Abstract
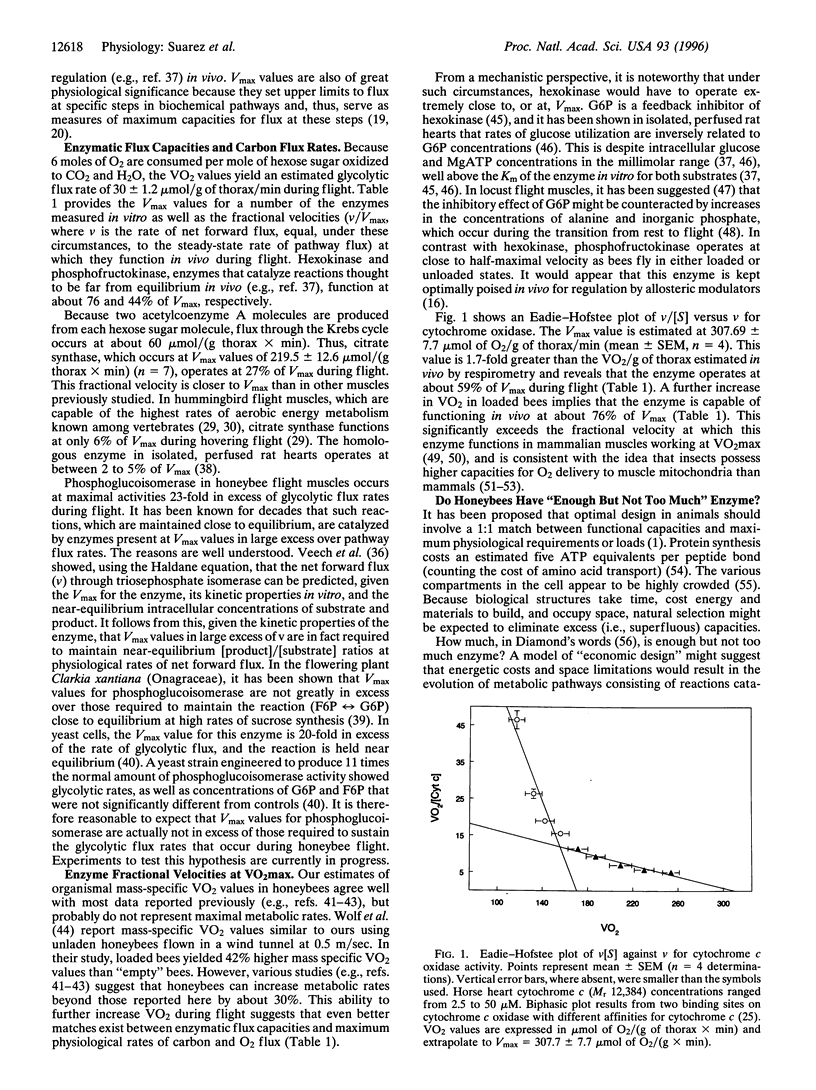
Honeybees rely primarily on the oxidation of hexose sugars to provide the energy required for flight. Measurement of VCO2 (equal to VO2, because VCO2/VO2 = 1.0 during carbohydrate oxidation) during flight allowed estimation of steady-state flux rates through pathways of flight muscle energy metabolism. Comparison of Vmax values for flight muscle hexokinase, phosphofructokinase, citrate synthase, and cytochrome c oxidase with rates of carbon and O2 flux during flight reveal that these enzymes operate closer to Vmax in the flight muscles of flying honeybees than in other muscles previously studied. Possible mechanistic and evolutionary implications of these findings are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardawi M. S., Majzoub M. F., Masoud I. M., Newsholme E. A. Enzymic and metabolic adaptations in the gastrocnemius, plantaris and soleus muscles of hypocaloric rats. Biochem J. 1989 Jul 1;261(1):219–225. doi: 10.1042/bj2610219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin K. M., Winder W. W., Terjung R. L., Holloszy J. O. Glycolytic enzymes in different types of skeletal muscle: adaptation to exercise. Am J Physiol. 1973 Oct;225(4):962–966. doi: 10.1152/ajplegacy.1973.225.4.962. [DOI] [PubMed] [Google Scholar]
- Benevolensky S. V., Clifton D., Fraenkel D. G. The effect of increased phosphoglucose isomerase on glucose metabolism in Saccharomyces cerevisiae. J Biol Chem. 1994 Feb 18;269(7):4878–4882. [PubMed] [Google Scholar]
- Blomstrand E., Ekblom B., Newsholme E. A. Maximum activities of key glycolytic and oxidative enzymes in human muscle from differently trained individuals. J Physiol. 1986 Dec;381:111–118. doi: 10.1113/jphysiol.1986.sp016316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney G. J., Taegtmeyer H., Newsholme E. A. Tricarboxylic acid cycle flux and enzyme activities in the isolated working rat heart. Biochem J. 1981 Dec 15;200(3):701–703. doi: 10.1042/bj2000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C. E. The steady-state kinetics of cytochrome c oxidation by cytochrome oxidase. Biochim Biophys Acta. 1990 Jun 26;1017(3):187–203. doi: 10.1016/0005-2728(90)90184-6. [DOI] [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. The activities of lipases and carnitine palmitoyltransferase in muscles from vertebrates and invertebrates. Biochem J. 1972 Dec;130(3):697–705. doi: 10.1042/bj1300697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochem J. 1972 Jan;126(1):49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M. Evolutionary physiology. The red flag of optimality. Nature. 1992 Jan 16;355(6357):204–206. doi: 10.1038/355204a0. [DOI] [PubMed] [Google Scholar]
- Dickinson M. H., Lighton J. R. Muscle efficiency and elastic storage in the flight motor of Drosophila. Science. 1995 Apr 7;268(5207):87–90. doi: 10.1126/science.7701346. [DOI] [PubMed] [Google Scholar]
- Dobson G. P., Yamamoto E., Hochachka P. W. Phosphofructokinase control in muscle: nature and reversal of pH-dependent ATP inhibition. Am J Physiol. 1986 Jan;250(1 Pt 2):R71–R76. doi: 10.1152/ajpregu.1986.250.1.R71. [DOI] [PubMed] [Google Scholar]
- England P. J., Randle P. J. Effectors of rat-heart hexokinases and the control of rates of glucose phosphorylation in the perfused rat heart. Biochem J. 1967 Dec;105(3):907–920. doi: 10.1042/bj1050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B. How crowded is the cytoplasm? Cell. 1982 Sep;30(2):345–347. doi: 10.1016/0092-8674(82)90231-8. [DOI] [PubMed] [Google Scholar]
- Garland T., Jr, Carter P. A. Evolutionary physiology. Annu Rev Physiol. 1994;56:579–621. doi: 10.1146/annurev.ph.56.030194.003051. [DOI] [PubMed] [Google Scholar]
- Goldfarb A. H., Bruno J. F., Buckenmeyer P. J. Intensity and duration of exercise effects on skeletal muscle cAMP, phosphorylase, and glycogen. J Appl Physiol (1985) 1989 Jan;66(1):190–194. doi: 10.1152/jappl.1989.66.1.190. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Bianconcini M. S., Parkhouse W. S., Dobson G. P. On the role of actomyosin ATPases in regulation of ATP turnover rates during intense exercise. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5764–5768. doi: 10.1073/pnas.88.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y., Sato K., Tsuchiya N., Thomas S., Fell D. A., Veech R. L., Passonneau J. V. Control of glucose utilization in working perfused rat heart. J Biol Chem. 1994 Oct 14;269(41):25502–25514. [PubMed] [Google Scholar]
- Kruckeberg A. L., Neuhaus H. E., Feil R., Gottlieb L. D., Stitt M. Decreased-activity mutants of phosphoglucose isomerase in the cytosol and chloroplast of Clarkia xantiana. Impact on mass-action ratios and fluxes to sucrose and starch, and estimation of Flux Control Coefficients and Elasticity Coefficients. Biochem J. 1989 Jul 15;261(2):457–467. doi: 10.1042/bj2610457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunoki M., Storlien L. H., MacDessi J., Oakes N. D., Kennedy C., Chisholm D. J., Kraegen E. W. Muscle glucose uptake during and after exercise is normal in insulin-resistant rats. Am J Physiol. 1993 Feb;264(2 Pt 1):E167–E172. doi: 10.1152/ajpendo.1993.264.2.E167. [DOI] [PubMed] [Google Scholar]
- Lee C. P., Gu Q., Xiong Y., Mitchell R. A., Ernster L. P/O ratios reassessed: mitochondrial P/O ratios consistently exceed 1.5 with succinate and 2.5 with NAD-linked substrates. FASEB J. 1996 Feb;10(2):345–350. doi: 10.1096/fasebj.10.2.8641569. [DOI] [PubMed] [Google Scholar]
- Lefebvre Y. A., Huber R. E. Solubilization, purification, and some properties of trehalase from honeybee (Apis mellifera). Arch Biochem Biophys. 1970 Oct;140(2):514–518. doi: 10.1016/0003-9861(70)90096-2. [DOI] [PubMed] [Google Scholar]
- Leite A., Neto J. A., Leyton J. F., Crivellaro O., el-Dorry H. A. Phosphofructokinase from bumblebee flight muscle. Molecular and catalytic properties and role of the enzyme in regulation of the fructose 6-phosphate/fructose 1,6-bisphosphate cycle. J Biol Chem. 1988 Nov 25;263(33):17527–17533. [PubMed] [Google Scholar]
- Lueck J. D., Fromm H. J. Kinetics, mechanism, and regulation of rat skeletal muscle hexokinase. J Biol Chem. 1974 Mar 10;249(5):1341–1347. [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Maximum catalytic activity of some key enzymes in provision of physiologically useful information about metabolic fluxes. J Exp Zool. 1986 Aug;239(2):159–167. doi: 10.1002/jez.1402390203. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Zammit V. A. Use of enzyme activities as indices of maximum rates of fuel utilization. Ciba Found Symp. 1979;(73):245–258. doi: 10.1002/9780470720561.ch14. [DOI] [PubMed] [Google Scholar]
- Parra J., Pette D. Effects of low-frequency stimulation on soluble and structure-bound activities of hexokinase and phosphofructokinase in rat fast-twitch muscle. Biochim Biophys Acta. 1995 Sep 6;1251(2):154–160. doi: 10.1016/0167-4838(95)00084-8. [DOI] [PubMed] [Google Scholar]
- Reynafarje B., Costa L. E., Lehninger A. L. O2 solubility in aqueous media determined by a kinetic method. Anal Biochem. 1985 Mar;145(2):406–418. doi: 10.1016/0003-2697(85)90381-1. [DOI] [PubMed] [Google Scholar]
- Robinson D. M., Ogilvie R. W., Tullson P. C., Terjung R. L. Increased peak oxygen consumption of trained muscle requires increased electron flux capacity. J Appl Physiol (1985) 1994 Oct;77(4):1941–1952. doi: 10.1152/jappl.1994.77.4.1941. [DOI] [PubMed] [Google Scholar]
- Rowan A. N., Newsholme E. A. Changes in the contents of adenine nucleotides and intermediates of glycolysis and the citric acid cycle in flight muscle of the locust upon flight and their relationship to the control of the cycle. Biochem J. 1979 Jan 15;178(1):209–216. doi: 10.1042/bj1780209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor B. Biochemical adaptations for flight in the insect. Biochem Soc Symp. 1976;(41):111–131. [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerzmann K., Hoppeler H., Kayar S. R., Weibel E. R. Oxidative capacity of muscle and mitochondria: correlation of physiological, biochemical, and morphometric characteristics. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1583–1587. doi: 10.1073/pnas.86.5.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez R. K. Hummingbird flight: sustaining the highest mass-specific metabolic rates among vertebrates. Experientia. 1992 Jun 15;48(6):565–570. doi: 10.1007/BF01920240. [DOI] [PubMed] [Google Scholar]
- Suarez R. K., Lighton J. R., Brown G. S., Mathieu-Costello O. Mitochondrial respiration in hummingbird flight muscles. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4870–4873. doi: 10.1073/pnas.88.11.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez R. K., Lighton J. R., Moyes C. D., Brown G. S., Gass C. L., Hochachka P. W. Fuel selection in rufous hummingbirds: ecological implications of metabolic biochemistry. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9207–9210. doi: 10.1073/pnas.87.23.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez R. K. Upper limits to mass-specific metabolic rates. Annu Rev Physiol. 1996;58:583–605. doi: 10.1146/annurev.ph.58.030196.003055. [DOI] [PubMed] [Google Scholar]
- Taylor C. R., Weibel E. R. Design of the mammalian respiratory system. I. Problem and strategy. Respir Physiol. 1981 Apr;44(1):1–10. [PubMed] [Google Scholar]
- Veech R. L., Raijman L., Dalziel K., Krebs H. A. Disequilibrium in the triose phosphate isomerase system in rat liver. Biochem J. 1969 Dec;115(4):837–842. doi: 10.1042/bj1150837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIS-FOGH T. DIFFUSION IN INSECT WING MUSCLE, THE MOST ACTIVE TISSUE KNOWN. J Exp Biol. 1964 Jun;41:229–256. doi: 10.1242/jeb.41.2.229. [DOI] [PubMed] [Google Scholar]
- Wegener G., Schmidt H., Leech A. R., Newsholme E. A. Antagonistic effects of hexose 1,6-bisphosphates and fructose 2,6-bisphosphate on the activity of 6-phosphofructokinase purified from honey-bee flight muscle. Biochem J. 1986 Jun 15;236(3):925–928. doi: 10.1042/bj2360925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Taylor C. R., Hoppeler H. The concept of symmorphosis: a testable hypothesis of structure-function relationship. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10357–10361. doi: 10.1073/pnas.88.22.10357. [DOI] [PMC free article] [PubMed] [Google Scholar]



