Abstract
Objective
We analyzed and evaluated enhanced chronic hepatitis C virus (HCV) surveillance in New York City (NYC), which involved detailed investigations on a sample of newly reported HCV patients.
Methods
Beginning in July 2009, we generated a simple random sample bimonthly from all patients newly reported with a positive HCV test. We administered questionnaires to clinicians and patients to collect clinical and epidemiological information on patients diagnosed from April 2009 to January 2011 and evaluated the staff resources required to conduct enhanced surveillance.
Results
Of 205 patients meeting inclusion criteria, 40 (19.5%) tested HCV ribonucleic acid (RNA) negative. For the remaining 165 patients, questionnaires were completed by 164 clinicians (99.4%) and 77 patients (46.7%). Many patients (54.0%) were born between 1945 and 1964, and most patients were Hispanic (32.7%) or non-Hispanic black (32.7%). Common risk factors were injection (43.0%) and intranasal (33.9%) drug use. One-third of patients were diagnosed in nontraditional medical settings including substance abuse/detoxification centers (25.0%), jail/prison (6.7%), and psychiatric facilities (1.8%). Of 98 patients with positive HCV RNA tests, 38.8% were immune to hepatitis A and 39.8% were immune to hepatitis B. Investigators required approximately 3.5 hours to complete each investigation and averaged 50 days from assignment to completion.
Conclusions
Although conducting enhanced HCV surveillance requires significant resources, investigating a representative sample provides detailed information about NYC's HCV population. Surveillance data have been used to plan educational initiatives for clinicians and patients, which may have led to increased awareness of HCV status, improved patient support, and better overall care.
Hepatitis C virus (HCV) is a major public health problem, with an estimated 1.3% of Americans and 1.8%–2.4% of New York City (NYC) residents chronically infected.1–4 Over time, HCV can lead to cirrhosis, liver disease, and hepatocellular carcinoma.5,6 Although a recent study demonstrated that HCV mortality currently exceeds human immunodeficiency virus (HIV) mortality in the United States, resources for HIV surveillance and treatment vastly exceed those dedicated to HCV.7 As newly approved, potentially more effective, and easier to tolerate HCV treatment regimens become available, it is expected that more patients will complete treatment, ultimately reducing overall HCV morbidity and mortality.8
In response to the increasing concern about HCV in the U.S., the Institute of Medicine (IOM) issued “Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C,” which calls on health departments to improve HCV surveillance. The purpose of improved surveillance is to better understand the local and national impact of HCV, increase awareness, and target resources to affected populations and risk groups. The IOM acknowledges that current funding is inadequate to support HCV surveillance needs and recommends that further funding be provided.1 While NYC receives funding for hepatitis surveillance, investigation of all newly reported cases is not feasible, as the NYC Department of Health and Mental Hygiene (DOHMH) receives approximately 10,000 newly reported HCV cases a year.9
Because investigating each case is not feasible, we developed enhanced chronic HCV surveillance using a sampling protocol to collect detailed information from a representative sample of patients. We describe the results of enhanced surveillance of a sample of patients and evaluate the usefulness and resources required.
METHODS
The NYC Health Code requires laboratories and clinicians to report positive HCV antibody tests with a high signal-to-cutoff ratio and positive recombinant immunoblot assays (RIBAs) to the health department. It is recommended that all antibody-positive patients receive ribonucleic acid (RNA) testing to determine if they are chronically infected; positive RNA tests are also reportable.10,11 Required data elements include date of birth, sex, patient name, and address; type of HCV test; and clinician name and address.
Beginning in July 2009, we randomly selected 20 patients every two months from all patients newly reported with positive HCV tests in the 2–3 months before the sampling date. Analysis included patients with diagnosis dates from April 2009 to January 2011. We excluded non-NYC residents and patients younger than 18 years of age or with no date of birth in the system. We also excluded laboratory and sampling errors discovered during investigation. Investigators identified laboratory errors through direct communication with the testing laboratory. A few sampling errors occurred because of incorrect reporting of patients' dates of birth. Once we corrected the dates of birth, we determined that these patients matched to reports with HCV diagnosis dates earlier than specified for our sampling time frame.
Investigators, mainly graduate student interns, contacted the clinician to ensure that the patient had been notified of his/her positive HCV test. If the clinician had not informed the patient, investigators urged the clinician to do so immediately. Investigators administered questionnaires to clinicians and patients to collect demographics, risk factors, reasons for HCV testing, clinical information, and laboratory results. The DOHMH did not order or perform any laboratory tests for this study but did collect any available results. If the patient did not have an RNA test, we reminded the clinician that RNA testing is recommended.
For RNA-positive patients, questionnaires included additional questions about antiviral treatment, hepatitis A and B immunity, support groups, and counseling. Investigators faxed the questionnaire to all clinicians listed on the laboratory reports and instructed them to return it within five business days; investigators followed up with the clinician by phone to encourage completion. If clinicians mentioned that the patient had seen another clinician who may know the patient better, we contacted that clinician as well. If the clinician did not complete the questionnaire, the investigator instead reviewed the patient's medical record. If we determined that a patient was HCV RNA-negative during the investigation process, we excluded that patient from further investigation and analysis.
One week after contacting the clinician, investigators attempted to contact the patient by phone (three attempts during one week). During the patient interview, if it was apparent that the patient was unaware of his/her positive HCV test, investigators discontinued the questionnaire and reminded the clinician to inform the patient of his/her positive test. After one month, investigators again contacted the patient. For patients who were unreachable by phone, we sent a letter and questionnaire by certified mail. If we received no response after two weeks, staff closed the investigation. At the conclusion of each investigation, investigators sent clinicians a patient education booklet called “Hepatitis C: The Facts” and a bulletin for clinicians on HCV diagnosis and management.12,13 Investigators also mailed the booklet to patients who requested it, although many patients had already received it as part of DOHMH's routine outreach.
We entered all data into Microsoft® Access and performed all analyses using SAS® software version 9.2.14 We set statistical significance at α=0.05. To establish representativeness of the sample, we compared sex, county of residence, and age for patients contacted as part of enhanced surveillance with all newly reported HCV patients in 2010 using Chi-square statistics. We compared demographics of patients interviewed and not interviewed using Fisher's exact test. If both clinicians and patients answered a question, we used a hierarchal algorithm with “yes” taking precedence, followed by “no,” and “unknown.” We examined discrepancies between clinician and patient answers for 18 selected variables (e.g., risk factor, insurance, and HIV status) to assess internal consistency. Additionally, we calculated the frequency of the response “unknown” to all 29 questions asked of clinicians, excluding sex, birth country, and race/ethnicity. DOHMH further evaluated the enhanced surveillance system by interviewing the surveillance coordinator and four investigators to ascertain the time necessary to complete investigations, challenges encountered in collecting information from patients and clinicians, and ways in which the data have been used for public health purposes. We did not assess time for training, reviewing investigations, and data entry.
We calculated response frequencies for questions asked of both clinicians and patients (165 responses), those asked only of clinicians (164 responses), and those asked only of patients (77 responses). Seven questions were only relevant for patients with positive HCV RNA tests; therefore, we calculated these frequencies out of the 98 HCV RNA-positive patients.
RESULTS
From April 2009 to January 2011, 21,177 newly reported patients tested positive for HCV in NYC, and of these we randomly selected 220 patients. We excluded 15 patients because they did not meet inclusion criteria. According to previously reported laboratory results and any additional results received through contact with clinicians, 98 (48%) of the remaining 205 patients had a positive HCV RNA test, 40 (19.5%) had a negative HCV RNA test, and 67 (32.5%) did not receive the RNA testing necessary to determine infection status even after DOHMH staff requested such testing (Figure). Thus, we limited analyses to 165 patients after excluding the 40 HCV RNA-negative patients.
Figure.
Flow diagram of enhanced chronic hepatitis C surveillance in New York City: New York City Department of Health and Mental Hygiene, 2011
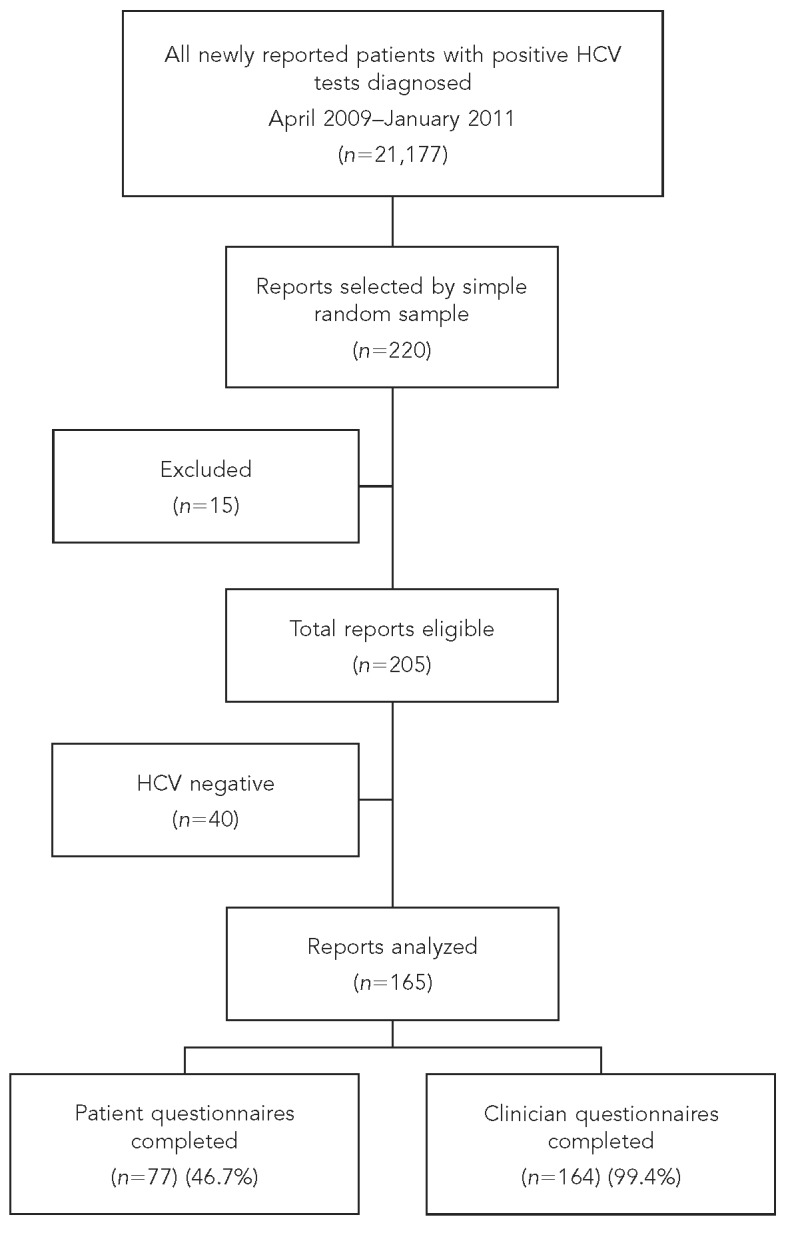
HCV = hepatitis C virus
Clinicians completed 135 of 165 (81.8%) questionnaires: 131 (79.9%) by fax and four (2.4%) by mail/phone. DOHMH staff completed 29 (17.7%) additional clinician questionnaires by medical record review, which resulted in completing 164 of 165 clinician questionnaires total (99.4%). Patients completed 77 (46.7%) of 165 interviews (Figure). Reasons for incomplete interviews were: unable to contact (65.9%), refusal (9.1%), homelessness (8.0%), deceased (6.8%), out of the country (3.4%), incarcerated (2.3%), cognitive issues (2.3%), or inpatient in a substance abuse/detoxification center or psychiatric facility (2.2%) (data not shown).
We calculated the frequency of the response “unknown” to each of the 29 questions asked of clinicians. The question with the greatest percentage of unknown responses was whether the patient had contact with someone with HCV (88.5% unknown), while the question with the lowest percentage was the type of facility in which the clinician diagnosed the patient (4.2% unknown). Among the 29 specified questions, the median percentage was 50.9% unknown. Discrepancies between clinician and patient answers occurred 2.6% of the time (data not shown).
Sex, county of residence, and age were not significantly different when comparing 165 patients sampled for enhanced surveillance with all 9,992 newly reported people with a positive HCV test in 2010 (data not shown). However, when we compared enhanced surveillance patients, those interviewed were less likely to be homeless (11.1% vs. 88.9%, p=0.03), an inpatient (21.4% vs. 78.6%, p=0.0001), or in jail/prison (9.1% vs. 90.9%, p=0.01) when diagnosed, and more likely to have private insurance (78.3% vs. 21.7%, p=0.02) than those not interviewed (data not shown).
As shown in Table 1, males accounted for 61.8% and those born between 1945 and 1964 accounted for 54.0% of the sample; the median age was 53 years. Most patients were Hispanic (32.7%) or non-Hispanic black (32.7%). The most common reasons for testing were HCV risk factors (47.3%), elevated liver function tests (LFTs) (29.1%), and asymptomatic, prenatal, or donor screening (26.1%). The most common risk factors were injection (43.0%) or intranasal (33.9%) drug use and having ever been in jail/prison (32.7%). Of the 165 patients, 118 (71.5%) had insurance, most of whom had Medicaid and/or Medicare (83.1%) (Table 1). One-third of patients were diagnosed in nontraditional health-care settings, such as substance abuse/detoxification centers (25.0%), jail/prison (6.7%) (Table 2), and psychiatric facilities (1.8%) (data not shown).
Table 1.
Enhanced HCV surveillance information from clinician and patient questionnaires: New York City, 2009–2011 (n=165)


aAlbania (1), Myanmar (1), China (1), Georgia Republic (1), Guinea (1), and South Korea (1)
bNot mutually exclusive
HCV = hepatitis C virus
HIV = human immunodeficiency virus
Table 2.
Enhanced HCV surveillance information from clinician questionnaires: New York City, 2009–2011 (n=164)
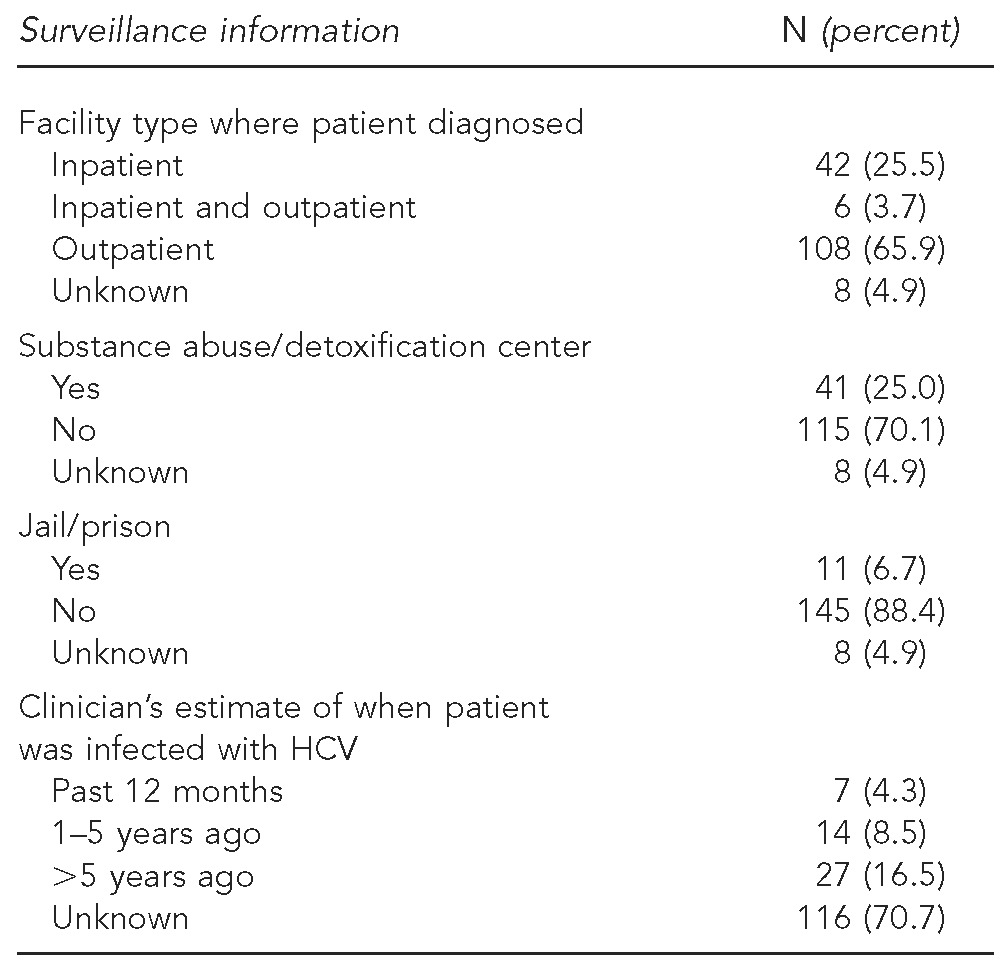
HCV = hepatitis C virus
Fifty-three patients (68.8%) had a primary care clinician and 49.4% requested the educational booklet. Of the 55 interviewed patients who were HCV RNA-positive, 17 (30.9%) who were not already attending a support group were interested in attending one (Table 3). More than half of clinicians reported that they counseled patients about alcohol use (61.2%) and HCV transmission (61.2%). Clinicians also reported that 38.8% of patients were immune to hepatitis A and 39.8% were immune to hepatitis B (Table 4).
Table 3.
Enhanced HCV surveillance information from patient questionnaires: New York City, 2009–2011 (n=77)
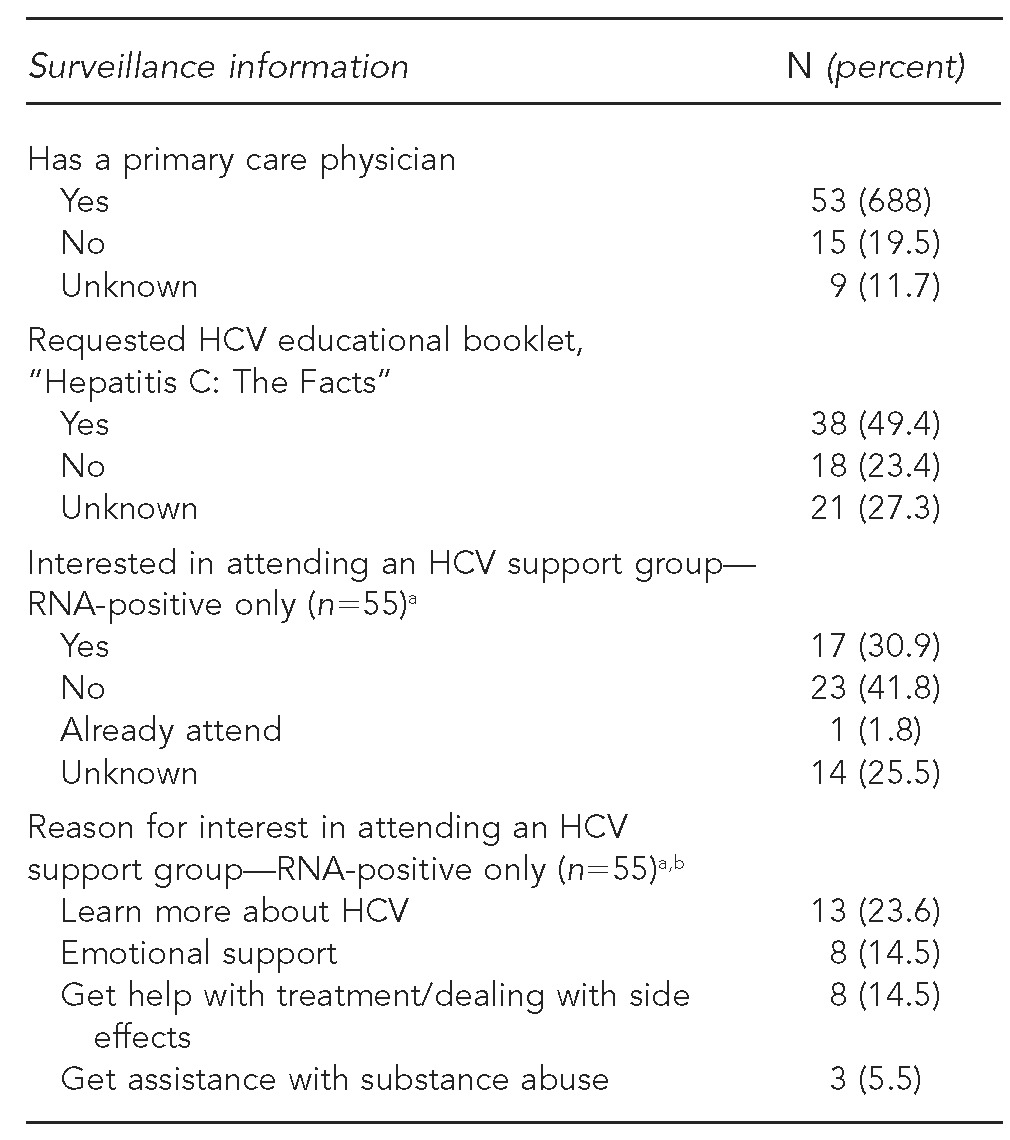
aCalculated out of 55 RNA-positive interviewed patients, as questions were only asked of patients
bNot mutually exclusive
HCV = hepatitis C virus
RNA = ribonucleic acid
Table 4.
Enhanced HCV surveillance information from clinician questionnaires for RNA-positive patients: New York City, 2009–2011 (n=98)
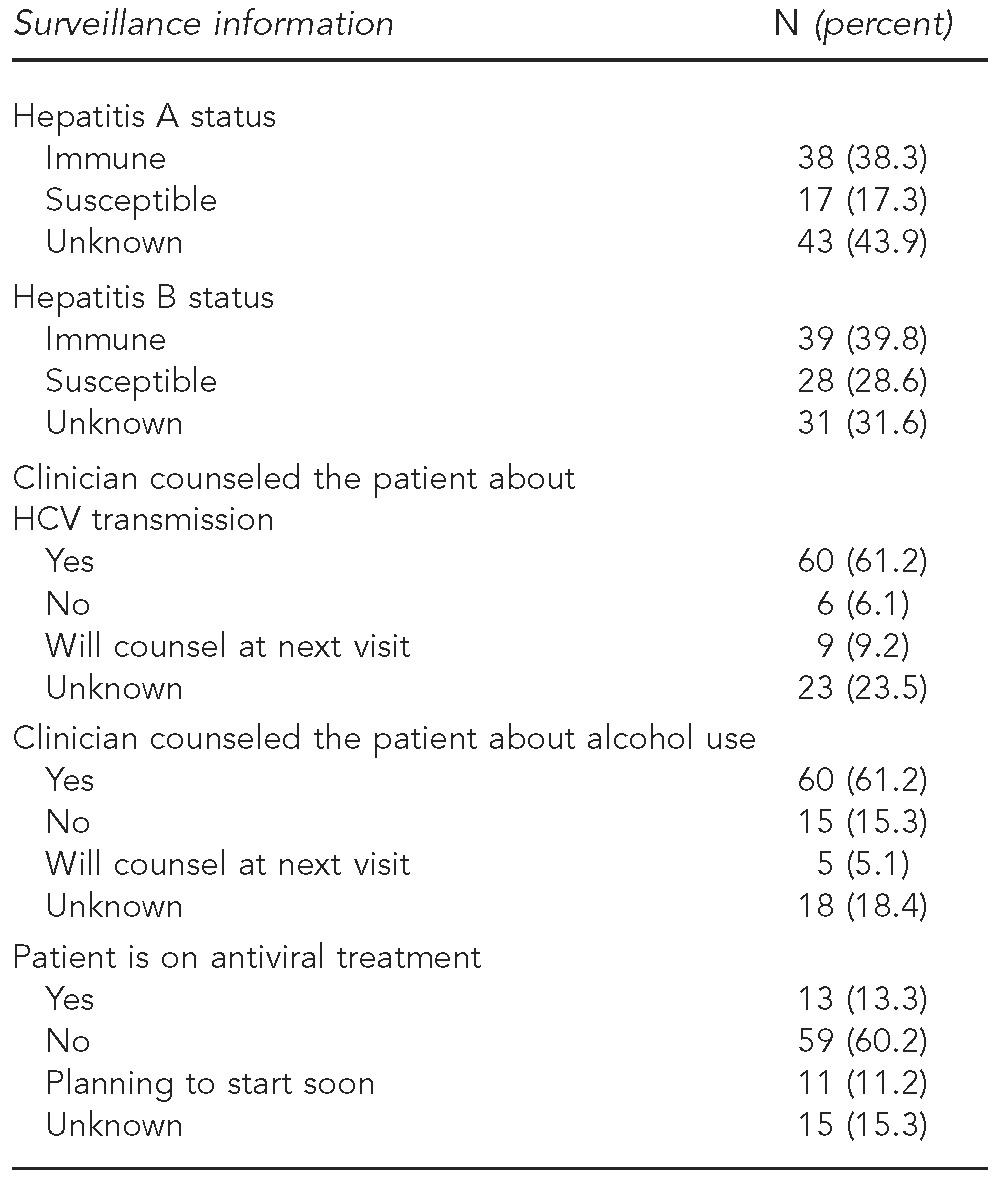
HCV = hepatitis C virus
RNA = ribonucleic acid
DOHMH staff estimated that they spent a median of 3.5 hours on each investigation. The median time from assignment to completion was 50 days. Staff indicated that the protocol was easy to follow but that patients were difficult to reach, and getting clinicians to complete the questionnaire required persistence and multiple phone calls. Investigators stated that they exhausted all opportunities to collect data from patients and clinicians; however, in many cases, patients had changed addresses since the report, had their phones disconnected, or were homeless and could not be interviewed. Staff also commented that the data collected were useful for characterizing patients for educational outreach as well as identifying what tests clinicians were ordering and why. They stated that analyzing a sample of cases allows DOHMH to obtain patient characteristics that are not available from laboratory reports alone and to identify unmet needs of the population. Staff deemed the data on risk factors and the type of facility making the HCV diagnosis especially valuable in devising new strategies for prevention and patient support.
DISCUSSION
Although resource intensive, NYC's enhanced HCV surveillance project provides a detailed look at a sample of newly reported people and suggests opportunities to increase education and improve medical care. Patients properly informed of their diagnoses can take steps to improve their own health, including obtaining appropriate vaccinations, abstaining from drinking alcohol and other hepatotoxic drugs, and possibly undergoing treatment. For health departments, having detailed surveillance data allows for planning and outreach. The sample was generally representative of the newly reported HCV population in NYC. Clinician and patient answers were relatively consistent, with questions answered differently only 2.6% of the time.
We encountered expected difficulties in reaching patients for interviews, as addresses and phone numbers were often incorrect. We do not believe the patient interview rate could have been raised even with additional staff. Difficulty in reaching clinicians with insight into patients' risk factors and medical history was compounded by the fact that 30% of interviewed patients did not have primary care clinicians and that many patients were tested in nontraditional settings, such as substance abuse/detoxification centers, jail/prison, and psychiatric facilities.
The enhanced surveillance data show that about half of the patients (47.3%) were tested for HCV due to risk factor histories, as per Centers for Disease Control and Prevention (CDC) guidelines.15 However, 40% of patients were tested for elevated LFTs or health problems, which suggests that many patients are not being diagnosed early enough, potentially limiting the possibility for medical management necessary to slow or prevent disease progression or prevent them from transmitting the infection to their close contacts.1,16 CDC recently proposed a one-time screening of people born between 1945 and 1964 (baby boomers) to identify undetected chronic HCV infections, which could lead to earlier diagnoses and potentially decrease the chances of complications such as cirrhosis and liver cancer.17
Unknown race/ethnicity for routine HCV surveillance data has previously been reported as 73.6% in NYC and 51%–52% nationally.18,19 With our enhanced HCV surveillance investigations, we reduced the proportion in our sample with unknown status to 7.3%. Non-Hispanic black people represented 32.7% of our sample compared with only 22.8% of the NYC population.20 This disparity should be addressed by considering educational campaigns targeted specifically toward non-Hispanic black people in NYC.
Half of interviewed patients requested the “Hepatitis C: The Facts” educational booklet, and more than one-quarter of RNA-positive patients not already attending an HCV support group were interested in doing so. This finding suggests that there are unmet educational needs in the HCV population. DOHMH developed a website that lists local HCV support groups and other patient resources and will continue distributing educational booklets to all newly reported patients.21 Increased educational initiatives specifically targeting substance abuse/detoxification centers should be considered, as injection (43%) and intranasal (33.9%) drug use were the most common risk factors and 25% of patients were diagnosed in these facilities.
The data demonstrate that some newly reported patients are not receiving basic recommended medical services. One-third of patients did not receive a follow-up RNA test to determine whether they were chronically infected and, therefore, eligible for treatment. A separate article describes this population and the importance of RNA testing in detail.22 The DOHMH is exploring options to increase the percentage of antibody-positive patients with known HCV infection status. Furthermore, nearly 40% of clinicians did not counsel their patients about HCV transmission or alcohol use. Alcohol is the most important modifiable risk factor to prevent progression of liver disease.23–27 Additionally, even though hepatitis A and B vaccination is recommended for HCV patients, more than half of patients had unknown status or were susceptible to one or both viruses.14,28,29
Although routine surveillance data on all people with HCV provide only basic information, we collected detailed demographic, clinical, and epidemiologic information by investigating a representative sample in enhanced surveillance. We used these detailed findings to develop educational materials, such as an HCV bulletin for primary care clinicians that details the need for RNA testing, counseling, and clinical management of HCV patients. Enhanced surveillance data on country of birth and primary language spoken also allowed us to prioritize translation of educational materials. DOHMH further described patients' unmet needs in terms of education, vaccination, support groups, and RNA testing to share with other stakeholders who address these needs.30,31
The 2010 IOM report's call to action proposes that health departments investigate all acute and chronic HCV and HBV cases. However, the volume of newly reported HCV cases annually in NYC would require 35,000 person-hours a year, which far exceeds available resources. Sampling, therefore, is the only feasible option. Similar sampling has been used for previous HCV projects and for surveillance on other high-volume diseases such as chronic hepatitis B, influenza, Lyme disease, and gonorrhea.30,32–35 We believe that the IOM's goals can be achieved through additional funding to continue and improve this type of enhanced surveillance system.
Limitations
This study had certain limitations. Unknown answers from clinicians were common, likely because patients were tested in non-routine care settings (e.g., detoxification and correctional facilities) where their clinicians may not know them very well. It is important to balance sample size with completeness, which is why NYC elected to investigate a small sample to achieve a high clinician completion rate (99%). Interviewing patients was also difficult; 53% of patients were not interviewed. This interview rate is not surprising given the population's mobility, and the rate is comparable with interview rates from other HCV studies ranging from 40%–68%.30,36,37 Because many clinicians responded “unknown” to questions and we could not interview half of the patients, we will continue to interview both clinicians and patients to obtain the most complete information possible. We were less successful in reaching incarcerated and homeless patients; thus, our interview data may not represent those with the greatest needs in terms of medical care and education. Surveillance data only include those reported to the health department with positive HCV tests; they do not include people untested and unaware of their HCV infection.
CONCLUSIONS
HCV surveillance involving the investigation of every case requires a large commitment of staff resources, but investigating a representative sample of new reports is a feasible alternative. Collecting key demographic and clinical information on a sample of individuals reported with HCV allows health departments and other stakeholders to develop HCV educational initiatives and target preventive services.
Acknowledgments
The authors thank the enhanced hepatitis C investigators who assisted on this project: Perminder Khosa, Janette Yung, Jennifer Baumgartner, Jennifer Brite, Victoria Tsai, and Timothy Wen.
Footnotes
This study was funded in part by a hepatitis surveillance grant from the Centers for Disease Control and Prevention (CDC), Emerging Infections Program. This study was also supported in part by an appointment to the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists (CSTE) and funded by CDC Cooperative Agreement #5U38HM000414. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the funding agencies.
All data used in this analysis were collected as part of routine public health surveillance activities. Therefore, this study was exempt from institutional review board review.
REFERENCES
- 1.Institute of Medicine. Washington: National Academies Press; 2010. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bornschlegel K, Berger M, Garg RK, Punsalang A, McKinney CM, Gwynn RC, et al. Prevalence of hepatitis C infection in New York City, 2004. J Urban Health. 2009;86:909–17. doi: 10.1007/s11524-009-9396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balter S. Stark JH, Kennedy J, Bornschlegel K, Konty K. Estimating the prevalence of hepatitis C infection in New York City using surveillance data. Epidemial Infect. 2013;9:1–8. doi: 10.1017/S0950268813000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (US) Hepatitis C information for health professionals: hepatitis C FAQs for health professionals. [cited 2012 Feb 27]. Available from: URL: http://www.cdc.gov/hepatitis/hcv/HCVfaq.htm.
- 7.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 8.Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216–24. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 9.New York City Department of Health and Mental Hygiene. Hepatitis A, B and C surveillance report: New York City, 2008 and 2009. [cited 2012 Mar 28]. Available from: URL: http://www.nyc.gov/html/doh/downloads/pdf/cd/cd-hepabc-surveillance-report-08-09.pdf.
- 10.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alter MJ, Kuhnert WL, Finelli L. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. MMWR Recomm Rep. 2003;52(RR-3):1–13. 15. [PubMed] [Google Scholar]
- 12.New York City Department of Health and Mental Hygiene. Hepatitis C: the facts. [cited 2013 Jun 3]. Available from: URL: http://www.nyc.gov/html/doh/downloads/pdf/cd/cd-hepc-bro.pdf.
- 13.New York City Department of Health and Mental Hygiene. Diagnosing and managing hepatitis C 2010. [cited 2012 Apr 12]. Available from: URL: http://www.nyc.gov/html/doh/downloads/pdf/chi/chi25-3.pdf.
- 14.SAS Institute, Inc. SAS®: Version 9.2. Cary (NC): SAS Institute, Inc.; 2008. [Google Scholar]
- 15.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR19):1–39. [PubMed] [Google Scholar]
- 16.Alter MJ, Seeff LB, Bacon BR, Thomas DL, Rigsby MO, Di Bisceglie AM. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Ann Intern Med. 2004;141:715–7. doi: 10.7326/0003-4819-141-9-200411020-00013. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (US) CDC announces first ever national hepatitis testing day and proposes that all baby boomers be tested once for hepatitis C [press release] 2012 May 18. [cited 2013 Jun 3]. Available from: URL: http://www.cdc.gov/nchhstp/newsroom/HepTestingRecsPressRelease2012.html.
- 18.Centers for Disease Control and Prevention (US) Viral hepatitis statistics and surveillance: table 4.4: reported cases of laboratory-confirmed, chronic (past or present) hepatitis C infection, by sex, race/ethnicity, age group, and case criteria—enhanced viral hepatitis surveillance sites, 2010. [cited 2012 Jun 29]. Available from: URL: http://www.cdc.gov/hepatitis/Statistics/2010Surveillance/Table4.4.htm.
- 19.Klevens RM, Miller J, Vonderwahl C, Speers S, Alelis K, Sweet K, et al. Population-based surveillance for hepatitis C virus, United States, 2006–2007. Emerg Infect Dis. 2009;15:1499–502. doi: 10.3201/eid1509.081050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.New York City Department of City Planning. Population: 2010 census data: table PL-P1 NYC: total population New York City and boroughs, 2000 and 2010. [cited 2012 Apr 10]. Available from: URL: http://www.nyc.gov/html/dcp/pdf/census/census2010/t_pl_p1_nyc.pdf.
- 21.New York City Department of Health and Mental Hygiene. Patient support groups. [cited 2013 Jun 5]. Available from: URL: http://nychepbc.org/resources/patient-support-groups.
- 22.McGibbon E, Bornschlegel K, Balter S. Half a diagnosis: gap in confirming infection among hepatitis C antibody-positive patients. Am J Med. doi: 10.1016/j.amjmed.2013.01.031. In press. [DOI] [PubMed] [Google Scholar]
- 23.Ascione A, Tartaglione T, Di Costanzo GG. Natural history of chronic hepatitis C virus infection. Dig Liver Dis. 2007;39(Suppl 1):S4–7. doi: 10.1016/s1590-8658(07)80003-x. [DOI] [PubMed] [Google Scholar]
- 24.Marcellin P, Pequignot F, Delarocque-Astagneau E, Zarski JP, Ganne N, Hillon P, et al. Mortality related to chronic hepatitis B and chronic hepatitis C in France: evidence for the role of HIV coinfection and alcohol consumption. J Hepatol. 2008;48:200–7. doi: 10.1016/j.jhep.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Westin J, Lagging LM, Spak F, Aires N, Svensson E, Lindh M, et al. Moderate alcohol intake increases fibrosis progression in untreated patients with hepatitis C virus infection. J Viral Hepat. 2002;9:235–41. doi: 10.1046/j.1365-2893.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- 26.Harris DR, Gonin R, Alter HJ, Wright EC, Buskell ZJ, Hollinger FB, et al. The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. Ann Intern Med. 2001;134:120–4. doi: 10.7326/0003-4819-134-2-200101160-00012. [DOI] [PubMed] [Google Scholar]
- 27.Frieden TR, Ozick L, McCord C, Nainan OV, Workman S, Comer G, et al. Chronic liver disease in central Harlem: the role of alcohol and viral hepatitis. Hepatology. 1999;29:883–8. doi: 10.1002/hep.510290308. [DOI] [PubMed] [Google Scholar]
- 28.Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-7):1–23. [PubMed] [Google Scholar]
- 29.Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP) MMWR Recomm Rep. 1991;40(RR-13):1–25. [PubMed] [Google Scholar]
- 30.Bornschlegel K, Crotty KJ, Sahl S, Balter S. Unmet needs among people reported with hepatitis C, New York City. J Public Health Manag Pract. 2011;17:E9–17. doi: 10.1097/PHH.0b013e3182053f1b. [DOI] [PubMed] [Google Scholar]
- 31.McGibbon E. Unmet need for hepatitis C PCR testing, New York City, 2009–2010. Presented at the CSTE Annual Conference; 2011 Jun 12–16; Pittsburgh. [Google Scholar]
- 32.Surveillance for chronic hepatitis B virus infection—New York City, June 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2012;61(01):6–9. [PubMed] [Google Scholar]
- 33.Samuel MC, Mocello AR, Gilson D, Bradbury KJ, Bolan G. California gonorrhea surveillance system: methodologic aspects and key results of a sample-based system. Public Health Rep. 2009;124(Suppl 2):87–97. doi: 10.1177/00333549091240S213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.New York State Department of Health. Lyme disease per 100,000 population. [cited 2012 May 30]. Available from: URL: http://www.health.ny.gov/statistics/chac/general/lyme.htm.
- 35.Smith PF, Hadler JL, Stanbury M, Rolfs RT, Hopkins RS. “Blueprint version 2.0:” updating public health surveillance for the 21st century. J Public Health Manag Pract. 2013;19:231–9. doi: 10.1097/PHH.0b013e318262906e. [DOI] [PubMed] [Google Scholar]
- 36.Notes from the field: hepatitis C virus infections among young adults—rural Wisconsin, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(19):358. [PubMed] [Google Scholar]
- 37.San Francisco Department of Public Health. Chronic hepatitis B and hepatitis C infection surveillance report 2010: San Francisco, California. [cited 2012 Jul 18]. Available from: URL: http://www.sfcdcp.org/publications.html.


