Abstract
Purpose
The present study examines the impact of typical aging and Parkinson’s disease (PD) on the relationship among breath pausing, syntax, and punctuation.
Methods
Thirty young adults, 25 typically aging older adults, and 15 individuals with PD participated. Fifteen participants were age- and sex-matched to the individuals with PD. Participants read a passage aloud two times. Utterance length, location of breath pauses relative to punctuation and syntax, and number of disfluencies and mazes were measured.
Results
Older adults produced shorter utterances, a smaller percentage of breaths at major boundaries, and a greater percentage of breaths at minor boundaries than young adults, but there was no significant difference between older adults and individuals with PD on these measures. Individuals with PD took a greater percentage of breaths at locations unrelated to a syntactic boundary than control participants. Individuals with PD produced more mazes than control participants. Breaths were significantly correlated with punctuation for all groups.
Conclusions
Changes in breath pausing patterns in older adults are likely due to changes in respiratory physiology. However, in individuals with PD, such changes appear to result from a combination of changes to respiratory physiology and cognition.
INTRODUCTION
Successful communication involves not only motor plans and movements of the speech system but also language planning and cognitive resources. Since speech is an overlaid function, speakers must balance metabolic demands of oxygen/carbon dioxide exchange with speech production (Bunn & Mead, 1971; Hoit, Lansing, & Perona, 2007). Balancing physiology with language involves, among other things, deciding when to pause to inhale (breath pause).
In speech, pauses can be used to mark the ends of prosodic phrases, changes in intonation, and syllable duration (Schirmer, Alter, Kotz, & Friederici, 2001; Steinhauer, 2003). Typical adults produce longer pauses at major prosodic boundaries (e.g., boundary of a group of into national phrases) than at minor ones (e.g., boundary for a single into national phrase) (Price, Ostendorf, Schattuck-Hufnagel, & Fong, 1991). Although prosodic structure is not the same as syntactic structure (Ferreira, 1993; Gee & Grosjean, 1983), prosodic boundaries typically coincide with syntactic boundaries (Price et al., 1991; Warren, 1996) with the longest pauses often occurring at major syntactic boundaries (Price et al., 1991). Similarly, it has been shown that, during speech, adults take more breaths at major syntactic boundaries (e.g., after independent and dependent clauses) than at minor syntactic boundaries (e.g, after a prepositional phrase) (Grosjean & Collins, 1979; Wang, Kent, Duffy, & Thomas, 2005; Winkworth, Davis, Ellis, & Adams, 1994).
This pattern of pausing longer and more often at major syntactic boundaries has importance from the perspective of successful communication. Listeners use pauses along with other prosodic cues to parse syntactic and semantic units in running speech. For example, Price and colleagues (1991) found that listeners could reliably disambiguate syntactically ambiguous sentences using only prosodic cues (syllable duration, pause duration, and boundary tones). In fact, some studies have suggested that pausing is more important to understanding syntactically complex sentences than pitch contour (Price et al., 1991; Shah, Baum, & Dwivedi, 2006). In an elicited production task, Shah and colleagues (2006) found that typical speakers produced longer pauses and longer syllable durations at syntactic boundaries but less consistently produced a falling intonation pattern at the same boundaries. Despite the lack of consistent into national cues, listeners were accurate in understanding the intended meaning of structurally ambiguous sentences from the production task. Interestingly, in the same study, individuals with left-hemisphere brain damage were found to insert pauses in syntactically inappropriate locations in some sentence types, resulting in lower listener accuracy in determining the intended meaning of those sentence types. Thus breath pauses that occur at syntactically inappropriate locations may contribute to poor intelligibility and reduced naturalness of speech (Hammen & Yorkston, 1994).
Although written language lacks acoustic markers of prosody such as pause duration and pre-boundary lengthening, research suggests that punctuation in written language functions similarly to prosody in spoken language. There is a relationship between commas in written language and prosodic boundaries in spoken language. In silent reading, longer pauses are taken at major syntactic boundaries than at minor ones (Stine, 1990). Further, commas elicit a similar event-related brain potential (ERP) response to that elicited by prosodic boundary marks (including pauses) in spoken language perception (Steinhauer, 2003). Similarly, in reading aloud, readers generally place prosodic breaks and breath pauses at locations with punctuation, with more significant breaks occurring at a period than at a comma. For example, it has been found that young adults tend to breathe more often at a period than at a comma when reading aloud (Conrad, Thalacker, & Schonle, 1983). As with spoken prosody, punctuation in written language is not the same as syntax but corresponds closely to syntax, with periods representing independent clause boundaries and commas representing dependent clause boundaries or minor syntactic boundaries such as between items in a list. Thus, the results from Conrad and colleagues (1983) match closely with those of other studies of breath pausing in spoken sentence production.
While the research discussed above has demonstrated a clear relationship between syntax and breath pausing in young adults, only a few studies have attempted to define this relationship in older adults and disordered speakers, such as individuals with Parkinson’s disease (PD). It is likely that this relationship may be altered in older adults and individuals with PD given the documented changes to the physiological properties of the respiratory system as a result of typical aging and disease (Bode, Dosman, Martin, Ghezzo, & Macklem, 1976; Frank, Mead, & Ferris, 1957; Huber, 2008; Huber & Darling, 2011; Mittman, Edelman, Norris, & Shock, 1965; Sadagopan & Huber, 2007; Solomon & Hixon, 1993). Typical aging and PD may also result in changes to cognition which could impact language skills and lead to problems planning breath pauses with language formulation (Birren & Schaie, 2001; Hayes, Davidson, Keele, & Rafal, 1998; Mather, 2010; Oliveira, Gurd, Nixon, Marshall, & Passingham, 1997; Sommers & Danielson, 1999; Tun, O’Kane, & Wingfield, 2002; Zgaljardic, Borod, Foldi, & Mattis, 2003; Zgaljardic et al., 2006). Although most studies have focused on comprehension of language rather than production (Kemper & Mitzner, 2001; Sommers & Danielson, 1999), some studies suggest that limitations in working memory and reduced inhibitory mechanisms may impair language skills relevant for production. Stine and colleagues have shown that during silent reading, older adults tended to pause for less time at the end of sentences and pause longer at minor syntactic boundaries than younger adults (Stine, 1990; Stine, Cheung, & Henderson, 1995). They suggest that these age-related differences in silent reading may be related to reduced working memory in older adults; the entire sentence is too long for older adults to maintain in working memory, and so they parse the sentence at minor boundaries, breaking it into smaller chunks (Stine, 1990; Stine et al., 1995). If a similar effect of working memory was found to be present for speech production, older adults would be expected to pause more often at minor boundaries and less often at major boundaries than young adults do. Looking specifically at production, Kemper and colleagues have shown a decline in syntactic complexity in spoken and written language with increasing age in healthy aging and have related the decline to working memory (Kemper, 1987; Kemper, Marquis, & Thompson, 2001). Results from a controlled production task similarly showed evidence for capacity-based limitations on the length, complexity, and content of older adults’ sentence productions (Kemper, Herman, & Chiung-Ju, 2004).
The impact of cognitive impairments on language has not been well studied in individuals with PD. However, some of the cognitive impairments in individuals with PD are similar to those seen with typical aging, although more severe. Thus, it is likely that cognitive impairments in individuals with PD also contribute to language impairments. Specifically, limitations in working memory, reduced inhibitory mechanisms, and difficulties with set switching are likely to play a role in impaired language skills (Brown & Marsden, 1990; Hayes et al., 1998). Individuals with PD have also been shown to have subtle language processing difficulties, in particular related to syntactic processing, which may make it more difficult for them to plan breath pauses with syntax (Friederici, Kotz, Werheid, Hein, & von Cramon, 2003; Grossman et al., 1993; Grossman et al., 2000; Grossman, Lee, Morris, Stern, & Hurtig, 2002; Hochstadt, 2009).
Several studies have demonstrated that individuals with dysarthria take breaths more often at minor syntactic locations than control participants (Bunton, 2005; Hammen & Yorkston, 1994; Wang et al., 2005). However, due to differences in the classification of breath pauses, the type of speech task used, and the variety of dysarthria types studied, the magnitude of the differences in breath pausing patterns between speakers with dysarthria and control participants varies widely across studies. Hammen & Yorkston (1994) demonstrated that, in a reading task, individuals with a variety of neurological disorders affecting speech production, including seven individuals with PD, took breaths at sentence boundaries only 39% of the time, as compared to 74% for control participants, a significant difference. Individuals with dysarthria took breaths within phrases or clauses 23% of the time, as compared to 2% for the control participants, also significantly different. Bunton (2005) found somewhat different results for conversational speech, though the group differences were in the same direction. Individuals with PD took breaths at structural boundaries between 50 and 71% of the time in conversational speech, while control subjects took breaths at structural boundaries between 75 and 87% of the time (Bunton, 2005). Bunton did not analyze these data statistically. Wang and colleagues (2005) obtained quite different results in their study of conversational speech by individuals with traumatic brain injury and a range of dysarthria characteristics. The reported that 9 out of 12 of their subjects took breaths at locations comparable to the control participants, but no statistical analysis was completed. Further, they reported high inter-subject variability with the percent of breaths taken at “appropriate breath locations” ranging from 0%–99% (Wang et al., 2005, p. 71). However, the variability Wang and colleagues reported may be due to the wide range of dysarthria types and speech impairment severity levels represented in the study patient sample.
The current literature on breath pausing and syntax is limited in that, to our knowledge, no studies have systematically investigated the relationship between breath pausing and syntax in typical older adults. In fact, studies that have included older adults have only included these subjects as the control group and did not contrast their data with that of a younger adult group. Although we do gain some information about older adults’ breath pausing patterns from the control group data, it is important to understand how older adults’ breath pauses compare with those of younger adults to fully understand the effects of typical aging and to set realistic goals for disordered speakers. For these reasons, the present study includes a young adult control group.
Another major limitation of some previous studies is the use of overly broad classification systems for recording the locations of breath pauses. For example, Bunton (2005) classified breath pauses broadly into one of two categories: structural (preceding a clause) or other (between single words or lists of items). Thus, valuable information about the use of breath pauses at more minor syntactic boundaries (e.g., at the beginning of a prepositional phrase) may have been lost. Older adults and individuals with PD have been shown to produce shorter utterances as compared to young adults (Hoit & Hixon, 1987; Huber, 2008). Shorter utterances, and thus more frequent breath pauses, may result in fewer breaths at major syntactic boundaries, but these breaths may still be tied to syntax in a predictable way. Therefore, when recording the position of breath pauses, it is important to distinguish between minor syntactic boundaries (e.g., following a subordinate clause) and locations unrelated to a syntactic boundary (e.g. within a prepositional phrase). This distinction is important because there may be very different consequences to overall intelligibility. Furthermore, when describing the relationship between syntax and breath pausing in older adults and speakers with dysarthria, it is important to be as precise as possible to allow for replication with new populations and for translation to clinical evaluation methods. Hammen & Yorkston (1994) provided the most clearly-defined classification system of breath pausing, defining breath pausing as either primary (at a sentence boundary), secondary (at a phrase or subordinate clause boundary), or other (within a phrase or clause). The present study adopts a similar three-way classification system for the syntactic location of breath pauses.
Conrad et al (1983) showed that young adults use punctuation as a cue for breath pausing when reading aloud. However, no current studies have examined whether older adults or individuals with PD use punctuation in the same way. The present study investigates this issue by analyzing breath pause locations in relation to punctuation in addition to syntax.
Research Question
The present study examines the impact of typical aging and PD on the syntactic location of breath pauses. Using a reading passage and a detailed classification of breath pauses, this study provides a comprehensive overview of typical aging, extends earlier work to a larger population of individuals with PD, and examines the relationship between breath pausing and punctuation in these populations. It is important to understand the relationship between breath pausing and syntax in order to set appropriate goals for individuals with dysarthria in therapy and to develop treatment strategies designed to improve intelligibility and naturalness of speech in individuals with motor speech disorders. Based on previous literature, our hypotheses were as follows:
Typically aging adults will produce shorter utterances than young adults. A smaller percentage of total breaths will be taken at major boundaries and a larger percentage of breaths will be taken at minor boundaries in older adults as compared to young adults. However, in general, breaths will be associated with syntactic boundaries and punctuation marks for both young adults and older adults.
Individuals with PD will produce shorter utterances than age- and sex-matched control participants. A smaller percentage of total breaths will be taken at major boundaries and a larger percentage of breaths will be taken at locations unrelated to a syntactic boundary by individuals with PD as compared to age- and sex-matched control participants. In addition to taking a larger percentage of breaths at locations unrelated to syntax, the individuals with PD will take a greater percentage of breaths at locations without punctuation as compared to control participants.
METHODS
Participants
Seventy-one monolingual, native speakers of American English participated in this study: 30 young adults (15 women and 15 men), 25 older adults (15 women and 10 men), and 15 individuals with PD (6 women and 9 men). A subset of the older adults (fourteen who were age- and sex-matched to the individuals with PD) were used as controls to compare to the individuals with PD. Data were collected from one additional female participant as a control to match F03PD, who was too young to match with any of the older adults. Thus, a total of 15 participants were age- and sex-matched to the individuals with PD. The mean ages of the participants were as follows: young women: 22 years (range: 20–35 years), older women: 72 years (range: 66–76 years), women with PD: 70 years (range: 51–80 years), age-matched women: 70 years (range: 50–76 years), young men: 23 years (range: 20–29 years), older men: 71 years (range: 66–82 years), men with PD: 74 years (range: 70–83 years), age-matched men: 72 years (range: 66–82 years). For age-matching, we strove to find healthy older adults within 2 years of age for each of the individuals with PD. The average age difference for the women with PD and the controls was 1 year (range: 0–3 years). The average age difference for the men with PD and the controls was 2.5 years (range: 0–8 years). There were two men with PD for whom matches were not as close as we would have preferred, 6 and 8 years difference. Information about time since diagnosis, medications, and overall speech severity for individuals with PD is presented in Table 1.
Table 1.
Detailed Information about Participants with Parkinson’s disease
| Participant | Age (years) | Time since diagnosis (years) | History of speech treatment | Drugs | Perceptual speech severity |
|---|---|---|---|---|---|
| F01PD | 73 | 1 | No | Mirapex | Mild |
| F02PD | 70 | 9 | No | Eldepryl, Wellbutrin, Zoloft | Normal- Mild |
| F03PD | 51 | 2 | No | Comtan, Elevail, Sinemet | Normal- Mild |
| F04PD | 74 | 5 | No | Bromocriptine, Eldepryl, Sinemet | Normal |
| F05PD | 80 | 6 | No | Eldepryl, Requip, Stalevo | Mild |
| F06PD | 76 | 1 | No | None | Normal |
| M01PD | 83 | 5 | No | None | Moderate |
| M02PD | 76 | 5 | No | Comtan, Requip, Sinemet | Moderate |
| M03PD | 69 | 3–4 | No | Permax, Stalevo | Normal- Mild |
| M04PD | 70 | 4 | No | Stalevo Sinemet Mirapex | Normal- Mild |
| M05PD | 75 | 3 | Yes | Carbidopa, Comtan, Permax | Moderate |
| M06PD | 73 | 10 | Yes | Lipitor, Metoprolol, Prozac, Sinemet | Mild |
| M07PD | 70 | 4 | No | Sinemet | Mild |
| M08PD | 82 | 4 | No | Amantadine, Flomax, Sinemet | Mild |
| M09PD | 70 | 5 | Yes | Amantadine, Sinemet | Mild |
Speech impairment ratings were made by three certified speech-language pathologists who were unaffiliated with the study and who were experienced in the assessment and treatment of individuals with motor speech disorders. To rate speech impairment, the speech-language pathologists listened to individual sentences taken from the reading passage. Ratings of loudness, speech rate, articulatory precision, breathiness, and hoarseness were made on a scale of 1 (normal) to 7 (severe) and then averaged to obtain an overall speech impairment rating. Normal was taken as a rating of 1, mild was a rating of 2–3, moderate a rating of 4–5, and severe a rating of 6–7. These rating descriptors were also provided to the clinicians, in addition to the numbers. Overall, the patients’ speech impairments ranged from mild to moderate, with reduced loudness and imprecise articulation most commonly rated as problematic.
At the time of the experiment, all participants reported being free from colds, infections, and allergy symptoms, having been non-smoking for at least the past five years, no history of respiratory problems, no history of neurological disease (except PD), no head or neck cancer or surgery, and no formal training in singing or speaking. All older adults and individuals with PD were required to be living independently in the community and ambulatory and had to pass the Mini-Mental State Exam (Folstein, Folstein, & McHugh, 1975) or the Cognitive-Linguistic Quick Test (CLQT) (Helm-Estabrooks, 2001). Young and older adults were determined by the first author to have normal speech, language, and voice, and demonstrated normal lung function by producing vital capacity (VC), forced vital capacity (FVC), and forced expiratory volume in one second (FEV1.0) at greater than or equal to 80% of expected values based on age, sex, height, weight, and ethnicity (VacuMed Discovery Handheld Spirometer). Individuals with PD were tested within 1–3 hours of taking their anti-Parkinsonian medications.
Equipment
Respiratory kinematic data were transduced with the Respitrace (Ambulatory Monitoring, Inc.). An elastic band placed around the rib cage (RC), just under the axilla, transduced RC movement. A second band placed around the abdomen (AB), below the last rib at the level of the participant’s umbilicus, transduced AB movement. Signals from the Respitrace bands were digitized through the analog-to-digital converter in the Optotrak system (Northern Digital Inc.). A microphone signal was digitized time-locked with the Respitrace signal.
Procedures and Speech Stimuli
Each participant read a short (68-word) reading passage two times at comfortable loudness and pitch (Sapienza & Stathopoulos, 1995). The reading passage was displayed on a computer screen in front of the participant. Participants were instructed to read the passage aloud and to be sure they were clear and audible to an experimenter sitting about four feet away.
Two researchers independently listened to the reading passages and marked any deviations from the passage produced by the participants. In cases where there was a discrepancy between the two transcriptions, the researchers came to consensus on what the participant said.
Measurements
A breath group was defined as all of the words produced on one breath, and utterance length was defined as the number of syllables produced on each breath group. The location of a breath was determined using the sum signal from the Respitrace which was computed by summing the calibrated rib cage and abdomen signals (Huber, 2007, 2008; Huber, Chandrasekaran, & Wolstencroft, 2005; Huber & Darling, 2011). A breath was defined as a sharp upward deflection in the sum signal. In general, there was little doubt as to the location of breaths. However, in a few cases, the microphone signal was used to corroborate the location of a breath since we did not expect participants would speak on inhalation. The microphone signal was also monitored perceptually for speech during inhalation. The location of breaths was then analyzed from the perspective of punctuation and syntax. While there was no difference in the number of breaths taken by individuals with PD and the control participants [F(1, 28.5) = .03, p = .858], older adults took significantly more breaths as compared to younger adults [F(1, 53) = 8.39, p = .006] (see Table 2). Because of the difference in the number of breaths in the older and younger adults, percent data were used rather than raw counts to reduce the effect of number of breaths.
Table 2.
Means and Standard Errors (in parentheses) for all Dependent Measures by Group
| Measurements | Young Adults | Older Adults | Age- and Sex- Matched Controls | Individuals with Parkinson’s Disease |
|---|---|---|---|---|
| Number of Breaths | 15.9 (0.49) | 13.4 (0.49) | 15.4 (0.72) | 14.9 (0.87) |
| Utterance Length | 14.2 (0.51) | 12.0 (0.39) | 12.6 (0.65) | 13.2 (0.74) |
| Percent of Breaths at Periods | 72.7 (1.62) | 64.2 (1.43) | 64.7 (1.86) | 63.1 (2.45) |
| Percent of Breaths at Commas | 24.1 (1.40) | 32.4 (1.17) | 31.6 (1.59) | 27.6 (2.09) |
| Percent of Breaths at Locations without Punctuation | 3.2 (0.68) | 3.4 (0.73) | 3.7 (0.94) | 9.2 (2.38) |
| Percent of Breaths at Major Boundaries | 74.9 (1.39) | 67.2 (1.56) | 67.4 (1.58) | 64.0 (2.39) |
| Percent of Breaths at Minor Boundaries | 23.8 (1.42) | 32.1 (1.27) | 32.0 (1.48) | 29.7 (2.03) |
| Percent of Breaths at Locations Unrelated to Syntax | 1.2 (0.44) | 0.8 (0.33) | 0.8 (0.34) | 6.3 (2.02) |
| Mazes | 0.3 (0.35) | 0.7 (0.078) | 0.1 (0.06) | 0.7 (0.23) |
| Disfluencies | 0.1 (0.09) | 0.2 (0.04) | 0.3 (0.14) | 0.4 (0.14) |
Breaths at Punctuation
The breaths taken at periods, commas, and at locations without punctuation were counted. For each participant in each trial, the percent of breaths taken at each location was computed by dividing the number of breaths at a location by the total number of breaths taken in the trial.
Breaths at Syntactic Location
Breaths were also analyzed according to syntax using the following categories.
Major syntactic boundary (i.e., after an independent clause)
Minor syntactic boundary (i.e., after a dependent clause or before a prepositional phrase)
Locations unrelated to a syntactic boundary (i.e., in the middle of a prepositional phrase, after a pronominal subject, etc.)
A copy of the reading passage, with syntactic boundaries indicated, is presented in Appendix A. For each trial, the percent of breaths taken at each syntactic location was computed by dividing the number of breaths at a particular location by the total number of breaths taken by the participant in that trial.
Error Analysis
The number of disfluencies (sound or single word repetitions) and mazes (multiple word repetitions, restarted utterances, and other deviations from the text) were counted. Breaths immediately before, during, or after mazes or disfluencies were not included in the counts associated with punctuation and syntax.
Statistical Analysis
The type of analysis used depended on the structure and distribution of the data. The data on the effects of aging were analyzed separately from the data regarding the effects of PD. For all analyses, the factors were age (young vs. older adult) or group (PD vs. control). The α level was set as α < 0.05 for all statistical tests. Additional information about the statistical analyses is presented in Appendix B.
For utterance length, number of breaths, percent of breaths at a period and comma, and the percent of breaths at the major and minor boundaries, the data were analyzed using linear mixed models with SAS procedure PROC MIXED because different subjects had different numbers of data points which prevented simply averaging values for each subject. Each subject was modeled with a random individual effect which is nested within the fixed-effect factor (either age or group). At most, two repeated measures were observed from each subject and their covariance structures were modeled as unstructured. For the tests of fixed effects, the Kenward-Roger method (Kenward & Roger, 1997) was used to compute the degrees of freedom of the corresponding t-test.
The numbers of mazes and disfluencies were counting measures of small values, and were modeled by Poisson distributions. The data were analyzed using generalized linear mixed models with SAS procedure PROC GLIMMIX. As above, each subject was modeled with a random individual effect which is nested within the fixed-effect factor (either age or group). At most two repeated measures were observed from each subject, and they were modeled as independent measures (for any specified subject). For the tests of fixed effects, the Kenward-Roger method (Kenward & Roger, 1997) was used to compute the degrees of freedom of the corresponding t-test.
The data for percent of breaths at locations without punctuation and percent of breaths at locations unrelated to syntax were positively skewed and contained many zero measures. Type 1 Tobit models (Amemiya, 1984) were used to model the truncated measures. The data were analyzed using SAS procedure PROC QLIM, and the effects were tested using the likelihood ratio test (χ2-test).
To examine the relationship between breath pauses and punctuation, linear regressions between the number of breaths at minor boundaries and the number of breaths at commas were fitted for each group. There are 26 locations defined as minor boundaries in the reading passage and ten of those are marked with a comma (see Appendix A). One major boundary occurred at a comma and that location was excluded from this analysis.
To establish inter-measurer reliability for utterance length and categorization of breaths, 2 young men, 2 young women, 2 older men, 2 older women, 2 men with PD, 1 woman with PD were randomly chosen for remeasurement. This accounted for about 15% of the subjects. Breath counts matched for every subject. For utterance length, a paired t-test was performed to test whether significant differences existed between the two measurers. The mean difference was 0.11 syllables, which is insignificant [t (363)=0.64, p=0.42]. Overall, reliability was good.
RESULTS
Means and standard errors for each group for dependent measure are presented in Table 2 to provide potential comparative values for clinical practice.
Typical Aging
Utterance Length
Older adults produced shorter utterances than young adults [F(1, 53) = 6.24, p = .016].
Effects of Punctuation
Older adults took a significantly smaller percent of breaths at periods [F(1, 53) = 8.33, p = .006] and a significantly larger percent of breaths at commas than young adults [F(1, 53) = 11.84, p = .001]. For the percent of breaths at locations without punctuation, there was no significant effect of age [χ2(1) = .06, p = .812].
Effects of Syntax
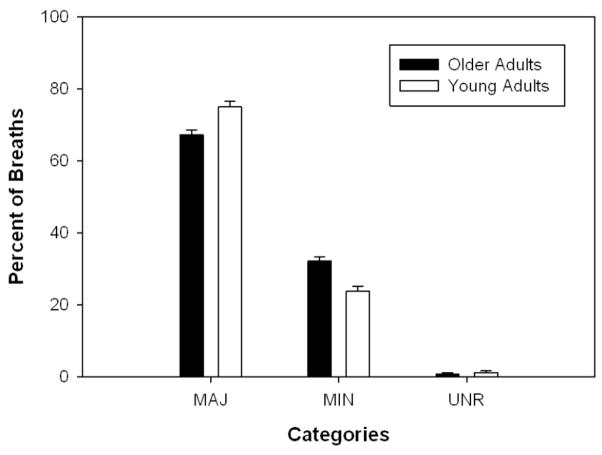
Older adults took a significantly smaller percent of breaths at major boundaries [F(1, 53) = 7.72, p = .008] and a significantly larger percent of breaths at minor boundaries [F(1, 53) = 10.64, p = .002] than young adults (see Figure 1). The linear regressions demonstrated strong positive relationships between breaths at minor boundaries and breaths at commas for both young (R2 = 0.90) and older adults (R2 = 0.91) (see Figure 2). Few subjects took breaths at minor boundaries which were not marked by commas. There was no significant effect of age for the percent of breaths at locations unrelated to syntax [χ2(1) = .03, p = .866] (see Figure 1).
Figure 1.
Percent of breaths at syntactic boundaries in younger and older adults.
Figure 2.
Scatter plot demonstrating the linear relationship between number of breaths at commas and number of breaths at minor boundaries for young adults, older adults, individuals with Parkinson’s disease (PD), and control participants.
Error Analyses
There were very few instances of mazes or disfluencies in the data from the young and older adults and no significant effect of age for the number of mazes [F(1, 91.99) = .00, p = .987] or disfluencies [F(1, 108) = .98, p = .341]. Seventy-six percent of the trials had no mazes in them and nineteen percent had one maze in them. Three young adults produced 2–3 mazes in one of the two trials. One female older adult produced a large number of mazes (12 and 13) for both trials of the reading passage. Ninety percent of the trials had no disfluencies and seven percent had one disfluency. The maximum number of disfluencies in any trial was three, produced by an older male.
Parkinson’s Disease
Utterance Length
For utterance length, there was no significant effect of group [F(1, 28.2) = .09, p = .764].
Effects of Punctuation
For the percent of breaths at periods, commas, and no punctuation, there were no significant effects of group (Periods: [F(1, 28.2) = .29, p = .596], Commas: [F(1, 26.9) = 2.02, p = .167], No Punctuation: [χ2(1) = 2.37, p = .124]).
Effects of Syntax
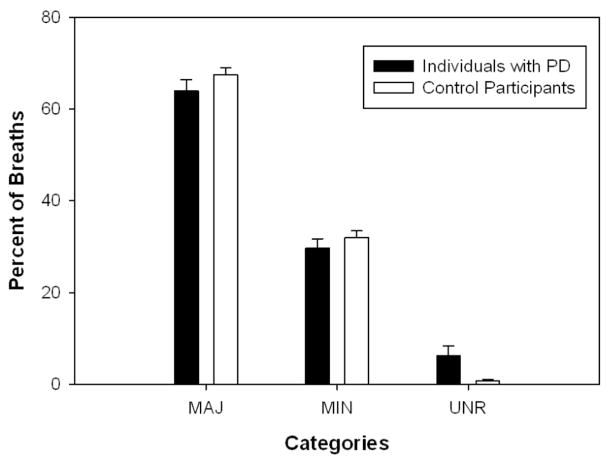
For the percent of breaths at major and minor boundaries, there were no significant effects of group (Major: [F(1, 28) = 1.11, p = .301], Minor: [F(1, 27.8) = .49, p = .489]). The linear regressions demonstrated strong positive relationships between breaths at minor boundaries and breaths at commas for both controls (R2 = 0.90) and individuals with PD (R2 = 0.89) (see Figure 2). For the percent of breaths at locations unrelated to syntax, there was a significant effect of group [χ2(1) = 4.38, p = .036]. Individuals with PD took a significantly larger percentage of breaths at locations unrelated to syntax as compared to control participants (see Figure 3).
Figure 3.
Percent of breaths at syntactic boundaries in the individuals with PD and the control participants.
Error Analyses
Individuals with PD produced significantly more mazes than control subjects [F(1, 56) = 7.84, p = .007]. For the number of disfluencies, there were no significant effects of group [F(1, 35.25) = .97, p = .331].
DISCUSSION
The present study examined the impact of typical aging and PD on the syntactic location of breath pauses. In general, the hypotheses about changes as a result of typical aging were supported. However, support for the hypotheses about changes as a result of PD was mixed.
Typical Aging
In general, a majority of breaths produced by both young adults and typically aging adults were located at major or minor syntactic boundaries, as expected. We hypothesized that typically aging adults would produce shorter utterances and take a smaller percentage of breaths at major boundaries and a greater percentage of breaths at minor boundaries than young adults. These hypotheses were supported by the results. Further, breaths at minor boundaries were highly associated with punctuation, as hypothesized. Interestingly, although 16 of the 26 minor syntactic boundaries in the text did not have any punctuation marking, almost all of the breath pauses at minor boundaries occurred at locations marked with commas. This suggests that commas are an important visual cue for breath pauses in reading, especially for speakers such as older adults who may need to take more breaths at minor boundaries. While changes in breath pausing patterns have been linked to negative changes in speech intelligibility and naturalness (Hammen & Yorkston, 1994), the typically aging adults were generally readily intelligible to listeners. It is likely that while typically aging adults take a greater percentage of breaths at minor boundaries, the fact that the majority of their breaths are still tied to syntax preserves speech intelligibility and naturalness.
Changes in breath pausing patterns in typical aging could be the result of either changes to respiratory physiology or changes to cognition affecting the ability to plan breath pauses relative to syntax. In this study, changes in breath pausing patterns in typically aging adults are likely the result of changes to respiratory physiology rather than changes in cognition because breath pauses were still closely tied to syntax despite the fact that typically aging adults produced shorter utterances than young adults. Changes to respiratory physiology in typically aging adults include decreased elasticity of the lungs, decreased compliance of the chest wall, lower elastic recoil forces, and decreased muscle mass (Berry, Vitalo, Larson, Patel, & Kim, 1996; Bode et al., 1976; Enright, Adams, Boyle, & Sherrill, 1995; Enright, Kronmal, Manolio, Schenker, & Hyatt, 1994; Knudson, Slatin, Lebowitz, & Burrows, 1976; Pfitzenmeyer et al., 1993; Sherrill, Lebowitz, Knudson, & Burrows, 1992; Tolep & Kelsen, 1993). These physiologic changes make it more difficult for aging adults to inspire to higher lung volumes and continue speaking to lower lung volumes. Thus, typically aging adults may produce shorter utterances as a result of a decreased ability to utilize expiratory muscles to produce longer utterance at lower lung volumes (Huber, 2008). Typically aging adults may also produce shorter utterances as a way to ensure breath pauses are produced at syntactically appropriate locations as opposed to speaking until they are forced to inspire at a syntactically inappropriate location, suggesting intact cognition or intact coordination of respiration with language formulation.
Parkinson’s disease
We hypothesized that individuals with PD would produce shorter utterances as compared to control participants. This hypothesis was not supported. Some previous studies have demonstrated significantly shorter utterances for individuals with PD as compared to control participants (Huber & Darling, 2011; Solomon & Hixon, 1993), but not all studies have reported significant differences (Bunton, 2005). High speaker variability, as observed in Bunton (2005), results in difficulties comparing results across studies. Comparisons of mean data indicate that the individuals with PD in our study and those in Solomon and Hixon (1993) produced comparable mean utterance lengths, 13.2 syllables and 13.6 syllables, respectively. However, control participants in the current study produced 12.6 syllables per breath group, while control participants in Solomon and Hixon (1993) produced 17.5 syllables per breath group. Thus, the discrepancy in the results across the current study and Solomon and Hixon is due to utterance length differences between the control participants and not the individuals with PD. The mean from the current study is at the low end of the range of values reported for utterance length in typically aging adults, from 12–18 syllables per breath group, in previous literature (Hoit & Hixon, 1987; Hoit, Hixon, Altman, & Morgan, 1989).
We hypothesized that individuals with PD would take a smaller percentage of breaths at major boundaries. This was not supported by our results. There were no significant differences between individuals with PD and control participants for breaths taken at major boundaries or minor boundaries. Two studies reported that individuals with dysarthria take fewer breaths at sentence or major boundaries than control participants (Bunton, 2005; Hammen & Yorkston, 1994). One of those studies, Hammen & Yorkston (1994), included participants with dysarthria due to traumatic brain injury, cerebrovascular accident, and amyotrophic lateral sclerosis, as well as PD. As individuals with different types of dysarthria exhibit different types of speech symptoms and different breath pausing patterns, differences between our study and theirs are not surprising. Bunton (2005), the only previous study to solely utilize individuals with PD, found that individuals with PD took breaths at structural boundaries between 50 and 71% of the time in conversational speech, while control subjects took breaths at structural boundaries between 75 and 87% of the time. In the current study, individuals with PD took breaths at major boundaries 63.1% of the time, which is consistent with Bunton’s results. However, control participants in the current study only took breaths at major boundaries 64.7% of the time, which is much lower than control participants in Bunton’s study. Therefore, much like our results for utterance length, the control participants drove the differences in findings across studies. This could be related to age differences between the control participants, the speech task examined, or the number of control participants included. Bunton only had 6 control participants while the current study had 15 control participants. The control participants in Bunton’s study were younger than the control participants in the current study, particularly the men. Previous studies from our laboratory have shown significant differences in the respiratory patterns utilized for reading and monologue in young adults, older adults, and individuals with Parkinson’s disease (Huber, 2007; Huber & Darling, 2011). Those respiratory patterns differences may also result in differences in breath pausing behavior and may also explain the differences in the control group means between the current study and Bunton (2005).
Differences in the severity of the speech impairments demonstrated by the participants may also be the cause of discrepancies between the current study and previous studies. In general, individuals with PD in the current study had mild speech severity with only 3 individuals with moderate speech severity. Most of the participants in Hammen & Yorkston (1994) were moderate with some participants having moderate-severe speech impairment. Therefore, significant differences in breath pausing patterns may only occur with more severe speech impairment. This hypothesis was supported by the results from the current study. Figure 4 depicts the percentage of breaths taken at the three syntactic boundaries for the mild and moderate patients. Although no statistical test was completed on these data due to large differences in subject numbers, it is clear that the individuals with moderate dysarthria took a smaller percentage of breaths at major boundaries and a larger percentage of breaths at locations unrelated to a syntactic boundary.
Figure 4.
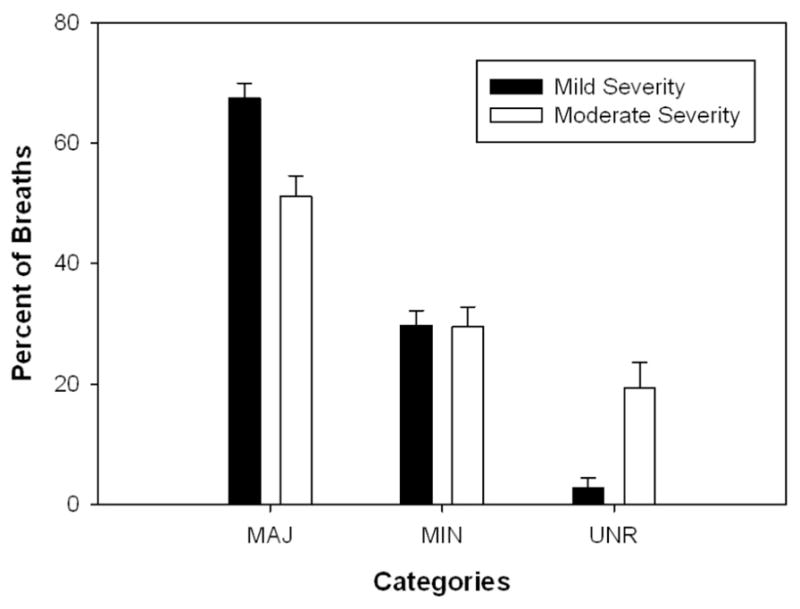
Percent of breaths at syntactic boundaries in individuals with normal or mild speech symptoms as compared to those with moderate speech symptoms.
We also hypothesized that individuals with PD would take a greater percentage of breaths at locations unrelated to a syntactic boundary than control participants. This was supported by our findings. This finding was stronger in the individuals with moderate speech impairments than in those with mild speech impairments (see Figure 4). While speech impairment and breath pausing appear to be related, as evidenced by our results, it is unclear to what extent speech impairment impacts breath pausing. A longitudinal study is needed to further substantiate the impact of speech impairment on breath pausing patterns.
Results for punctuation followed results for syntax closely. There were no significant group differences for breaths at periods or commas. Again, very few subjects took breaths at minor boundaries that were not marked with a comma, suggesting that commas serve as an important visual cue for breath pausing. However, there was no significant difference for breath at no punctuation, but individuals with PD took a greater percentage of breaths at locations unrelated to syntax. This suggests that the syntactic-based breath categorization scheme may be more sensitive to the kinds of difficulties individuals with PD have than a punctuation-based categorization scheme.
In this study, changes in breath pausing patterns in individuals with PD are likely the result of both changes to respiratory physiology and cognition. The fact that utterance length was not shorter than the control participants, but the percent of breaths at locations unrelated to syntax was higher for individuals with PD suggests that respiratory physiological change cannot be the sole cause. Since the individuals with PD in this study were, with one exception, older adults, respiratory changes which occur with typical aging will affect their speech production. Additionally, increased chest wall rigidity and reduced inspiratory and expiratory muscle strength may exacerbate the typical aging-related changes in individuals with PD (De Pandis et al., 2002; Haas, Trew, & Castle, 2004; Inzelberg et al., 2005; Sabate, Gonzalez, Ruperez, & Rodriguez, 1996; Solomon & Hixon, 1993). Individuals with PD have also been shown to have subtle language processing difficulties, in particular related to syntactic processing, which may make it more difficult for them to plan breath pauses with syntax (Friederici et al., 2003; Grossman et al., 1993; Grossman et al., 2000; Grossman et al., 2002). Problems with syntactic processing may underlie our finding that individuals with PD took a greater percentage of breaths at locations unrelated to a syntactic boundary. The finding that individuals with PD produced more mazes than older adults further supports the hypothesis that individuals with PD experience subtle changes to language formulation. This finding was particularly striking given that the task was a reading task where little language formulation was required. In support of the hypothesis that both respiratory physiologic and cognitive changes underlie impairments in the coordination of breathing and language in PD, previous data from our lab have shown that individuals with PD have difficulty coordinating respiratory movements and language formulation in extemporaneous speech (Huber & Darling, 2011). Individuals with PD demonstrate weaker relationships between lung volume initiation and utterance length in extemporaneous speech than in reading, suggesting that these individuals do not plan utterances prior to inhalation. By not consistently planning utterances prior to inhalation, individuals with PD are forced to stop speaking wherever they are in their utterance when they are driven by the physiologic need to breathe. Therefore, by not planning the appropriate respiratory support for their utterances, individuals with PD likely increase the likelihood that breaths will occur at locations unrelated to a syntactic boundary. Taken together, these data suggest that changes to cognition and language skills may impact how well individuals with PD maintain the relationship between breath pauses and syntax.
Clinical Implications
This study provides a normative data set from typically aging adults, demonstrating the relationship between syntax and breath pausing. While typically aging adults tend to breathe at syntactically appropriate locations, they do often breathe at minor boundaries while still preserving intelligibility. Thus, clinicians should focus on training individuals with PD to breathe at both major and minor syntactic boundaries, not just major syntactic boundaries. Our results also suggest that direct treatment of breath pausing patterns may not be warranted until moderate impairments in speech production are evident in individuals with PD. However, given the preliminary nature of the data suggesting that speech impairment is related to breath pausing patterns, it would be advisable to monitor breath pausing patterns at all stages of the disease, particularly since unnatural pausing patterns are likely to impact naturalness and intelligibility of speech.
Summary
This study examined alterations in breath pausing patterns with typical aging and PD. Typically aging adults take a greater percentage of breaths at minor boundaries and occasional breaths at locations unrelated to a syntactic boundary without negatively impacting speech intelligibility and naturalness. It is currently unknown what percentage of breaths at locations unrelated to a syntactic boundary is needed before a reduction in speech intelligibility or naturalness can be perceived. Future research on the relationship between breath pauses and intelligibility would help to inform clinicians when to address breath pausing issues in individuals with dysarthria. In addition, it is not known whether older adults and individuals with PD would show the same breath pausing patterns in spontaneous speech. In particular, it is unclear whether minor syntactic boundaries would be utilized as often when visual cues (e.g., commas) are not available. Further research is needed to answer this question.
This study also demonstrated that individuals with PD take a greater percentage of breath pauses at locations unrelated to a syntactic boundary than control participants. Our results suggest that breath pausing patterns are more impaired in individuals with moderate speech impairment. However, some of the statistically significant differences were small (in particular the comparison between individuals with PD and controls for percent of breaths at locations unrelated to syntax). These small statistical differences, along with some of non-significant findings, may be due to the small number of participants with PD in the study and the inclusion of participants with milder dysarthria. Future research should observe breath pausing patterns using larger sample sizes, more severe speakers, and longitudinal designs to further determine the impact of speech impairment and disease severity on breath pausing patterns.
This study suggests that changes in breath pausing patterns in typically aging adults are due to changes in respiratory physiology. However, in individuals with PD, the data suggest that such changes may result from a combination of changes to respiratory physiology and changes to cognition. However, the current study cannot distinguish the extent to which physiological and cognitive mechanisms are involved in breath pausing in individuals with PD. Future research should also seek to further investigate the physiological and cognitive factors that affect breath pausing patterns in individuals with PD.
Acknowledgments
This research was funded by the National Institutes of Health, National Institute on Deafness and Other Communication Disorders, grant # 1R03DC05731, a Research Support Incentive Grant from the Center on Aging and the Life Course at Purdue University, and a Summer Faculty Support Grant from Purdue University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders, the National Institutes of Health, the Center on Aging and the Life Course, or Purdue University.
Appendix A: Papa Passage with Syntactic Locations Marked
Papa [MIN] was a great man. [MAJ] Working all his life [MIN] as a carpenter, [MIN] he built homes [MIN] for other people. [MAJ] Papa [MIN] was an excellent craftsman. [MAJ] Anyone who worked with Papa [MIN] knew that he was an honest man. [MAJ] Papa [MIN] gave himself to his work, [MIN] toiling daily [MIN] for small amounts of money. [MAJ] No one [MIN] disliked Papa. [MAJ] In fact, [MIN] neighbors [MIN] used to bring Papa apples [MIN], pears [MIN], and other fruits [MIN], especially around the holidays. [MAJ]
I remember Papa [MIN] for his kind ways. [MAJ] What I remember [MIN] was the manner [MIN] in which Papa dressed, [MIN] the way he carried himself. [MAJ] Papa [MIN] was such a strong man. [MAJ] Devoted to his family [MIN], especially his children [MIN], Papa [MIN] worked night and day [MIN] to provide for us. [MAJ] Although we never showed Papa our appreciation [MIN] on a daily basis [MIN], I know that he felt our love [MAJ], or so I hope.
Appendix B: Models and SAS Codes
Models and SAS Codes
1. Linear Mixed Modes
where Yijk is the k-th measure of j-th subject with factor level i, μ is the overall mean, αi is the fixed effect of the factor, βj(i) is the independent individual random effect distributed as N (0, ), and εijk is the error distributed as N(0, ) with cov(εijk, εijl) = ρklσkσl. The SAS codes for such a model is as follows,
proc mixed data=oayadata;
class group person;
model y = group/ddfm=kr s;
random intercept/subject=person g v;
repeated/type=un sub=person r;
run; quit;
2. Generalized Linear Mixed Modes
where Yijk is the k-th measure of j-th subject with factor level i, μis the overall mean, αi is the fixed effect of the factor, βj(i) is the independent individual random effect distributed as N (0, ). The SAS codes for such a model is as follows,
proc glimmix data=oayadata;
class group person;
model y = group/ddfm=kr s dist=poisson;
random intercept/subject = person g v;
run; quit;
3. Type 1 To bit Modes
where Yijk is the k-th measure of j-th subject with factor level i, μis the overall mean, αi is the fixed effect of the factor, and εijk is the independent error distributed as N (0, σ2). The SAS codes for such a model is as follows,
proc qlim data=oayadata;
model y = group;
endogenousy ~ censored(lb=0);
test group=0/lr;
run; quit;
References
- Amemiya T. Tobit models: a survey. Journal of Econometrics. 1984;24:3–61. [Google Scholar]
- Berry JK, Vitalo CA, Larson JL, Patel M, Kim MJ. Respiratory muscle strength in older adults. Nursing Research. 1996;45(3):154–159. doi: 10.1097/00006199-199605000-00006. [DOI] [PubMed] [Google Scholar]
- Birren JE, Schaie KW, editors. Handbook of the Psychology of Aging. 5. San Diego: Academic Press; 2001. [Google Scholar]
- Bode FR, Dosman J, Martin RR, Ghezzo H, Macklem PT. Age and sex differences in lung elasticity, and in closing capacity in nonsmokers. Journal of Applied Physiology. 1976;41(2):129–135. doi: 10.1152/jappl.1976.41.2.129. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Cognitive function in Parkinson’s disease: from description to theory. Trends in Neuroscience. 1990;13(1):21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Bunn J, Mead J. Control of ventilation during speech. Journal of Applied Physiology. 1971;31:870–872. doi: 10.1152/jappl.1971.31.6.870. [DOI] [PubMed] [Google Scholar]
- Bunton K. Patterns of lung volume use during an extemporaneous speech task in persons with Parkinson’s disease. Journal of Communication Disorders. 2005;38:331–348. doi: 10.1016/j.jcomdis.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Conrad B, Thalacker S, Schonle P. Speech respiration as an indicator of integrative contextual processing. Folia Phoniatrica. 1983;35:220–225. doi: 10.1159/000265766. [DOI] [PubMed] [Google Scholar]
- De Pandis MF, Starace A, Stefanelli F, Marruzzo P, Meoli I, De Simone G, et al. Modification of respiratory function parameters in patients with severe Parkinson’s disease. Neurological Sciences. 2002;23(Suppl 2):S69–70. doi: 10.1007/s100720200074. [DOI] [PubMed] [Google Scholar]
- Enright PL, Adams AB, Boyle PJR, Sherrill DL. Spirometry and maximal respiratory pressure references from healthy Minnesota 65- to 85-year-old women and men. Chest. 1995;108:663–669. doi: 10.1378/chest.108.3.663. [DOI] [PubMed] [Google Scholar]
- Enright PL, Kronmal RA, Manolio TA, Schenker MB, Hyatt RE. Respiratory muscle strength in the elderly. American Journal of Respiratory and Critical Care Medicine. 1994;149(2):430–438. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]
- Ferreira F. Creation of prosody during sentence production. Psychological Review. 1993;100:233–253. doi: 10.1037/0033-295x.100.2.233. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini Mental State.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frank NR, Mead J, Ferris BG., Jr The mechanical behavior of the lungs in healthy elderly persons. The Journal of Clinical Investigation. 1957;36:1680–1687. doi: 10.1172/JCI103569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA, Werheid K, Hein G, von Cramon DY. Syntactic comprehension in Parkinson’s disease: Investigating early automatic and late integrational processes using event-related brain potentials. Neuropsychology. 2003;17:133–142. [PubMed] [Google Scholar]
- Gee JP, Grosjean F. Performance structures: a psycholinguistic and lingustic appraisal. Cognitive Psychology. 1983;15:411–458. [Google Scholar]
- Grosjean F, Collins M. Breathing, Pausing, and Reading. Phonetica. 1979;36:98–114. doi: 10.1159/000259950. [DOI] [PubMed] [Google Scholar]
- Grossman M, Carvell S, Gollomp S, Stern MB, Reivich M, Morrison D, et al. Cognitive and physiological substrates of impaired sentence processing in Parkinson’s disease. Journal of Cognitive Neuroscience. 1993;5:480–498. doi: 10.1162/jocn.1993.5.4.480. [DOI] [PubMed] [Google Scholar]
- Grossman M, Kalmanson J, Bernhardt N, Morris J, Stern MB, Hurtig HI. Cognitive resource limitations during sentence comprehension in Parkinson’s disease. Brain and Language. 2000;73(1):1–16. doi: 10.1006/brln.2000.2290. [DOI] [PubMed] [Google Scholar]
- Grossman M, Lee C, Morris J, Stern MB, Hurtig HI. Assessing resource demands during sentence processing in Parkinson’s disease. Brain and Language. 2002;80:603–616. doi: 10.1006/brln.2001.2630. [DOI] [PubMed] [Google Scholar]
- Haas BM, Trew M, Castle PC. Effects of respiratory muscle weakness on daily living function, quality of life, activity levels, and exercise capacity in mild to moderate Parkinson’s disease. American Journal of Physical Medicine and Rehabiliation. 2004;83:601–607. doi: 10.1097/01.phm.0000133436.61009.02. [DOI] [PubMed] [Google Scholar]
- Hammen VL, Yorkston KM. Respiratory patterning and variability in dysarthric speech. Journal of Medical Speech-Language Pathology. 1994;2(4):253–261. [Google Scholar]
- Hayes AE, Davidson MC, Keele SW, Rafal RD. Toward a functional analysis of the basal ganglia. Journal of Cognitive Neuroscience. 1998;10:178–198. doi: 10.1162/089892998562645. [DOI] [PubMed] [Google Scholar]
- Helm-Estabrooks N. Cognitive Linguistic Quick Test: Harcourt Assessment. 2001. [Google Scholar]
- Hochstadt J. Set-shifting and the on-line processing of relative clauses in Parkinson’s disease: results from a novel eye-tracking method. Cortex. 2009;45(8):991–1011. doi: 10.1016/j.cortex.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Hixon TJ. Age and speech breathing. Journal of Speech and Hearing Research. 1987;30:351–366. doi: 10.1044/jshr.3003.351. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Hixon TJ, Altman ME, Morgan WJ. Speech breathing in women. Journal of Speech and Hearing Research. 1989;32(2):353–365. doi: 10.1044/jshr.3202.353. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Lansing RW, Perona KE. Speaking-related dyspnea in healthy adults. Journal of Speech, Language, and Hearing Research. 2007;50:361–374. doi: 10.1044/1092-4388(2007/026). [DOI] [PubMed] [Google Scholar]
- Huber JE. Effects of cues to increase sound pressure level on respiratory kinematic patterns during connected speech. Journal of Speech, Language, and Hearing Research. 2007;50:621–634. doi: 10.1044/1092-4388(2007/044). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE. Effects of utterance length and vocal loudness on speech breathing in older adults. Respiratory Physiology and Neurobiology. 2008;164(3):323–330. doi: 10.1016/j.resp.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE, Chandrasekaran B, Wolstencroft JJ. Changes to respiratory mechanisms during speech as a result of different cues to increase loudness. Journal of Applied Physiology. 2005;98:2177–2184. doi: 10.1152/japplphysiol.01239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE, Darling M. Effect of Parkinson’s disease on the production of structured and unstructured speaking tasks: Respiratory physiologic and linguistic considerations. Journal and of Speech, Language and Hearing Research. 2011;54(1):33–46. doi: 10.1044/1092-4388(2010/09-0184). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzelberg R, Peleg N, Nisipeanu P, Magadle R, Carasso R, Weiner P. Inspiratory muscle training and the perception of dyspnea in Parkinson’s disease. Canadian Journal of Neurological Sciences. 2005;32:213–217. doi: 10.1017/s0317167100003991. [DOI] [PubMed] [Google Scholar]
- Kemper S. Life-span changes in syntactic complexity. Journal of Gerontology. 1987;42:323–328. doi: 10.1093/geronj/42.3.323. [DOI] [PubMed] [Google Scholar]
- Kemper S, Herman R, Chiung-Ju L. Sentence production by young and older adults in controlled contexts. Journal of Gerontology: Psychological Sciences. 2004;59B(5):220–224. doi: 10.1093/geronb/59.5.p220. [DOI] [PubMed] [Google Scholar]
- Kemper S, Marquis J, Thompson M. Longitudinal change in language production: Effects of aging and dementia on grammatical complexity and propositional content. Psycology and Aging. 2001;16:600–614. doi: 10.1037//0882-7974.16.4.600. [DOI] [PubMed] [Google Scholar]
- Kemper S, Mitzner TL. Language production and comprehension. In: Birren JE, Warner Schaie K, editors. Handbook of the Psychology of Aging. 5. San Diego: Academic Press; 2001. [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Knudson R, Slatin R, Lebowitz M, Burrows B. The maximal expiratory flow-volume curve. American Review of Respiratory Disease. 1976;113:587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- Mather M. Aging and cognition. WIREs Cognitive Science. 2010 May-Jun;1:346–362. doi: 10.1002/wcs.64. [DOI] [PubMed] [Google Scholar]
- Mittman C, Edelman NH, Norris AH, Shock NW. Relationship between chest wall and pulmonary compliance with age. Journal of Applied Physiology. 1965;20:1211–1216. [Google Scholar]
- Oliveira RM, Gurd JM, Nixon P, Marshall JC, Passingham RE. Micrographia in Parkinson’s disease: the effect of providing external cues. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:429–433. doi: 10.1136/jnnp.63.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfitzenmeyer P, Brondel L, d’Athis P, Lacroix S, Didier J, Gaudet M. Lung function in advanced age: Study of ambulatory subjects aged over 75 years. Gerontology. 1993;39:267–275. doi: 10.1159/000213542. [DOI] [PubMed] [Google Scholar]
- Price PJ, Ostendorf M, Schattuck-Hufnagel S, Fong C. The use of prosody in syntactic disambiguation. The Journal of the Acoustical Society of America. 1991;90:2956–2970. doi: 10.1121/1.401770. [DOI] [PubMed] [Google Scholar]
- Sabate M, Gonzalez I, Ruperez F, Rodriguez M. Obstructive and restrictive pulmonary dysfunctions in Parkinson’s disease. Journal of Neurological Sciences. 1996;138:114–119. doi: 10.1016/0022-510x(96)00003-2. [DOI] [PubMed] [Google Scholar]
- Sadagopan N, Huber JE. Effects of loudness cues on respiration in individuals with Parkinson’s disease. Movement Disorders. 2007;22:651–659. doi: 10.1002/mds.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza CM, Stathopoulos ET. Speech task effects on acoustic and aerodynamic measures of women with vocal nodules. Journal of Voice. 1995;9(4):413–418. doi: 10.1016/s0892-1997(05)80203-6. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Alter K, Kotz SA, Friederici AD. Lateralization of prosody during language production: A lesion study. Brain and Language. 2001;76:1–17. doi: 10.1006/brln.2000.2381. [DOI] [PubMed] [Google Scholar]
- Shah A, Baum S, Dwivedi V. Neural substrates of linguistic prosody: evidence from syntactic disambiguation in the productions of brain-damaged patients. Brain and Language. 2006;96:78–89. doi: 10.1016/j.bandl.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Sherrill DL, Lebowitz M, Knudson R, Burrows B. Continuous longitudinal regression equations for pulmonary function measures. European Respiratory Journal. 1992;5:452–462. [PubMed] [Google Scholar]
- Solomon NP, Hixon TJ. Speech breathing in Parkinson’s disease. Journal of Speech and Hearing Research. 1993;36:294–310. doi: 10.1044/jshr.3602.294. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Danielson SM. Inhibitory processes and spoken word recognition in young and older adults: The interaction of lexical competition and semantic content. Psychology and Aging. 1999;14:458–472. doi: 10.1037//0882-7974.14.3.458. [DOI] [PubMed] [Google Scholar]
- Steinhauer KM. Electrophysiological correlates of prosody and punctuation. Brain and Language. 2003;86:142–164. doi: 10.1016/s0093-934x(02)00542-4. [DOI] [PubMed] [Google Scholar]
- Stine EAL. On-line processing of written text by younger and older adults. Psychology and Aging. 1990;5(1):68–78. doi: 10.1037//0882-7974.5.1.68. [DOI] [PubMed] [Google Scholar]
- Stine EAL, Cheung H, Henderson D. Adult age differences in the on-line processing of new concepts in discourse. Aging and Cognition. 1995;2(1):1–18. [Google Scholar]
- Tolep K, Kelsen SG. Effect of aging on respiratory skeletal muscles. Clinics in Chest Medicine. 1993;14:363–378. [PubMed] [Google Scholar]
- Tun PA, O’Kane G, Wingfield A. Distraction by competing speech in young and older adults. Psychology and Aging. 2002;17:453–467. doi: 10.1037//0882-7974.17.3.453. [DOI] [PubMed] [Google Scholar]
- Wang YT, Kent RD, Duffy JR, Thomas JE. Dysarthria in traumatic brain injury: A breath group and into national analysis. Folia Phoniatrica et Logopaedica. 2005;57:59–89. doi: 10.1159/000083569. [DOI] [PubMed] [Google Scholar]
- Warren P. Prosody and parsing: an introduction. Language and Cognitive Processes. 1996;11:1–16. [Google Scholar]
- Winkworth AL, Davis PJ, Ellis E, Adams RD. Variability and consistency in speech breathing during reading: Lung volumes, speech intensity, and linguistic factors. Journal of Speech and Hearing Research. 1994;37:535–556. doi: 10.1044/jshr.3703.535. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson’s disease: Relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ, Gordon MF, Geigin A, et al. An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson’s disease. Journal of Clinical and Experimental Neuropsychology. 2006;28:1127–1144. doi: 10.1080/13803390500246910. [DOI] [PMC free article] [PubMed] [Google Scholar]