Abstract
Engineered nanoparticles (NPs) are in the same size category as atmospheric ultrafine particles, <100 nm. Per given volume, both have high numbers and surface areas compared to larger particles. The high proportion of surface atoms/molecules can give rise to a greater chemical as well as biological activity, for example the induction of reactive oxygen species in cell-free medium as well as in cells. When inhaled as singlet particles, NPs of different sizes deposit efficiently in all regions of the respiratory tract by diffusion. A major difference to larger size particles is the propensity of NPs to translocate across cell barriers from the portal of entry (e.g., the respiratory tract) to secondary organs and to enter cells by various mechanisms and associate with subcellular structures. This makes NPs uniquely suitable for therapeutic and diagnostic uses, but it also leaves target organs such as the central nervous system (CNS) vulnerable to potential adverse effects (e.g., oxidative stress). Neuronal transport of NPs has been described, involving retrograde and anterograde movement in axons and dendrites as well as perineural translocation. This is of importance for access of inhaled NPs to the central nervous system (CNS) via sensory nerves existing in the nasopharyngeal and tracheobronchial regions of the respiratory tract. The neuronal pathway circumvents the very tight blood brain barrier. In general, translocation rates of NP from the portal of entry into the blood compartment or the CNS are very low. Important modifiers of translocation are the physicochemical characteristics of NPs, most notably their size and surface properties, particularly surface chemistry. Primary surface coating (when NPs are manufactured) and secondary surface coating (adsorption of lipids/proteins occurring at the portal of entry and during subsequent translocation) can significantly alter NP biokinetics and their effects. Implications of species differences in respiratory tract anatomy, breathing pattern and brain anatomy for extrapolation to humans of NP effects observed in rodents need to be considered. Although there are anecdotal data indicating a causal relationship between long-term ultrafine particle exposures in ambient air (e.g., traffic related) or at the workplace (e.g., metal fumes) and resultant neurotoxic effects in humans, more studies are needed to test the hypothesis that inhaled nanoparticles cause neurodegenerative effects. Some, but probably not the majority of NPs, will have a significant toxicity (hazard) potential, and this will pose a significant risk if there is a sufficient exposure. The challenge is to identify such hazardous NPs and take appropriate measures to prevent exposure.
Keywords: Nanoparticle, inhalation, translocation, brain, neurodegeneration
1. Introduction
Engineered nanoparticles (NPs, <100 nm in size in any dimension) are manufactured at an increasing rate, characterized by diverse chemistries, different crystalline or amorphous states, and different shapes, and feature desirable superior mechanical, chemical, electrical or optical properties for use in multiple applications, including diverse consumer and industrial products, and for medical purposes. However, in the wake of these exciting developments and discoveries are mounting concerns that inadvertent or unavoidable exposures of humans and the environment to NPs will give rise to undesirable effects for human and environmental health. That is because the same properties that make NPs so desirable for numerous applications may also enhance their potential to induce toxicity in living systems. For example, the exponentially increasing surface area per unit volume with decreasing nanoparticle size is equivalent to an increasing ratio of the number of particle's surface atoms (or molecules) to the number of total atoms (or molecules) of the particle. This greater surface area per volume has the potential to make the smaller NPs chemically more reactive and to render them biologically more active per given mass. Obviously, this general surface area concept is modified by other particle properties, e.g., chemistry or shape, so that even small chemical alterations or defects of the NP surface can change its activity (Jiang et al., 2008). A specific concern is that any contact with NPs – whether by inhalation, ingestion or dermal – is thought to result in significant uptake and internal exposure of sensitive organs, such as the central nervous system (CNS), and cause permanent damage (Minkel, 2007). These concerns are based on studies showing high toxicity of some NPs when administered at very high doses; however, a high hazard potential is not equivalent to a high risk; thus an understanding of NP biokinetics relevant to exposure levels is essential. Some of the underlying concepts are discussed in this paper.
2. Concepts of inhalation nanotoxicology
Among the different routes of exposure to NPs, the respiratory tract is considered to be a major portal-of-entry because of the likelihood that NPs will become airborne during handling. Results from numerous in vitro and in vivo toxicological studies as well as epidemiological studies of susceptible populations revealed adverse effects of ambient atmospheric ultrafine particles (<100 nm) in toxicological studies and in susceptible parts of the population (EPA, 2004). Knowledge acquired from these studies as well as from in vitro and in vivo studies with NPs clearly shows that the interactions of NPs with the organism, cells and tissues can be very different from those of larger particles. Table 1 contrasts some of these differences, but also shows similarities with respect to physicochemical properties and biological/toxicological effects, assuming for the latter the respiratory tract as portal-of-entry. Although NPs are considered to be particles <100 nm in diameter by toxicologists and material scientists, this definition should not imply a rigid division between nano-sized particles and larger ones, but more so a vague transitional phase in physicochemical terms that is material-specific. For example, 240 nm polystyrene particles deposited in the alveolar region were not found to translocate across the alveolo-capillary barrier into the blood circulation; however, when coated with lecithin these particles did translocate and appeared in blood monocytes (Kato et al., 2003). Thus, even particles larger than 100 nm can translocate across the alveolo-capillary barrier depending on surface modifications. Therefore this transitional phase between NPs <100 nm and particles >500 nm is left open in Table 1.
Table 1.
Particle Size Differences: Findings from in vivo and in vitro studies with emphasis on respiratory tract
| Nanoparticles (<100 nm) | Larger Particles (>500 nm) | |
|---|---|---|
| Physico-chemical Interactions: | ||
| Ratio: number or surface area/volume | high | low |
| Agglomeration in air, liquids | Likely (dependent on medium; surfaec) | less likely |
| Deposition in respiratory tract | diffusion; throughout resp. tract | sedimentation, impaction, interception; throughout resp. tract |
| Protein/lipid adsorption in vitro | very effective and important for bio-kinetics and effects | less effective |
| Translocation to secondary target organs: | yes | generally not (to liver under “overload”) |
| Clearance | ||
| — mucociliary | probably yes | efficient |
| — alv. macrophages | poor | efficient |
| — epithelial cells | yes | mainly under overload |
| — lymphatic | yes | under overload |
| — blood circulation | yes | under overload |
| — sensory neurons (uptake + transpon) | yes | under overload |
| Proteinl/lipid adsorption in vivo | yes | some |
| Cell entry/uptake | yes (caveolae: clathrin; lip. rafts diffusion) | yes (primarily phagocytic cells) |
| — mitochondria | yes | no |
| — nucleus | yes (<40 nm) | no |
| Effects (caveat: dose, particle chemistry): | ||
| at secondary target organs | yes | no |
| at portal of entry (resp. tract) | yes | yes |
| — inflammation | yes | yes |
| — oxidative stress | yes | yes |
| — activation of signaling pathways | yes | yes |
| — genotoxicity, carcinogenicity | probably yes | some |
Some fundamental differences between NPs and larger particles include deposition behavior in the respiratory tract and mechanisms related to clearance, cell entry and translocation to secondary organs. With respect to the type of effects that have been observed in studies with nano-sized particles and larger particles, there does not appear to be a major difference. However, one key difference is related to the uniqueness of NPs to translocate from the respiratory tract to secondary target organs. Thus, effects such as inflammation, oxidative stress and molecular cell activation are likely to occur not only in the primary organ of entry, but also in secondary target organs. Such effects are unlikely to occur with larger particles, except under lung particle overload conditions (silicosis of the liver, spleen, bone marrow [Eide et al., 1984; Slavin et al., 1985]) and in the case of asbestos-induced mesothelioma and associated lympho-hematogenic spread of asbestos fibers (Brown, 1974). An important caveat to keep in mind is that Table 1 refers to the particles themselves and not to any soluble fractions, either of the particle or of adsorbed materials.
2.1 Dose and dose rate as key concepts of nanotoxicology
The aforementioned studies on particle lung overload in rats (Morrow, 1992) demonstrate that overwhelming the capacity of alveolar macrophages to phagocytize and clear retained particles from the alveolar region of the lung results in severe lung injury by mechanisms that are not operational at lower doses. Dose, dose rate and dose metrics are critical determinants of effects which need to be considered when designing toxicological studies. This is indicated in Table 1 with an important cautionary note regarding the issue of dose. High doses administered as a bolus in an animal study (e.g., via intratracheal instillation) or to cell cultures can readily identify a NP as hazardous on the basis of observed significant inflammatory/oxidative stress responses. Although such studies are valuable and may be used for ranking the toxicity of newly developed NPs against a reference or benchmark particle, observed effects may not be directly extrapolated as occurring under in vivo exposure conditions (Driscoll et al., 2000). A key difference is the dose rate (dose per unit time) in addition to the amount of the delivered dose (dose per unit surface area of the respiratory tract). The mechanisms underlying effects induced by a high dose rate (bolus delivery) are likely very different from those induced when the same dose is delivered by inhalation over days, weeks or months. Results from bolus type dose delivery should not be used for purposes of risk assessment; however, when designed as dose-response studies including reasonably low doses, they can be very valuable as hypothesis forming or proof of principle studies, to be validated in vivo.
As an example, the oxidative stress-inducing capacity of nano-TiO2 (~25 nm primary particle size with ~150 nm aggregates [Jiang et al., 2008]) was described in a well-designed in vitro dose-response study using mouse brain microglia cells, showing that concentrations of 10 ppm of nano-TiO2 induced reactive oxygen species (Long et al., 2006). This is an intriguing finding; however, the result was unfortunately misinterpreted by the popular press, despite the cautionary tone of the study's authors, with the eye-catching headline “Nanoparticles in sun creams can stress brain cells” (Ball, 2006). Instead, the results need to be considered in the context of the biokinetics of NPs from the portal of entry to the brain. Even if nano-TiO2 in skin care products could enter the blood circulation by crossing the skin barrier – which has not yet been demonstrated despite concerted efforts (Lademann et al., 2007) – it would be only miniscule amounts. In order to get to the brain, the very tight blood brain barrier (BBB) must be overcome next, which is another event of low probability. Any amount of nano-TiO2 that may travel from skin to brain will result in orders of magnitude lower concentrations than used in in vitro studies. Thus, confirmation of in vitro results through realistic in vivo studies is mandatory to test hypotheses generated from in vitro studies.
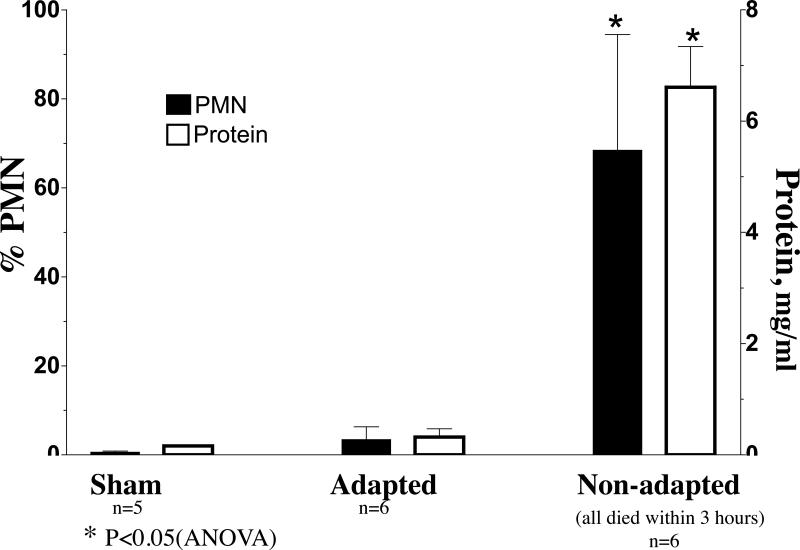
The generally held view that a non-cytotoxic in vitro dose is realistic and expected to occur under relevant in vivo exposure conditions needs to be critically assessed. The relevancy of doses and the impact of dose rate should carefully be considered and discussed each time. The usually very low dose rates experienced in vivo are likely to induce adaptive physiologic (often protective) responses which can render the organism unresponsive to even high doses. For example, a 15-minute inhalation exposure of rats to highly toxic PTFE (polytetrafluoroethylene) fumes (ultrafine particles, ~20 nm) caused severe lung damage and mortality; however, exposures for 5 minutes on 3 consecutive days resulted in adaptive responses (e.g., up-regulation of antioxidants) that completely protected the rats from the toxicity of a subsequent 15-minute exposure, whereas all simultaneously exposed non-adapted rats died (Figure 1; Johnston et al., 2000).
Figure 1.
Lung lavage neutrophils and protein response in PTFE-fume adapted and non-adapted F-344 rats. Adapted rats were exposed for 3 days for 5 min. each day to PTFE fumes, followed on Day 4 by a 15-min. exposure. Non-adapted rats were sham-exposed on 3 days for 5 min. and PTFE-fume exposed for 15 min. on day 4. Control rats were sham-exposed on all days for the same duration. Mean particle size was ~20 nm at concentration of 5×105 part/cm3 (~50 μg/m3).
With respect to the BBB, on-going research is aimed at developing strategies to overcome this hurdle so NPs administered into the blood circulation can be used for efficient drug delivery to the brain (Kreuter, 2007). One strategy involves adsorbing specific proteins, peptides or surfactants onto the surface of biodegradable NPs in order to prolong particle circulation in blood, as well as augment specific interactions with endothelial transport receptors, thereby optimizing NP crossing potential without altering normal BBB function. For example, a recent study showed that only cholesterol-terminated polyethylene glycol nanoparticles (150-200 nm) functionalized with a tyrosine aminotransferase peptide were shown to localize after tail-vein injection in the neural stroma of the rat hippocampus (Liu et al., 2008). In addition, the upper respiratory tract can also serve as a portal-of-entry for NPs to the blood and to the CNS, which will be discussed in the following section.
3. NP Translocation to the Brain and Effects
3.1 Deposition of inhaled NPs and translocation pathways
The deposition of inhaled NPs in the respiratory tract is governed by random motion due to bombardment by gas molecules, known as Brownian motion or diffusion. Deposition by this mechanism is greatest for the smallest NPs, as shown in Figure 2 for the adult human respiratory tract, assuming nose breathing at 10 L/min (ICRP, 1994). Thus, about 85% of airborne NPs of about 1 nm in size will be deposited by this diffusional deposition in the upper respiratory tract, whereas in the tracheobronchial and alveolar regions of the lower respiratory tract, peaks of deposition are for NPs of around 5 nm (~35%) and of 20 nm (~50%), respectively. Of course, if the primary particles of NPs are agglomerated, the deposition efficiency is a function of the larger agglomerate size. Gravitational (sedimentation) and inertial (impaction) forces increasingly become determinants of deposition for particle sizes above 200 nm.
Figure 2.
Predicted deposition fraction of inhaled particles by region in the human respiratory tract during nasal breathing. Diffusion is the main mechanism by which nano-sized particles (<0.1 μm) deposit. Based on ICRP (1994)
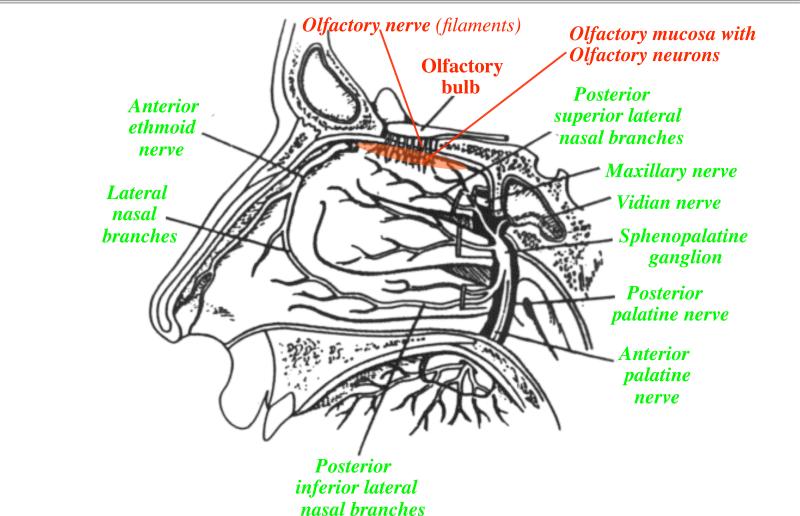
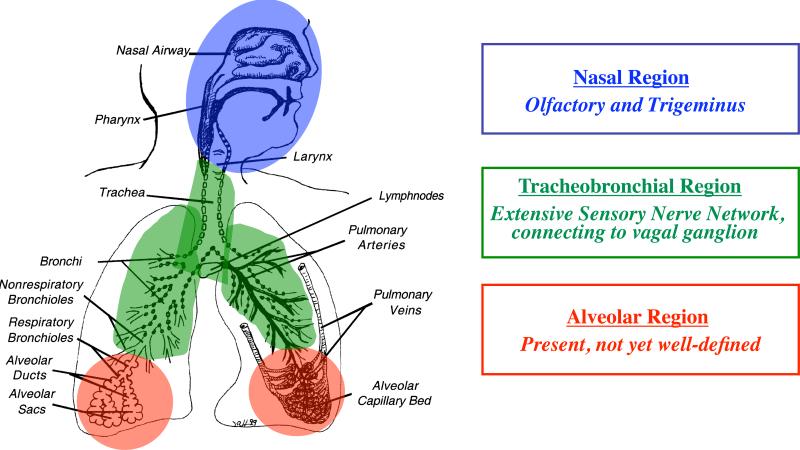
The high diffusional deposition of the smallest NPs in the upper respiratory tract has significant biological/toxicological implications because NPs of this size range behave similarly to smell molecules in the inspired air that are directed at the olfactory mucosa (Figure 3). For efficient smell recognition, numerous neurons embedded in the olfactory mucosa are connected to the nasal lumen through their dendrites. The significance of sensory nerve structures lies in the potential that neuronal axons and dendrites can transport nanoparticles in retrograde and anterograde directions (Adams and Bray, 1983). Indeed, this was demonstrated for the olfactory nerve by the recent confirmation of a little noticed earlier discovery that nanoparticles deposited in the nasal cavity can translocate with amazing velocity along this sensory neuronal pathway to the olfactory bulb (DeLorenzo, 1970; Oberdörster et al., 2004). The nasopharyngeal airways and the tracheobronchial airways are supplied with sensory nerves as well, whereas the presence of sensory nerves in the alveolar region of the lung is less defined (Figure 4). These sensory nerves have either direct (olfactory and trigeminus nerve) or indirect (tracheobronchial, via vagus) connections to specific areas of the CNS.
Figure 3.
Outline of human nasal cavity indicating olfactory and trigeminal nerve supply of nasal olfactory region and turbinates. (Modified from Illum, 2000).
Figure 4.
Sensory nerves in the respiratory tract, consisting of dense networks in the upper respiratory tract and tracheobronchial region and some in the alveolar region.
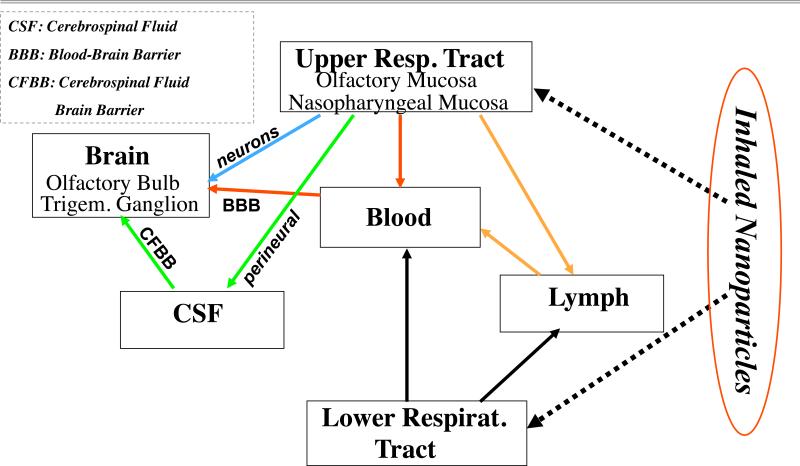
Thus, translocation of NPs depositing in the respiratory tract can occur along different routes, as indicated in the previous paragraphs and illustrated in Fig. 5. NPs depositing in the upper and lower respiratory tract may translocate directly to the blood compartment or via the nasal and lung lymphatic circulation. Based on studies published thus far, translocation rates for NPs into the blood circulation appear to be very low (Kreyling et al., 2002).
Figure 5.
From respiratory tract to brain: Potential translocation pathways of nanoparticles after deposition in the upper and lower respiratory tract.
Subsequent translocation of blood-borne NPs across the tight junctions of the BBB in vivo has not been conclusively demonstrated; although experimental drug delivery via intravenously administered NPs to the brain has been reported (Kreuter, 2007; Liu et al., 2008), it is still an open question as to whether the NPs in those studies did localize in brain tissue or had just entered the endothelial cells of the CNS vasculature and the drug was released to the brain from there. NP translocation across the BBB is more likely to occur at sites where this barrier is less well developed or injured, and thus leaky, i.e, at the circumventricular areas. For example, destroying the integrity of the BBB by osmotic pressure or by extremely high doses of metal NPs (30 mg/kg, i.v. or 50 mg/kg i.p. of ~50 nm Cu or Ag or 60 nm Al particles) can deliver NPs to the brain (Sharma, 2007). The doses used in these studies cannot be achieved under realistic in vivo conditions, and results need to be interpreted with caution. The mechanisms of NP transfer across the intact BBB remain elusive and there are still open questions as to the long-term consequences of NP accumulation in the CNS and their fate within CNS structures.
Translocation to CNS structures from the upper respiratory tract via neuronal pathways is now well established but seems to be of low efficiency. Table 2 summarizes studies that demonstrated olfactory nerve translocation of different nano-sized particles following dosing of several animal species by intranasal instillation or whole body inhalation. Estimates from instillation and inhalation studies of the amount translocated from deposits on the nasal olfactory mucosa range from <1% to more than 10%. This is apparently dependent on NP surface chemistry, size (primary particle vs. agglomerate), dose and exposure method as discussed below. It is interesting to note that a loss in olfactory function is a common feature of neurodegenerative diseases such as Parkinson's, Alzheimer's and Huntington's Disease (Barrios et al., 2007; Doty, 2008; Kovács, 2004; Moberg and Doty, 1997), raising the possibility that olfactory translocation of inhaled NPs and olfactory neuropathology might be etiologically linked.
Table 2.
Olfactory Nerve Nanoparticle Translocation to Brain
| NP-Type | Species | Authors/Year |
|---|---|---|
| 30 nm Polio-virus (inst) | Chimpanzee | Bodian and Howe, 1941 |
| 50 nm Silver-coated gold (inst) | Squirrel monkey | DeLorenzo, 1970 |
| 36 nm Carbon (inhal) | Rat | Oberdörster et al., 2004 |
| 30 nm Mn-oxide (inhal) | Rat | Elder et al., 2006 |
| 76 nm Co-polymer (inst) | Rat | Zhang et al, 2006 |
| 30 – 110 nm gold (inhal) | Rat | Yu et al, 2007 |
| 80 nm Rutile/155 nm Anatase TiO2 (inst)* | Mouse | Wang et al., 2008a,b |
inst = intranasal instillation inhal = whole body inhalation
Note: Extremely high instilled doses of 500 μg and 1 mg/mouse were used.
An interesting translocation pathway depicted in Figure 5 is suggested by the study of Czerniawska (1970), which describes the appearance of radioactive nanogold (198Au) particles in the cerebrospinal fluid (CSF) following submucosal injection into the nasal olfactory area of rabbits. This author observed highest radioactivity in the CSF adjacent to the cribriform plate of the skull, the area where olfactory nerve axons emerge and connect to the olfactory bulb (Figure 6). High 198Au activity was also measured in CSF surrounding the olfactory bulb and the corpus callosum cistern. Czerniawska (1970) confirmed from these results earlier findings that there is a direct connection between the olfactory mucosa and the CSF (Orosz et al., 1957). 198Au activity in CSF can be interpreted as input from perineural transport of nanogold particles which has been described as a very rapid translocation route from nose to brain (Illum, 2000). In order to access the brain from the CSF compartment, the cerebrospinal fluid brain barrier has to be overcome (Figure 5). An interesting alternative to this interpretation is that nanogold particles after axonal transport to the olfactory bulb cross into the CSF space and are distributed to different brain areas (Segal, 2000). Further research is needed to identify the biokinetics of NPs within the CNS and associated structures.
Figure 6.
Nanogold (198Au) activity of cerebrospinal fluid (CSF) collected at different locations of the brain surface one or two hours after nasal olfactory submucosal injection in rabbits. (based on results of Czerniawska, 1970).
The importance of the CSF compartment for delivery of nanoparticles and nanomedicines from the nose to the brain was recently demonstrated when researchers showed that a neuroprotective drug encapsulated within 80 nm MPEG-PLA NPs was significantly and directly transported from the olfactory mucosa to the olfactory bulb, CSF, and other brain regions, i.e., bypassing systemic transport from the blood (Zhang et al., 2006). CSF fluid had the highest direct transport percentage of the drug after nasal NP delivery, it was even higher than in the olfactory bulb; all CNS compartments received 1.6 – 3.3-fold more of the drug when bound to the NP than when the drug was delivered in solution (Figure 7).
Figure 7.
“Direct Transport Percentage” to CNS regions and Cerebrospinal Fluid (CSF) of drug loaded into co-polymer nanoparticles ( ) or as solution (
) or as solution ( ) during 6 hrs following intranasal instillation in rats. (redrawn from results by Zhang et al., 2006).
) during 6 hrs following intranasal instillation in rats. (redrawn from results by Zhang et al., 2006).
3.2 Neuronal NP translocation to olfactory bulb and CNS effects
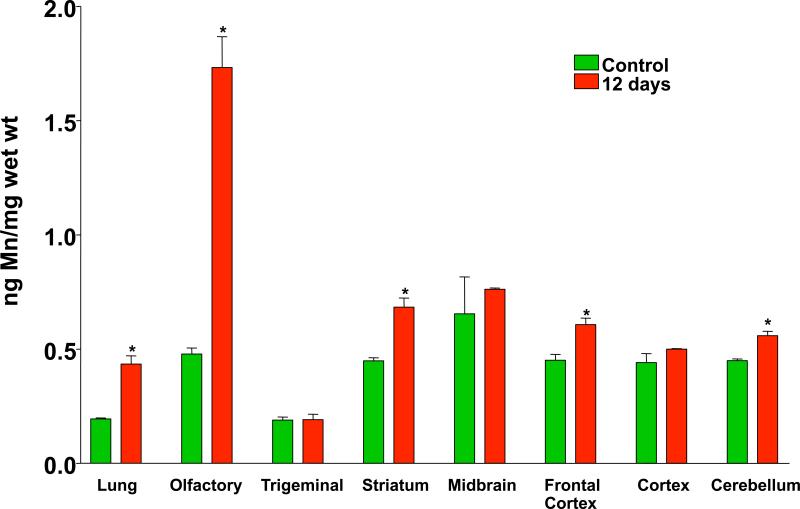
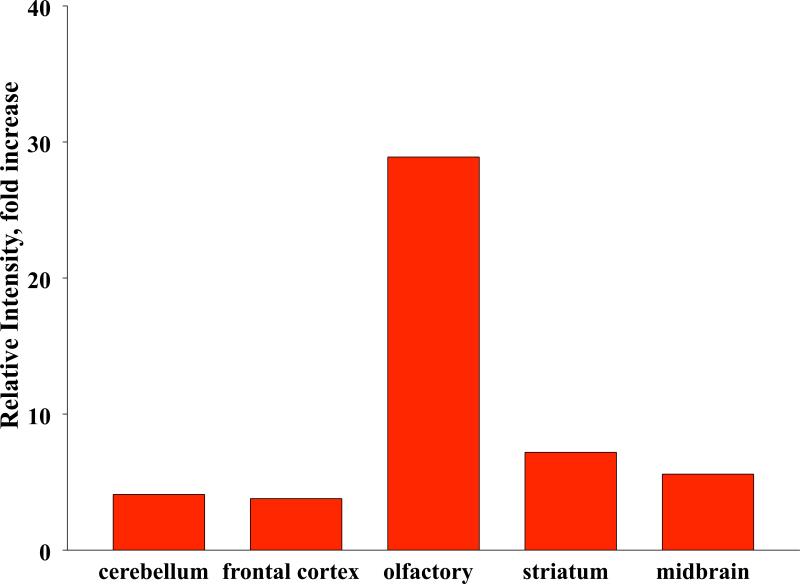
Elder et al. (2006) carried out several inhalation studies in rats with Mn-oxide nanoparticles (33 nm count median diameter in the airborne state) to test olfactory nerve translocation and the potential of such exposure to induce effects. After occlusion of the right nostrils of the rats, they were exposed to Mn-oxide NPs by inhalation on 2 consecutive days for 6 hours each day. Only the left olfactory bulb showed a large increase in Mn, with no increase of Mn in the right olfactory bulb (Figure 8). This result confirmed the olfactory neuronal pathway of NP translocation from nose to brain and excluded the possibility that a significant contribution may have come from blood-borne Mn. An estimation of the amount depositing on the olfactory mucosa using a computerized Multiple Path Particle Deposition Model (Asgharian et al., 1999) showed that the amount of Mn translocated to the olfactory bulb was 11% of the deposit on the olfactory mucosa. In a subsequent 12-day (6 hrs/day) inhalation exposure with both nostrils open, Mn concentration in the olfactory bulbs increased almost 4-fold, with slight increases also in other brain regions, whereas lung Mn concentrations only increased a little more than 2-fold (Figure 9). Inflammatory responses were also observed in those brain regions that had increased Mn levels, with the olfactory bulb showing an almost 30-fold increase in TNFa (Figure 10). This may not be surprising, given that manganese is well recognized as a neurotoxicant and that dissolution of Mn-oxide NPs in brain tissue may reach very high local intracellular levels. It should be mentioned hat these inhalation exposures were performed at airborne concentrations of Mn that are similar to what is generated by arc welding (0.01-5 mg/m3). Indeed, altered locomotion has been described in a cohort of welders (Finkelstein et al., 2007).
Figure 8.
Inhalation of ultrafine (~30 nm) Mn-oxide particles in rats with right nostril occluded. Accumulation of Mn in right and left olfactory bulb 24 hours after a 6- hr. exposure to ultrafine Mn-oxide particles (n=3-5, mean ± SD). Green = left olfactory bulb; red = right olfactory bulb. Eleven percent of nano-Mn-oxide depositing on the olfactory mucosa was estimated to have translocated to the olfactory bulb. (Elder et al., 2006).
Figure 9.
Mn concentration in lung and brain regions of rats following 12 days of ultrafine Mn-oxide exposure (mean ± SD; *significant increases vs. control). (465 μg/m3; 17×106 part./cm3; CMD: 31 nm; GSD: 1.77). (Elder et al., 2006).
Figure 10.
Changes of TNF alpha expression in brain regions after 12 day exposure (as specified in Fig. 9) to ultrafine Mn-oxide in rats (fold increase over control).
Obviously, the inflammatory response induced in rats by Elder et al. (2006) through realistic and relevant inhalation exposures to Mn-oxide NPs should be regarded as a serious health concern. However, it would be premature to suggest that all NPs have the same neuro-inflammatory potential. There are many significant differences among NPs in terms of physicochemical characteristics that influence both NP biokinetics as well as responses, and this has to be evaluated on a case-by-case basis. However, particular caution has to be exercised to avoid situations where exposures to high concentrations of airborne NPs occur. Prudence calls for personal protection equipment and engineering controls to guard against such exposures.
There is an urgent need for identifying hazardous NPs and characterizing exposures and the biokinetics of NPs. For example, highly reactive inhaled materials such as the aforementioned PTFE fumes comprising nano-sized particles (Figure 1) which induce severe acute lung injury, are likely to also damage CNS structures. Indeed, gene array analysis of olfactory bulb tissue of PTFE-exposed rats revealed increased levels of inflammatory and oxidative stress-related genes (Figure 11, unpublished data). A large decrease in glutamate transporter stands out, possibly indicating an adverse effect on excitatory neurotransmitter removal which may result in toxic glutamate buildup and dysregulation of glutathione levels in the extracellular space of CNS tissue. The specificity of this response and its implications need to be examined in further studies. Of course, because of the high acute pulmonary and associated systemic toxicity the observed gene expression changes in the olfactory bulb may just be a manifestation of the general high systemic toxicity induced in the PTFE-exposed rats. Exposures of humans to metal and polymer fumes (all consisting of nano-sized particles) are well known to induce CNS symptoms such as headaches; however, there are no data in humans demonstrating translocation of fume particles to the brain.
Figure 11.
Microarray analysis of rat olfactory bulb tissue after PTFE fume exposure (4 hrs. after a 15 min. exposure at 2.6×106 particles/cm3, ~20 nm).
As pointed out above, significant differences among NPs with regard to their translocation kinetics to and effects in the CNS should be expected because of the vast differences in physico-chemical properties of different NPs. For example, in contrast to the result with inhaled Mn-oxide NP (Figures 8 and 9) only a very small fraction of gold NPs deposited by intranasal instillation onto the olfactory mucosa was translocated to the olfactory bulb in rats (Figure 12). This was found with different sizes of nanogold particles that were coated with rat serum albumin to avoid agglomeration when these particles were suspended in physiological saline (Rinderknecht et al., 2007). There appears to be a size dependency, though, with the larger 20 nm gold particles translocating less than the 2 and 5 nm particles. Surface coating also appears to affect translocation rate since more of the uncoated 35 nm Mn-oxide particles translocated to the olfactory bulb compared to the 35 nm PEG-coated Q-dots and similar-sized albumin-coated Au particles. Overall, the translocation rates at 24-hours post exposure were less than 0.01% of the instilled dose. This low translocation rate may be due to several factors: (i) intranasal instillation may not dose all areas of the olfactory mucosa; (ii) in contrast, inhalation of singlet NPs optimizes deposition on the olfactory mucosa in terms of total amount and evenness of distribution; and (iii) perhaps more importantly, nasal instillation is equivalent to a bolus administration to the nasal mucosa, where efficient ciliary clearance mechanisms exist. The high dose rate of instillation is very different from the low dose rate of inhalation which represents continuous dosing over many hours.
Figure 12.
Nanoparticle translocation to olfactory bulb 24 hrs. after left intranasal instillation in rats. Percent of total instilled dose of nanogold particles (10 μg) of different sizes coated with rat serum albumin (RSA), and Cd-Se quantum dots (5 μg Cd) with different surface modification measured in the olfactory bulb. Intranasally instilled nano-Mn-oxide (10 μg) (see Fig. 9) is shown for comparison.
The importance of dose rate for affecting not only the response but also NP biokinetics is supported by results from studies in which the same NP was administered by both methods. As described above, Elder et al., (2006) estimated that 11% of Mn-oxide NPs deposited on the olfactory mucosa by inhalation had translocated to the olfactory bulb in rats. In a separate study, the same Mn oxide particles were administered by intranasal instillation and only 0.01% of the intranasally administered amount accumulated in the olfactory bulb (Figure 12). Moreover, intranasal instillation of differently coated CdSe-ZnS polymer-capped quantum dots (Elder et al., 2007) in rats revealed even lower translocation rates which were similar to the nanogold particles (Figure 12). In contrast, estimates of nose-to-olfactory bulb translocation from another short-term inhalation study in rats with inhaled carbon NPs (count median diameter 36 nm) were ~20%, although this value is probably associated with considerable uncertainty(Oberdörster et al., 2004).
A reasonable conclusion from these diverse results of nose-to-brain translocation seems to be that the physiological route of inhalation (assuming nasal breathing) is a more effective way of dosing the olfactory mucosa with NPs for subsequent translocation to the brain than intranasal instillation. Of course, this conclusion has to be considered in the context of the deposition efficiency of inhaled NPs in the nose, which is very high for 1 nm particles but an order of magnitude lower for 70 nm particles (Figure 2). As discussed before, NP translocation to the CSF seems to be more efficient than directly to the olfactory bulb. Regardless, highest NP concentrations in the CNS – which could be either toxic or therapeutic – are expected to be achieved with the smallest inhaled NPs, whereas larger inhaled NPs predictably result in higher concentrations in other parts of the body.
3.3 NP surface properties affecting translocation
Particle size is only one of several NP properties that determine their kinetics and effects in the organism. Other physicochemical characteristics, in particular those related to NP surface, are decisive as is illustrated by the concept of differential adsorption. This concept states that physicochemical properties of NPs and conditions at the portal-of-entry determine adsorption of proteins/lipids on the NP surface, which in turn determines their biokinetics and effects (Figure 13). For example, NPs depositing in the respiratory tract will come into contact with the epithelial lining fluid containing proteins and lipids that can adsorb onto the NPs. This alters NP properties and, as a result, affects their biokinetic behavior and cell entry. Subsequent translocation to the interstitium, lymph or blood circulation may result in successive protein coatings. This secondary coating process is dynamic and depends on adsorption and desorption constants of the different proteins and their relative concentrations in the surrounding milieu and, therefore, changes with time. Upon cell entry of NPs, coating with the cytoskeletal proteins, actin and vimentin has been observed (Ehrenberg and McGrath, 2005), raising the question that this may affect cytoskeletal networks.
Figure 13.
Concept of differential adsorption for NP (corona formation) stating that physicochemical properties of NPs and the milieu at the portal-of-entry alter NP surface properties and determine their fate with respect to translocation to secondary organs and cell entry. (Modified from Müller and Heinemann, 1989 as referenced in Müller and Keck, 2004).
Cedervall et al. (2007) have identified a number of human plasma proteins that bind to co-polymer NPs. They found different classes of proteins, most prominently apolipoproteins, albumin, fibrinogen and others, which formed a protein corona on NPs during incubation with plasma. It is of interest to note that in other studies coating of polymer NPs with apolipoprotein E was found to enhance delivery across the BBB of drug-loaded NPs, probably via LDL receptors on endothelial cells of brain capillaries (Kreuter, 2007). An interesting finding with respect to NP-protein association and corona formation was reported by Linse et al. (2007) who found that under specific in vitro conditions different types of NPs accelerated fibrillation of an amyloid protein (Figure 14). A general hypothesis derived from these proof-of-principle studies is that NPs may be an etiological factor for amyloid diseases, such as Parkinson's, Alzheimer's, and Creutzfeld-Jakob diseases and dialysis-related amyloidosis. Proving this hypothesis that NPs may cause protein fibrillation under realistic in vivo conditions would have far-reaching consequences, in particular with respect to underlying mechanisms of neurodegenerative diseases (Colvin and Kulinowski, 2007). The necessity for more research in this area is obvious.
Figure 14.
In vitro studies of NPs incubated with human β2 microglobulin resulting in protein corona formation on particles (Linse et al., 2007). This nanoparticle-protein association led to nucleation and acceleration of protein fibrillation (unfolding) by the nanoparticles. (shown here for Co-polymer NP; was also observed by the authors with QDs, Ceria, CNTs).
3.4 NP biokinetics based on low translocation rates
From studies like these, researchers become increasingly aware of the concept that coating of NPs with specific proteins, lipids or polymers can significantly alter their kinetics in the organism and their interactions with cells. Studies evaluating this concept of differential adsorption revealed significant differences between gold particles coated with either PEG or albumin, administered either to the lower respiratory tract or directly intravenously into rats (Rinderknecht et al., 2007). These studies confirmed that NP biokinetics can be modified by their coating as well as the portal-of-entry, as is schematically illustrated in Figures 15 for two target organs: brain and liver. Of course, major differences between the two routes of exposure exist with respect to the input into the blood compartment. While both represent bolus-type dosing, input of NPs into the blood compartment from deposits in the respiratory tract occurs (i) at a much lower overall dose; (ii) at a very low dose rate; and (iii) into the pulmonary venous circulation (oxygenated blood, entering systemic arterial circulation) which is in contrast to systemic venous circulation of i.v. injection. The secondary coating of NPs along this translocation pathway will very likely affect their fate once they enter the blood circulation, as discussed above. In addition, NP size also modifies NP kinetics, as will be described in a detailed forthcoming paper.
Figure 15a and b.
Concept: Portal-of-entry and nanoparticle coating affect NP biokinetics. Depicted is a simplified scheme with only the brain and liver as target organs following dosing of the respiratory tract or the blood compartment with pegylated or albumin-coated gold NPs. Thickness of the arrows reflect approximate amount of translocation.
Protein coating of NPs during incubation in biological media depends on particle surface properties and on the components in the biological medium. For example, it is conceivable that incubation of NPs in blood plasma from humans, rats, or mice leads to different NP-protein complexes. Whether this causes differences with respect to NP biodistribution and effects between these species is unknown. However, the possibility of such differences might be an important factor to be considered for extrapolation of results from rodent studies to humans.
Although translocation rates of NP from the portal-of-entry to secondary organs may be very low, a continuous exposure may result in significant accumulation of NPs in a secondary target organ. Thus, it is important to obtain data on the retention characteristics of NPs in both primary and secondary target organs, including NP elimination pathways. Clearance from the upper and lower respiratory tract via muco-ciliary function are likely to transfer some of the NPs deposited there to the GI-tract with subsequent elimination via feces (Semmler et al., 2004; Semmler-Behnke et al., 2007). NPs translocating into the blood circulation can be eliminated via the kidneys; this elimination route seems to be limited by size to about 5 nm (Choi et al., 2007). However, Singh et al. (2006) and Lacerda et al. (2008) reported that even 20-30 nm thick multi-walled carbon nanotubes, 0.5–2 μm in length, were excreted in urine when intravenously administered to rats. They explain this by a hydrodynamic lining up of the nanotubes so they can pass through the glomerular pores. Blood-borne NPs accumulating in the liver are most likely eliminated via hepato-biliary excretion into the GI tract, a pathway that is well known for removal of some metals (Clarkson et al., 1988).
With regard to the CNS, no data on NP elimination are available yet. It is conceivable that the CSF via its connections to the nasal lymphatic system (Czerniawska, 1970) and to the blood circulation could be an excretory pathway for the brain, which needs to be investigated in future studies. Indeed, Segal et al. (2000) concluded from their review on CSF barriers that the CSF may both act as a compartment for distribution of substances to many brain regions, but also can act as elimination route for waste products into the blood circulation since the brain has no lymphatics. Figure 16 highlights known elimination pathways in an overall scheme of NP biokinetics following different routes of exposure.
Figure 16.
Exposure and biokinetics of nano-sized particles, emphasizing known elimination pathways (thick red arrows) or unknowns (?) (modified from Oberdörster et al., 2005).
4. Conclusions
Engineered NPs (<100 nm in any of 3 dimensions) show high diversity with respect to physicochemical properties, which in turn affect their interactions with organisms, cells and subcellular compartments. Upon inhalation, airborne NPs have a high deposition efficiency in the upper and lower respiratory tract, with maxima of deposition for different NP sizes in each region. Deposition is highest in the nasal compartment for the smallest NPs due to diffusional movement. Key considerations for NPs include:
The potential of NPs to translocate from the site of deposition in the respiratory tract to secondary organs via the blood circulation. However, results to date show that only a very small fraction seems to translocate.
Coating of the surface (primary when manufactured; secondary when interacting with biological media in the organism) can significantly impact biokinetics and effects.
NPs can access the CNS via different pathways, including neuronal and paraneuronal (upper respiratory tract; CSF) and the systemic blood circulation.
Neuronal translocation of NPs appears very low after non-physiologic dosing of the upper respiratory tract, but appears to be greater following inhalation.
Depending on NP chemistry and reactivity, significant inflammatory responses in the CNS may be induced following inhalation exposure.
At present, neurotoxicity caused by NPs following relevant inhalation exposure is only established for Mn oxide NPs in an animal model, and many open questions need to be resolved before more definitive statements about adverse CNS effects can be made. Research needs include evaluation of mechanisms of NP uptake and axonal translocation, changes of NP surface chemistry during neuronal transport, retention and distribution kinetics and elimination pathways in the CNS. Until more is known about these specific open questions and specific protective measures can be established, exposure to airborne NPs of unknown reactivity should be minimized. Consumer products giving rise to airborne NPs when used should not be marketed without prior appropriate toxicity testing.
Acknowledgements
The research described in this paper was supported in part by grants from the U.S. Department of Defense AFOSR (MURI FA9550-04-1-0430), U.S. EPA (RD-83172201 and PM Center Grant RD 832415), NSF SGER Grant (BES-0427262), and NIEHS (P30 ESO1247).
References
- 1.Adams RJ, Bray D. Rapid transport of foreign particles microinjected into crab axons. Nature. 1983;303:718–720. doi: 10.1038/303718a0. [DOI] [PubMed] [Google Scholar]
- 2.Asgharian B, Miller F, Subramaniam RP. Dosimetry software to predict particle deposition in humans and rats. CIIT Activities. 1999;19(3) [Google Scholar]
- 3.Ball P. Nanoparticles in sun creams can stress brain cells. Nature (News) 2006 doi: 10.1038; published on line June 16, 2006 ( http://www.nature.com/news/2006/060616/full/news060612-14.html)
- 4.Barrios FA, Gonzalez L, Favila R, Alonso ME, Salgado PM, Diaz R, Fernandez-Ruiz J. Olfaction and neurodegeneration in HD. Neuroreport. 2007;18(1):73–76. doi: 10.1097/WNR.0b013e3280102302. [DOI] [PubMed] [Google Scholar]
- 5.Bodian D, Howe HA. Experimental studies on intraneural spread of poliomyelitis virus. Bull. Johns Hopkins Hosp. 1941;69:248–267. [Google Scholar]
- 6.Brown A. Lymphohematogenous spread of asbestos. Environ. Health Perspect. 1974;9:203–204. doi: 10.1289/ehp.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cedervall T, Linse I, Lindman S, Berggård T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. PNAS. 2007;104:2050–2055. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nature Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarkson TW, Friberg L, Nordberg GF, Sager PR, editors. Biological Monitoring of Toxic Metals. Plenum Press; 1988. p. 686. [Google Scholar]
- 10.Colvin VL, Kulinowski KM. Nanoparticles as catalysts for protein fibrillation. PNAS. 2007;104(21):8679–8680. doi: 10.1073/pnas.0703194104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czerniawska A. Experimental investigations on the penetration of 198Au from nasal mucous membrane into cerebrospinal fluid. Acta Otolaryng. 1970;70:58–61. doi: 10.3109/00016487009181859. [DOI] [PubMed] [Google Scholar]
- 12.De Lorenzo AJ. The olfactory neuron and the blood-brain barrier. In: Wolstenholme G, Knight J, editors. Taste and Smell in Vertebrates. Churchill, London: 1970. pp. 151–176. [Google Scholar]
- 13.Doty RL. The olfactory vector hypothesis of neurodegenerative disease: Is it viable? Ann. Neurol. 2008;63:7–15. doi: 10.1002/ana.21327. [DOI] [PubMed] [Google Scholar]
- 14.Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdörster G, Salem H, Schlesinger RB. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: Uses and limitations. Toxicol. Sci. 2000;55:24–35. doi: 10.1093/toxsci/55.1.24. [DOI] [PubMed] [Google Scholar]
- 15.EPA EPA Health Criteria Document: Air Quality Criteria for Particulate Matter. 2004. EPA/600/P-99/002aF, October, 2004.
- 16.Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Ito Y, Finkelstein J, Oberdörster G. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elder A, Corson N, Gelein R, Mercer P, Rinderknecht A, Finkelstein J, Oberdörster G. Tissue Distribution in Rats of Surface-Modified Quantum Dots Following Lung or Intravenous Exposure. Am. J. Respir. Crit. Care Med. 2007;175:A246. [Google Scholar]
- 18.Eide J, Gylseth B, Skaug V. Silicotic lesions of the bone marrow: histopathology and microanalysis. Histopathology. 1984;8:693–703. doi: 10.1111/j.1365-2559.1984.tb02381.x. [DOI] [PubMed] [Google Scholar]
- 19.Ehrenberg M, McGrath JL. Binding between particles and proteins in extracts: implications for microrheology and toxicity. Acta Biomaterialia. 2005;1:305–315. doi: 10.1016/j.actbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein Y, Milatovic D, Aschneer M. Modulation of cholinergic systems by manganese. NeuroToxicology. 2007;28:1003–1014. doi: 10.1016/j.neuro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 21.ICRP International Commission on Radiological Protection. Human respiratory model for radiological protection. Ann. ICRP. 1994;24:1–300. [PubMed] [Google Scholar]
- 22.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Europ. J. Pharmacol. Sci. 2000;11:1–18. doi: 10.1016/s0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J, Oberdörster G, Elder A, Gelein R, Meercer P, Biswas P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology. 2008;3 doi: 10.1080/17435390701882478. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston CJ, Finkelstein JN, Mercer P, Corson N, Gelein R, Oberdörster G. Pulmonary effects induced by ultrafine PTFE particles. Toxicol. Appl. Pharmacol. 2000;168:208–215. doi: 10.1006/taap.2000.9037. [DOI] [PubMed] [Google Scholar]
- 25.Kovács T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res. Rev. 2004;3:215–232. doi: 10.1016/j.arr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Kreuter J. Nanoparticles — a historical perspective. Int. J. Pharmaceut. 2007;331:1–10. doi: 10.1016/j.ijpharm.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, Oberdörster G, Ziesenis A. Ultrafine insoluble iridium particles are negligibly translocated from lung epithelium to extrapulmonary organs. J. Tox. Environ. Health. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 28.Lacerda L, Soundararajan A, Singh R, Pastorin G, Al-Jamal KT, Turton J, Frederik P, Herrero MA, Li S, Bao A, Emfietzoglou D, Mather S, Phillips WT, Prato M, Bianco A, Goins B, Kostarelos K. Dynamic imaging of functionalized multi-walled carbon nanotube systemic circulation and urinary excretion. Adv. Mater. 2008;20:225–230. [Google Scholar]
- 29.Lademann J, Richter H, Teichmann A, Otberg N, Blume-Peytavi U, Luengo J, Weiβ B, Schaefer UF, Lehr C-M, Wepf R, Sterry W. Nanoparticles – An efficient carrier for drug delivery into the hair follicles. Europ. J. Pharm. Biopharm. 2007;66:159–164. doi: 10.1016/j.ejpb.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Linse S, Cabaleiro-Lago C, Xue W-F, Lynch I, Lindman S, Thulin E, Radford SE, Dawson KA. Nucleation of protein fibrillation by nanoparticles. PNAS. 2007;104:8691–8696. doi: 10.1073/pnas.0701250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, Guo K, Lu J, Venkatraman SS, Luo D, Ng KC, Ling E-A, Moochhala S, Yang Y-Y. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG-TAT for drug delivery across the blood-brain barrier. Biomaterials. 2008;29:1509–1517. doi: 10.1016/j.biomaterials.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV12): implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006;40:4346–4352. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- 33.Minkel JR. The truth about nanopollution. Popular Science. 2007 Jan;270(1):62. 2007. [Google Scholar]
- 34.Moberg PJ, Doty RL. Olfactory function in Huntington's disease patients and at-risk offspring. Int. J. Neurosci. 1997;89(1-2):133–139. doi: 10.3109/00207459708988468. [DOI] [PubMed] [Google Scholar]
- 35.Morrow PE. Contemporary Issues in Toxicology – Dust overloading of the lungs: Update and appraisal. Toxicol. Appl. Pharmacol. 1992;113:1–12. doi: 10.1016/0041-008x(92)90002-a. [DOI] [PubMed] [Google Scholar]
- 36.Müller RH, Keck CM. Drug delivery to the brain realization by novel drug carriers. J. Nanosci. Nanotechnol. 2004;4:471–483. doi: 10.1166/jnn.2004.078. [DOI] [PubMed] [Google Scholar]
- 37.Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhalat. Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 38.Oberdörster G, Oberdörster E, Oberdörster J. Invited Review: Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orosz A, Foldes I, Kosa C, Toth G. Radioactive isotope studies of the connection between the lymph circulation of the nasal mucosa, the cranial cavity and cerebrospinal fluid. Acta Physiologica Academiae Scientiarum Hungaricae. 1957;2:75–81. [PubMed] [Google Scholar]
- 40.Rinderknecht A, Elder A, Prud'homme R, Gindy M, Harkema J, Oberdörster G. Surface functionalization affects the role of nanoparticle disposition. Am. J. Respir. Crit. Care Med. 2007;175:A246. [Google Scholar]
- 41.Segal MB. The Choroid Plexuses and the Barriers Between the Blood and the Cerebrospinal Fluid. Cell. and Mol. Neurobiol. 2000;20(No. 2):183–196. doi: 10.1023/A:1007045605751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semmler M, Seitz J, Erbe F, Mayer P, Heyder J, Oberdörster G, Kreyling WG. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung including transient translocation into secondary organs. Inhalat. Toxicol. 2004;16:453–459. doi: 10.1080/08958370490439650. [DOI] [PubMed] [Google Scholar]
- 43.Semmler-Behnke M, Takenaka S, Fertsch S, Wenk A, Seitz J, Mayer P, Oberdörster G, Kreyling WG. Efficient elimination of inhaled nanoparticles from the alveolar region: evidence for interstitial uptake and subsequent re-entrainment onto airways epithelia. Environ. Health Perspect. 2007;115:728–733. doi: 10.1289/ehp.9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma HS. Nanoneuroscience: emerging concepts on nanoneurotoxicity and nanoneuroprotection. Nanomedicine. 2007;2:753–758. doi: 10.2217/17435889.2.6.753. [DOI] [PubMed] [Google Scholar]
- 45.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. PNAS. 2006;103:3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slavin RE, Swedo JL, Brandes D, Gonzalez-Vitale JC, Osornio-Vargas A. Extrapulmonary silicosis: a clinical, morphologic, and ultrastructural study. Hum. Pathol. 1985;16:393–412. doi: 10.1016/s0046-8177(85)80233-1. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Liu Y, Jiao F, Lao F, Li W, Gu Y, et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. Toxicology. 2008a;254(1-2):82–90. doi: 10.1016/j.tox.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Chen C, Liu Y, Jiao F, Li W, Lao F, et al. Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol. Letter. 2008b;183(1-3):72–80. doi: 10.1016/j.toxlet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Yu LE, Yung L-YL, Ong C-N, Tan Y-L, Balasubramaniam KS, Hartono D, Shui G, Wenk MR, Ong W-Y. Translocation and effects of gold nanoparticles after inhalation exposure in rats. Nantoxicology. 2007;1:235–242. [Google Scholar]
- 50.Zhang Q, Zha L, Zhang Y, Jiang W, Lu W, Shi Z, Jiang X, Fu S. The brain targeting efficiency following nasally applied MPEG-PLA nanoparticles in rats. J. Drug Targeting. 2006;14:281–290. doi: 10.1080/10611860600721051. [DOI] [PubMed] [Google Scholar]