (17054)
51 limit 50 to 42ochran language (14353)
52 limit 51 to yr=”2006 –Current” (5606)
53 limit 52 to (controlled clinical trial or meta analysis or randomized controlled trial) (690)
54 exp Technology Assessment, Biomedical/ or exp Evidence-based Medicine/ use mesz (63489)
55 exp Biomedical Technology Assessment/ or exp Evidence Based Medicine/ use emez (523373)
56 (health technology adj2 assess$).ti,ab. (3059)
57 exp Random Allocation/ or exp Double-Blind Method/ or exp Control Groups/ or exp Placebos/ use mesz (379638)
58 Randomized Controlled Trial/ or exp Randomization/ or exp RANDOM SAMPLE/ or Double Blind Procedure/ or exp Triple Blind Procedure/ or exp Control Group/ or exp PLACEBO/ use emez (901804)
59 (random* or RCT).ti,ab. (1255504)
60 (placebo* or sham*).ti,ab. (414042)
61 (control* adj2 clinical trial*).ti,ab. (35063)
62 meta analysis/ use emez (58594)
63 (meta analy* or metaanaly* or pooled analysis or (systematic* adj2 review*) or published studies or published literature or medline or embase or data synthesis or data extraction or 42ochrane).ti,ab. (252855)
64 or/53–63 (2164699)
65 52 and 64 (1348)
66 remove duplicates from 65 (960)
▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪▪
CINAHL.
| # | Query | Results |
|---|---|---|
| S43 | S39 and S42 Limiters – Published Date from: 20060101-20121231; English Language |
411 |
| S42 | S40 or S41 | 157006 |
| S41 | random* or sham*or rct* or health technology N2 assess* or meta analy* or metaanaly* or pooled analysis or (systematic* N2 review*) or published studies or medline or embase or data synthesis or data extraction or 43ochrane or control* N2 clinical trial* | 148913 |
| S40 | (MH “Random Assignment”) or (MH “Random Sample+”) or (MH “Meta Analysis”) or (MH “Systematic Review”) or (MH “Double-Blind Studies”) or (MH “Single-Blind Studies”) or (MH “Triple-Blind Studies”) or (MH “Placebos”) or (MH “Control (Research)”) | 83970 |
| S39 | S33 and S38 | 6361 |
| S38 | S34 or S35 or S36 or S37 | 66000 |
| S37 | homecare OR homemaker service* OR home nurs* OR meals on wheels | 9390 |
| S36 | ((home OR domicil* OR communit*) N2 (visit* OR care OR caring OR caregiver* OR healthcare OR assist* OR aid* OR agenc* OR service* OR rehabilitation)) | 57389 |
| S35 | (MH “Home Health Agencies”) OR (MH “Home Health Care Information Systems”) | 4318 |
| S34 | (MH “Home Health Aides”) OR (MH “Home Health Care+”) | 27543 |
| S33 | S5 or S8 or S11 or S15 or S19 or S22 or S27 or S32 | 223005 |
| S32 | S28 or S29 or S30 or S31 | 71626 |
| S31 | chronic* N2 disease* or chronic* N2 ill* | 43890 |
| S30 | comorbid* OR co-morbid* OR multimorbid* OR multi-morbid* OR (complex* N1 patient*) OR (multiple N2 (condition* OR disease* OR patient*)) | 30356 |
| S29 | (MH “Comorbidity”) | 16703 |
| S28 | (MH “Chronic Disease”) | 23713 |
| S27 | S23 or S24 or S25 or S26 | 8821 |
| S26 | chronic N2 bronchitis or emphysema | 1823 |
| S25 | (MH “Emphysema”) | 886 |
| S24 | chronic obstructive N2 disease* or chronic obstructive N2 disorder* or copd or coad | 7394 |
| S23 | (MH “Pulmonary Disease, Chronic Obstructive+”) | 5374 |
| S22 | S20 or S21 | 16228 |
| S21 | pressure N1 ulcer* or bedsore* or bed N1 sore* or skin N1 ulcer* OR pressure N1 wound* OR decubitus | 9608 |
| S20 | (MH “Skin Ulcer+”) | 14882 |
| S19 | S16 or S17 or S18 | 70413 |
| S18 | diabetes or diabetic* or niddm or t2dm | 70413 |
| S17 | (MH “Diabetic Patients”) | 3551 |
| S16 | (MH “Diabetes Mellitus, Type 2+”) | 18307 |
| S15 | S12 or S13 or S14 | 38366 |
| S14 | stroke or tia or transient ischemic attack or cerebrovascular apoplexy or cerebrovascular accident or cerebrovascular infarct* or brain infarct* or CVA | 37868 |
| S13 | (MH “Cerebral Ischemia, Transient”) | 1907 |
| S12 | (MH “Stroke”) OR (MH “Stroke Patients”) | 25741 |
| S11 | S9 OR S10 | 18910 |
| S10 | myocardi*failure OR myocardial decompensation OR myocardial insufficiency OR cardiac failure OR cardiac decompensation or cardiac insufficiency OR heart failure OR heart decompensation OR heart insufficiency | 18898 |
| S9 | (MH “Heart Failure+”) | 14423 |
| S8 | S6 OR S7 | 8118 |
| S7 | atrial N1 fibrillation* OR atrium N1 fibrillation* OR auricular N1 fibrillation* | 8118 |
| S6 | (MH “Atrial Fibrillation”) | 6503 |
| S5 | S1 OR S2 OR S3 OR S4 | 30205 |
| S4 | TI myocardi* N2 infarct* or TI heart N2 infarct* or TI cardiac N2 infarct* OR TI coronary N2 infarct* or TI arterioscleros* or TI atheroscleros* | 9678 |
| S3 | coronary artery disease OR cad OR heart attack* | 7725 |
| S2 | (MH “Myocardial Infarction+”) | 19236 |
| S1 | (MH “Coronary Arteriosclerosis”) | 4653 |
Wiley Cochrane.
| ID | Search | Hits |
|---|---|---|
| #1 | MeSH descriptor Coronary Artery Disease explode all trees | 2183 |
| #2 | MeSH descriptor Myocardial Infarction explode all trees | 7746 |
| #3 | (myocardi* or heart or cardiac or coronary) NEAR/2 (atheroscleros* or arterioscleros* or infarct*):ti or (coronary artery disease or cad or heart attack*):ti | 8469 |
| #4 | MeSH descriptor Atrial Fibrillation explode all trees | 2102 |
| #5 | (atrial NEAR/2 fibrillation* or atrium NEAR/2 fibrillation* or auricular NEAR/2 fibrillation*):ti | 2310 |
| #6 | MeSH descriptor Heart Failure explode all trees | 4710 |
| #7 | (myocardi* NEAR/2 (failure or decompensation or insufficiency)):ti or (heart NEAR/2 (failure or decompensation or insufficiency)):ti or (cardiac NEAR/2 (failure or decompensation or insufficiency)):ti | 5252 |
| #8 | MeSH descriptor Stroke explode all trees | 3899 |
| #9 | MeSH descriptor Ischemic Attack, Transient explode all trees | 466 |
| #10 | (stroke or tia or transient ischemic attack or cerebrovascular apoplexy or cerebrovascular accident or cerebrovascular infarct* or brain infarct* or CVA):ti | 9902 |
| #11 | MeSH descriptor Diabetes Mellitus, Type 2 explode all trees | 6993 |
| #12 | (diabetes or diabetic* or niddm or t2dm):ti | 16585 |
| #13 | MeSH descriptor Skin Ulcer explode all trees | 1572 |
| #14 | (pressure or bed or skin) NEAR/2 (ulcer* or sore* or wound*):ti | 669 |
| #15 | (decubitus or bedsore*):ti | 98 |
| #16 | MeSH descriptor Pulmonary Disease, Chronic Obstructive explode all trees | 1754 |
| #17 | (chronic obstructive NEAR/2 (lung* or pulmonary or airway* or airflow or respiratory):ti | 2415 |
| #18 | (copd or coad):ti | 3319 |
| #19 | (chronic airflow obstruction):ti | 72 |
| #20 | MeSH descriptor Emphysema explode all trees | 91 |
| #21 | (chronic NEAR/2 bronchitis) or emphysema:ti | 1183 |
| #22 | MeSH descriptor Chronic Disease explode all trees | 9875 |
| #23 | (chronic* NEAR/2 disease* or chronic* NEAR/2 ill*):ti | 1670 |
| #24 | MeSH descriptor Comorbidity explode all trees | 1941 |
| #25 | (comorbid* OR co-morbid* OR multimorbid* OR multi-morbid* OR (complex* NEXT patient*) OR “patient* with multiple” OR (multiple NEAR/2 (condition* OR disease*))):ti | 649 |
| #26 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25) | 68126 |
| #27 | MeSH descriptor Home Care Services explode all trees | 1872 |
| #28 | MeSH descriptor Home Care Agencies explode all trees | 7 |
| #29 | MeSH descriptor Home Health Aides explode all trees | 17 |
| #30 | MeSH descriptor House Calls explode all trees | 215 |
| #31 | ((home or domicil* or communit*) NEAR/2 (care or caring or caregiver* or healthcare or assist* or aid* or agenc* or service* or rehabilitation)):ti or ((home or domicil* or communit*) NEAR/2 (care or caring or caregiver* or healthcare or assist* or aid* or agenc* or service* or rehabilitation)): ab | 2169 |
| #32 | (homecare or homemaker service*):ti and (homecare or homemaker service*):ab | 8 |
| #33 | (#27 OR #28 OR #29 OR #30 OR #31 OR #32) | 3650 |
| #34 | (#26 AND #33), from 2006 to 2012 | 335 |
CRD.
| Line | Search | Hits | ||
|---|---|---|---|---|
| ❐ | 1 | MeSH DESCRIPTOR coronary artery disease EXPLODE ALL TREES | 230 | Delete |
| ❐ | 2 | (coronary artery disease or cad or heart attack*):TI | 213 | Delete |
| ❐ | 3 | ((myocardi* or heart or cardiac or coronary) adj2 (atheroscleros* or arterioscleros* or infarct*)):TI | 224 | Delete |
| ❐ | 4 | MeSH DESCRIPTOR Atrial Fibrillation EXPLODE ALL TREES | 225 | Delete |
| ❐ | 5 | (((atrial or atrium or auricular) adj 1 fibrillation*):TI | 0 | Delete |
| ❐ | 6 | ((atrial or atrium or auricular) adj 1 fibrillation*):TI | 168 | Delete |
| ❐ | 7 | MeSH DESCRIPTOR heart failure EXPLODE ALL TREES | 418 | Delete |
| ❐ | 8 | ((myocardi* or heart or cardiac) adj2 (failure or decompensation or insufficiency)):TI | 280 | Delete |
| ❐ | 9 | MeSH DESCRIPTOR stroke EXPLODE ALL TREES | 549 | Delete |
| ❐ | 10 | MeSH DESCRIPTOR Ischemic Attack, Transient EXPLODE ALL TREES | 32 | Delete |
| ❐ | 11 | (stroke or tia or transient ischemic attack or cerebrovascular apoplexy or cerebrovascular accident or cerebrovascular infarct* or brain infarct* or CVA):TI | 622 | Delete |
| ❐ | 12 | MeSH DESCRIPTOR Diabetes Mellitus, Type 2 EXPLODE ALL TREES | 511 | Delete |
| ❐ | 13 | (diabetes or diabetic* or niddm or t2dm):TI | 1223 | Delete |
| ❐ | 14 | MeSH DESCRIPTOR Skin Ulcer EXPLODE ALL TREES | 253 | Delete |
| ❐ | 15 | ((pressure or bed or skin) adj2 (ulcer* or sore* or wound*)):TI | 73 | Delete |
| ❐ | 16 | ( decubitus or bedsore*): TI | 0 | Delete |
| ❐ | 17 | MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive EXPLODE ALL TREES | 237 | Delete |
| ❐ | 18 | (chronic obstructive adj2 (lung* or pulmonary or airway* or airflow or respiratory)): TI | 219 | Delete |
| ❐ | 19 | (copd or coad):TI | 108 | Delete |
| ❐ | 20 | (chronic airflow obstruction):TI | 0 | Delete |
| ❐ | 21 | MeSH DESCRIPTOR Emphysema EXPLODE ALL TREES | 10 | Delete |
| ❐ | 22 | ((chronic adj2 bronchitis) or emphysema):TI | 47 | Delete |
| ❐ | 23 | MeSH DESCRIPTOR Chronic Disease EXPLODE ALL TREES | 687 | Delete |
| ❐ | 24 | ((chronic* adj2 disease*) or (chronic* adj2 ill*)):TI | 252 | Delete |
| ❐ | 25 | MeSH DESCRIPTOR Comorbidity EXPLODE ALL TREES | 146 | Delete |
| ❐ | 26 | (comorbid* OR co-morbid* OR multimorbid* OR multi-morbid* OR (complex* adj 1 patient*) OR “patient* with multiple” OR (multiple adj2 (condition* OR disease*))):TI | 22 | Delete |
| ❐ | 27 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 | 4656 | Delete |
| ❐ | 28 | MeSH DESCRIPTOR home care services EXPLODE ALL TREES | 375 | Delete |
| ❐ | 29 | MeSH DESCRIPTOR home care agencies EXPLODE ALL TREES | 1 | Delete |
| ❐ | 30 | MeSH DESCRIPTOR home health aides EXPLODE ALL TREES | 2 | Delete |
| ❐ | 31 | MeSH DESCRIPTOR house calls EXPLODE ALL TREES | 32 | Delete |
| ❐ | 32 | (((home or domicil* or communit*) adj2 (visit* or care or caring or caregiver* or healthcare or assist* or aid* or agenc* or service* or rehabilitation))) FROM 2006 TO 2012 | 785 | Delete |
| ❐ | 33 | #28 OR #29 OR #30 OR #31 OR #32 | 1057 | Delete |
| ❐ | 34 | #27 AND #33 | 190 | Delete |
| ❐ | 35 | #27 AND #33 | 190 | Delete |
Appendix 2: GRADE Tables
Table A1: GRADE Evidence Profile for Comparison of In-Home Care and Usual Care: Mortality.
| No. Of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| All-cause mortality – heart failure patients | |||||||
| 5 (RCTs) | Serious limitations (-1)a | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | n/a | ⊕⊕ Low |
| All-cause mortality – chronic disease | |||||||
| 1 (RCTs) | No serious limitations | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | n/a | ⊕⊕⊕ Moderate |
| Combined all-cause mortality and hospitalizations | |||||||
| 3 (RCTs) | Serious limitations (-1)c | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕⊕ Moderate |
| Cardiovascular-specific mortality | |||||||
| 2 (RCTs) | Serious limitations (-1)c | No serious limitations | No serious limitations | Serious limitations (-1)b | Undetected | n/a | ⊕⊕ Low |
Table A2: GRADE Evidence Profile for Comparison of In-Home Care and Usual Care: Hospital Utilization.
| No. Of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Unplanned hospitalizations | |||||||
| 2 (RCTs) | Serious limitations (-1)c | No serious limitations | No serious limitations | Serious limitations(-1)b | Undetected | n/a | ⊕⊕ Low |
| Heart failure-specific hospitalizations | |||||||
| 2 (RCTs) | Serious limitations (-1)c | No serious limitations | No serious limitations | Serious limitations(-1)b | Undetected | n/a | ⊕⊕ Low |
| Mean number of unplanned hospitalizations | |||||||
| 1 (RCTs) | Serious limitations (-1)c | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕⊕ Moderate |
| Mean number of heart failure-specific hospitalizations | |||||||
| 2 (RCTs) | Serious limitations (-1)c | No serious limitations | No serious limitations | Serious limitations(-1)b | Undetected | n/a | ⊕⊕ Low |
| Length of stay | |||||||
| 2 (RCTs) | Serious limitations (-1)c | No serious limitations | No serious limitations | Serious limitations(-1)b | Undetected | n/a | ⊕⊕ Low |
| Mean number of emergency department visits | |||||||
| 1 (RCT) | Serious limitations (-1)c | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕⊕ Moderate |
Table A3: GRADE Evidence Profile for Comparison of In-Home Care and Usual Care: Health-Related Quality of Life and Functional Status.
| No. Of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| General well-being – physical | |||||||
| 1 (RCT) | Very serious limitations (–2)d | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕ Low |
| General well-being – mental | |||||||
| 1 (RCT) | Very serious limitations (–2)d | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕ Low |
| Heart failure-specific well-being – nurse-led | |||||||
| 2 (RCTs) | Very serious limitations (–2)e | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕ Low |
| Heart failure-specific well-being – pharmacist | |||||||
| 1 (RCT) | Very serious limitations (–2)f | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕ Low |
| COPD-specific well-being | |||||||
| 1 (RCT) | Very serious limitations (–2)g | No serious limitations | No serious limitations | Very serious limitations (–2)g | Undetected | n/a | Indeterminate |
| Activities of daily living | |||||||
| 1 (RCT) | Serious hlimitations (–1)h | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕⊕ Moderate |
| Mobility | |||||||
| 1 (RCT) | Serious limitations (–1)h | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕⊕ Moderate |
| Instrumental activities of daily living | |||||||
| 1 (RCT) | Serious limitations (–1)h | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕⊕ Moderate |
Table A4: GRADE Evidence Profile for Comparison of In-Home Care and Usual Care: Physiological Measures.
| No. Of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Hemoglobin A1c | |||||||
| 2 (RCTs) | Very serious limitations (–2)d | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕ Low (Qualitative assessment)i |
| Systolic blood pressure | |||||||
| 2 (RCTs) | Very serious limitations (–2)d | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕ Low (Qualitative assessment)i |
| Diastolic blood pressure | |||||||
| 2 (RCTs) | Very serious limitations (–2)d | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕ Low (Qualitative assessment)i |
| Lipids (low density lipoprotein and total cholesterol) | |||||||
| 2 (RCTs) | Very serious limitations (–2)d | No serious limitations | No serious limitations | No serious limitations | Undetected | n/a | ⊕⊕ Low (Qualitative assessment)i |
Abbreviations: RCT, randomized controlled trial.
Allocation concealment was not identified and post-randomization exclusions may have biased results.
Imprecision based on sample size calculation.
Allocation concealment was not identified.
Allocation concealment was not identified and losses to follow-up may have biased results.
Allocation concealment was not identified, lack of blinding, and losses to follow-up may have biased results.
Lack of blinding and post-randomization exclusions may have biased results.
Lack of blinding and allocation concealment was not identified, imprecision (small sample size and confidence interval crosses threshold).
Losses to follow-up may have biased results.
Unable to meta-analyze results across the 2 studies.
Appendix 3: Summary Tables
Table A5: Summary of Study Characteristics (N = 12 Studies).
| Author, Year | Study Location | Cohort | Study Design | Length of Follow-Up (Length of Interventiona) | HC / UC | Losses to Follow-Up (HC / UC) |
|---|---|---|---|---|---|---|
| Spencer et al, 2011 (26) | Medical records, Detroit, USA | T2 DM | Parallel RCT | 6 mo (6 mo) | 84 / 99 | 56 / 57b |
| Aguado et al, 2010 (28) | University hospital, Spain | HF | Parallel RCT | Up to 2 y (n/a) | 42 / 64 | –c |
| Gilmore et al, 2010 (33) | Outpatient clinic, Louisiana, USA | COPD | FT RCT | 1–3 mo (n/a) | 10/17d | – |
| Gray et al, 2010 (35) | FHT, Ottawa, Canada | Chronice | Parallel RCT | 1–1.5 y (12–18 mo) | 120 / 121 | – |
| Allen et al, 2009 (34) | Acute care, Ohio, USA | Strokef | Parallel RCT | 6 mo (6 mo) | 190 / 190 | –g |
| Brotons et al, 2009 (29) | 4 hospitals (U+C), Spain | HF | Parallel RCT | 1 y (1 y) | 144 / 139 | 144 / 138c |
| Gitlin et al, 2009 (36) | Community, Philadelphia, USA | Chronic | Parallel RCT | Up to 5.25 y (6 mo) | 160 / 159 | – |
| Wongpiriyayothar et al, 2008 (30) | Hospital clinic, Thailand | HF | Parallel RCT | Up to 3 mo (n/a) | 48 / 48 | 48 / 45 |
| Holland et al, 2007 (27) | 3 hospitals, UK | HF | Parallel RCT | 6 mo (n/a) | 169 / 170 | 148 / 143c,h |
| Iraurqui et al, 2007 (31) | Tertiary care hospital, Spain | HF | Parallel RCT | 1 y (n/a) | 137 / 142 | – |
| Gitlin et al, 2006 (37) | Community, Philadelphia, USA | Chronic | Parallel RCT | 6 (6 mo) | 160 / 159 | 154 / 146 |
| Inglis et al, 2006 (32) | Tertiary centre, Australia | HF | Parallel RCT | Up to 10 y (n/a)i | 149 / 148 | – |
Abbreviations: C, community hospital; chronic, chronic disease; COPD, chronic obstructive pulmonary disease; FHT, Family Health Team; FT, factorial RCT; HbA1c, hemoglobin A1c; HC, home care; HF, heart failure; HRQOL, health-related quality of life; mo, month; RCT, randomized controlled trial; T2 DM, type 2 diabetes mellitus; U, University hospital; UC, usual care; y, year.
Length of intervention information may be indicated by n/a if the HC intervention was a single visit or a few visits (e.g., 2–3 visits), and refers to the application of the intervention and does not refer to longer-term surveillance (e.g., the addition of telephone follow-up).
Reduced sample size for HbA1c, primary outcome. This is the number with complete data at baseline and 6-month follow-up.
Reduced sample size for HRQOL outcome (Aguado et al, 2010 (28), HC: 14 / UC: 23; Brotons et al, 2009 (29), HC: 101 / UC: 97; Holland et al, 2007 (27), HC: 78 / 80).
Study subjects after losses to follow-up.
For this particular study, 4 chronic diseases were specified: coronary artery disease, diabetes, congestive heart failure, COPD.
Ischemic stroke.
Reduced sample size for physiologic measures (HC: 175 / UC: 163).
Post-randomization exclusions (HC: 149 / UC: 144), plus reduced sample size at the end of follow-up (HC: 148 / UC: 143).
Reduced length of follow-up for the primary outcome of combined all-cause mortality and hospitalizations and separately for hospitalizations, median: 4.2 y, range: 3 to 6 y.
Table A6: Detailed Description of Home Care Intervention (N = 12 Studies).
| Author, Year | Components of Home Care | Type of Providersa | Frequency | Duration |
|---|---|---|---|---|
| Spencer et al, 2011 (26) | Promotion of healthy lifestyle and DM self-management education activities + 1 TC / 2 wks CHWs also provided community DM education classes and escorted PCP clinic visits, in-home care: goal setting, progress support, communication skills, facilitated referrals |
CHWs / family health advocates | 2 visits / mo | 60 min |
| Aguado et al, 2010 (28) | Education in relevant aspects of disease and self-management Elements of education included patient’s habits, understanding of medication, and preventive activities |
2 physician-trained nurses | 1 visit | 2 h |
| Gilmore et al, 2010 (33) | Educational support for disease management and evaluation of the patient’s general health environment Structured assessment form to summarize ADL, medications, and living space; evaluation of the home environment, medication access, and family or personnel assistance |
Respiratory therapist | 1 visit | 20–30 min |
| Gray et al, 2010 (35) | To ensure disease management and strong social supports + TC Patient care plan priorities based on 5 dimensions of care: disease management, medical review, education and self-care, social support and community integration, psychological issues Providers working with family physicians, educational classes, 22 patients received a telehealth / remote monitoring of clinical factors |
3 nurse practitioners, pharmacist | NP for 18 mo, P for 12 mob as needed | 1 h for NP |
| Allen et al, 2009 (34) | Comprehensive assessment, PT as needed, education for lifestyle modification, medication use, social services, education to recognize signs and symptoms of recurrence, self-management + 1 TC / wk (1st mo) and then 1 TC / mo (up to 6 mo) | Advanced practice nurse care manager | Initial visit and then as necessary | 1–2 h |
| Brotons et al, 2009 (29) | Intensive, including disease education, warning symptom recognition, assessment of medication adherence and lifestyle habits, medical history review, functional status and vital sign examination + TC / 15 days Additional information provided prior to hospital discharge, worked with PCP or cardiologist |
Nurses | Monthly | 40–45 min |
| Gitlin et al, 2009 (36) and Gitlin et al, 2006 (37) | Aimed to compensate for declining abilities by home environment and task performance modifications during the active phase (6 mo) + 3 TC (OT) during the maintenance phase (6–12 mo) OTs for environmental barriers and support, goal setting, cognitive, behavioural, and environmental strategies; PTs for balance and muscle strength exercises and fall recovery techniques |
OT, PT | OT: 4 + 1 TC, plus PT: 1 (active phase), 1 final OT visit (maintenance phase) | OT: 90 min, PT: 90 min |
| Wongpiriyayothar et al, 2008 (30) | Patient education and plan to enhance patient’s symptom monitoring and management skills + 2 TC / weekly Educational booklet also provided, coaching strategies used |
Advanced practice nurse | 2 visits 1 week apart | 1st: 2 h, 2nd: 45–60 min |
| Holland et al, 2007 (27) | Patient education on disease, medication, healthy lifestyle, signs and symptoms, removed discontinued drugs, educational booklet Worked with PCP and local pharmacist for use of drug adherence aid; community pharmacists were not independent prescribers to modify drug regimen; standardized visit form |
17 community pharmacists | 2 visits | 1st: 72 min 2nd: 50 min |
| Iraurqui et al, 2007 (31) | Educational program about disease facts and management (symptoms, lifestyle, diet, therapy), with special emphasis Home attention included physician visits and clinical exam, tests and analyses when needed therapeutic review; information manual, TC available for queries |
Nurses | 3 visits @ 2, 5, 10 days | 1 hr |
| Inglis et al, 2006 (32) | Comprehensive assessment, physical exam, reviewed medication adherence and disease knowledge, assessed social supports, remedial counselling, strategies, and monitoring action + TC at 6 mo (routine and surveillance) Report shared with PCP and cardiologist, community pharmacist contacted to help manage medications |
Nurse and P, or cardiac nurse | 1 visit | 60–90 min |
Abbreviations: ADL, activities of daily living; CHW, community health workers; DM, diabetes; HC, home care; h, hours; min, minutes; mo, months; NP, nurse practitioner; OT, occupational therapist; P, pharmacist; PCP, primary care provider; PT, physical therapist; TC, telephone call; wks, weeks.
Type of providers who delivered the in-home care.
Intervention period reduced to 12 months for those recruited last.
Table A7: Detailed Summary of Study Design Characteristics (N = 12 Studies).
| Author, Year | Study Population | Description of HC / UC | Results | Other Comments |
|---|---|---|---|---|
| Spencer et al, 2011 (26) | ≥ 18 y, physician dx T2 DM, AA or L/H, geographic defined, identified from MR | HC: Culturally defined HB CHW intervention for T2 DM in low income inner city AA and Latinos UC: Contacted once per mo to update contact information |
Mean age: 52.5 y; high school graduate: 60%; insulin use: 28% % change from baseline, at 6 mo, HbA1c, HC: n = 56, –0.8 (–1.2, –0.4, P < 0.01)a vs. UC: n = 57, 0.0 (–0.4, 0.4, ns)a; LDL, HC: n = 51, –10 (–17, –2, P < 0.05)a vs. UC: n = 55, –4 (–12, 4, ns)a; SBP, HC: n = 54, –2 (–6, 2, ns)a vs. UC: n = 65, –3 (–6, 1, ns)a; DBP, HC: n = 54, 0 (–3, 3, ns)a vs. UC: n = 65, –2 (–5, 1, ns)a |
Community living, all participants received REACH related to living a healthy lifestyle and diet, and at designated health care facilities; LFU 28/164 (17.1%) |
| Aguado et al, 2010 (28) | Patients admitted to hospital with systolic HF, class II to IV NYHA and < 45% on EC or in prior 6 mo | HC: HB education visit for discharged HF patients UC: no educational component Both: conventional discharge care and outpatient care by attending physicians |
Mean age: 77.6 y; secondary school education: 63%; NYHA class II: 46%; comorbidities, hypertension (59%), DM (39%), COPD (31%), CVA (15%) At 24 mo, all-cause mortality, HC: 20/42 (46.7%) vs. 35/64 (55.4%), P = 0.448; mean (SD) ED visits, HC: 0.68 (0.90) vs. UC: 2.00 (1.97), P = 0.001; mean (SD) unplanned hospitalizations, HC: 0.68 (0.94) vs. UC: 1.71 (1.67), P = 0.003; mean (SD) MLWHFQ score, HC: 11.9 (10.5) vs. UC: 18.3 (16.2); mean (SD) SF-36 physical score, HC: 50 (5) vs. UC: 44 (3); mean (SD) SF-36 mental score, HC: 52 (7) vs. UC: 44 (6) |
Intervention 1 week after discharge; LFU for outcome of HRQOL, HC: 28/42 (66.6%) vs. UC: 41/64 (64%), compliance with medication in HB group; 0 LFU for primary study outcomes; reduced SS for HRQOL, HC: 14 and UC: 23 |
| Gilmore et al, 2010 (33) | ≥ 18 y, confirmed spirometry, physician dx COPD, moderate to severe by GOLD, ≥ 4th grade reading literacy | HC: HB education visit for moderate to severe COPD UC: clinic visit with no educational component Both: information on medication use, physician initiated patient education related to inhalers and indications for medications |
Mean age: 58 y; mean (SD) education: 10.4 (2.5) y; mean (SD) FEW 45.2% (15.7) At 30–90 days, mean (SD) overall SGRQ change from baseline, HC: 1.79 (8.76) vs. UC: 0.55 (9.9) (ns) |
Outpatient pulmonary clinic, designed to examine education support by both a standardized home visit and COPD educational guide, additional information of SGRQ domains, additional information on knowledge and self-efficacy, LFU, 10/37 (27%) |
| Gray et al, 2010(35) | ≥ 50 y, at risk of functional decline, physical deterioration, or needing emergency services | HC: HB team care program (APTCare) UC: usual medical care Both: PCP visits |
Mean age: 72 y; high school education or higher: 61%; mean number of chronic conditionsb, HC: 2.7 vs. UC: 2.4 (without SDs) Mean (95% CI) ED visits, HC: 7.84 (6.9–8.8) vs. UC: 7.81 (6.9–8.7); mean (95% CI) all-cause hospitalizations, HC: 0.40 (0.3–0.5) vs. 0.46 (0.3–0.6) (without SDs) |
Community living, primary outcome: composite of quality of care for 4 chronic conditions of CAD, DM, HF, COPD (152 of 241 (63.1%) had 1 of 4 chronic conditions), mean LFU: 14.3 mo, additional information on appointments with physicians and day surgeries; ED visits: deceased patients were assumed to have each had 1 ED visit |
| Allen et al, 2009(34) | Ischemic stroke dx, NIHSS ≥ 1, discharged home, geographic region, no other dominant illness, English speaking, no planned endarterectomy | HC: comprehensive care management UC: organized stroke department care Both: UC and enhanced discharge planning |
Mean age: 68 y; diabetes: 36%; mean number of comorbidities: 0.7 At 6 mo, all-cause mortality, HC: 9/190 (4.5%) vs. UC: 7/190 (3.5%) (ns); mean LOS, HC: 1.6 vs. UC: 1.4a days; mean HRQOL total score, HC: 196 vs. UC: 199; % HbA1c > 6.5%, HC: 28.3 vs. 22.8; % SBP > 140 mm Hg, HC: 31.5 vs. UC: 30.0; % DBP > 90 mm Hg, HC: 5.6 vs. UC: 5.2; % total CHL > 180 mg/dL, HC: 35.4 vs. UC: 30.8 |
Intervention within 1 week of discharge; outcomes selected to reflect the process of care management – 5 domains; no SDs for HRQOL and physiological measures; HC: 175 / UC: 163 for physiological outcomes |
| Brotons et al, 2009 (29) | Hospitalized for suspected HF per Framingham, HF dx at hospital discharge in 1st or 2nd position (any age) | HC: intensive HB care UC: referred to PCP and/or cardiologist |
Mean (SD) age: 76.3 (8.2) y; NYHA class IV at hospitalizations, 51%; comorbidities, hypertension (76%), DM (42%), COPD (27%), with baseline differences for COPD At 1 y, combined, HC: 60/144 (41.7%) vs. UC: 75/138 (54.3%), P = 0.043; all-cause mortality, HC: 26/144 (18.1%) vs. 29/138 (21%) (ns); CVD mortality, HC: 19/144 (13.2%) vs. UC: 20/138 (14.5%); HF hospitalizations, HC: 52/144 (36.1%) vs. UC: 62/138 (44.9%) (ns); mean HF hospitalizations, HC: 1.01 vs. UC: 1.30 (ns) (without SDs); mean (SD) MLWHFQ score, HC: 18.57 (13.1) vs. UC: 31.11 (23.9), P < 0.001 |
Monthly visits after discharge; reduced sample size for HRQOL: 198 (70.2%); additional information on patient satisfaction and adherence to treatment; combined: hospitalization due to worsening of HF |
| Gitlin et al, 2009 (36) and Gitlin et al, 2006 (37) | Community-living adults, ambulatory, ≥ 70 y, cognitively intact, ≥ 1 functional difficulties, English speaking | HC: ABLE program UC: Home safety education booklet at study end |
Mean age: 79 y; less than a high school education: 31%, high school education: 32.3%, more than a high school education: 36.7%; comorbidities, hypertension (71%), CVD (39%), DM (23%) Up to 5.25 y, all-cause mortality, HC: 34/160 (21.3%) vs. UC: 42/159 (26.4%) At 6 mo, ADL, HC: 1.58 (0.54) vs. UC: 1.66 (0.63), P = 0.03a; mobility, HC: 2.35 (0.72) vs. UC: 2.41 (0.80), P = 0.15; IADL, HC: 1.97 (0.69) vs. UC: 2.07 (0.77), P = 0.04 [HC: n = 154 / UC:n = 146] |
Risk groups created by mortality risk, ↑ scores indicate ↑ risk, mean of 7 health conditions, additional information on 6 and 12 mo measures of fear of falling, functional self-efficacy, home hazards, and control-oriented strategies |
| Wongpiriyayothar et al, 2008 (30) | HF, ≥ 40 y, class II NYHA, stable medication use, ability to communicate, geographic area, not living alone | HC: HB program on symptom alleviation and well-being UC: HF booklet at end of study follow-up Both: received care from hospital health care providers |
Mean age: 60 y; finished primary school: 89% At 12 weeks, mean SF-36 physical score, HC: 78.3 vs. UC: 60.4, P < 0.001; mean SF-36 mental score, HC: 77.7 vs. UC: 58.6, P < 0.001 (without SDs) |
Intervention within 1 week of outpatient visit; not clear what is the primary outcome; additional information on symptom severity, as described in the text: many patients had comorbid diseases and > 1 CVD dx, no baseline info on NYHA |
| Holland et al, 2007 (27) | HF, > 18 y, taking ≥ 2 drugs | HC: HB community pharmacist-led UC: usual care |
Mean age: 77 y; NYHA class III, HC: 34.9% vs. UC: 32.6%; NYHA class IV, HC: 32.2% vs. UC: 34% At 6 mo, all-cause mortality, 30/149 (20.1%) vs. UC: 24/144 (16.6%), P = 0.54; mean (SD) MLWHFQ score, HC: 47.7 (26.3), n = 78 vs. UC: 44.5 (27.9), n = 80 (P = 0.32) |
Intervention within 2 weeks of discharge, post-randomization exclusions (HC: 20, UC: 26), additional information on EQ-5D and drug adherence |
| Iraurqui et al, 2007 (31) | HF, no COPD, severe cognitive deficits, psychiatric, or terminal disease, family support, geographic area | HC: HB educational program UC: PCP Both: PCP |
Mean age: 75.8 y; primary schooling or less: 89%; comorbidities, hypertension (68%), DM (36%) At 1 y, combined CI, HC: 62/137 (45.3%) vs. 75/142 (52.8%), P = 0.232; all-cause mortality CI, HC: 22/137 (16.1%) vs. 21/142 (14.8%), P = 0.769; CVD mortality, HC: 16/137 (11.7%) vs. UC; 18/142 (12.7%) (ns); unplanned hospitalization CI, HC: 59/137 (43.1%) vs. UC: 71/142 (50%), P = 0.280; mean (SD) hospitalizations, HC: 8.6 (7.2) vs. UC: 10.1 (12.9) (ns); mean (SD) HF hospitalizations, HC: 8.5 (6.4) vs. UC: 8.4 (11.6); mean (SD) LOS, HC: 8.4 (7.7) vs. UC: 9.6 (13) days (ns); ED visits, HC: 59/137 (43.1%) vs. UC: 57/142 (40.1%) (ns); HF ED visits, HC: 7/137 (5.1%) vs. 10/142 (7%) (ns) |
Intervention up to 15 days later; subgroup analysis with emphasis on non-adherers |
| Inglis et al, 2006 (32) | ≥ 55 y, HF dx, class II, III, IV NYHA, impaired systolic function (≤ 55%), hx ≥ 1 admission for acute HFc | HC: HB care UC: PCP and outpatient care Both: postdischarge planning, PCP, outpatient cardiology review |
Mean age: 75 y; NYHA class II, HC: 47% vs. UC: 44%; NYHA class III, HC: 45% vs. UC: 45%; comorbidities, hypertension (58%), COPD (36%), DM (29%) At 7.5 y, all-cause mortality, HC: 114/149 (76.5%) vs. 132/148 (89.1%), P = 0.0006; up to 10 y, mean (SD) LOS, HC: 8.2 (5.5) vs. UC: 8.8 (6.5) days (ns) At a median of 4.2 y, combined, HC: 130/149 (87%) vs. UC: 135/148 (91%); unplanned hospitalizations, HC: 112/149 (75%) vs. UC: 118/148 (80%) (ns) |
Minimum follow-up of 7.5 y, and up to 10 y, mean Charlson index score, HC: 2.9 (1.4) vs. UC: 2.8 (1.4), additional outcome information (e.g., median, event-free, hospital survival) |
Abbreviations: AA, African American; ABLE, Advancing Better Living for Elders; ADL, Activities of Daily Living; APTCare, Anticipatory and Preventive Team Care; CHW, community health workers; COPD, chronic obstructive pulmonary disease; CHL, cholesterol; CVD, cardiovascular disease; DBP, diastolic blood pressure; EC, echocardiography; dx, diagnosed; DM, diabetes mellitus; ED, emergency department; EQ-5D, EuroQoL; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; HB, home-based; HbA1c; hemoglobin A1c; HC, home care; HF, heart failure; HRQOL, health-related quality of life; hx, history; IADL, Instrumental Activities of Daily Living; LFU, length of follow-up; L/H, Latino/Hispanic; LOS, length of stay; mo, months; MLWHFQ, Minnesota Living With Heart Failure Questionnaire; MR, medical records; ns, nonsignificant; NIHSS, National Institutes of Health Stroke Scale; NYHA, New York Heart Association; PCP, primary care physician; QoL, quality of life; REACH, Racial and Ethnic Approaches to Community Health; SBP, systolic blood pressure; SD, standard deviation; SF-36, Medical Outcomes Study Short Form Health Survey; SGRQ, St George’s Respiratory Questionnaire; SS, sample size; T2 DM, type 2 diabetes mellitus; UC, usual care; y, years.
Adjusted for covariates.
Chronic conditions included diabetes, congestive heart failure, chronic anxiety, depression, or other mental illnesses, chronic obstructive pulmonary disease, coronary artery disease, neurologic conditions, hypertension, anemia, arthritis or back problems, cancer, asthma, cerebrovascular disease, ischemic heart disease, atrial fibrillation, peripheral vascular disease.
Acute HF defined as pulmonary congestion/edema and acute dyspnea at rest.
Table A8: Summary of Study Outcomes (Primary and Secondary) by Chronic Disease Population for Included Studies (N = 12 Studies).
| Clinical | Other | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | Combineda | All-cause Mortality | HF Mortality | All-cause HP | HF HP | LOS | ED Visits | HF ED Visits | HrQOL | Disease-specific | Functional status |
| Heart Failure | |||||||||||
| Aguado et al, 2010 (28) | ✓b | ✓b | ✓ | ✓b | ✓ | ||||||
| Brotons et al, 2009 (29) | ✓b | ✓ | ✓ | ✓ | ✓ | ||||||
| Wongpiriyayothar et al, 2008c(30) | ✓ | ||||||||||
| Holland et al, 2007 (27) | ✓ | ✓b | ✓ | ||||||||
| Iraurqui et al, 2007 (31) | ✓b | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Inglis et al, 2006 (32) | ✓b | ✓ | ✓ | ✓ | ✓ | ||||||
| Stroke | |||||||||||
| Allen et al, 2009d (34) | ✓ | ✓ | ✓ | ✓ | |||||||
| COPD | |||||||||||
| Gilmore et al, 2010 (33) | ✓b | ||||||||||
| T2 DM | |||||||||||
| Spencer et al, 2011 (26) | ✓b | ||||||||||
| Chronic | |||||||||||
| Gray et al, 2010d (35) | ✓ | ✓ | |||||||||
| Gitlin et al, 2009e (36) | ✓ | ||||||||||
| Gitlin et al, 2006 (37) | ✓b | ||||||||||
Abbreviations: COPD, chronic obstructive pulmonary disease; ED, emergency department; HF, heart failure; HP, hospitalizations; HRQOL, health-related quality of life; LOS, length of stay; T2 DM, type 2 diabetes mellitus.
Combined outcome of unplanned all-cause hospitalizations and all-cause mortality, except for Brotons (2009), (29) which included hospitalizations due to worsening of heart failure.
Primary outcome(s). Sample size calculation based on hospitalizations for Aguado et al, 2010 (28).
Primary outcome is not known.
Primary outcome was not relevant to this evidence-based analysis.
Primary outcome was based on a previous analysis of functional difficulties, self-efficacy, and fear of falling at 6 and 12 months.
Table A9: Risk of Bias for 12 Randomized Controlled Trials for the Comparison of Home Care versus Usual Care.
| Author, Year | Allocation Concealmenta | Blindingb | Complete Accounting of Patients and Outcome Eventsc | Selective Reporting Bias | Other Limitations |
|---|---|---|---|---|---|
| Spencer et al, 2011 (26) | Limitations | No limitations | Limitations | – | SSC? |
| Aguado et al, 2010 (28) | Limitations | No limitations | No limitations/Limitations | – | – |
| Gilmore et al, 2010 (33) | Limitations | Limitations | No limitations | – | – |
| Gray et al, 2010 (35) | No limitations | Limitations | No limitations | – | – |
| Allen et al, 2009 (34) | No limitations | No limitations | No limitations/Limitations | – | Baseline differenced |
| Brotons et al, 2009 (29) | Limitations | No limitations/Limitations | No limitations/Limitations | – | Baseline differenced |
| Gitlin et al, 2009 (36) | No limitations | No Limitations | No limitations | – | SSCe |
| Wongpiriyaythar et al, 2008 (30) | Limitations | Limitations | No Limitations | – | Primary outcome? |
| Holland et al, 2007 (27) | No limitations | No limitations/Limitations | Limitationsf | – | – |
| Iraurqui et al, 2007 (31) | No limitations | No limitations | No Limitations | – | – |
| Gitlin et al, 2006 (37) | No limitations | No limitations | Limitations | – | – |
| Inglis et al, 2006 (32) | Limitations | No limitations | No limitations | – | Baseline differencesd |
Abbreviations: CHL, cholesterol; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HRQOL, health-related quality of life; LDL, low density lipoprotein; SBP, systolic blood pressure; SSC, sample size calculation.
Absence of information.
Spencer et al, 2011 (26), abstraction of HbA1c from medical records was not performed by personnel unaware of group assignment; Brotons et al, 2009 (29), no limitations for primary clinical outcomes, possible limitations for secondary outcome, HRQOL; Holland et al, 2007 (27), lack of blinding is a limitation for HRQOL outcome but not for mortality outcome; Gitlin et al, 2009 (36), no limitations for mortality outcome.
Complete accounting of patients refers to losses to follow-up being described, and for outcome events, having performed an intent-to-treat analysis. Losses to follow-up may have biased results [HbA1c, SBP, DBP, LDL: Spencer et al, 2011 (26); HRQOL: Aguado et al, 2010 (28), Brotons et al, 2009 (29); HbA1c, SBP, DBP, total CHL: Allen et al, 2009 (34)].
Baseline differences: Allen et al, 2009 (34), in terms of percent with diabetes and mean hospital days in previous year; Brotons et al, 2009 (29), in terms of COPD; Inglis et al, 2006 (32), in terms of prior acute myocardial infarction, left bundle-branch block, and blood urea concentration.
Sample size calculation based on a previous study of the same patients, with the primary outcomes of the previous study not included in the current study.
Post-randomization exclusions is a source of bias.
Suggested Citation
This report should be cited as follows: Health Quality Ontario. In-home care for optimizing chronic disease management in the community: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2013 September;13(5):1-65. Available from: http://www.hqontario.ca/en/documents/eds/2013/full-report-OCDM-in-home-care.pdf.
Indexing
The Ontario Health Technology Assessment Series is currently indexed in MEDLINE/PubMed, Excerpta Medica/EMBASE, and the Centre for Reviews and Dissemination database.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to: EvidenceInfo@hqontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Conflict of Interest Statement
All reports in the Ontario Health Technology Assessment Series are impartial. There are no competing interests or conflicts of interest to declare.
Peer Review
All reports in the Ontario Health Technology Assessment Series are subject to external expert peer review. Additionally, Health Quality Ontario posts draft reports and recommendations on its website for public comment prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
About Health Quality Ontario
Health Quality Ontario (HQO) is an arms-length agency of the Ontario government. It is a partner and leader in transforming Ontario’s health care system so that it can deliver a better experience of care, better outcomes for Ontarians and better value for money.
Health Quality Ontario strives to promote health care that is supported by the best available scientific evidence. HQO works with clinical experts, scientific collaborators and field evaluation partners to develop and publish research that evaluates the effectiveness and cost-effectiveness of health technologies and services in Ontario.
Based on the research conducted by HQO and its partners, the Ontario Health Technology Advisory Committee (OHTAC) — a standing advisory sub-committee of the HQO Board — makes recommendations about the uptake, diffusion, distribution or removal of health interventions to Ontario’s Ministry of Health and Long-Term Care, clinicians, health system leaders and policy-makers.
This research is published as part of Ontario Health Technology Assessment Series, which is indexed in CINAHL, EMBASE, MEDLINE, and the Centre for Reviews and Dissemination. Corresponding OHTAC recommendations and other associated reports are also published on the HQO website. Visit http://www.hqontario.ca for more information.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, HQO and/or its research partners reviews the available scientific literature, making every effort to consider all relevant national and international research; collaborates with partners across relevant government branches; consults with clinical and other external experts and developers of new health technologies; and solicits any necessary supplemental information.
In addition, HQO collects and analyzes information about how a health intervention fits within current practice and existing treatment alternatives. Details about the diffusion of the intervention into current health care practices in Ontario add an important dimension to the review. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social, and legal issues relating to the intervention assist in making timely and relevant decisions to optimize patient outcomes.
The public consultation process is available to individuals and organizations wishing to comment on reports and recommendations prior to publication. For more information, please visit: http://www.hqontario.ca/en/mas/ohtac_public_engage_overview.html.
Disclaimer
This report was prepared by HQO or one of its research partners for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research. It also incorporates, when available, Ontario data and information provided by experts and applicants to HQO. It is possible that relevant scientific findings may have been reported since completion of the review. This report is current to the date of the literature review specified in the methods section, if available. This analysis may be superseded by an updated publication on the same topic. Please check the HQO website for a list of all publications: http://www.hqontario.ca/en/mas/mas_ohtas_mn.html.
Health Quality Ontario
130 Bloor Street West, 10th Floor
Toronto, Ontario
M5S 1N5
Tel: 416-323-6868
Toll Free: 1-866-623-6868
Fax: 416-323-9261
Email: EvidenceInfo@hqontario.ca
ISSN 1915-7398 (online)
ISBN 978-1-4606-1238-5 (PDF)
© Queen’s Printer for Ontario, 2013
List of Tables
| Table 1: Body of Evidence Examined According to Study Design |
| Table A1: GRADE Evidence Profile for Comparison of In-Home Care and Usual Care: Mortality |
| Table A2: GRADE Evidence Profile for Comparison of In-Home Care and Usual Care: Hospital Utilization |
| Table A3: GRADE Evidence Profile for Comparison of In-Home Care and Usual Care: Health-Related Quality of Life and Functional Status |
| Table A4: GRADE Evidence Profile for Comparison of In-Home Care and Usual Care: Physiological Measures |
| Table A5: Summary of Study Characteristics (N = 12 Studies) |
| Table A6: Detailed Description of Home Care Intervention (N = 12 Studies) |
| Table A7: Detailed Summary of Study Design Characteristics (N = 12 Studies) |
| Table A8: Summary of Study Outcomes (Primary and Secondary) by Chronic Disease Population for Included Studies (N = 12 Studies) |
| Table A9: Risk of Bias for 12 Randomized Controlled Trials for the Comparison of Home Care versus Usual Care |
List of Figures
| Figure 1: Citation Flow Chart |
| Figure 2: Combined All-Cause Mortality and Readmissions/Hospitalizationsa,b,c,d, |
| Figure 3: All-Cause Mortalitya,b |
| Figure 4: Cardiovascular-Specific Mortalitya,b,* |
| Figure 5: Unplanned Readmissions/Hospitalizationsa,b,c,d |
| Figure 6: Heart Failure-Specific Readmissions/Hospitalizationsa,b,c |
| Figure 7: Mean Number of Unplanned Readmissions/Hospitalizationsa,b,c |
| Figure 8: Mean Number of Heart Failure-Specific Readmissions/Hospitalizationsa,b,c,* |
| Figure 9: Mean Length of Hospital Staya,b,c |
| Figure 10: Mean Number of Emergency Department Visitsa,b,c |
| Figure 11: General Well-Being (assessed using SF-36)a,b,c,d,e,f,g |
| Figure 12: Heart Failure-Specific Well-Being (MLWHFQ)a,b,c,d,e |
| Figure 13: COPD-Specific Well-Being (SGRQ)a,b,c,d,e |
| Figure 14: Activities of Daily Livinga,b,c |
| Figure 15: Mobilitya,b,c |
| Figure 16: Instrumental Activities of Daily Livinga,b,c |
List of Abbreviations
- CCAC
Community Care Access Centre
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- DBP
Diastolic blood pressure
- ED
Emergency department
- HbA1c
Hemoglobin A1c
- HC
Home care
- HQO
Health Quality Ontario
- HRQOL
Health-related quality of life
- LDL
Low density lipoprotein
- LOS
Length of stay
- MD
Mean difference
- MLWHFQ
Minnesota Living With Heart Failure Questionnaire
- NPHS
National Population Health Survey
- OHTAC
Ontario Health Technology Advisory Committee
- RCT
Randomized controlled trial
- RR
Relative risk
- SBP
Systolic blood pressure
- SD
Standard deviation
- SE
Standard error
- SF-36
Medical Outcomes Study Short Form 36-Item Health Survey
- SGRQ
St George’s Respiratory Questionnaire
- UC
Usual care
Footnotes
Iraurgui is used throughout the text as a shortened form of the name Aldamiz-Echevarria Iraurgui.
References
- 1.Coyte PC, McKeever P, et al. Home care in Canada: passing the buck. Can J Nurs Res. 2001 Sep;33(2):11–25. [PubMed] [Google Scholar]
- 2.Health Canada. Home care in Canada 1999: an overview [Internet] [[updated 2010 Nov 3; cited 2012 Jun 13]]. Available from: http://www.hc-sc.gc.ca/hcs-sss/pubs/home-domicile/1999-home-domicile/index-eng.php .
- 3.Health Council of Canada. Seniors in need, caregivers in distress: what are the home care priorities for seniors in Canada? [Internet]. Toronto, ON: Health Council of Canada; 2012 Apr 1. [[cited 2012 Jun 22]. 64 p. ISBN 978-1-926961-38-5]. Available from: http://www.healthcouncilcanada.ca/tree/HCC_HomeCare_FA.pdf .
- 4.Rochon PA, Bronskill SE, Gruneir A, Liu B, Johns A, Lo AT, et al. Older women’s health. In: Bierman AS, editor. [Toronto: Joint publication of the Institute for Clinical Evaluative Sciences; 2011 [cited 2012 Jun 26]. 188 p];Project for an Ontario women’s health evidence-based report [Internet] Available from: http://www.powerstudy.ca/sites/powerstudy.ca/files/older_womens_health.pdf . [Google Scholar]
- 5.Toronto Central Community Care Access Centre. Transforming the experience of clients and caregivers. Toronto: Toronto Central Local Health Integration Network. [2011 [cited 2012 Jun 12]. 20 p]. Available from: http://www.ccacont.ca/Upload/toronto/General/TC%20CCAC%20Annual%20Report2010_11.pdf .
- 6.Ministry of Health and Long-Term Care (Health Data Branch). intelliHEALTH ONTARIO [Internet] [[updated 2012; cited 2013 Jan 31]]. Available from: www.intellihealth.moh.gov.on.ca .
- 7.Holland R, Battersby J, Harvey I, Lenaghan E, Smith J, Hay L. Systematic review of multidisciplinary interventions in heart failure. Heart. 2005 Jul;91(7):899–906. doi: 10.1136/hrt.2004.048389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor SJ, Bestall JC, Cotter S, Falshaw M, Hood SG, Parsons S, et al. Clinical service organisation for heart failure. Cochrane Database of Syst Rev 2005, Issue 2. doi: 10.1002/14651858.CD002752.pub2. Art.No.: CD002752. DOI: 10.1002/14651858.CD002752.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonseth J, Guallar-Castillon P, Banegas JR, Rodriguez-Artalejo F. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: a systematic review and meta-analysis of published reports. Eur Heart J. 2004 Sep;25(18):1570–95. doi: 10.1016/j.ehj.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Langhorne P, Widen-Holmqvist L, et al. Early Supported Discharge Trialists. Early supported discharge after stroke. J Rehabil Med. 2007;39(2):103–8. doi: 10.2340/16501977-0042. [DOI] [PubMed] [Google Scholar]
- 11.Trachtenberg M, Ryvicker M. Research on transitional care: from hospital to home. Home Healthc Nurse. 2011 Nov;29(10):645–51. doi: 10.1097/NHH.0b013e318234175d. [DOI] [PubMed] [Google Scholar]
- 12.Utens CM, Goossens LM, Smeenk FW, van Schayck OC, van Litsenburg W, Janssen A, et al. Effectiveness and cost-effectiveness of early assisted discharge for chronic obstructive pulmonary disease exacerbations: the design of a randomised controlled trial. BMC Public Health. 2010 Oct 18;10:618. doi: 10.1186/1471-2458-10-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira DS, Maltais F, Bourbeau J. Home-based pulmonary rehabilitation in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16(2):134–43. doi: 10.1097/MCP.0b013e32833642f2. [DOI] [PubMed] [Google Scholar]
- 14.Wingham J, Dalal HM, Sweeney KG, Evans PH. Listening to patients: choice in cardiac rehabilitation. Eur J Cardiovasc Nurs. 2006;5(4):289–94. doi: 10.1016/j.ejcnurse.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Review Manager (RevMan) [Computer program]. Copenhagen (DK): The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.. Version 5.1. [Google Scholar]
- 16.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011 Apr;64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Harrison MB, Graham ID, Lorimer K, Vandenkerkhof E, Buchanan M, Wells PS, et al. Nurse clinic versus home delivery of evidence-based community leg ulcer care: a randomized health services trial. BMC Health Serv Res. 2008 Nov 26;8:243. doi: 10.1186/1472-6963-8-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart S, Carrington MJ, Marwick TH, Davidson PM, MacDonald P, Horowitz JD, et al. Impact of home versus clinic-based management of chronic heart failure: the WHICH? (Which Heart Failure Intervention Is Most Cost-Effective & Consumer Friendly in Reducing Hospital Care) multicenter, randomized trial. J Am Coll Cardiol. 2012 Oct 2;60(14):1239–48. doi: 10.1016/j.jacc.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Goodman C. Literature searching and evidence interpretation for assessing health care practices. [1996. 81p. SBU Report No. 119E];Stockholm (SE): Swedish Council on Technology Assessment in Health Care. doi: 10.1017/s0266462300008321. [DOI] [PubMed] [Google Scholar]
- 20.Wong CX, Carson KV, Smith BJ, Wong CX, Carson KV, Smith BJ. Home care by outreach nursing for chronic obstructive pulmonary disease. [Art. No.: CD000994. DOI: 10.1002/14651858.CD000994.pub2];Cochrane Database of Syst Rev 2011, Issue 3. doi: 10.1002/14651858.CD000994.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Markle-Reid M, Browne G, Weir R, Gafni A, Roberts J, Henderson SR. The effectiveness and efficiency of home-based nursing health promotion for older people: a review of the literature. Med Care Res Rev. 2006 Oct;63(5):531–69. doi: 10.1177/1077558706290941. [DOI] [PubMed] [Google Scholar]
- 22.Jonsdottir H. Nursing care in the chronic phase of COPD: a call for innovative disciplinary research. J Nurs Healthc Chronic Illn. 2008 Jul;17(7B):272–90. doi: 10.1111/j.1365-2702.2007.02271.x. [DOI] [PubMed] [Google Scholar]
- 23.McCusker J, Verdon J. Do geriatric interventions reduce emergency department visits? A systematic review. J Gerontol A Biol Sci Med Sci. 2006;61A(1):53–62. doi: 10.1093/gerona/61.1.53. [DOI] [PubMed] [Google Scholar]
- 24.Raman G, DeVine D, Lau J. Non-pharmacological interventions for post-discharge care in heart failure. [2008 [cited 2012 Jun 26]. 113 p];Rockville (MD): Tufts-New England Medical Center Evidence-based Practice Centre. Available from: http://www.ahrq.gov/clinic/ta/hospdischrg/hospdischg.pdf . [Google Scholar]
- 25.Bernabei R, Landi F, Gambassi G, Sgadari A, Zuccala G, Mor V, et al. Randomised trial of impact of model of integrated care and case management for older people living in the community. BMJ. 1998 May 2;316(7141):1348–51. doi: 10.1136/bmj.316.7141.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer MS, Rosland AM, Kieffer EC, Sinco BR, Valerio M, Palmisano G, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health. 2011;101(12):2253–60. doi: 10.2105/AJPH.2010.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holland R, Brooksby I, Lenaghan E, Ashton K, Hay L, Smith R, et al. Effectiveness of visits from community pharmacists for patients with heart failure: HeartMed randomised controlled trial. BMJ. 2007;334(7603):1098–101. doi: 10.1136/bmj.39164.568183.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguado O, Morcillo C, Delas J, Rennie M, Bechich S, Schembari A, et al. Long-term implications of a single home-based educational intervention in patients with heart failure. Heart Lung. 2010;39(6):S14–22. doi: 10.1016/j.hrtlng.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Brotons C, Falces C, Alegre J, Ballarin E, Casanovas J, Cata T, et al. Randomized clinical trial of the effectiveness of a home-based intervention in patients with heart failure: the IC-DOM study. Rev Esp Cardiol. 2009;62(4):400–8. doi: 10.1016/s1885-5857(09)71667-6. [DOI] [PubMed] [Google Scholar]
- 30.Wongpiriyayothar A, Pothiban L, Liehr P, Senaratana W, Sucumvang K. Effects of home-based care program on symptom alleviation and well-being among persons with chronic heart failure. Thai J Nurs Res. 2008 Jan;12(1):25–39. [Google Scholar]
- 31.Aldamiz-Echevarría Iraurgui B, Muñiz J, Rodríguez-Fernández JA, Vidán-Martínez L, Silva-César M, Lamelo-Alfonsín F, et al. [Randomized controlled clinical trial of a home care unit intervention to reduce readmission and death rates in patients discharged from hospital following admission for heart failure]. Rev Esp Cardiol. 2007 Jan 1;60(9):914–22. doi: 10.1157/13109644. [DOI] [PubMed] [Google Scholar]
- 32.Inglis SC, Pearson S, Treen S, Gallasch T, Horowitz JD, Stewart S. Extending the horizon in chronic heart failure: effects of multidisciplinary, home-based intervention relative to usual care. Circulation. 2006;114(23):2466–73. doi: 10.1161/CIRCULATIONAHA.106.638122. [DOI] [PubMed] [Google Scholar]
- 33.Gilmore TW, Walter RE, Davis TC, Wissing DR. Educational strategies to improve quality of life in patients with COPD. Resp Care Educ Ann. 2010;19(Fall):13–31. [Google Scholar]
- 34.Allen K, Hazelett S, Jarjoura D, Hua K, Wright K, Weinhardt J, et al. A randomized trial testing the superiority of a postdischarge care management model for stroke survivors. J Stroke Cerebrovasc Dis. 2009;18(6):443–52. doi: 10.1016/j.jstrokecerebrovasdis.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray D, Armstrong CD, Dahrouge S, Hogg W, Zhang W. Cost-effectiveness of anticipatory and preventive multidisciplinary team care for complex patients: evidence from a randomized controlled trial. Can Fam Physician. 2010 Jan;56(1):e20–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Gitlin LN, Hauck WW, Dennis MP, Winter L, Hodgson N, Schinfeld S. Long-term effect on mortality of a home intervention that reduces functional difficulties in older adults: results from a randomized trial. J Am Geriatr Soc. 2009;57(3):476–81. doi: 10.1111/j.1532-5415.2008.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gitlin LN, Winter L, Dennis MP, Corcoran M, Schinfeld S, Hauck WW. A randomized trial of a multicomponent home intervention to reduce functional difficulties in older adults. J Am Geriatr Soc. 2006 May;54(5):809–16. doi: 10.1111/j.1532-5415.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- 38.Stewart S, Horowitz JD. Home-based intervention in congestive heart failure: long-term implications on readmission and survival. Circulation. 2002 Jun 18;105(24):2861–6. doi: 10.1161/01.cir.0000019067.99013.67. [DOI] [PubMed] [Google Scholar]
- 39.Dahrouge S, Hogg W, Lemelin J, Liddy C, Legault F. Methods for a study of Anticipatory and Preventive multidisciplinary Team Care in a family practice. Can Fam Physician. 2010 Feb;56(2):e73–83. [PMC free article] [PubMed] [Google Scholar]
- 40.Hogg W, Lemelin J, Dahrouge S, Liddy C, Armstrong CD, Legault F, et al. Randomized controlled trial of anticipatory and preventive multidisciplinary team care: for complex patients in a community-based primary care setting. Can Fam Physician. 2009 Dec;55(12):e76–85. [PMC free article] [PubMed] [Google Scholar]
- 41.Berry C, McMurray J. A review of quality-of-life evaluations in patients with congestive heart failure. Pharmacoeconomics. 1999 Sep;16(3):247–71. doi: 10.2165/00019053-199916030-00003. [DOI] [PubMed] [Google Scholar]
- 42.Wyrwich KW, Spertus JA, Kroenke K, Tierney WM, Babu AN, Wolinsky FD. Clinically important differences in health status for patients with heart disease: an expert consensus panel report. Am Heart J. 2004 Apr;147(4):615–22. doi: 10.1016/j.ahj.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 43.Rector TS, Tschumperlin LK, Kubo SH, Bank AJ, Francis GS, McDonald KM, et al. Use of the Living With Heart Failure questionnaire to ascertain patients’ perspectives on improvement in quality of life versus risk of drug-induced death. J Card Fail. 1995 Jun;1(3):201–6. doi: 10.1016/1071-9164(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 44.Jones PW, Spencer S, Adie S. The St George Respiratory Questionnaire manual version 2.1. London, U.K.: Respiratory Medicine, St George’s Hospital Medical School. 2003 May;29:1–16 p. [Google Scholar]


