Abstract
This is the first study of the effect of topiramate on linguistic behavior and verbal recall using a computational linguistics system for automated language and speech analysis to detect and quantify drug-induced changes in speech recorded during discourse level tasks. Healthy volunteers were administered a single, 100 mg oral dose of topiramate in two double-blind, randomized, placebo-controlled, crossover studies. Subjects’ topiramate plasma levels ranged from 0.23–2.81ug/mL. We found a significant association between topiramate levels and impairment on measures of verbal fluency elicited during a picture description task, correct number of words recalled on a paragraph recall test, and reaction time recorded during a working memory task. Using the tools of clinical pharmacology and computational linguistics, we elucidated the relationship between the determinants of a drug’s disposition as reflected in plasma concentrations and their impact on cognitive functioning as reflected in spoken language discourse.
Keywords: topiramate, cognition, verbal fluency, spontaneous speech, reaction time, plasma concentration
INTRODUCTION
Topiramate, a second-generation antiepileptic drug (AED), with formal indications for partial and generalized seizures and migraine prophylaxis, is being increasingly prescribed for a variety of other conditions including obesity, pain, bipolar disorder, and alcoholism. Despite its widespread use, topiramate is associated with adverse effects on attention and memory (1–3). Topiramate is also associated with a unique cognitive signature affecting language use in a subset of patients (3, 4), who often describe their impairment as “a word finding difficulty” (3, 4).
Topiramate’s broad spectrum of applications is likely a consequence of its multiple mechanisms of action that include modification of Na+ or Ca2+ dependent action potentials, enhancement of GABA-mediated receptors, and inhibition of kainate-mediated conductance at glutamate receptors of the AMPA/kainate type as well as carbonic anhydrase (CA II & IV) (5, 6). Yet the mechanisms by which topiramate’s effect on the brain impacts an individual’s cognition are poorly understood.
Moreover, not all individuals complain of topiramate-induced cognitive impairment, and the percent of those who are affected varies with the study and population under consideration (7). For example, between 11–20% of patients with refractory epilepsy treated with topiramate report some type of cognitive adverse event (8, 9). There is strong evidence that the number and magnitude of both subjective and objective accounts of topiramate-induced cognitive deficits can be partially attributed to the effects of polytherapy, titration rate, and maintenance dose in both patients and healthy adults (10, 11) yet these factors do not fully capture the majority of the inter-individual variability in the cognitive response to topiramate.
It has been postulated that the topiramate-induced language impairment is secondary to changes in frontal lobe or executive function rather than exerting direct effects on linguistic processing (2, 12). This hypothesis is supported by topiramate-induced decreases in neuropsychological measures of generative (phonemic) verbal fluency, working memory and attention (1–3, 13–19) that persist through the titration period (2, 20, 21) and improve after topiramate is discontinued (2, 12). Topiramate’s effect on verbal fluency may actually reflect a widespread disruption of language specific networks that include regions within the frontal and parietal cortices (22) and cerebellum (23, 24).
Current investigations into topiramate’s effect on language and cognition are limited by the use of laboratory-based neuropsychological assessment tools that do not capture the interplay of cognitive processes that underlie complex behaviors such as spontaneous speech. For example, standard neuropsychological measures have been criticized due to their poor ecological predictive validity outside the context of a controlled assessment setting (25). Therefore, it is not surprising that traditional neuropsychological tests such as confrontation naming or generative verbal fluency correlate poorly with subjective patient reports of word finding difficulties (26, 27).
Automatic computerized classification of spontaneous speech into predefined categories based on its prosodic characteristics (e.g., duration and frequency of hesitations, intonation, rhythm) has been the subject of much study in computational linguistics (28–30). Multiple investigators, including our group, have successfully applied automated speech and language analysis tools to the assessment of individuals with mild cognitive impairment (31), aphasia in children (32), and frontotemporal lobar degeneration (33). However, these tools have not yet been applied to characterize effects of medications on cognition.
Our primary objective was to demonstrate the relationship between topiramate and nonlaboratory based measures of linguistic behavior (e.g., spontaneous speech fluency) and verbal recall. We applied an innovative System for Automated Language and Speech Analysis (SALSA), coupled with standard neuropsychological tests, to studies of healthy volunteers who received a single 100 mg oral dose of topiramate in a randomized, double blind, crossover, placebo-controlled design. Our central hypotheses were: a) topiramate adversely affects individual performance on measures of linguistic behavior and verbal recall compared to a no-drug baseline and b) the magnitude of topiramate’s effect is proportional to its plasma concentration.
In the first study, a single 2 mg oral dose of lorazepam was chosen as an active comparator to 100 mg topiramate. Unlike topiramate, lorazepam produces its cognitive effects via a mild generalized sedation, which will characterize more generalized effects on verbal fluency and recall. In the second study, we examined the effects of 100 mg oral topiramate on the reaction times (RT) recorded during performance of a working memory task (34). Here we postulated that, when compared to a no-drug baseline, topiramate induces cognitive changes via effects on frontal lobe function that are reflected in the subject’s behavioral performance (i.e., RTs). In both studies, we measured drug concentration at the time of the task performance. Plasma concentration is a more direct indicator of drug exposure than dose due to differences in pharmacokinetic processes such as absorption, distribution, elimination, and metabolism. Since patients with epilepsy are, by the very nature of their disorder, prone to cognitive impairments(35), including those affecting language, any untoward cognitive effects of pharmacological seizure control may prove particularly debilitating. The characterization and quantification of linguistic behavior, including speech, memory, and executive functions and their relationships to topiramate exposure in healthy adults is necessary to lay the groundwork for determining the mechanisms leading to individual intolerability and discontinuation of drug therapy in patients with epilepsy.
METHODS
Subjects
Twenty-five native English-speaking, healthy volunteers (8 women, 17 men) between 18 and 50 years of age were recruited from two sites, the University of Minnesota (n=14; 6 women, 8 men) and the University of Florida (n=11; 2 women, 9 men). Exclusion criteria included histories of significant cardiovascular, endocrine, hematopoietic, hepatic, neurologic, psychiatric, or renal disease; current or a history of drug or alcohol abuse within the past 5 years; the use of concomitant medications known to affect topiramate or lorazepam, or that alter cognitive function including antidepressants, anxiolytics, psychostimulants such as Ritalin, prescribed analgesics, and antipsychotics; prior hypersensitivity to topiramate, lorazepam or related compounds; a positive pregnancy test (administered to all women before the start of each of each test session); use of any investigational drug within the previous thirty days; non-native speakers of English; diagnosed with a speech and/or language impairment/disability; uncorrectable low vision; a dominant left-hand (to control for brain lateralization of language).
Study Design
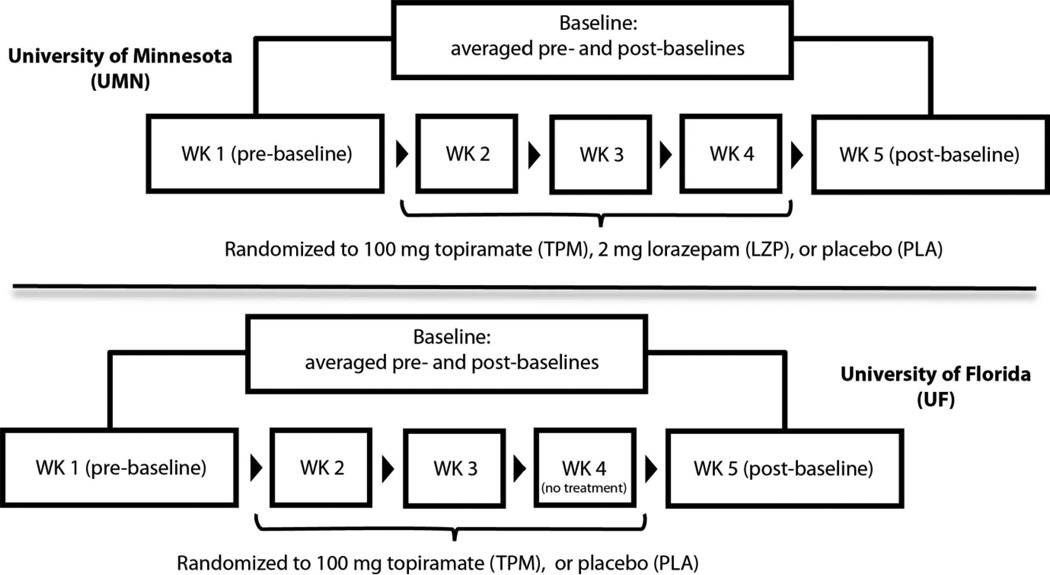
The University of Minnesota and University of Florida served as study sites. In a randomized, double blind, crossover design, Minnesota subjects received 100 mg oral topiramate, 2 mg oral lorazepam, and an inactive placebo (three-period crossover) whereas Florida subjects received topiramate and placebo (two-period crossover). One baseline (no treatment) period was pre-pended, and another was appended, to each crossover design. Traditional neuropsychological tests were administered in each period. In addition, we employed SALSA to precisely and objectively quantify various measures of language on discourse level tests of verbal recall and spontaneous speech. The Institutional Review Boards at each site approved the respective protocol. The study design for each site is presents in Figure 1.
Figure 1.
Design for randomized, double-blind, placebo-controlled, crossover studies at the University of Minnesota (UMN) and University of Florida (UFL).
Session 1. Subjects signed an IRB approved consent form after which they supplied a brief demographic, medical, and medication history. A neuropsychological test battery (Baseline 1) lasting approximately 1 hour was administered after which subjects were randomly assigned to a study treatment sequence (at Minnesota: topiramate, lorazepam, placebo in random order; at Florida: topiramate, placebo in random order).
Sessions 2–4. Subjects were administered their randomized drug, with neuropsychological testing performed between 1 and 1.5 hours after drug ingestion. Vital signs were recorded and a blood sample was drawn immediately after testing to establish plasma drug levels. Florida subjects did not undergo any testing during Session 4.
Session 5. Subjects returned for a second neuropsychological baseline (Baseline 2). All language-based tests were audio-recorded using an array microphone at a 16 kHz sampling rate for subsequent computerized analysis. All tests were administered by a single, trained examiner at each site.
Neuropsychological measures
The neuropsychological test battery included:
Word-level Language/Verbal Tests: Controlled Oral Word Association Test (COWA) (36): a measure of generative phonemic word fluency requiring three, 60-second trials to generate words (no proper nouns) beginning with specific letters.
Category (or Semantic) Fluency (37): subjects name as many items from a particular category, e.g., animals, as they can within a 60-second time period. Switching is a variation of category fluency requiring alternate retrieval of items from two different categories, e.g., furniture/musical instruments.
Discourse-level Language/Verbal Tests: MCG Paragraph Memory (38): A test of verbal recall; after being read a short story, the subject is asked to recall the story exactly as it was heard (IR1). The story is read again, and the subject is once gain asked to recall (IR2). In addition, a 30-minute delayed recall of the story is obtained (DR). Four parallel versions of this test exist.
Picture Description Task (Standard): a. Boston Diagnostic Aphasia Examination (“Cookie Theft”), b. Minnesota Test for Differential Diagnosis of Aphasia, c. Kentucky Aphasia Test, d. Nichols-Brookshire “Rescue” Picture Description Task, e. Nichols-Brookshire “Birthday” Picture Description Task
Minnesota Adaptive Picture Description Stimulus (MAPDS). The novel MAPDS task was designed specifically for the current study to minimize practice effects over multiple visits while maintaining comparable linguistic complexity and thematic content and was administered immediately after the standard picture description tasks.
Computerized speech and language analysis
Computerized linguistic analysis of speech and language functioning was performed on speech samples recorded during Discourse Level Language/Verbal tests using SALSA (39). SALSA is designed to analyze both what the subjects said (content) and how they said it (manner) in response to tasks such as a picture description or a story recall. SALSA uses automatic speech recognition technology to create a precise alignment between the verbatim transcription of the speech sample and the audio signal. Based on the output of this system, we defined the following variables for the picture description (standard and MAPDS) and MCG Paragraph Memory tests:
- Picture Description and MCG Paragraph Memory Tasks
- Word count – Raw count of words produced by the subject in response to the task.
- Disfluency rate – The ratio of disfluent events including filled pauses (um’s and ah’s), false starts, and repetitions to the total number of words in the sample.
- MCG Paragraph Memory Task Only
- Words Correctly Recalled (“Correct Words”) – the number of morphologically normalized words present in the story and recalled by the subject.
- All speech and language variables obtained during story recall were transformed using the ratio of total number of words in the story due to differences in word length across versions of he MCG Paragraph Memory (38).
- Picture Description Task Only
- Correct Units of Information – The number of words and phrases used in describing a picture that are commonly used in healthy controls in response to the same stimuli. Correct Units of Information was first developed for manual analysis of aphasic speech (40, 41). We adapted and automated this approach for computerized analysis.
Blood sampling and drug analysis
A single blood sample was collected into EDTA containing tubes immediately following each 2 to 3 hour-long testing session (i.e., 2 to 3 hours after dose). The time of drug administration and the time of the blood draw were recorded. Samples were immediately centrifuged and the plasma frozen until analysis. Topiramate plasma levels were established through a simultaneous liquid chromatograph-mass spectrometry (LCMS) assay, developed by Subramanian, Birnbaum and Remmel (2008). The limit of quantification (LOQ) was 0.375 mg/mL and a limit of detection (LOD) was defined as a signal to noise ratio (S/N) of 3:1 or less. Accuracies for the assay were ±6.34%, ±1.14%, and ±11.20% for the low, middle, and high QCs respectively. A coefficient of variance for within- and between-day variability was <9.56% and <8.9%, respectively.
Plasma concentrations of lorazepam were measured by a method developed by Zhu and Luo (42). Analyses were performed on a coupled high performance liquid chromatographelectrospray tandem mass spectrometer (Waters Micromass Quatro Ultima). The LOQ for this assay was 1 ng/mL, and the run time for each sample was six (6) minutes. The assay was validated according to FDA guidelines (43). Peak area ratios (analyte/internal standard) were used for measuring sample concentration from extracted matrix. LOQ was determined based on a s/n greater than 5. LOD had a s/n of at least 3. Validation of the assay was performed by preparing standard curves in triplicate (within-run) for each day on five different days (between run, N=15). The percentage coefficient of variation (CV) was calculated from the average standard deviation of quality control samples at each level (N=15). The estimated variability was determined from calculated concentrations of multiple triplicate weighted (1/x2) standard curves. Accuracy was calculated by dividing the grand mean by the target concentration and multiplying by 100.
Statistical Analysis for Neuropsychological and Speech Measures
For each neuropsychological measure and each speech measure, the pre- and post-drug baseline scores were averaged to correct for any practice effects that were associated with repeated testing across the entire study. Treatment effects were characterized as change scores, i.e., (drug session score - average baseline score)/average baseline score, for each measure for each participant, associated with drug conditions.
Spearman rank correlations between drug blood levels and each neuropsychological and speech change score corresponding to the drug sessions were computed. Neuropsychological and speech change scores were analyzed as independent repeated measures ANOVA with drug group (topiramate, lorazepam, placebo) as the primary factor of interest. Tukey’s HSD was used to adjust type I error rates for the multiple comparisons among the three drug groups. All ANOVAs were adjusted for site (Minnesota, Florida) and treatment order (8 possible orders), and included a random effect for participant to control for within-person correlation across the several sessions.
To avoid unnecessary inflation of the type I error rate, two neuropsychological measures were identified a priori as our primary variable: COWA and category fluency. These tests were chosen because of their established sensitivity to topiramate (44). All remaining neuropsychological measures were considered secondary variables. All speech measures were considered exploratory, with no a priori hypotheses established. Reported p-values shown are not adjusted for multiple analyses.
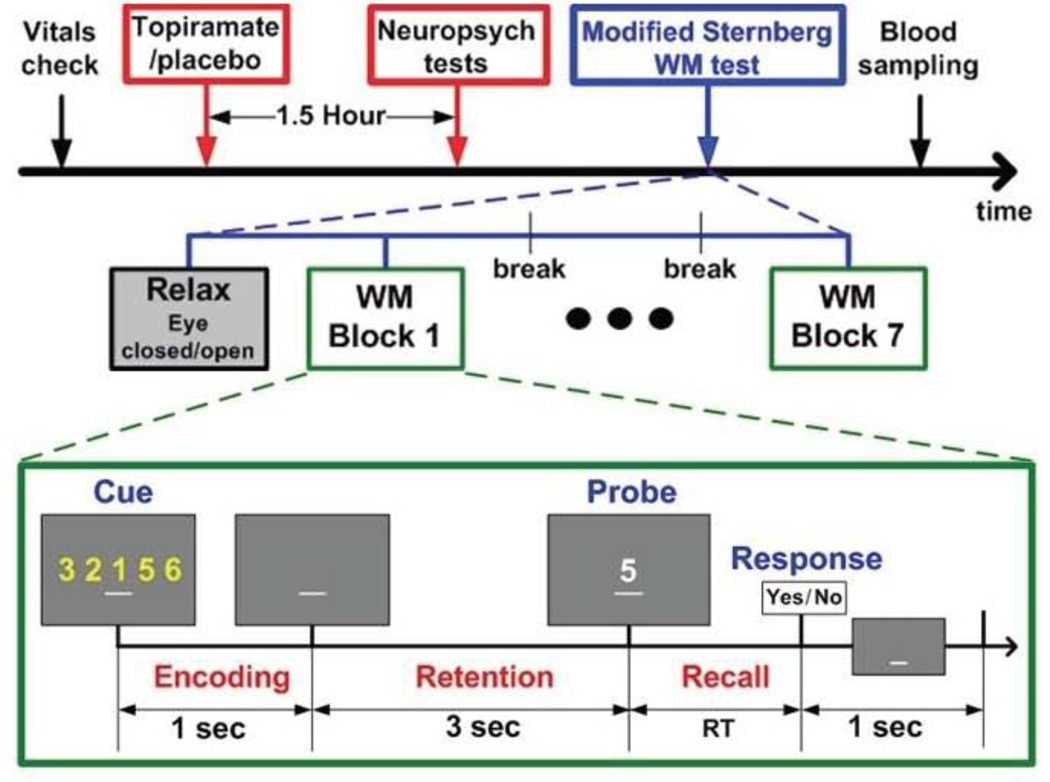
Working Memory Paradigm
A modified working memory task (34)(Figure 2) was presented during each session to each subject at Florida and EEG was recorded simultaneously (results of EEG will be reported elsewhere). A set of digits (0 to 9) appeared on a CRT monitor for 1 second, followed by a 3-second retention period, after which the probe digit was presented. Subjects were instructed to press a “yes” (index finger in the dominant hand) or “no” (middle finger) button to indicate whether or not the probe stimulus belonged to the previously viewed set. Memory-load was a function of the size of the digit set and was 1, 3 or 5.
Figure 2.
University of Florida study timeline and working memory paradigm.
Behavioral analysis
Behavioral performance was quantified by (1) reaction time (RT), reflecting the time from the onset of the probe digit to the key press response and, (2) error rate, the number of responses divided by the total number of trials where subjects pressed the “No” or “Yes” button when deciding whether or not the probe digit belonged to the cue set. Behavioral data were analyzed in a 3×3 factorial ANOVA considering each memory load as a factor with three drug sessions: average of two baselines, 1st drug, and 2nd drug. Post-hoc analyses were performed when necessary to evaluate the statistical significance for the behavioral measures over memory loads or drug conditions.
RESULTS
Twenty subjects completed the two studies carried out at the two sites (7 women; 13 men; age (mean ± sd) = 28 ± 10 years; weight =76.4 ± 13.8 kg). Five additional subjects were recruited and signed informed consent but are not included in the analysis. Participants not completing the trial included one subject who developed paresthesia during session 2; one subject who was discovered to have a history of drug abuse; one who withdrew without providing explanation; and two subjects who were excluded administratively due to the presence of small, but detectable, amounts of topiramate in their plasma during placebo or baseline sessions. Individual topiramate plasma levels resulting from a 100 mg oral dose ranged between 0.23–2.28 µg/mL with a mean ± sd of 1.47 ± 0.57 µg/mL. Lorazepam plasma levels resulting from a single 2 mg oral dose ranged between 1.55ng– 22.69 ng/mL with a mean ± sd of 14.81 ± 5.49 ng/mL.
Table 1 presents the group means of scores, and Table 2 the Spearman correlations (ρ) of plasma topiramate and lorazepam levels with scores from the neuropsychological testing primary outcome measures and the exploratory speech measures.
Table 1.
Behavioral responses to TPM, LZP, and PLA, relative to a no-treatment baseline, as captured by neuropsychological and speech measures.
| Change score of TPM* from baseline |
Change score of LZP** from baseline |
Change score of PLA from baseline |
||||||
|---|---|---|---|---|---|---|---|---|
| Predicted group mean (SE) |
Test of change from baseline p value |
Predicted group mean (SE) |
Test of change from baseline p value |
Test of LZP vs. TPM p value |
Predicted group mean (SE) |
Test of change from baseline p value |
Test of PLA vs. TPM p value |
|
| Gen. Verbal Fluency | ||||||||
| Phonemic (FAS/BHR) | −0.24 (0.04) | <0.0001 | −0.05 (0.05) | 0.35 | 0.0031 | −0.003 (0.04) | 0.94 | <0.0001 |
| Category | ||||||||
| Animals/Clothing | −0.09 (0.06) | 0.10 | 0.13 (0.07) | 0.10 | 0.05 | −0.01 (0.06) | 0.83 | 0.47 |
| Boy/Girl names | −0.24 (0.06) | 0.0002 | 0.05 (0.07) | 0.43 | 0.0006 | −0.02 (0.06) | 0.79 | 0.0009 |
| Switching | −0.20 (0.05) | 0.0002 | 0.02 (0.07) | 0.80 | 0.04 | 0.002 (0.05) | 0.97 | 0.01 |
| MCG IR 1 | ||||||||
| Word Count | −0.14 (0.08) | 0.09 | −0.21 (0.12) | 0.08 | 0.88 | 0.08 (0.08) | 0.36 | 0.16 |
| Correct Words | −0.21 (0.10) | 0.04 | −0.23 (0.14) | 0.11 | 0.99 | 0.24 (0.10) | 0.02 | 0.007 |
| Disfluency rate | 0.11 (0.17) | 0.55 | −0.56 (0.22) | 0.01 | 0.01 | −0.09 (0.18) | 0.63 | 0.48 |
| MCG IR 2 | ||||||||
| Word Count | −0.19 (0.06) | 0.003 | −0.15 (0.08) | 0.06 | 0.90 | 0.13 (0.06) | 0.08 | 0.0003 |
| Correct Words | −0.10 (0.07) | 0.16 | −0.30 (0.10) | 0.007 | 0.29 | 0.33 (0.07) | 0.0001 | 0.001 |
| Disfluency rate | 0.04 (0.12) | 0.74 | −0.35 (0.17) | 0.04 | 0.12 | −0.19 (0.12) | 0.13 | 0.29 |
| MCG Delayed Recall | ||||||||
| Word Count | −0.08 (0.07) | 0.30 | −0.18 (0.10) | 0.09 | 0.69 | 0.12 (0.07) | 0.11 | 0.13 |
| Correct Words | −0.09 (0.08) | 0.28 | −0.27 (0.12) | 0.04 | 0.49 | 0.37 (0.08) | 0.0001 | 0.001 |
| Disfluency rate | 0.32 (0.26) | 0.22 | −0.15 (0.30) | 0.61 | 0.11 | 0.11 (0.26) | 0.68 | 0.44 |
| Picture Descript-Standard | ||||||||
| Word Count | 0.14 (0.08) | 0.11 | 0.10 (0.12) | 0.39 | 0.96 | 0.17 (0.09) | 0.07 | 0.98 |
| Correct Information Units | 0.27 (0.09) | 0.004 | 0.15 (0.11) | 0.19 | 0.52 | 0.25 (0.09) | 0.008 | 0.97 |
| Disfluency rate | 0.66 (0.24) | 0.01 | 0.14 (0.26) | 0.59 | 0.009 | 0.16 (0.24) | 0.51 | 0.002 |
| Picture Descript-MAPDS | ||||||||
| Word Count | 0.002 (0.11) | 0.99 | 0.19 (0.13) | 0.14 | 0.20 | 0.19 (0.11) | 0.09 | 0.08 |
| Correct Information Units | −0.02 (0.09) | 0.81 | −0.02 (0.13) | 0.85 | 1.00 | 0.19 (0.10) | 0.07 | 0.14 |
| Disfluency rate | 1.12 (0.83) | 0.19 | 0.80 (0.84) | 0.35 | 0.12 | 0.89 (0.83) | 0.29 | 0.15 |
TPM, topiramate; LZP, lorazepam; PLA, placebo
IR1, immediate recall 1; IR2, immediate recall 2
N= 20
LZP was only administered to UMN participants only (N=11)
Table 2.
Correlations between individual behavioral responses and plasma concentrations of TPM and LZP
| Test | Correlation of change score with TPM* plasma level |
Correlation of change score with LZP** plasma level |
|
|---|---|---|---|
| Rho (p value) | Rho (p value) | ||
| Gen. Verbal Fluency | |||
| Phonemic (FAS/BHR) | −0.38 (0.10) | +0.56 (0.07) | |
| Category | |||
| Animals/Clothing | +0.10 (0.69) | +0.24 (0.48) | |
| Boy/Girl names | +0.05 (0.82) | −0.23 (0.50) | |
| Switching | −0.01 (0.98) | −0.25 (0.47) | |
| MCG IR 1 | |||
| Word Count | −0.51 (0.02) | +0.32 (0.34) | |
| Correct Words | −0.65 (0.002) | +0.41 (0.21) | |
| Disfluency rate | +0.14 (0.55) | +0.46 (0.15) | |
| MCG IR 2 | |||
| Word Count | −0.31 (0.19) | +0.46 (0.15) | |
| Correct Words | −0.51 (0.02) | +0.52 (0.10) | |
| Disfluency rate | +0.17 (0.47) | +0.54 (0.09) | |
| MCG Delayed Recall | |||
| Word Count | −0.69 (0.001) | +0.39 (0.26) | |
| Correct Words | −0.68 (0.001) | +0.55 (0.10) | |
| Disfluency rate | +0.09 (0.69) | +0.47 (0.17) | |
| Picture Descript-Stand | |||
| Word Count | −0.22 (0.34) | +0.22 (0.52) | |
| Correct Information Units | +0.07 (0.78) | +0.15 (0.66) | |
| Disfluency rate | +0.49 (0.03) | +0.35 (0.30) | |
| Picture Descript-MAPDS | |||
| Word Count | −0.20 (0.39) | −0.10 (0.77) | |
| Correct Info Units | −0.40 (0.08) | +0.15 (0.68) | |
| Disfluency rate | +0.58 (0.007) | +0.48 (0.16) | |
| Reaction Time (RT) | load 1: | +0.83 (0.00) | |
| 3: | +0.94 (0.00) | ||
| 5: | +0.76 (0.02) |
TPM was administered at both sites (N=20);
LZP was administered to participants at UMN only (N=11)
TPM, topiramate; LZP, lorazepam
Word-level Language/Verbal Tests
Controlled Oral Word Association (COWA): There was a main effect of drug administration on number of words generated (F2,29 = 16.92, p < 0.0001) during the COWA. Relative to baseline, topiramate resulted in a significant decrease in the proportional number of words generated after presentation of the letters FAS or BHR (p<0.0001), whereas neither lorazepam nor placebo had an effect. COWA performance did not vary significantly as a function of plasma topiramate or lorazepam levels.
Category fluency: Subjects had significantly more difficulty generating boys/girls names (F2.29 = 12.35, p = 0.0001) following topiramate (p=0.0002) than lorazepam or placebo, neither of which differed from baseline. Animal/clothing naming was not affected by either drug compared to placebo (F2,29 = 3.16, p = 0.0575). The ability to switch between categories (F2,29 = 5.80, p = 0.0076) was also negatively impacted by topiramate (p=0.01), but not by lorazepam or placebo. As with COWA, there was no relationship between topiramate plasma levels and category fluency performance.
Discourse level language/verbal tests
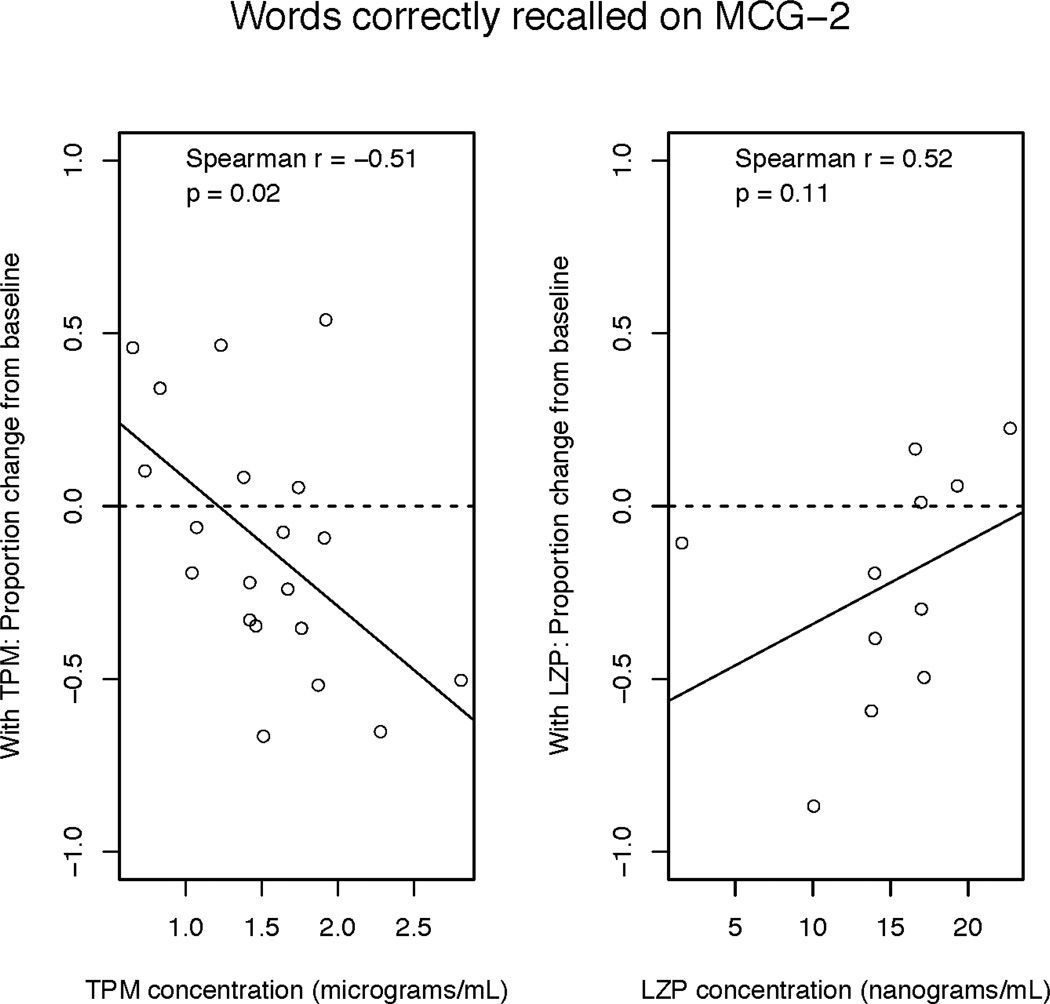
Words Correctly Recalled (“Correct Words”) from the MCG Paragraph Memory: The number of correct words recalled during the first learning trial (Immediate Recall 1 – IR1) was affected by drug treatment (F2,29 = 6.46, p = 0.005). Following topiramate and lorazepam administration, subjects recalled fewer correct words during IR1 and recalled significantly more following placebo (p=0.02). Similar patterns held after the second learning trial (Immediate Recall 2 – IR2; F2.29 = 15.26, p < 0.0001) and following a 30-minute delay (Delayed Recall – DR; F2,28 = 11.76, p = 0.0002). As seen in Figure 3 and Table 2, topiramate and lorazepam also had differential effects on the correlation between proportion of correct words and drug plasma levels: During all three recall conditions, the proportion of correct words recalled was negatively, and significantly, associated with higher topiramate plasma levels. However, in the lorazepam condition, better recall was always positively associated with higher lorazepam plasma levels, though the correlation did not reach statistical significance.
Figure 3.
Proportion of correct words (relative to baseline) recalled from MCG Paragraph Memory versus plasma drug level. The superimposed solid line represents the least squares regression line.
Disfluency rate from the MCG Paragraph Memory: Drug treatment also significantly affected the disfluency rate during IR1 (F2,29 = 5.04, p = 0.013). Lorazepam caused a significant, overall decrease in disfluency rate (more fluent compared to baseline) during IR1 (p=0.01). Subjects with the highest lorazepam plasma levels had the highest disfluency rates across all three trials, though the association was not statistically significant. During the topiramate condition, subjects had a tendency to be less fluent than at baseline, but the effect was not significant during any of the 3 recall conditions. In their first story recall trial (IR1), subjects on topiramate were significantly less fluent (i.e., higher number of disfluencies) than when taking lorazepam (p=0.01). As with lorazepam, there was no significant correlation between the magnitude of the disfluency rate and topiramate plasma levels, though the strength of the association was much smaller for topiramate than lorazepam.
Picture Description: The disfluency rate generated from the standard picture description task was significantly affected by drug administration (F2,29 = 9.27, p = 0.0008) with topiramate causing a significant increase in disfluency rate (less fluent compared to baseline, p=0.01). No changes were observed during lorazepam or placebo. Disfluency rates generated by both the standard and MAPDS picture description tasks were significantly correlated with plasma topiramate levels (p=0.03 and p=0.007, respectively).
Reaction time
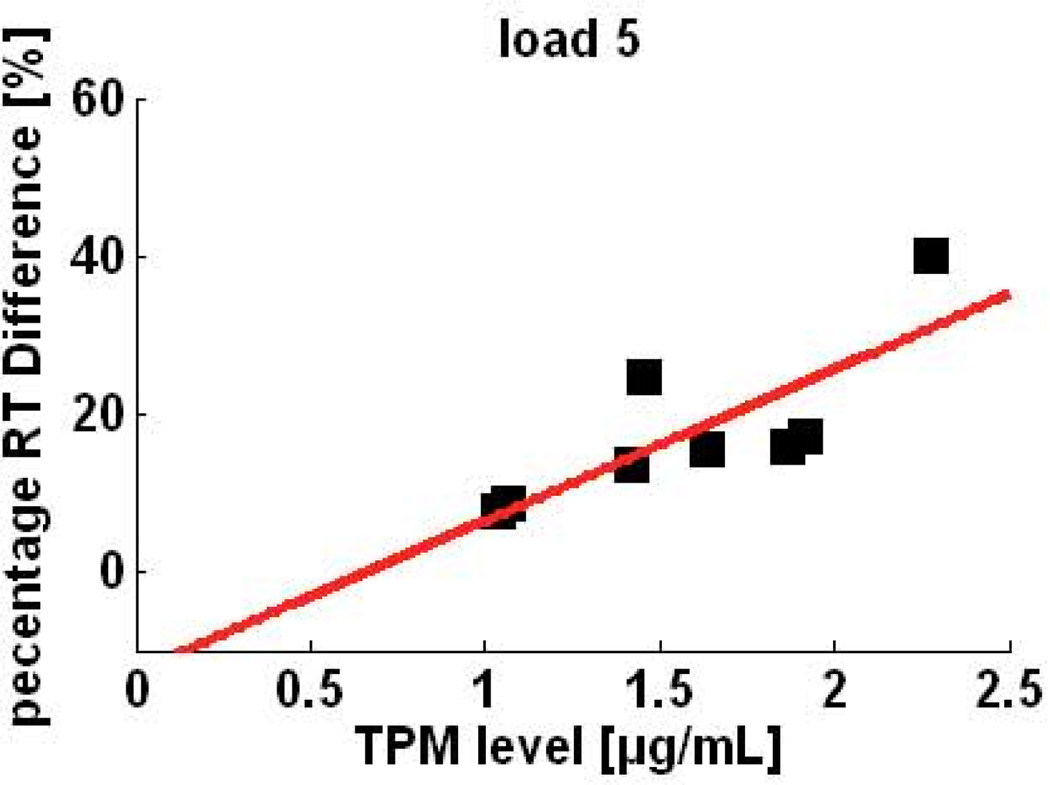
Reaction time (RT) and error rate: Subjects responded faster and more accurately for smaller memory loads across all treatments (see Figure 4). Topiramate tended to increase RT and error rate. RTs were significantly affected by both the memory load (F2,72 = 12.6, p < .001) and drug treatment (F2,72 = 6.8, p = .002) but there was no significant interaction between the two factors(F4,72 = 0.16, p = 0.96). Tukey HSD pair-wise comparisons revealed that RT was significantly longer for higher memory load (p < .001 for load 5, and p = .027 for load 3) when compared to lower memory load (load 1). Topiramate was significantly different from placebo (p=0.003) as well as from baseline (p=0.016). As shown in Figure 4, despite the relatively small number of subjects, there was a highly significant, positive correlation between topiramate plasma levels and percent change in RT from baseline for all memory loads tested (1: ρ = 0.83, p=0.00; 3: ρ = 0.94, p=0.00; 5: ρ = 0.76, p = 0.02).
Figure 4.
Percent change in reaction time from baseline versus plasma TPM levels of individuals during the highest memory load condition of a modified Sternberg (working memory) task.
Error rate was increased during the topiramate condition relative to baseline. Although no statistically significant effects were observed, there was a trend for a higher error rate with increased memory load.
DISCUSSION
We provide the first report of a significant negative association between individual topiramate plasma concentrations and verbal fluency of discourse level speech as measured by a novel system for automated speech and language analysis (i.e., SALSA). We also demonstrate a strong, positive association between topiramate plasma levels and reaction time during a working memory task.
In these two randomized, placebo controlled studies, we employed an innovative, multidisciplinary framework that combines the tools of clinical pharmacology and computational linguistics to elucidate the relationships between the determinants of a drug’s disposition as reflected in plasma concentrations and their consequent impact on cognitive functioning as reflected in spoken language discourse. This approach, based on individual plasma drug level rather than dose, constitutes an important first step towards the goal of optimizing drug therapy and increasing compliance by reducing a patient’s risk of developing language-related adverse side effects.
Range of plasma concentrations
Despite the fact that topiramate is not extensively metabolized (45), we found large range of plasma concentrations (0.23–2.28 µg/mL) resulting from a single oral dose (100mg) in subjects from both study sites. In support of these results, a recent study our group (Marino & Birnbaum) in collaboration with Cirulli et al (2012) from Duke found up to a 55-fold variation in topiramate plasma levels sampled from 158 healthy volunteers who were given an acute, 100mg oral dose of topiramate (46).
The pharmacokinetic and physical properties of topiramate favor our assumption that the reported plasma levels obtained between 2–3 hours after drug administration are a good approximation of topiramate brain concentrations. Topiramate absorption is relatively quick (1–4h) and its protein binding is low (15%). There is also a strong correlation between plasma and CSF total and unbound concentrations, where total topiramate concentration in CSF is 85% of that in the plasma (47). Given that there does not seem to be a saturable carrier mechanism restricting topiramate transport across the blood brain barrier (47), and it is a small, water-soluble compound at physiologic pH, topiramate probably moves freely in the extracellular space once it has crossed the blood-brain barrier (48).
Plasma concentrations and traditional neuropsychological performance
We observed that individual differences in drug plasma levels are strongly associated with the disfluency rate of subjects asked to describe a picture following a single, 100 mg oral dose of topiramate. There were no significant relationships between topiramate plasma levels and other characteristics of speech and language elicited on the picture description tasks including word count, speaking rate, mean silent pause duration, or correct units of information. These findings indicate a relatively subtle topiramate effect on cognition reflected in the manner of speech production rather than its content, suggesting greater impairment in frontal motor/executive networks than in semantic networks.
There was also no statistically significant relationship between topiramate plasma levels and the traditional clinical word-level measures of generative fluency, i.e., COWA, despite the significant decline of generative fluency performance in response to topiramate at the group level. Studies designed to characterize drug-induced effects on cognition rely primarily on standard neuropsychological test batteries that typically include verbal and category generative fluency tests such as COWA and category fluency. In the context of ordinary language and communication, however, words are retrieved because of their semantic, rather than their phonemic, relationship to each other(49). Moreover, generative verbal fluency tasks are restricted to measuring the speed and accuracy of reaction to a stimulus prompt presented in an artificial experimental task, and cannot adequately reflect the fluency of spontaneous speech produced in everyday activities.
It should also be noted that Loring et al.’s (2011) recent parallel group, three-dose study on severely obese subjects reports a small (−0.23) but significant negative association between serum levels and total score on the Computerized Neuropsychological Test Battery (CNTB). However, the CNTB does not include measures of verbal fluency. Cirulli et al (2012) did include a test of phonemic fluency in their battery (i.e., COWA), and while administration of 100mg of TPM exerted a strong, negative impact on COWA performance, the relationship between TPM plasma concentrations and the magnitude of the scores was relatively minor (r2 = 0.12, n=135).
Topiramate also negatively affected memory as reflected in the decrease in the proportion of correct words recalled from a paragraph memory task relative to baseline. During IR1 of the MCG Paragraph Memory task, topiramate significantly impaired the recall of correct words across all subjects. Furthermore, individual performance was significantly lower in those with higher topiramate plasma levels during each of the recall conditions (IR1, IR2, DR).
In clinical practice, the largest number of prescriptions for topiramate are written for migraine prophylaxis rather than for epilepsy. Since topiramate dosages for migraine are typically lower than those used for epilepsy, this might explain why topiramate is generally is better tolerated by migraineurs than patients with epilepsy.
We did not expect to observe higher lorazepam concentrations with better prose passage recall. Although the magnitude of the correlation was large, it was not statistically significant, most likely because of the relatively small sample size. It is worthwhile to note that following topiramate, however, subjects informally reported a “blocking” of the information in the story being read. This suggests that rather than having difficulty recalling the information during retrieval, topiramate interfered with committing the details of the story to memory during encoding. In addition, subjects appeared anxious when told that they would be asked to repeat a story verbatim; therefore it is possible that the paradoxical effect of improved story recall associated with lorazepam may be a function of lorazepam’s anxiolytic properties. However, given the relatively small number of subjects that received lorazepam, this finding needs to be replicated with a larger sample.
Plasma concentrations and behavioral measures during a working memory task
There appears to be a strong, positive relationship between topiramate levels and RT in response to working memory loads of increasing difficulty in the Sternberg task. This suggests a concentration-dependent effect of topiramate on frontal lobe systems involved in working memory.
Conclusion
The ability to tailor drug therapy to a specific individual is directly dependent on our ability to measure the effects of medications on the individual’s physiologic and cognitive states. SALSA enables the delineation and quantification of ecologically relevant features of natural language as they occur during spontaneous speech. Our cutting-edge approach challenges the existing paradigms of neuropsychological assessment by shifting the focus from the description of isolated language/neuropsychological impairments towards a characterization of language production and its interaction with other cognitive systems. This approach allows us to effectively address clinical questions while elucidating mechanism.
Limitations
The use of healthy volunteers is not thought to be a significant limitation of this study. We felt is was first necessary to establish whether or not SALSA was sensitive to topiramate-induced changes in natural language and speech production, and drug-induced changes in cognition are typically easier to establish in the absence of cognitive impairment associated with the underlying brain substrate giving rise to a patient’s epilepsy. Nevertheless, the single-dose paradigm limits our ability to generalize our findings to long-term chronic effects of topiramate therapy in patients. Our next step will be a dose-ranging study to further explore the relationship between topiramate concentrations and the magnitude of its effect on language which will enable us to design future steady-state dosing studies in patients with epilepsy.
HIGHLIGHTS.
A single, 100mg dose of topiramate significantly impairs verbal fluency and recall
Topiramate plasma levels are positively related to disfluency rate in discourse
Topiramate and lorazepam have differential effects on paragraph memory recall
System for automated language and speech analysis used to quantify verbal fluency
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health/National Institute for Neurological Diseases and Stroke [K 01 NS050309 to S.E.M.]; National Institute for Aging [R01 AG026490 to A.K.B.] and University of Minnesota/Academic Health Center Faculty Research Development Award [to S.E.M., S.V.S.P., A.K.B.]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
References
- 1.Aldenkamp AP, De Krom M, Reijs R. Newer antiepileptic drugs and cognitive issues. Epilepsia. 2003;44(Suppl 4):21–29. doi: 10.1046/j.1528-1157.44.s4.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee HW, Jung DK, Suh CK, Kwon SH, Park SP. Cognitive effects of low-dose topiramate monotherapy in epilepsy patients: A 1-year follow-up. Epilepsy Behav. 2006 Jun;8(4):736–741. doi: 10.1016/j.yebeh.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Thompson PJ, Baxendale SA, Duncan JS, Sander JW. Effects of topiramate on cognitive function. J Neurol Neurosurg Psychiatry. 2000 Nov;69(5):636–641. doi: 10.1136/jnnp.69.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mula M, Trimble MR, Thompson P, Sander JW. Topiramate and word-finding difficulties in patients with epilepsy. Neurology. 2003 Apr 8;60(7):1104–1107. doi: 10.1212/01.wnl.0000056637.37509.c6. [DOI] [PubMed] [Google Scholar]
- 5.Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia. 2000;41(Suppl 1):S35–S39. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- 6.Shank RP, Maryanoff BE. Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate. CNS Neurosci Ther. 2008 Summer;14(2):120–142. doi: 10.1111/j.1527-3458.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppola F, Rossi C, Mancini ML, Corbelli I, Nardi K, Sarchielli P, et al. Language disturbances as a side effect of prophylactic treatment of migraine. Headache. 2008 Jan;48(1):86–94. doi: 10.1111/j.1526-4610.2007.00860.x. [DOI] [PubMed] [Google Scholar]
- 8.Bootsma HP, Coolen F, Aldenkamp AP, Arends J, Diepman L, Hulsman J, et al. Topiramate in clinical practice: long-term experience in patients with refractory epilepsy referred to a tertiary epilepsy center. Epilepsy Behav. 2004 Jun;5(3):380–387. doi: 10.1016/j.yebeh.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Tatum WOt, French JA, Faught E, Morris GL, 3rd, Liporace J, Kanner A, et al. Postmarketing experience with topiramate and cognition. Epilepsia. 2001 Sep;42(9):1134–1140. doi: 10.1046/j.1528-1157.2001.41700.x. [DOI] [PubMed] [Google Scholar]
- 10.Meador KJ. Cognitive outcomes and predictive factors in epilepsy. Neurology. 2002 Apr 23;58(8) Suppl 5:S21–S26. doi: 10.1212/wnl.58.8_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- 11.Loring DW, Williamson DJ, Meador KJ, Wiegand F, Hulihan J. Topiramate dose effects on cognition: A randomized double-blind study. Neurology. 2011 Jan 11;76(2):131–137. doi: 10.1212/WNL.0b013e318206ca02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kockelmann E, Elger CE, Helmstaedter C. Significant improvement in frontal lobe associated neuropsychological functions after withdrawal of topiramate in epilepsy patients. Epilepsy Res. 2003 May;54(2–3):171–178. doi: 10.1016/s0920-1211(03)00078-0. [DOI] [PubMed] [Google Scholar]
- 13.Martin R, Kuzniecky R, Ho S, Hetherington H, Pan J, Sinclair K, et al. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology. 1999 Jan 15;52(2):321–327. doi: 10.1212/wnl.52.2.321. [DOI] [PubMed] [Google Scholar]
- 14.Ojemann LM, Ojemann GA, Dodrill CB, Crawford CA, Holmes MD, Dudley DL. Language Disturbances as Side Effects of Topiramate and Zonisamide Therapy. Epilepsy Behav. 2001 Dec;2(6):579–584. doi: 10.1006/ebeh.2001.0285. [DOI] [PubMed] [Google Scholar]
- 15.Meador KJ, Loring DW, Hulihan JF, Kamin M, Karim R. Differential cognitive and behavioral effects of topiramate and valproate. Neurology. 2003 May 13;60(9):1483–1488. doi: 10.1212/01.wnl.0000063308.22506.19. [DOI] [PubMed] [Google Scholar]
- 16.Kockelmann E, Elger CE, Helmstaedter C. Cognitive profile of topiramate as compared with lamotrigine in epilepsy patients on antiepileptic drug polytherapy: relationships to blood serum levels and comedication. Epilepsy Behav. 2004 Oct;5(5):716–721. doi: 10.1016/j.yebeh.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Blum D, Meador K, Biton V, Fakhoury T, Shneker B, Chung S, et al. Cognitive effects of lamotrigine compared with topiramate in patients with epilepsy. Neurology. 2006 Aug 8;67(3):400–406. doi: 10.1212/01.wnl.0000232737.72555.06. [DOI] [PubMed] [Google Scholar]
- 18.Salinsky MC, Storzbach D, Spencer DC, Oken BS, Landry T, Dodrill CB. Effects of topiramate and gabapentin on cognitive abilities in healthy volunteers. Neurology. 2005 Mar 8;64(5):792–798. doi: 10.1212/01.WNL.0000152877.08088.87. [DOI] [PubMed] [Google Scholar]
- 19.Romigi A, Cervellino A, Marciani MG, Izzi F, Massoud R, Corona M, et al. Cognitive and psychiatric effects of topiramate monotherapy in migraine treatment: an open study. Eur J Neurol. 2008 Feb;15(2):190–195. doi: 10.1111/j.1468-1331.2007.02033.x. [DOI] [PubMed] [Google Scholar]
- 20.Fritz N, Glogau S, Hoffmann J, Rademacher M, Elger CE, Helmstaedter C. Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy Behav. 2005 May;6(3):373–381. doi: 10.1016/j.yebeh.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Froscher W, Schier KR, Hoffmann M, Meyer A, May TW, Rambeck B, et al. Topiramate: a prospective study on the relationship between concentration, dosage and adverse events in epileptic patients on combination therapy. Epileptic Disord. 2005 Sep;7(3):237–248. [PubMed] [Google Scholar]
- 22.De Ciantis A, Muti M, Piccolini C, Principi M, Di Renzo A, De Ciantis R, et al. A functional MRI study of language disturbances in subjects with migraine headache during treatment with topiramate. Neurol Sci. 2008 May;29(Suppl 1):S141–S143. doi: 10.1007/s10072-008-0906-5. [DOI] [PubMed] [Google Scholar]
- 23.Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006 Feb;129(Pt 2):306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- 24.Schweizer TA, Alexander MP, Susan Gillingham BA, Cusimano M, Stuss DT. Lateralized cerebellar contributions to word generation: a phonemic and semantic fluency study. Behav Neurol. 2010;23(1–2):31–37. doi: 10.3233/BEN-2010-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaytor N, Schmitter-Edgecombe M, Burr R. Improving the ecological validity of executive functioning assessment. Arch Clin Neuropsychol. 2006 Apr;21(3):217–127. doi: 10.1016/j.acn.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Heller RB, Dobbs AR. Age differences in word finding in discourse and nondiscourse situations. Psychol Aging. 1993 Sep;8(3):443–450. [PubMed] [Google Scholar]
- 27.Rohrer JD, Knight WD, Warren JE, Fox NC, Rossor MN, Warren JD. Word-finding difficulty: a clinical analysis of the progressive aphasias. Brain. 2008 Jan;131(Pt 1):8–38. doi: 10.1093/brain/awm251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shriberg E. Higher level features in speaker recognition. In: Muller C, editor. Speaker Classification I of Lecture Notes in Computer Science / Artificial Intelligence. Heidelberg / Berlin / New York: Springer; 2007. pp. 241–259. [Google Scholar]
- 29.Shriberg E, Ferrer L, Kajarekar S, Venkataraman A, Stolcke A. Modeling prosodic feature sequences for speaker recognition. Speech Communication. 2005;46(3–4):455–472. [Google Scholar]
- 30.Shriberg E, Stolcke A. Interspeech. Brisbane, Australia: 2008. The case for automatic higher-level features in forensic speaker recognition. [Google Scholar]
- 31.Roark B, Hosom J, Mitchell M, Kaye JA. Automatically derived spoken language markers for detecting mild cognitive impairment. International Conference on Technology and Aging (ICTA) 2007 [Google Scholar]
- 32.Hosom JP, Shriberg L, Green JR. Diagnostic assessment of childhood apraxia of speech using automatic speech recognition (ASR) methods. Journal of Medical Speech - Language Pathology. 2004 Dec;12(4):167–171. [PMC free article] [PubMed] [Google Scholar]
- 33.Pakhomov SV, Smith GE, Chacon D, Feliciano Y, Graff-Radford N, Caselli R, et al. Computerized analysis of speech and language to identify psycholinguistic correlates of frontotemporal lobar degeneration. Cogn Behav Neurol. 2010 Sep;23(3):165–177. doi: 10.1097/WNN.0b013e3181c5dde3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sternberg S. Memory-scanning: mental processes revealed by reaction-time experiments. Am Sci. 1969 Winter;57(4):421–457. [PubMed] [Google Scholar]
- 35.Motamedi G, Meador K. Epilepsy and cognition. Epilepsy Behav. 2003 Oct;4(Suppl 2):S25–S38. doi: 10.1016/j.yebeh.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. 4th edition. Oxford University Press; 2004. [Google Scholar]
- 37.Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. 3rd edition. Iowa City: AJA Associates; 1983. [Google Scholar]
- 38.Meador KJ, Loring DW, Abney OL, Allen ME, Moore EE, Zamrini EY, et al. Effects of carbamazepine and phenytoin on EEG and memory in healthy adults. Epilepsia. 1993 Jan-Feb;34(1):153–157. doi: 10.1111/j.1528-1157.1993.tb02389.x. [DOI] [PubMed] [Google Scholar]
- 39.Pakhomov SV, Smith GE, Marino S, Birnbaum A, Graff-Radford N, Caselli R, et al. A computerized technique to assess language use patterns in patients with frontotemporal dementia. J Neurolinguistics. 2010 Mar 1;23(2):127–144. doi: 10.1016/j.jneuroling.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicholas LE, Brookshire RH. A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. J Speech Hear Res. 1993 Apr;36(2):338–350. doi: 10.1044/jshr.3602.338. [DOI] [PubMed] [Google Scholar]
- 41.Yorkston KM, Beukelman DR. An analysis of connected speech samples of aphasic and normal speakers. Journal of Speech and Hearing Disorders. 1980 Feb;45(1):27–36. doi: 10.1044/jshd.4501.27. [DOI] [PubMed] [Google Scholar]
- 42.Zhu H, Luo J. A fast and sensitive liquid chromatographic-tandem mass spectrometric method for assay of lorazepam and application to pharmacokinetic analysis. J Pharm Biomed Anal. 2005 Sep 1;39(1–2):268–274. doi: 10.1016/j.jpba.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 43.FDA, Guidance fo Industry: Bioanalytical Method Validation. Services USDoHaH. Rockville MD: 2001. [Google Scholar]
- 44.Salinsky M, Storzbach D, Oken B, Spencer D. Topiramate effects on the EEG and alertness in healthy volunteers: a different profile of antiepileptic drug neurotoxicity. Epilepsy Behav. 2007 May;10(3):463–469. doi: 10.1016/j.yebeh.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Bourgeois BF. Pharmacokinetics and metabolism of topiramate. Drugs Today (Barc) 1999 Jan;35(1):43–48. doi: 10.1358/dot.1999.35.1.522947. [DOI] [PubMed] [Google Scholar]
- 46.Cirulli ET, Urban TJ, Marino SE, Linney KN, Birnbaum AK, Depondt C, et al. Genetic and environmental correlates of topiramate-induced cognitive impairment. Epilepsia. 2012 Jan;53(1):e5–e8. doi: 10.1111/j.1528-1167.2011.03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christensen J, Hojskov CS, Dam M, Poulsen JH. Plasma concentration of topiramate correlates with cerebrospinal fluid concentration. Ther Drug Monit. 2001 Oct;23(5):529–535. doi: 10.1097/00007691-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Reife RA. Topiramate. In: Shorvon S, Dreifuss F, Fish D, Thomas D, editors. The treatment of epilepsy. Oxford: Blackwell Science Ltd; 1996. pp. 471–481. [Google Scholar]
- 49.Ross TP, Calhoun E, Cox T, Wenner C, Kono W, Pleasant M. The reliability and validity of qualitative scores for the Controlled Oral Word Association Test. Arch Clin Neuropsychol. 2007 May;22(4):475–488. doi: 10.1016/j.acn.2007.01.026. [DOI] [PubMed] [Google Scholar]