Abstract
The therapeutic effects of probiotic treatment in alcoholic liver disease (ALD) have been studied in both patients and experimental animal models. Although the precise mechanisms of the pathogenesis of ALD are not fully understood, gut-derived endotoxin has been postulated to play a crucial role in hepatic inflammation. Previous studies have demonstrated that probiotic therapy reduces circulating endotoxin derived from intestinal Gram-negative bacteria in ALD. In this study, we investigated the effects of probiotics on hepatic tumor necrosis factor-α (TNFα) production and inflammation in response to chronic alcohol ingestion. Mice were fed Lieber deCarli liquid diet containing 5% alcohol for 8 weeks and Lactobacillus rhamnosus GG (LGG) was supplemented in the last weeks. Eight-week alcohol feeding caused a significant increase in hepatic inflammation as shown by histological assessment and hepatic tissue myeloperoxidase activity assay. Two-weeks of LGG supplementation reduced hepatic inflammation and liver injury and markedly reduced TNFα expression. Alcohol feeding increased hepatic mRNA expression of Toll-like receptors and CYP2E1 and decreased nuclear factor erythroid 2-related factor 2 expression. LGG supplementation attenuated these changes. Using human peripheral blood monocytes-derived macrophages we also demonstrated that incubation with ethanol primes both lipopolysaccharide- and flagellin-induced TNFα production, and LGG culture supernatant reduced this induction in a dose dependent manner. In addition, LGG treatment also significantly decreased alcohol-induced phosphorylation of p38 MAP kinase. In conclusion, probiotic LGG treatment reduced alcohol-induced hepatic inflammation by attenuation of TNFα production via inhibition of TLR4 and TLR5-mediated endotoxin activation.
INTRODUCTION
Although the exact mechanisms of pathogenesis of alcoholic liver disease (ALD) are still not fully understood, accumulating studies have suggested that gut bacteria play a crucial role in the development of ALD. Gram-negative bacteria-derived endotoxemia, in conjunction with impaired gut integrity, may be one of the mechanisms for activating pro-inflammatory pathways leading to ALD [1]. Significantly increased lipopolysaccharide (LPS) levels were found in ALD patients at different stages [2-4] and in experimental animal models of ALD [5-7]. LPS stimulates hepatic Kupffer cells to produce pro-inflammatory cytokines and chemokines, resulting in reactive oxygen species ultimately leading to a vicious positive feedback loop that perpetuates liver inflammation.
LPS induces tumor necrosis factor α (TNFα) production by hepatic Kupffer cells via pattern recognition receptors such as Toll-like receptors (TLRs). Nine human TLRs have been identified [8]; each recognizes specific patterns of ligands in the microbial components. Among them, TLR4, which has been well-defined, recognizes Gram-negative bacteria-produced LPS. TLR1, 2 and 6 recognize microbial lipopeptides, while TLR3, 7, 8 and 9 bind to either single or double stranded RNA or bacterial or viral DNA. TLR5 is involved in the recognition of another major bacteria produced-exdotoxin: flagellin. The binding of endotoxin by TLR4 and TLR5 leads to activation of mitogen-activated protein kinase (MAPK) and nuclear factor κB (NFκB), resulting in the production of pro-inflammatory mediators such as TNFα.
There is as yet no FDA-proved drug for ALD. Other than abstinence, liver transplantation is the only choice for end-stage ALD treatment. Understanding the mechanisms of ALD may lead to the development of new therapeutic strategies. Clinical and experimental animal studies suggest the beneficial effects of probiotics in the prevention and treatment of ALD [6, 7, 9, 10]. A recent report from our laboratory shows that oral administration of Lactobacillus rhamnosus GG (LGG) attenuates established alcohol-induced hepatic steatosis and liver injury in a mouse model of ALD [7]. Indeed, probiotics change the gut microbiota profile and thereby alter the gut lumen favoring an anti-inflammatory milieu; such changes result in decreased production of pro-inflammatory bacterial products and improved barrier integrity leading to a decreased endotoxin release and liver protection. Although the effects of LGG on intestine gene expression have been studied in a dextran sodium sulfate-induced colitis mouse model [11], the protective effects of LGG in the alcoholic liver disease are associated with mucus layer protection by up-regulation of multiple mucus layer inducers and stabilizers, such as intestinal trefoil factor, which depends on hypoxia-inducible factor activation. The aim of present study was to further explore the mechanisms underlying the protective effects of LGG against ALD. Specifically, we investigated the effects of LGG on TLR activation and hepatic inflammation in a mouse model of ALD, and the effects of LGG culture supernatant on LPS and flagellin-induced TLR activation in vitro using macrophages. We showed that LGG inhibited TLR activation leading to the attenuation of pro-inflammatory TNFα production via p38 MAPK pathway.
MATERIALS AND METHODS
Animal model
Male C57BL/6N mice were obtained from Harlan (Indianapolis, IN). Mice were pair-fed liquid diets (Lieber DeCarli) containing either alcohol (alcohol-fed, AF) or isocaloric maltose–dextrin (pair-fed, PF), as described previously [7]. LGG (109 CFU/mouse/day) was added to the diet in the last two weeks of the experiment. Mice were maintained on the treatments for a total of 8 weeks. Liver samples were collected and fixed in Formalin for histology study or snap-frozen in liquid nitrogen and stored in −80 °C for later use. All mice were treated according to the protocols reviewed and approved by the Institutional Animal Care and Use Committee of the University of Louisville.
Liver histology
Formalin-fixed paraffin tissue sections were processed for staining with hematoxylin and eosin and then studied by light microscopy.
Liver TNFα levels and MPO activity assay
Liver samples were homogenized (50 mg/ml) in RIPA buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 2mM EDTA, 4 mM Na3VO4, 40 mM NaF, 1% Triton X-100, 1 mM Phenylmethylsulfonyl Fluoride (PMSF), 1% protease inhibitor cocktail). TNFα levels were measured using the Infinity Assay Kit (BD, Sparks, MD) according to the manufacturer’s instructions. For hepatic myeloperoxidase (MPO) activity assay, liver samples were homogenized (50 mg/ml) in 0.5% hexadecyltrimethylammonium bromide in 10 mM 3-(N-morpholino) propanesulfonic acid (MOPS) and centrifuged at 15,000 g for 40 min. The suspension was then sonicated three times, each for 30 s with an interval of 30 s. An aliquot of supernatant was mixed with a solution of 1.6 mM tetramethylbenzidine and 1 mM hydrogen peroxide. Activity was measured spectrophotometrically as the change in A650 at 37°C with a Spectramax microplate reader (Molecular Devices, Biotek). Results are expressed as milliunits of MPO activity per milligram of protein, as determined by the Bradford assay.
Cell culture and treatment
Cells were maintained at 37 °C in a humidified atmosphere with 5% CO2. Monocyte-derived macrophages were generated as previously described [12, 13]. In brief, peripheral blood monocytes (PBMCs), obtained from healthy donors as described previously [14], were washed twice with saline and suspended in RPMI 1640 buffer supplemented with 5% heat-activated fetal bovine serum (FBS), 0.01 M HEPES, and 50 μg/ml gentamicin (R5). PBMCs were plated in Costar 6 –well plates and allowed to mature for 4 days. After the maturation period, cells were washed twice in Dulbecco’s PBS with 0.2% FBS, and resuspended at 5 × 106/ml in R5. PBMC-derived macrophages were treated with 25mM ethanol for 3 days with and without the addition of LGG culture supernatant (LGG-s) derived from 109 CFU/ml bacteria at three concentrations of 0.5 μl/ml, 1 μl/ml and 2 μl/ml. After incubation with flagellin (Sigma-Adrich, St Louis, MO, 100 mg/ml) or LPS (Sigma-Aldrich, 100 ng/ml) for 4 hours, the cells were harvested.
Real time RT-PCR assay
The mRNA levels of TNFα, Cyp2E1 and TLRs were assessed by real-time RT-PCR. In brief, the total RNA was isolated with Trizol according to manufacturer’s protocol (Invitrogen, Carlsbad, CA) and reverse-transcribed using GenAmp RNA PCR kit (Applied Biosystems, Foster City, CA). The sequences of forward and reverse primers are listed in Table 1. Quantitative real time RT-PCR was performed on an ABI 7500 thermocycler (Applied Biosystems) and SYBR green PCR Master Mix was used. The relative quantities of target transcripts were calculated from duplicate samples after normalization to β-actin. Relative mRNA expression was calculated using the ΔΔCt method.
Table1.
Primer Sequences for Real-Time PCR
| Gene | Sequences (Forward/Reverse 5′-3′) | |
|---|---|---|
| TNFα | aggggacattcctgtgttcc | ttaccctgtttccccattcc |
| Cyp2E1 | tgggaacagcacacagtgac | gctggccctccttttaacac |
| TLR4 | gatctgagcttcaaccccttg | tgccatgccttgtcttcaat |
| TLR5 | ctccagacgcctcatctcac | tggcatatgttccaagcgta |
| β-actin | gagaccttcaacacccc | atagctcttctccagggagg |
Immunoblotting analysis
Tissues and cultured cells were homogenized and immunoblotting was performed as previously described [7]. Membranes were probed using antibodies (Cell Signaling, Danvers, MA) against Nrf2, phospho-p38 and total-p38, and Cyp2E1. β-actin was probed as a loading control. The protein bands were visualized by an enhanced chemiluminescence detection system (GE Healthcare, Piscataway, NJ) and quantified by densitometry analysis.
Statistics
All data are expressed as mean ± SEM. The data were analyzed by one way ANOVA and Newman-Keuls multiple-comparison test. Differences between groups were considered significant at P<0.05.
RESULTS
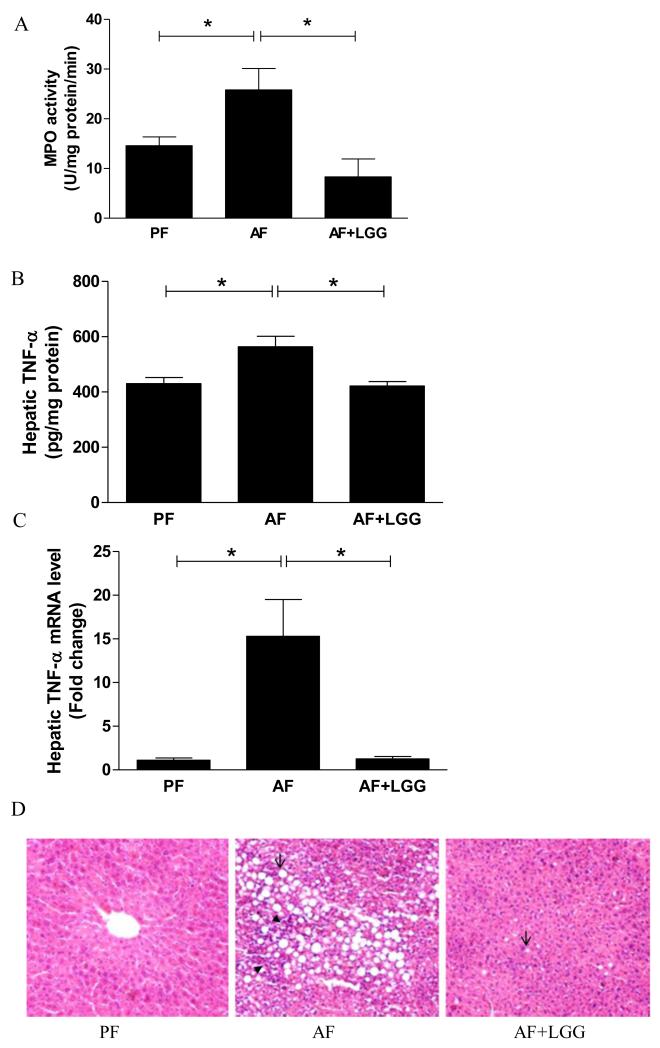
Previously, we showed that LGG supplementation attenuated alcohol-induced hepatic steatosis [7]. Here we demonstrated that LGG supplementation reduced alcohol-induced hepatic inflammation. The hepatic MPO activity was significantly higher in alcohol-fed animals compared with pair-fed controls, indicating an increased liver inflammation by alcohol exposure. The alcohol-increased MPO activity was completely abolished by LGG supplementation (Fig 1A). Likewise, the protein level of the hepatic inflammatory cytokine, TNFα, was significantly increased by alcohol exposure, and this increase was abolished by LGG supplementation (Fig 1B). Alcohol exposure markedly up-regulated the mRNA level of TNFα by 15 fold, and again, this effect was completely prevented by LGG supplementation (Fig 1C). Histological measurement showed that alcohol exposure significantly increased, and LGG supplementation decreased, hepatic fat and inflammatory foci accumulation (Fig 1D).
Figure 1.
Effects of LGG supplementation on hepatic inflammation. Mice were fed alcohol or isocaloric maltose dextrin for 8 weeks and LGG was supplemented in the last two weeks. A. MPO activity; B. TNFα protein levels and C. TNFα mRNA expression. D. H&E staining of liver sections. Arrows indicate fat drops; and arrowheads denote inflammation foci. PF: Pair-fed; AF: Alcohol-fed; AF+LGG: Alcohol-fed+LGG. *significantly different.
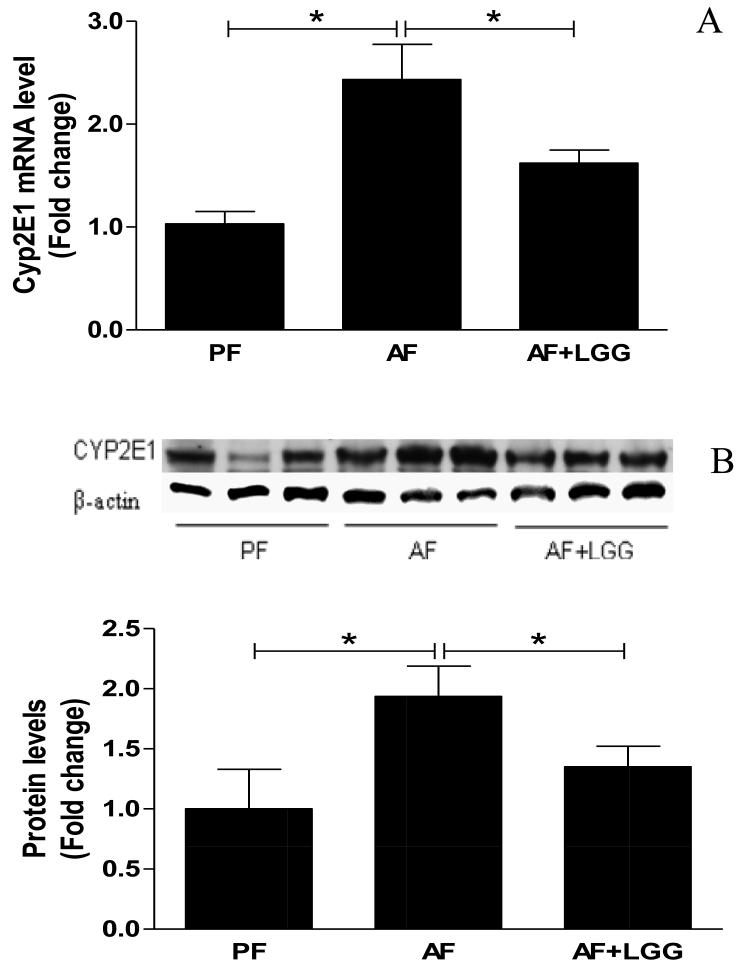
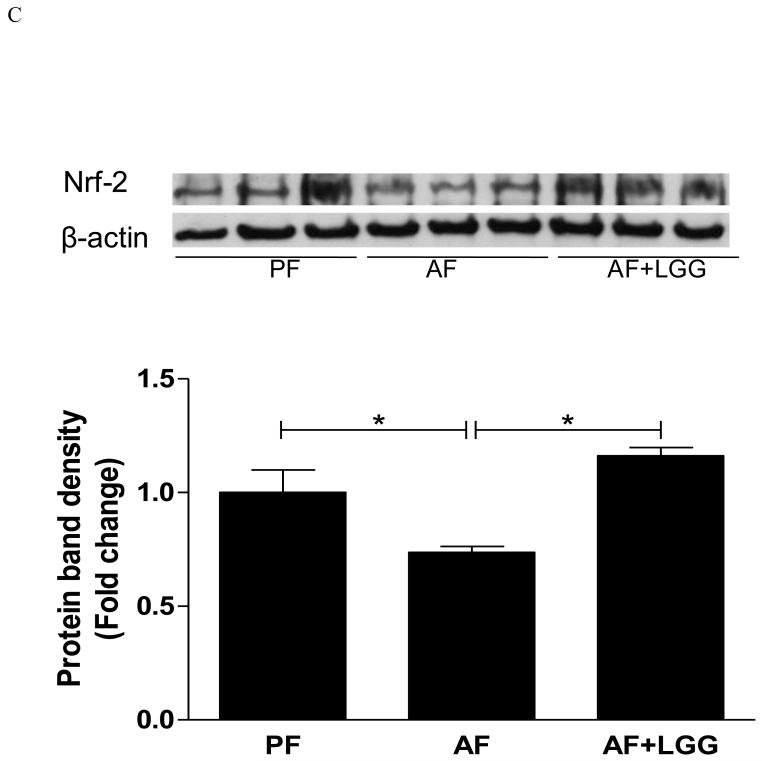
CYP2E1 plays an important role in the alcohol-mediated oxidative stress, lipid metabolism and peroxidation in the development of ALD. Numerous studies have demonstrated that hepatic levels of CYP2E1 are significantly increased during alcohol intoxication [15-17]. Therefore, we determined CYP2E1 expression at both mRNA and protein levels. Alcohol feeding significantly increased CYP2E1 mRNA expression, which was normalized by LGG supplementation (Fig 2A). Western blotting revealed a similar increase in protein level of CYP2E1 by alcohol and attenuation by LGG-supplementation (Fig 2B). Nuclear factor erythroid 2-related factor 2 (Nrf2) is critical in cellular anti-oxidative defenses. To evaluate the effects of LGG on alcohol-induced hepatic oxidative stress, Nrf2 protein expression was determined. Eight-weeks of alcohol feeding caused a significant decrease in Nrf2 protein expression, and LGG supplementation increased the Nrf2 protein levels (Fig 2C), indicating an effect of LGG on antioxidant defense.
Figure 2.
Effects of LGG supplementation on hepatic Cyp2e1 and Nrf2 expression. Mice were treated as described in Fig 1. Cyp2E1 mRNA (A) and protein levels (B). Nrf2 protein expression. *significantly different.
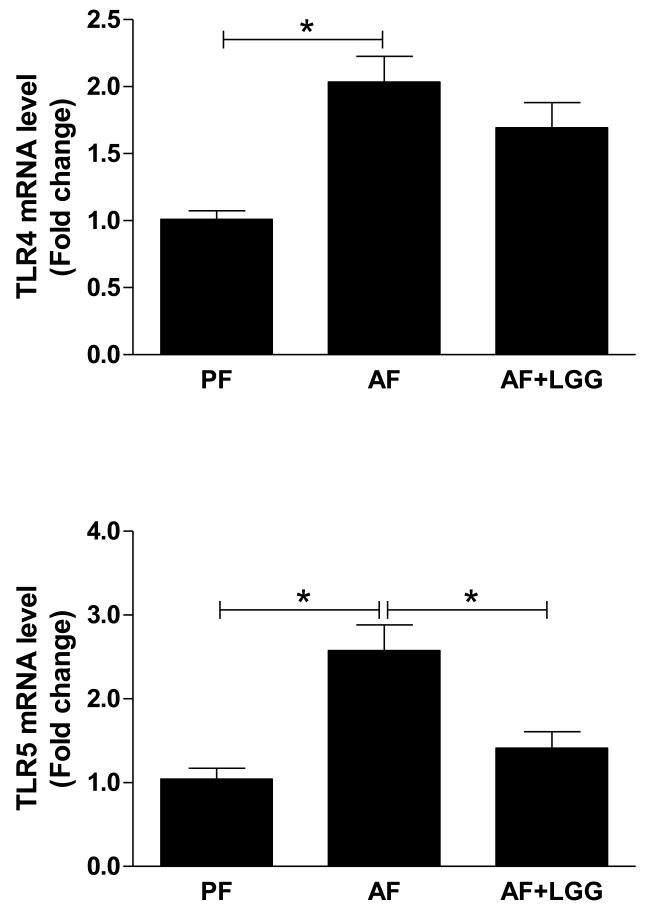
TLRs are critically involved in the activation of adapted immune response to various pathogens including alcohol. The resting liver usually expresses low levels of TLRs, possibly in order to reduce the hepatic response to the intestine-derived endotoxin [18]. Over-production of pro-inflammatory cytokines due to chronic stimulation of TLRs may lead to liver disease [18]. Measurement of the TLR mRNA levels revealed that alcohol exposure induced an increase in TLR4 and TLR5, which recognize Gram-negative bacteria-derived endotoxin LPS and flagellin, respectively. Importantly, LGG supplementation attenuated the increase of both TLR4 and TLR5 mRNA levels, and this reduction seems more potent for TLR5 (Fig 3). We also observed an upregulation of TLR1, 2, 3, 7, 8, and 9 mRNA levels by chronic alcohol ingestion as previously described [15], and an attenuation by LGG supplementation (data not shown).
Figure 3.
Effects of LGG supplementation on hepatic TLR mRNA expression. Mice were treated as described in Fig 1. *significantly different.
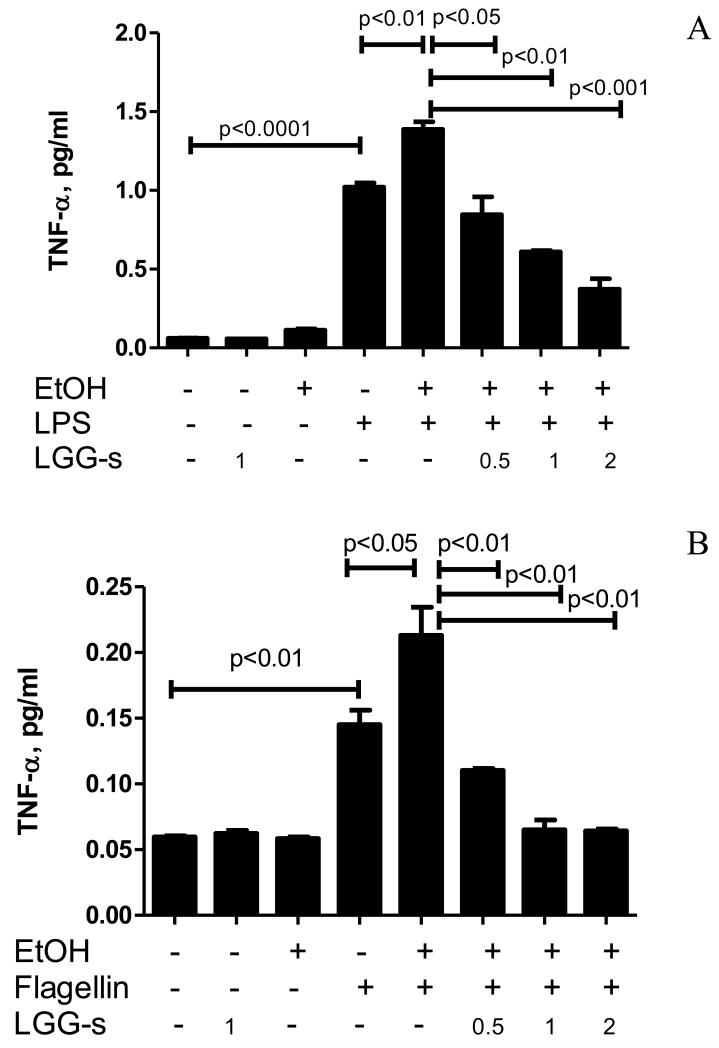
In particular, TLR4 is the most studied TLR in ALD. TLR4 knockout mice showed decreased hepatic steatosis and inflammation, and significantly reduced liver triglyceride, TNFα, and IL-6 serum levels after chronic alcohol feeding [19, 20], suggesting a key role for TLR4-mediated signals in alcoholic liver disease. TLR5 is another important TLR in the innate immune response, which specifically binds its ligand-flagellin produced by Gram-negative bacteria in the intestine. To determine the effects of alcohol and LGG on bacterial LPS and flagellin-induced inflammatory cytokine regulation, human PBMC-derived macrophages, which express both TLR4 and TLR5, were exposed to alcohol and LGG cultural supernatant (LGG-s), and challenged by LPS and flagellin. Media levels of TNFα were measured. Ethanol exposure did not induce significant TNFα production compared to control, but significantly augmented LPS and flagellin-induced TNFα expression. Pretreatment with LGG-s alone had no effect of TNFα production but significantly reduced ethanol primed and LPS- or flagellin-induced production of TNFα in a dose dependent manner (Fig 4). Taken together, ethanol exposure primed PBMC-derived macrophages and enhanced the effects of LPS and flagellin on TNFα production, which was attenuated by LGG-s pretreatment.
Figure 4.
Effects of LGG-supernatant on endotoxin-induced TNFα production in macrophages. PBMCs from healthy individuals were incubated for 4 days to mature to macrophages, as described in Materials and Methods. LGG bacteria were cultured to reach 109CFU/ml, and the supernatant was obtained by centrifugation and filtration through 0.22 μm filter. The macrophages were treated with 25mM ethanol for 3 days in the presence or absence of LGG-s at a concentration of 0.5 μl/ml, 1 μl/ml, and 2 μl/ml, as indicated. Cells were then challenged by LPS (A) or flagellin (B) for 4 hours. Media TNFα was evaluated by ELISA. *significantly different.
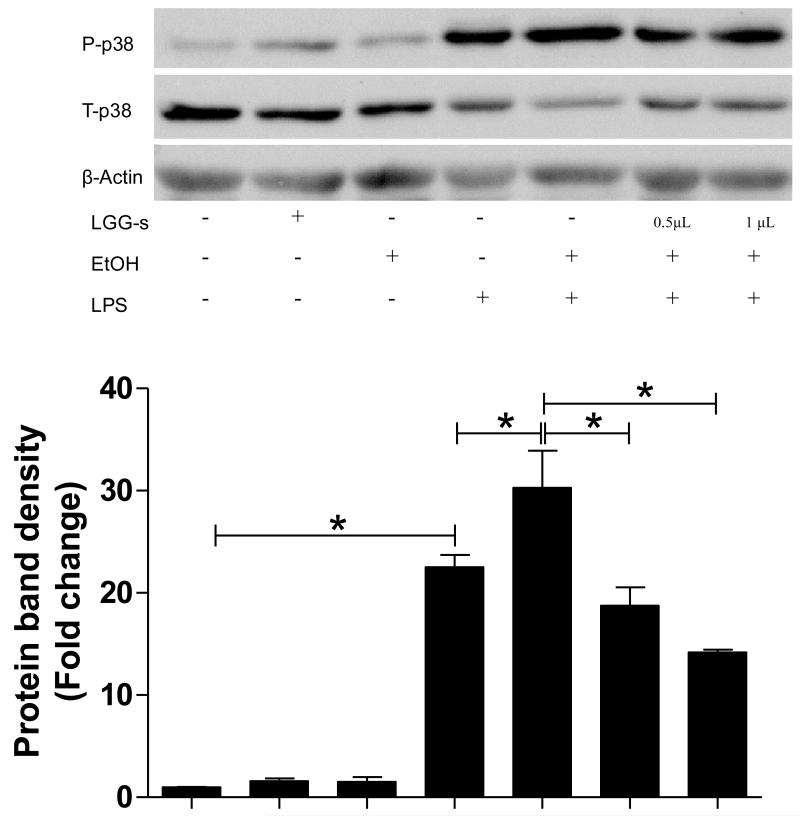
Endotoxin/TLR-induced reactive oxygen species (ROS) production plays an important role in the stimulation of downstream signaling leading to activation of MAP kinases and NFκB during chronic alcohol exposure. There was no significant change in the phosphorylation of p38 MAP kinase by 3-day ethanol or LGG-s exposure in PBMC-derived macrophages, while 4-h exposure of LPS induced a marked activation of p38 by 20 folds. Ethanol pre-exposure further increased LPS-stimulated phosphorylation of p38, and decreased total p38 MAPK protein levels. Importantly, LGG-s pretreatment prevented the ethanol-primed and LPS-induced p38 MAPK phosphorylation (Fig 5).
Figure 5.
Effects of LGG-s on p38 MAP kinase activation. PBMC-derived macrophages treated as in Figure 4 were analyzed for phosphorylation of p38 MAPK by immunoblotting. The protein bands were quantified by densitometry analysis, normalized to β-actin. *significantly different.
DISCUSSION
Our earlier studies demonstrated that LGG treatment attenuated chronic alcohol-induced liver steatosis and injury, which was associated with the improvement of intestinal barrier function and decreased exdotoxemia [7]. ALD is characterized by chronic inflammation, which is considered to be due to elevated inflammatory activators, such as LPS derived from Gram-negative bacteria in the gut. However, alcohol-mediated liver injury cannot be mimicked by chronic low-dose LPS in the absence of ethanol [21]. Numerous studies have indicated that chronic alcohol administration seems to prime the inflammatory cell activation. Our previous work [22] demonstrated that PBMCs from patients with alcoholic hepatitis produce more pro-inflammatory mediators, such as TNFα, in response to LPS. Hence, the effects of LGG supplementation on TNF production in response to chronic alcohol feeding in vivo and in alcohol-primed macrophages in vitro were examined in the present study.
A major finding of this study was that two-weeks of LGG supplementation attenuated chronic alcohol-induced TNFα production and hepatic inflammation. Moreover, LGG culture supernatant markedly reduced LPS-inducible TNFα expression in human PBMC-derived macrophages. Previous studies have shown that probiotics inhibit intestinal epithelial cell inflammatory signaling in experimental colitis models [23-28]. Probiotics may also directly modulate immune cell functions [29]. Normalized neutrophil function [30] and a decrease in systemic inflammation markers caused by LPS in rats [31] have been observed. Here, we showed that LGG ameliorated chronic alcohol-induced hepatic inflammation in mice, and LGG culture supernatant attenuated the alcohol-primed and endotoxin-induced TNFα production in macrophages. Increased TNFα expression in response to LPS requires the activation of a set of transcription factors binding to the promoter region of TNFα [32, 33]. Activation of TNFα expression in most types of macrophages requires recruitment of NFκB and early growth response 1 (Egr-1) [32, 33].
One of the mechanisms by which pathogens sensitize cells to LPS-induced liver injury is induction of CYP2E1 [34]. Accumulating evidence has demonstrated that alcohol-induced CYP2E1 expression plays an important role in ALD. Activation of CYP2E1 is associated with increased lipid peroxidation, mitochondrial dysfunction and hepatotoxicity [35]. CYP2E1 overexpressing transgenic mice had higher ALT levels and greater liver injury compared with the control mice [36]. Inhibition of CYP2E1 decreased fatty liver, and genetic depletion of CYP2E1 completely inhibited alcohol-induced hepatic steatosis. In the current study, we demonstrated that LGG supplementation markedly attenuated the chronic alcohol-induced significant increase of hepatic CYP2E1 expression in both mRNA and protein levels, which correlated with hepatic TNFα production and liver inflammation. On the other hand, activation of CYP2E1 generates ROS during its catalytic cycle. Our recent study has shown that LGG supplementation, indeed, reduced chronic alcohol exposure-induced hepatic superoxide formation [7], which was in agreement with previous studies in a rat model of ALD [9].
ROS-induced oxidative stress is a major contributing factor in alcoholic liver injury. Nrf2 plays a critical role in cellular anti-oxidant defenses through modulation of several major anti-oxidant responsive element (ARE)-dependent anti-oxidative mechanisms, including NADPH dehydrogenase-quinone-1 (NQO1), glutathione-S-transferase (GST) and glutathione reductase (GR). Loss of Nrf2 results in a significantly reduced ability to detoxify acetaldehyde, a toxic alcohol metabolite, and hepatic steatosis [37]. Chronic alcohol exposure induces an increase in hepatic Nrf2 expression, which is associated with CYP2E1 induction by alcohol [38]. However, our current study showed a decrease in Nrf2 protein level in the livers of alcohol exposed mice. This is likely due to the difference in alcohol-treatment period. In our study, the chronic alcohol exposure time was eight weeks, while only four weeks of alcohol treatment was applied in the study described by Gong et al. [38]. Nrf2, as a stress responsive protein, is increased by alcohol to combat alcohol induced, CYP2E1 mediated -oxidative stress. However, this is likely a short term solution. Long term alcohol exposure may cause the loss of this adaptive ability. Nevertheless, our results, in which LGG supplementation normalized alcohol-induced decrease in Nrf2, indicate that LGG has the potential to suppress alcohol-induced oxidative stress in the liver.
Initial studies in our laboratory showed that increased priming of human monocytes and Kupffer cells by alcohol led to exaggerated LPS-induced expression of TNFα [39]. Notably, the current study demonstrated that ethanol pretreatment sensitized both LPS- and flagellin-induced TNFα production in macrophages. Both LPS and flagellin are Gram-negative bacterium-derived endotoxins, and are the ligands for TLR4 and TLR5, respectively. Although LPS-TLR4 is the major and well-studied mechanism in ALD, other endotoxins appear to be responsible for some of the pathological changes observed in ALD. Several specific ligands to different TLRs have been tested for the ability to increase TNFα production in alcohol-fed mice [40]. Interestingly, although there was no change in TLR5 mRNA level in response to alcohol, the mice exposed to alcohol were more sensitive to flagellin challenge compared with control mice. The significant increase of hepatic TLR5 by alcohol exposure in our study is likely due to the longer time of exposure to alcohol.
Alcohol-induced TLR5 increase may be dependent not only on gut-derived endotoxin but also on the degree of oxidative stress in the liver, as demonstrated by Gustot, et al. [40]. The increase in TLR5 was normalized by LGG supplementation, again indicating the potential of LGG to combat oxidative stress in alcohol treated liver. Notably, our in vivo observation was confirmed by in vitro experiments. Our initial experiments using RAW 264.7 cells showed the response only to LPS, but not to flagellin for TNF production (data not shown). This is due to the murine macrophages expressing TLR4 but not TLR5 [41, 42]. However, human PBMC-derived macrophages express both TLR4 and TLR5 [43-45] and therefore will be activated by both LPS and flagellin. Thes significant attenuation by LGG-s in alcohol-primed, LPS- or flagellin-induced, TNFα production suggests that LGG culture supernatant may have a potential anti-inflammatory effect through activation of TLRs in response to gut bacteria-derived endotoxins.
The MAPK signaling cascade plays an essential role in the initiation of many cellular processes such as proliferation and apoptosis and inflammation [46]. Recognition of endotoxin, such as LPS and flagellin, by TLRs activates MAPK signaling pathways, resulting in TNFα production. Chronic alcohol exposure increases p38 MAP kinase activity, which contributes to TNFα expression in Kupffer cells, possibly by stabilization of TNFα mRNA via interaction with tristetraprolin [47, 48]. Inhibition of p38 activation completely abrogated alcohol exposure-mediated stabilization of TNFα mRNA. Ethanol treatment also induces ERK1/2 signaling leading to EGR-1 and NFκB transcriptional activation which contributes to the enhanced TNFα transcription [49, 50]. However, although the activity of JNK was decreased by chronic alcohol exposure in mice without any effect on TNFα mRNA level, acute alcohol exposure increased JNK phosphorylation and AP-1 binding in monocytes. While ethanol exposure did not affect p38 phosphorylation in PBMC-derived macrophages, LPS treatment increased p-p38, and ethanol pretreatment exaggerated LPS-induced phosphorylation of p38. The discrepancy in MAPK activation by alcohol in various studies may be due to the different treatment time and dose. Chen, et al. [51] showed that acute alcohol exposure resulted in an activation of ERK1/2, but ethanol did not modulate agonist-induced activation of ERK1/2 unless hepatocytes were treated with ethanol for at least 16 h. Nevertheless, the fact that probiotic supernatant pretreatment significantly reduced alcohol-primed LPS-induced phosphorylation of p38 in macrophages implies that LGG-s inhibition of TNFα production is likely via the decrease of p38 MAPK activation.
Our recent study[52] demonstrated that the LGG culture supernatant has beneficial effects in preventing binge alcohol-induced liver injury. The beneficial effects of probiotic supernatants have been demonstrated in several other pathogenic conditions [27, 28, 53], and it has been postulated that the secreted factors by probiotics are major contributors of the probiotic beneficial effects. Although several active ingredients have been identified in probiotic supernatant, more studies on the detailed identification of probiotic secreted factors warrant further investigation.
In conclusion, this study shows the protective effects of orally administered LGG against alcohol-induced liver inflammation in a mouse model. The molecular mechanisms associated with these protective effects include prevention of alcohol-induced oxidative stress by prevention of the decreased Nrf2 responsiveness, suppression CYP2E1 expression, inactivation of TLR4 and TLR5, and inhibition of p38 MAP kinase phosphorylation leading to decreased NFκB activation and TNFα production.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. JHepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 3.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. JHepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 4.Fukui H, Matsumoto M, Bode C, Bode JC, Tsujita S, Tsujii T. Endotoxaemia in patients with liver cirrhosis and upper gastrointestinal bleeding: detection by the chromogenic assay with plasma Tween 80 pretreatment. JGastroenterolHepatol. 1993;8:577–581. doi: 10.1111/j.1440-1746.1993.tb01656.x. [DOI] [PubMed] [Google Scholar]
- 5.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 6.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) ProcSocExpBiolMed. 1994;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Kirpich I, Liu Y, Ma Z, Barve S, McClain CJ, Feng W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. The American journal of pathology. 2011;179:2866–75. doi: 10.1016/j.ajpath.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual review of immunology. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 9.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163–172. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, Cave M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–82. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Przybyszewski J, Mitra D, Becker C, Brehm-Stecher B, Tentinger A, MacDonald RS. Soy protein diet, but not Lactobacillus rhamnosus GG, decreases mucin-1, trefoil factor-3, and tumor necrosis factor-alpha in colon of dextran sodium sulfate-treated C57BL/6 mice. The Journal of nutrition. 2011;141:1239–46. doi: 10.3945/jn.110.137414. [DOI] [PubMed] [Google Scholar]
- 12.Mukundan L, Bishop GA, Head KZ, Zhang L, Wahl LM, Suttles J. TNF receptor-associated factor 6 is an essential mediator of CD40-activated proinflammatory pathways in monocytes and macrophages. JImmunol. 2005;174:1081–1090. doi: 10.4049/jimmunol.174.2.1081. [DOI] [PubMed] [Google Scholar]
- 13.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. JImmunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr., Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. AmJPathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- 15.Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ. The Type of Dietary Fat Modulates Intestinal Tight Junction Integrity, Gut Permeability, and Hepatic Toll-Like Receptor Expression in a Mouse Model of Alcoholic Liver Disease. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kessova I, Cederbaum AI. CYP2E1: biochemistry, toxicology, regulation and function in ethanol-induced liver injury. CurrMolMed. 2003;3:509–518. doi: 10.2174/1566524033479609. [DOI] [PubMed] [Google Scholar]
- 17.Song BJ. Ethanol-inducible cytochrome P450 (CYP2E1): biochemistry, molecular biology and clinical relevance: 1996 update. Alcohol ClinExpRes. 1996;20:138A–146A. doi: 10.1111/j.1530-0277.1996.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 18.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–20. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol ClinExpRes. 2011;35:1509–1518. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deaciuc IV, Fortunato F, D’Souza NB, Hill DB, Schmidt J, Lee EY, McClain CJ. Modulation of caspase-3 activity and Fas ligand mRNA expression in rat liver cells in vivo by alcohol and lipopolysaccharide. Alcohol ClinExpRes. 1999;23:349–356. [PubMed] [Google Scholar]
- 22.McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 23.Menard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut. 2004;53:821–828. doi: 10.1136/gut.2003.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osman N, Adawi D, Molin G, Ahrne S, Berggren A, Jeppsson B. Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMCGastroenterol. 2006;6:31. doi: 10.1186/1471-230X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peran L, Camuesco D, Comalada M, Nieto A, Concha A, Adrio JL, Olivares M, Xaus J, Zarzuelo A, Galvez J. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. IntJColorectal Dis. 2006;21:737–746. doi: 10.1007/s00384-005-0773-y. [DOI] [PubMed] [Google Scholar]
- 26.Peran L, Sierra S, Comalada M, Lara-Villoslada F, Bailon E, Nieto A, Concha A, Olivares M, Zarzuelo A, Xaus J, Galvez J. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. BrJNutr. 2007;97:96–103. doi: 10.1017/S0007114507257770. [DOI] [PubMed] [Google Scholar]
- 27.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. AmJPhysiol Cell Physiol. 2006;290:C1018–C1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 28.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn U, Hamelmann E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. ClinExpAllergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 30.Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. JHepatol. 2008;48:945–951. doi: 10.1016/j.jhep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Li N, des RC, Fang M, Liboni K, McMahon R, Caicedo RA, Neu J. Lactobacillus rhamnosus GG decreases lipopolysaccharide-induced systemic inflammation in a gastrostomy-fed infant rat model. JPediatrGastroenterolNutr. 2006;42:545–552. doi: 10.1097/01.mpg.0000221905.68781.4a. [DOI] [PubMed] [Google Scholar]
- 32.Tsai EY, Falvo JV, Tsytsykova AV, Barczak AK, Reimold AM, Glimcher LH, Fenton MJ, Gordon DC, Dunn IF, Goldfeld AE. A lipopolysaccharide-specific enhancer complex involving Ets, Elk-1, Sp1, and CREB binding protein and p300 is recruited to the tumor necrosis factor alpha promoter in vivo. MolCell Biol. 2000;20:6084–6094. doi: 10.1128/mcb.20.16.6084-6094.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. JBiolChem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, Wang X, Cederbaum AI. Lipopolysaccharide-induced liver injury in rats treated with the CYP2E1 inducer pyrazole. AmJPhysiol GastrointestLiver Physiol. 2005;289:G308–G319. doi: 10.1152/ajpgi.00054.2005. [DOI] [PubMed] [Google Scholar]
- 35.Jarvelainen HA, Fang C, Ingelman-Sundberg M, Lukkari TA, Sippel H, Lindros KO. Kupffer cell inactivation alleviates ethanol-induced steatosis and CYP2E1 induction but not inflammatory responses in rat liver. JHepatol. 2000;32:900–910. doi: 10.1016/s0168-8278(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 36.Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- 37.Lamle J, Marhenke S, Borlak J, von WR, Eriksson CJ, Geffers R, Manns MP, Yamamoto M, Vogel A. Nuclear factor-eythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology. 2008;134:1159–1168. doi: 10.1053/j.gastro.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Gong P, Cederbaum AI. Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology. 2006;43:144–153. doi: 10.1002/hep.21004. [DOI] [PubMed] [Google Scholar]
- 39.Gobejishvili L, Barve S, Joshi-Barve S, McClain C. Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2008;295:G718–24. doi: 10.1152/ajpgi.90232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Deviere J, Le MO. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 41.Mizel SB, Honko AN, Moors MA, Smith PS, West AP. Induction of macrophage nitric oxide production by Gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. JImmunol. 2003;170:6217–6223. doi: 10.4049/jimmunol.170.12.6217. [DOI] [PubMed] [Google Scholar]
- 42.Rowlett RM, Chrestensen CA, Nyce M, Harp MG, Pelo JW, Cominelli F, Ernst PB, Pizarro TT, Sturgill TW, Worthington MT. MNK kinases regulate multiple TLR pathways and innate proinflammatory cytokines in macrophages. AmJPhysiol GastrointestLiver Physiol. 2008;294:G452–G459. doi: 10.1152/ajpgi.00077.2007. [DOI] [PubMed] [Google Scholar]
- 43.Bachmann M, Horn K, Poleganov MA, Paulukat J, Nold M, Pfeilschifter J, Muhl H. Interleukin-18 secretion and Th1-like cytokine responses in human peripheral blood mononuclear cells under the influence of the toll-like receptor-5 ligand flagellin. Cell Microbiol. 2006;8:289–300. doi: 10.1111/j.1462-5822.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 44.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. JImmunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 45.Kathrani A, Holder A, Catchpole B, Alvarez L, Simpson K, Werling D, Allenspach K. TLR5 risk-associated haplotype for canine inflammatory bowel disease confers hyper-responsiveness to flagellin. PLoSOne. 2012;7:e30117. doi: 10.1371/journal.pone.0030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–2364. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. AmJPhysiol GastrointestLiver Physiol. 2002;282:G6–15. doi: 10.1152/ajpgi.00328.2001. [DOI] [PubMed] [Google Scholar]
- 48.Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathway. JBiolChem. 2001;276:41930–41937. doi: 10.1074/jbc.M107181200. [DOI] [PubMed] [Google Scholar]
- 49.Nagy LE. Stabilization of tumor necrosis factor-alpha mRNA in macrophages in response to chronic ethanol exposure. Alcohol. 2004;33:229–233. doi: 10.1016/j.alcohol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Nagy LE. Molecular aspects of alcohol metabolism: transcription factors involved in early ethanol-induced liver injury. AnnuRevNutr. 2004;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Ishac EJ, Dent P, Kunos G, Gao B. Effects of ethanol on mitogen-activated protein kinase and stress-activated protein kinase cascades in normal and regenerating liver. BiochemJ. 1998;334(Pt 3):669–676. doi: 10.1042/bj3340669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G32–41. doi: 10.1152/ajpgi.00024.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Segawa S, Fujiya M, Konishi H, Ueno N, Kobayashi N, Shigyo T, Kohgo Y. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoSOne. 2011;6:e23278. doi: 10.1371/journal.pone.0023278. [DOI] [PMC free article] [PubMed] [Google Scholar]