Abstract
Objective
People with knee osteoarthritis (OA) are thought to walk with high loads at the knee which are yet to be quantfied using modeling techniques that account for subject specific EMG patterns, kinematics and kinetics. The objective was to estimate medial and lateral loading for people with knee OA and controls using an approach that is sensitive to subject specific muscle activation patterns.
Methods
16 OA and 12 control (C) subjects walked while kinematic, kinetic and EMG data were collected. Muscle forces were calculated using an EMG-Driven model and loading was calculated by balancing the external moments with internal muscle and contact forces
Results
OA subjects walked slower and had greater laxity, static and dynamic varus alignment, less flexion and greater knee adduction moment (KAM). Loading (normalized to body weight) was no different between the groups but OA subjects had greater absolute medial load than controls and maintained a greater %total load on the medial compartment. These patterns were associated with body mass, sagittal and frontal plane moments, static alignment and close to signficance for dynamic alignment. Lateral compartment unloading during mid-late stance was observed in 50% of OA subjects.
Conclusions
Loading for control subjects was similar to data from instrumented prostheses. Knee OA subjects had high medial contact loads in early stance and half of the OA cohort demonstared lateral compartment lift-off. Results suggest that interventions aimed at reducing body weight and dynamic malalignment might be effective in reducing medial compartment loading and establishing normal medio-lateral load sharing patterns.
Keywords: Medial load, Lateral load, Musculoskeletal modeling, EMG, Muscle Force
Introduction
Articular loads during walking are implicated in the pathogenesis of medial knee osteoarthritis (OA)[1, 2]. Abnormal loading due to altered joint kinematics following meniscus or anterior cruciate ligament (ACL) injuries [3, 4], or due to obesity, varus malalignment [2, 5] and high external knee adduction moment (KAM) [6, 7] could lead to cartilage degeneration. Multiple studies have also shown that people with knee OA walk with greater muscle co-contraction which has been thought to be associated with increased loading and accelerated cartilage damage[8–10].
KAM during walking, represents the net torque around the knee joint in the frontal plane, and is used as a surrogate measure of medial compartment loading[1, 2]. Concequently, a number of gait retraining interventions have focused on reducing KAM [11]. A decrease in KAM is assumed to indicate a reduction in medial loading [12, 13]. However, a case-study using instrumented knee prosthesis showed that a reduction in 1st peak KAM does not guarantee a reduction in medial contact load [14]. Also, a reduction in KAM does not provide any information about changes in the relative distribution of the loading between medial and lateral compartments. People with knee OA are thought to walk with relatively greater loads over the medial comartment compared to the lateral [15]. Clinical utlility of KAM reduction techniques would be limited if medio-lateral load sharing remains unchanged. Furthermore, since KAM derived from inverse dynamics is a net moment, it does not explicitly account for the greater muscle co-contraction exhibited by people with knee OA, and may lead to under-estimations of medial knee loading [16, 17].
Data from instrumented knee prostheses have provided valuable insight into the knee loading patterns and are useful for validating modeling techniques, but these prostheses cannot be used in all individuals [18, 19]. It is challenging to infer articular loading for individuals with and without knee OA using these datasets. Post-total knee arthroplasty (TKA), the movement patterns are different compared to those pre-TKA or a healthy subject [20, 21]. The TKA procedure involves realignment of the mechanical axis and post-surgery these individuals typically experience reduced pain and instability which may reduce muscle co-contraction during daily activities thereby altering joint loads [22].
Due to the limitations of KAM and instrumented knee prostheses, numerous mathematical modeling techniques have been utilized to estimate knee contact loads [23–26]. None of these models account for subject specific electromyography (EMG) patterns while estimating articular loading in people with knee OA, who walk with high muscle co-contraction, presumably leading to higher muscle forces and greater joint loading [17, 27]. Using a case comparison study, we have recently demonstrated the feasibility of using an EMG-Driven modeling approach [28] combined with a moment-balancing algorithm[29] in knee OA, where mechanical and neuromuscular changes influence articular loading [30].
In this paper, we use the EMG-Driven modeling approach to compute tibiofemoral joint contact loads in a larger sample of people with knee OA and matched controls, while also evaluating the association of quadriceps strength and radiographic features of knee OA with the observed loading patterns. Hence, the aims of this paper are (1) to estimate articular loads at the knee during walking in people with knee OA compared to controls and, (2) to analyze the effect of mechanical and functional factors on joint loading in people with knee OA. We hypothesized that people with knee OA will have higher medial contact loads and lower lateral contact loads compared to matched Control subjects, and medial contact loads will be related to static knee varus (from radiographs) and peak dynamic varus (during walking).
Patients and Methods
Subjects
Data from sixteen subjects with medial knee OA (Kellgren-Lawrence ≥ 2) and twelve healthy controls (Kellgren-Lawrence ≤ 1) recruited from the community, as a part of a larger study on neuromuscular control were used. The OA subjects did not have any self-reported ligament or meniscus injuries and were diagnosed based on radiographic and clinical criteria established by the American College of Rheumatology[31]. The more symptomatic knee was used for the OA subjects while the dominant leg defined by kicking preference was evaluated for the controls. The protocol was approved by the institutional human subjects review board and all subjects signed an approved consent form.
Functional Measures
The self-report Knee Injury and Osteoarthritis Outcome Score (KOOS) was used covering 5 dimensions of function: Pain, Symptoms, Activities of Daily Living (ADL), Sports and Recreation Function (Sport), and Knee-Related Quality of Life (QOL)[32]. All are scored from 0 to 4, and the scores reported as a percentage score (0 = extreme knee problems, 100 = no problems) [32].The KOOS is a valid, reliable, and responsive measure of overall knee joint function in people with OA[33].
Quadriceps Strength
Quadriceps muscle force (in Newton) was measured during three maximal voluntary isometric contractions (MVIC) on an isokinetic dynamometer (Kin Com Isokinetic International, Harrison, TN 37341) with the knee flexed to 90°. The trial with the highest volitional force was used.
Radiographic Measures
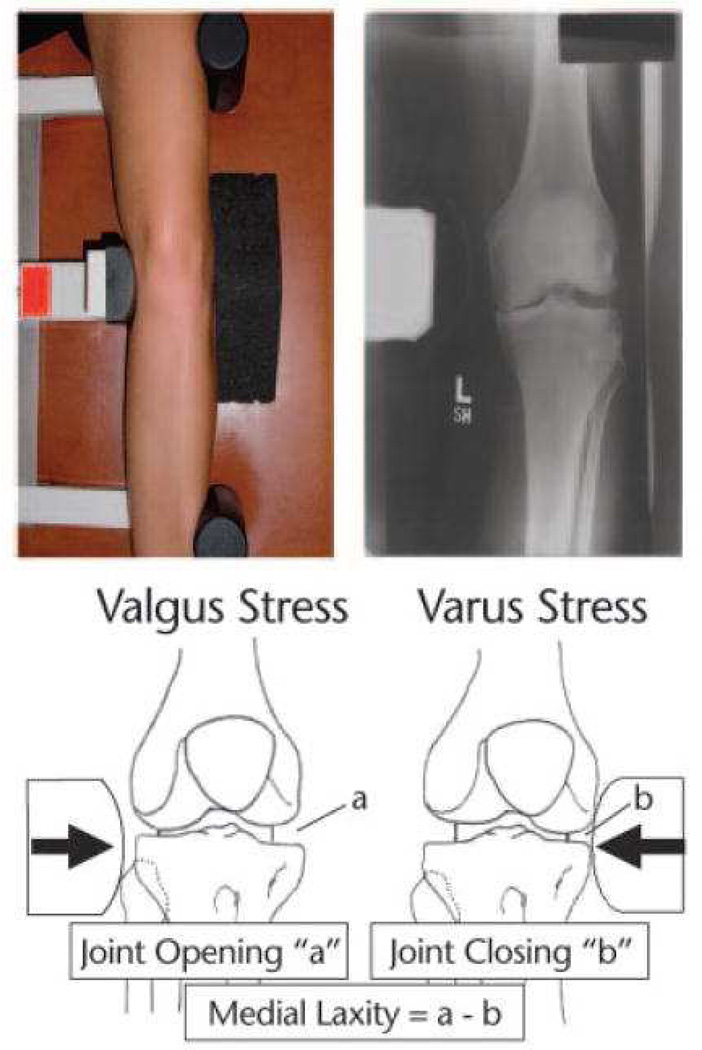
Medial joint space was measured on a posterior-anterior weight bearing semi-flexed radiograph as the narrowest distance between the femur and tibia [34]. Alignment was assessed using a standing, anterior- posterior radiograph in which the hip, knee, and ankle joints were visible. Alignment was determined by the angle (varus <180°, valgus >180°) of the mechanical axes of the femur and tibia [35]. Medial and lateral joint laxity was measured using the “open space” technique during varus and valgus stress radiographs[36] (Fig. 1). Subjects were positioned supine in a Telos Stress device (Austin Assoc., Fallston, MD) with the knee flexed 20° and the patella facing anteriorly. Varus or valgus stress was created by 150 N of force applied by the Telos device at the joint line. Joint spaces were measured and laxity was calculated as shown in Figure 1.
Figure 1.
Setup for varus stress radiograph on left lower extremity, with corresponding radiograph (top). For the varus stress radiograph (shown), a consistent 150-N force was applied to the medial knee joint line. For the valgus stress radiograph (not shown), the force was applied to the lateral joint line. Calculation of medial laxity (bottom).
Kinematics, Kinetics and EMG
Subjects walked at their self-selected speed over-ground for 10 trials. Kinematic data were collected at 120 Hz using a passive 8-camera system (VICON MX, Oxford Metrics, UK) and ground reaction force data recorded at 1080 Hz from one force platform (Bertec Corp, Worthington, OH). Muscle activity was recorded concurrently at 1080 Hz using a 16-channel system (Motion Lab Systems, Baton Rouge, LA) and signals bandpass filtered between 20–50 Hz in the hardware. Preamplified surface electrodes (20 mm inter-electrode distance, 12 mm disk diameter, input impedance 108 Ohms, CMRR > 10dB) were placed on the mid-bellies, parallel to the muscle fibers of semintendinosis (ST), biceps femoris (BFL), vastus medialis (VM), vastus lateralis (VL), rectus femoris (RF), and medial (MG) and lateral (LG) heads of the gastrocnemius, after skin preparation with alcohol rub, abrading and shaving [37]. For the musculoskeletal model, which has 10 muscles, activation for the semimembranosus (SM) was assumed to be equal to the ST, while activation for the BFL and biceps short head (BFS) were assumed equal. Vastus intermedius (VI) was taken as the average of VM and VL. EMG data for each muscle were also collected at rest and MVIC (seperate from the quadriceps strength test) for signal normalization purposes.
Euler angles (X-Y-Z) using right handed coordinate systems, and inverse dynamics were used to calculate joint kinematics and kinetics. All data were processed in Visual3D (C-motion, Germantown, MD). Stance phase variables for the sagittal plane included, knee flexion excursion (change in knee angle from heel strike to peak knee flexion), extension excursion (change in knee angle from peak knee flexion to peak knee extension), peak external knee flexion moment (KFM) and peak external knee extension moment (KEM). In the frontal plane, 1st and 2nd peak KAM and adduction angles at 1st and 2nd peak KAM were calculated. All angles were expressed in degrees and all moments normalized to subject’s height (Ht) and body weight (BW) and expressed as a percentage.
EMG-Driven Model
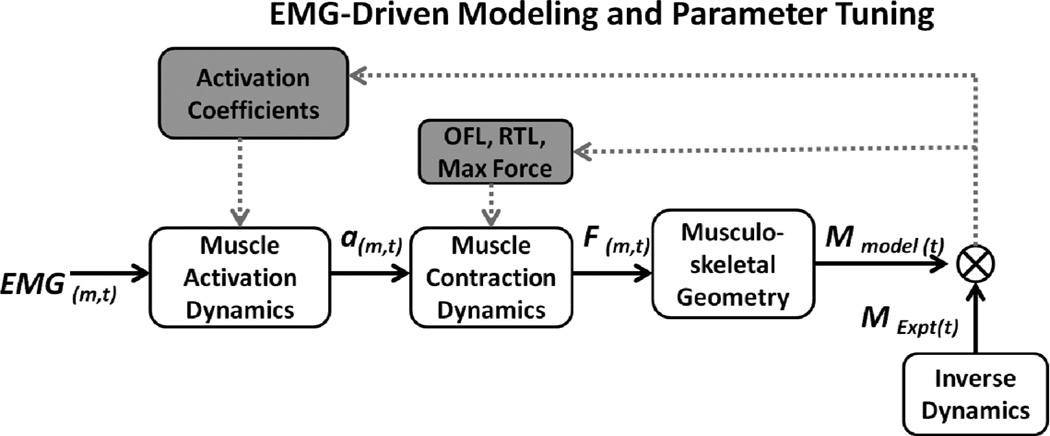
The EMG-Driven musculoskeletal model has been described in detail elsewhere and a summary of the methods with a flowchart (Figure 2) is provided here [28, 38]. EMG were converted to a parameter called muscle activation, taking into account electromechanical delay, the time-varying nature of EMG and factors in EMG-force relationship [28]. Muscles with small cross-sectional area, specifically, tensor fascia lata, sartorius and gracilis were not included as they have a relatively small contribution to the total muscle force and prone to cross-talk when recordings are made with surface elctrodes[29]. A model allowing sagittal and frontal plane knee motion, was built in Software for Interactive Musculoskeletal Modeling (SIMM, MusculoGraphics, Inc., care of Motion Analysis Corporation 3617 Westwind Blvd. Santa Rosa, CA 95403) [39] and used to calculate the muscle-tendon lengths, as well as, flexion-extension and varus-valgus moment arms for each muscle during each frame of stance. The model was scaled to individual subject anthropometrics using measurements from long cassette radiographs. The muscle activations and scaled muscle tendon-lengths were input into a Hill-type muscle model which takes into account the force-length and force-velocity relationships when determining individual muscle forces. The muscle forces from all muscles were multiplied by their sagittal plane moment arms and summed to compute the net moment in the sagittal plane. An optimization algorithm [40] was applied which iteratively adjusted model parameters to minimize the difference between the model-computed joint moment and the moment calculated using inverese dynamics. The parameters included optimal fiber length, tendon slack length, maximum muscle force and coefficients involved in the transformation of EMG to muscle activation. This optimization process has been described in detail elsewhwere [28]. Data for one walking trial was used to optimize the model (i.e., determine optimal muscle parameters). These optimized parameters were then used to predict muscle forces and sagittal plane knee moments for the other walking trials. The three trials with the smallest root mean square difference and the largest R2 between the model predicted sagittal plane moment and the moment computed from inverse dynamics were used for analysis. The joint contact loads presented in this study are from the trials that were predicted using the model and did not include the trial used in parameter optimization process.
Figure 2.
EMG for muscle, m, at time, t, was transformed into muscle activation (a) to activate a Hill-type muscle model (muscle contraction dynamics). The force, F, for each muscle was then multiplied by its sagittal plane moment arm according to the musculoskeletal geometry obtained using Software for Interactive Musculoskeletal Modeling (SIMM). Individual muscle moments were then summed at each point in time to obtain the model estimated sagittal plane knee moment, Mmodel(t). The knee moment was also calculated using inverse dynamics from video-based motion capture data, MExpt(t). EMG-driven model parameters including activation coefficients, optimal fiber length, OFL, resting tendon length, RTL, and the maximum isometric force for each muscle were iteratively adjusted to minimize the sum-squared difference between the model estimated moment and the moment computed from inverse dynamics(represented by the crossed circle). The process of optimally adjusting model parameters is depicted by the grey shaded boxes and arrows.
Estimation of medial and lateral contact load and load distribution
Joint contact loads for the medial and lateral compartment were estimated using the algorithm developed by Winby et al. [29]. Each muscle in the model has the potential to generate an internal moment in the frontal plane about a medial and lateral contact point relative to the center of the joint. Muscle moment arms about the medial and lateral contact points were calculated using SIMM. The internal muscle moments about the medial contact point are summed and oppose the external KAM calculated about an axis originating in the medial compartment. A positive residual moment indicates that muscle forces were sufficient to counter the external loads (i.e. KAM) and this will require a lateral compressive force (i.e. joint contact) to maintain static equilibrium at the joint. In contrast, a negative residual moment indicates a tensile force contributed by passive structures – such as ligaments and capsule, and are needed to maintain equilibrium. The process is repeated for the lateral compartment to calculate the requisite medial contact force to maintain equilibrium. The medial and lateral contact points were assumed to lie at 25% of the scaled inter-condylar width from the center of the knee. The contact load for the medial and lateral compartments was calculated in Newton, and also normalized to body weight (BW) and as a percentage of total loading. Medial and lateral loading at 1st peak KAM in the 1st half of stance (initial contact to zero crossing of the anterior-posterior[AP] ground reaction force [GRF]) and 2nd peak KAM during the 2nd half of stance (zero crossing of the AP GRF to the end of preswing) were used for analysis.
Statistics
RMS and R2 values between sagittal plane knee moment calculated from inverse dynamics and predicted from forward dynamics using the model, for the 3 predicted (not optimized) trials for each subject, were calculated as a measure of the model fit. Group means for Control and OA groups are reported.
One way ANOVA was used to evaluate differences in demographic, functional, radiographic and strength variables, and articular loads (in % total load). Analysis of Covariance, adjusting for walking speed, was used to compare kinematic, kinetic variables, and articular loads (in BW and in Newton). Multiple linear regression models were used to assess the strength of the relationships of mass, static alignment, laxity, quadriceps strength, peak sagittal moment, peak KAM and adduction angles at peak KAM with medial contact loads (in Newton).
Results
The OA subjects were older and had greater weight but the differences in age, weight and BMI were not statistically significant (Table 1).
Table 1.
Age, Mass and BMI for the Osteoarthritis (OA) and Control groups
| Variable | Controls (n = 12) | Osteoarthritis (n = 16) | p- value |
|---|---|---|---|
| Age (years)* | 59.5 (10.4) | 65.2 (9.5) | 0.145 |
| Mass (kg)* | 81.6 (19.2) | 85.1 (15.7) | 0.599 |
| BMI (kg/m2)* | 28.4 (5.2) | 28.6 (4.3) | 0.891 |
| Males:Females | 6:6 | 8:8 |
Mean (Standard Deviation) for both groups
Function, Strength, Radiograph and Walking Speed
The OA subjects were more disabled on all subscales of KOOS (p <0.001) (Table 2). The OA group had lower quadriceps strength but it was not statistically significant (p=0.16, Table 2). The OA subjects had smaller medial joint space (p <0.001), greater varus (p <0.001) and greater medial laxity (p=0.001) but the difference in lateral laxity (p=0.166) was not statistically significant between the groups (Table 2). OA subjects walked slower compared to controls (p=0.007, Table 2).
Table 2.
KOOS, Quadriceps Force, Walking Speed, and Radiographic parameters for Osteoarthritis and Control groups.
| Variables* | Controls (n = 12) | Osteoarthritis (n = 16) | p-value |
|---|---|---|---|
| Functional Variables | |||
| KOOS- symptoms | 98.5 (96.7, 100.3) | 62.3 (54.1, 70.5) | <0.001 |
| KOOS- pain | 99.8 (99.3, 110.3) | 64.2 (56.0, 72.4) | |
| KOOS- ADL | 100.0 (100.0, 100.0) | 71.6 (63.0, 80.2) | |
| KOOS- Sports/Recreation | 96.7 (91.5, 101.8) | 42.2 (31.6, 52.8) | |
| KOOS- Quality of Life | 99 .0 (97.4, 100.5) | 41.0 (29.0, 53.0) | |
| Quadriceps force (N/BMI) | 26.0 (18.7, 33.2) | 22.4 (17.6, 27.2) | 0.160 |
| Walking speed (m/sec) | 1.55 (1.41, 1.69) | 1.34 (1.27, 1.41) | 0.007 |
| Radiographic Variables | |||
| Medial joint space (mm) | 4.4 (3.9, 4.9) | 1 (0.1, 1.9) | <0.001 |
| Alignment (degrees) | 178.6 (176.8, 180.3) | 174.4 (172.1, 176.7) | <0.001 |
| Medial laxity (mm) | 3.3 (2.4, 4.2) | 5.5 (4.6, 6.4) | 0.001 |
| Lateral laxity (mm) | 4.7 (3.2, 5.1) | 3.4 (2.7, 4.1) | 0.166 |
Mean (95 % Confidence Intervals) for both groups
KOOS = Knee injury and Osteoarthritis Outcome Score, ADL = Activities of Daily Living
Model Fit
The sagittal plane knee moment predicted by the model matched well with the moment calculated using inverse dynamics. The average RMS (Mean±SD) and R2 Mean±SD) values between the predicted and calculated sagittal plane knee moments for the OA group were 12±6 (RMS) and 0.81±0.09 (R2). The values for the control group were 9.7±3.2 (RMS) and 0.89±0.04 (R2).
Kinematics, Kinetics and Articular Loads
During the 1st half of stance, the OA group had greater varus (p=0.015) and KAM (p = 0.027) (Table 3). The differences in flexion excursion (p = 0.203) and flexion moment (p = 0.757) were not statistically significant. During the 2nd half of stance, the OA group had greater varus (p=0.005) and KAM (p = 0.067) approached statistical significance (Table 3). Differences in extension excursion (p = 0.211) and flexion moment (p = 0.247) were not statistically significant (Table 3).
Table 3.
Kinematic and Kinetic variables for stance phase of gait for subjects with Knee Osteoarthritis and Controls.
| Variables* | Controls (n = 12) |
Osteoarthritis (n = 16) |
p- value** |
|
|---|---|---|---|---|
| 1st half of stance | Flexion excursion(degrees) | 15.3 (13.6, 17.0) | 12.1 (9.92, 14.2) | 0.203 |
| Peak varus(degrees) | 1.2 (−0.9, 3.4) | 6.4 (4.0, 8.6) | 0.004 | |
| Peak sagittal moment (%BW*Ht) | 5.24 (4.23, 6.26) | 5.00 (4.00, 6.01) | 0.757 | |
| Peak frontal moment (%BW*Ht) | −2.35 (−2.83, −1.87) | −3.3 (−3.83, −2.73) | 0.027 | |
| 2nd half of stance | Extension excursion (degrees) | 17.7 (14.6, 20.8) | 12.7 (8.8, 15.9) | 0.211 |
| Peak varus (degrees) | −0.7 (−3.2, 1.8) | 4.6 (1.9, 7.2) | 0.015 | |
| Peak sagittal moment (%BW*Ht) | −1.13 (−1.67, −0.60) | −0.42 (−0.94, 0.10) | 0.247 | |
| Peak frontal moment (%BW*Ht) | −1.69 (−2.19, −1.18) | −2.7 (−3.33, −2.08) | 0.067 | |
Mean (95 % Confidence Intervals) for all variables. BW= body weight, Ht = height.
p-values after adjusting for walking speed
The average medial and lateral loading patterns (in BW) for the OA and control groups are shown in Figure 3. For contact loads (in BW), during the 1st and 2nd halves of stance, the OA subjects had higher medial (p=0.201, p=0.666) and lower lateral loads (p=0.251, p=0.093) but the differences were not statistically significant (Table 4, Fig 2a–b). For contact loads (in Newton), during the 1st half of stance, the OA group had approximately 250N higher medial loading, which was significant, (p=0.014) but the lateral loading (p=0.726) was no different (Table 4). During the 2nd half of stance, the OA group had approximately 350N higher medial loading which was not significant (p=0.098) and neither was lateral loading (p=0.162) (Table 4). For medial and lateral loading expressed as a percentage of total load, during the 1st half of stance, the OA group had a greater percentage of load on the medial compartment (~74% vs. ~66%) which was close to significance (p=0.058) (Table 4). During the 2nd half of stance, the OA group had a greater percentage (~90% vs 82%) of load on the medial compartment but it was not statistically significant (p = 0.101). (Table 4).
Figure 3.
a–c Medial Condylar Load (a), Lateral Condylar Load (b) and Total Load (c) for Osteoarthritis (Black) and Control(grey) subjects with loading over the whole stance phase in body weights (Left panel) and the loading at 1st and 2nd peak knee adduction moment(Right panel). Error bars indicate 95 % confidence intervals.
Table 4.
Peak Medial, Lateral and Total contact loads (at 1st peak knee adduction moment during the 1st half of stance and 2nd peak knee adduction moment during the 2nd half of stance) for Osteoarthritis (n = 16) and Control (n = 12) subjects.
| Variables* | C | OA | C | OA | C | OA |
|---|---|---|---|---|---|---|
| in BWs | In Newton | In % Total | ||||
| At 1st peak KAM during the 1st half of stance | ||||||
| Medial Load | 2.37 (2.12, 2.61) | 2.57 (2.24, 2.90) | 1860 (1615, 2104) | 2108 (1837, 2380) | 66.0 (60.3, 71.6) | 74.5 (67.8, 81.1) |
| p-value | 0.187 | 0.014 | 0.058 | |||
| Lateral Load | 1.30 (0.95, 1.65) | 0.93 (0.67, 1.18) | 1001 (750, 1251) | 792 (556, 1028) | 34.0 (28.2, 39.7) | 25.5 (18.9, 32.1) |
| p-value | 0.666 | 0.726 | 0.058 | |||
| Total Load | 3.67 (3.16, 4.17) | 3.50 (3.06, 3.93) | 2860 (2445, 3276) | 2901 (2472, 3329) | ||
| p-value | 0.952 | 0.173 | ||||
| At 2nd peak KAM during the 2nd half of stance | ||||||
| Medial Load | 1.80 (1.44, 2.16) | 2.07 (1.80, 2.34) | 1369 (1184,1553) | 1702 (148, 1928) | 81.9 (75.5, 88.3) | 89.6 (82.7, 96.5) |
| p-value | 0.201 | 0.110 | 0.101 | |||
| Lateral Load | 0.45 (0.19, 0.70) | 0.12 (−0.21, 0.46) | 320 (179, 462) | 89 (−205, 383) | 18.1 (11.7, 24.5) | 10.4 (3.5, 17.3) |
| p-value | 0.093 | 0.162 | 0.101 | |||
| Total Load | 2.24 (1.69, 2.80) | 2.20 (1.80, 2.60) | 1689 (1432, 1946) | 1792 (1468, 2117) | ||
| p-value | 0.362 | 0.965 | ||||
Mean (95 % Confidence intervals)
adjusted for walking speed
BW = Body Weight, OA = Osteoarthritis, KAM = Knee Adduction Moment
Results from the linear regression (Table 5) revealed that during the 1st half of stance, increases in mass (p = 0.007), external flexion moment (p = 0.047)and KAM (p = 0.002) were associated with an increase in medial contact load (in Newton). During the 2nd half of stance, increase in static varus (p = 0.019) was associated with an increase in medial contact load (in Newton) whereas dynamic varus (p = 0.061) was close to significance.
TABLE 5.
Results from Multiple Linear Regression for medial contact loads (in Newton) during early and late stance (n = 12 for Controls, n = 16 for knee osteoarthritis).
| Dependent Variable |
Covariates | B (Standard Error) | β | p-value |
|---|---|---|---|---|
| Medial Contact Load at 1st peak KAM during the 1st half of stance | Mass | 16.50 (5.04) | .48 | 0.007 |
| Mechanical axis | 2.45 (28.48) | .02 | 0.933 | |
| Medial Laxity | −30.99 (45.47) | −.11 | 0.508 | |
| Lateral Laxity | −54.27 (49.45) | −.15 | 0.294 | |
| Quadriceps strength | −7.48 (7.07) | −.14 | 0.311 | |
| Peak Sagittal Moment | 107.87 (48.74) | .38 | 0.047 | |
| 1st peak KAM | −527.68 (13702) | −.86 | 0.002 | |
| Adduction Angle at 1st peak KAM | 30.85 (32.94) | .26 | 0.367 | |
| Medial Contact Load at 2nd peak KAM during the 2nd half of stance | Mass | 2.50 (6.05) | .11 | 0.686 |
| Mechanical axis | −84.69 (31.23) | −.85 | 0.019 | |
| Medial Laxity | 8.27 (48.32) | .04 | 0.867 | |
| Lateral Laxity | 65.36 (57.31) | .26 | 0.276 | |
| Quadriceps strength | 6.99 (8.63) | .19 | 0.434 | |
| Peak Sagittal Moment | −10.53 (79.08) | −.03 | 0.896 | |
| 2nd peak KAM | −135.30 (124.11) | −0.36 | 0.297 | |
| Adduction Angle at 2nd peak KAM | 62.17 (30.08) | 0.77 | 0.061 | |
KAM = Knee Adduction Moment
Discussion
Analyses of magnitude and patterns of articular loading, as well as factors that affect loading, are critical to understanding some of the mechanisms related to initiation and progression of knee OA. This study presents novel data demonstrating that people with medial knee OA walk with greater absolute load over the medial compartment, while also maintaining a medio-lateral loading distribution with a relatively higher proportion of the load over the medial compartment. The medial compartment loading was found to be associated with body mass, static alignment, and flexion and adduction moments. The results present possible targets for therapeutic interventions aimed at improving medio-lateral loading patterns in this population to slow cartilage degeneration.
The average peak total loading for our control subjects was in the range of 2 – 4.5 BWs which agrees with the previous report (3 – 4.4 BWs) using similar techniques [29]. The values reported from the instrumented knee studies range from 2.1 – 3.5 BWs and 1500 – 3000 N [18, 19, 41–43]. Our values range from 1500 – 4600 N. Difference in loading between the subjects in this study and those measured from instrumented knee studies may be in part due to differences in the subjects tested. The subjects in the instrumented knee studies tend to be older, had knee OA prior to surgery and walked slower compared to our subjects who were younger, more active and walked faster.
Peak medial loading reported using mathematical models range from 2.3 – 2.4 BWs [15, 26, 44]. Our data for average peak medial loading range from 0.8 – 3 for the control subjects which is close to previous predictions. For medio-lateral load distribution, the instrumented knee studies have reported medial compartment loading ranging from 53 – 92% of the total load [42, 45, 46]. Our data for the healthy subjects ranged betweeen 53–100% of the total load and in general, appear to be consistent with these published reports.
The subjects with knee OA had greater self-reported disability, medial laxity and varus malalignment and lower medial joint space compared to the control group. These results show that there were significant structural differences at the knee between the two groups. Although the OA group did not have significantly higher BMI compared to the Control group, the varus alignment could lead to greater medial compartmental loading. Further studies will need to be done to investigate if the difference of 250–350 N in the medial compartment loads over repeated cycles is associated with progressive cartilage damage. Eight of our sixteen OA subjects showed unloading of the lateral comparment in mid to late stance. It has been suggested that people with medial knee OA might show lateral condylar lift-off if they are not able to counter the higher external adduction loads [1, 47]. Our data suggest this postulate may be true as we found that half of our OA subjects demonstrated lateral compartment lift-off. Loading the medial compartment to 100% along with higher KAM, might very well overload the medical condylar cartilage. Repetitive high loading of the medial comparment could lead to a more rapid progression of this subgroup of people with knee OA who have lateral compartment lift-off. It has been reported that, varus thrust during walking, which could be the visual evidence for lateral comparment unloading, is related to a four fold increase in risk for progression[47].
A movement pattern with increased loading over the medial compartment, while unloading the lateral compartment, in the OA subjects could possibly be due to a failure of the neuromuscular system as quadriceps strength and passive laxity were not associated with loading. Also we did not find any differences in quadriceps strength between the two groups. These finding suggest that the control of muscle forces during dynamic activites could also be important in addition to muscle strength. Further studies are needed to ascertain if neuromuscular retraining intervention could be used in people with knee OA who demonstrate lateral comparment unloading to obtain a more balanced loading at the knee and potentially slow the progression of the disease.
It was interesting to note that the unloading occured during mid to late stance during 2nd peak KAM and not during the early weight acceptance phase where 1st peak KAM occurs. The magnitude of the 1st peak KAM is a reliable biomechanical marker of OA progression [1, 6] and has also been the focus of interventions which aim at reducing loading by diminishing the 1st peak of KAM [11]. In our subjects, OA group had higher KAM and also higher medial compartment loading at the same time. The medial load was related to mass, KAM, static malalignment (from x-ray) and had a close to significance relationship with dynamic malalignment (as measured by frontal plane knee angle), which could be due to the small sample size. The data suggest that the 1st peak loading could potentially be reduced by a lowering the body weight and 2nd peak loading could be reduced by interventions aimed at reducing dynamic frontal plane malalignment like gait retraining and bracing. Recently a study that involved a subject with an instrumented knee demonstrated that walking with a medial thrust and with a walking pole could reduce medial compartmental loading [48] but it is difficult to predict if these strategies would work for people with knee OA. For the 2nd peak of KAM, gait modifications like increased toe-out angle [49] and greater abduction moment at hip [50] have been shown to reduce the second peak of KAM. Also, the recent study which demonstrated that reduction in 1st peak of KAM does not correspond to a reduction in medial contact load at the same time; also showed that a reduction on 2nd peak KAM did correspond to a reduction in medial contact loads during that phase of stance[14]. KAM by itself may not be sufficient to describe medial compartment loads and it has to be considered in the context of sagittal plane moments [14]. For example if the KAM decreases and the knee flexoion moment decreases then one can be fairly, although not 100% confident, the medial contact force has decreased. If both increase it is likely that medial contact force will also increase. When the moments change in opposite directions it is difficult to infer what might be occurring at the joint and this is an advantage that modeling has over inferring joint contact forces from inverse dynamics alone. Results from the linear regression analyses in this study also support these findings, which showed that both peak sagittal and frontal plane moments were positively related to medial loading in the 1st half of stance, with an increase in both of these moments being related to increased medial contact loads. Again, it needs to be seen if gait modification strategies investigated in individuals with instrumented knee prostheses would be effective in people with knee OA. The modeling approach used in this study may lend insight when evaluating whether these interventions are effective in unloading the medial and loading the lateral compartment in people with medial knee OA in the presence of abnormal muscle activation patterns, static and dynamic malalignment and other impairments. It would also be interesting to study the effect of surgical re-alignment procedures including high tibial osteotomy, unicompartmental arthroplasty ant TKA on medio-lateral load sharing patterns.
The data from the modeling approach have to be interpreted in light of certain limitations associated with the method. Knee ligaments and a number of smaller muscles like Tensor Fascia Lata, Gracilis and Sartorius were not included in this model. Though these muscles do not contribute significantly to sagittal plane stability, they may contribute to balancing the external load in the frontal plane. It is possible therefore that loading magnitudes and patterns might have been influenced by the addition of these muscles. Also, the inclusion of lateral muscles, especially TFL, might have prevented isolated lateral compartment unloading noted for a few of our healthy subjects. It is also important to note here that our results are based on a small sample size, likely leading to insufficient statistical power for some of the analyses.
In conclusion, the EMG driven musculoskeletal model predicted different joint loads the people with and without knee osteoathritis. Previous predictions of higher medial loading in people with knee OA were confirmed. Lateral compartment lift-off was also seen in people with knee OA as previously suggested. Reducing joint loading in people with knee OA is an important step towards slowing progreesion of the disease. In addition, knowledge of the distribution of the total load between the medial and lateral compartments is clinically relevant when critically evaluating the efficacy of an intervention. The EMG-Driven model utilized in this study is sensitive to patient specific gait mechanics and muscle activation patterns and thus may be a useful instrument in evaluating interventions aimed at reducing medial loading and slowing progreesion of knee OA.
Acknowledgements
Funding by NIH P20 RR16458, NIH S10RR022396, ACR REF Health Professional Graduate Student Research Preceptorship, International Society of Biomechanics Doctroal Dissertation Grant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
All authors contributed to conception, data analysis, data interpretation, mansucript draft and final approval. Data acquisition was done by Kumar.
Authors Kumar (Deepak.kumar@ucsf.edu) and Rudolph (krudolph@une.edu) take full responsibility for the integrity of this work as a whole.
Conflict of Interest
None.
Contributor Information
Deepak Kumar, Email: deepak.kumar@ucsf.edu.
Kurt T. Manal, Email: manal@udel.edu.
Katherine S. Rudolph, Email: krudolph@une.edu.
References
- 1.Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 2.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, et al. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41:1233–1240. doi: 10.1002/1529-0131(199807)41:7<1233::AID-ART14>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 3.Frost HM. Perspectives: a biomechanical model of the pathogenesis of arthroses. Anat Rec. 1994;240:19–31. doi: 10.1002/ar.1092400103. [DOI] [PubMed] [Google Scholar]
- 4.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3:261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 5.Messier SP, Legault C, Loeser RF, Van Arsdale SJ, Davis C, Ettinger WH, et al. Does high weight loss in older adults with knee osteoarthritis affect bone-on-bone joint loads and muscle forces during walking? Osteoarthritis Cartilage. 2011;19:272–280. doi: 10.1016/j.joca.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennell KL, Bowles KA, Wang Y, Cicuttini F, Davies-Tuck M, Hinman RS. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheum Dis. 2011;70:1770–1774. doi: 10.1136/ard.2010.147082. [DOI] [PubMed] [Google Scholar]
- 8.Hubley-Kozey CL, Hill NA, Rutherford DJ, Dunbar MJ, Stanish WD. Co-activation differences in lower limb muscles between asymptomatic controls and those with varying degrees of knee osteoarthritis during walking. Clin Biomech (Bristol, Avon) 2009;24:407–414. doi: 10.1016/j.clinbiomech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Rudolph KS, Schmitt LC, Lewek MD. Age-related changes in strength, joint laxity, and walking patterns: are they related to knee osteoarthritis? Phys Ther. 2007;87:1422–1432. doi: 10.2522/ptj.20060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutherford DJ, Hubley-Kozey CL, Stanish WD, Dunbar MJ. Neuromuscular alterations exist with knee osteoarthritis presence and severity despite walking velocity similarities. Clin Biomech (Bristol, Avon) 2011;26:377–383. doi: 10.1016/j.clinbiomech.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Simic M, Hinman RS, Wrigley TV, Bennell KL, Hunt MA. Gait modification strategies for altering medial knee joint load: a systematic review. Arthritis Care Res (Hoboken) 2011;63:405–426. doi: 10.1002/acr.20380. [DOI] [PubMed] [Google Scholar]
- 12.Shakoor N, Block JA. Walking barefoot decreases loading on the lower extremity joints in knee osteoarthritis. Arthritis Rheum. 2006;54:2923–2927. doi: 10.1002/art.22123. [DOI] [PubMed] [Google Scholar]
- 13.Erhart-Hledik JC, Elspas B, Giori NJ, Andriacchi TP. Effect of variable-stiffness walking shoes on knee adduction moment, pain, and function in subjects with medial compartment knee osteoarthritis after 1 year. J Orthop Res. 2012;30:514–521. doi: 10.1002/jor.21563. [DOI] [PubMed] [Google Scholar]
- 14.Walter JP, D'Lima DD, Colwell CW, Jr, Fregly BJ. Decreased knee adduction moment does not guarantee decreased medial contact force during gait. J Orthop Res. 2010;28:1348–1354. doi: 10.1002/jor.21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt LC, Rudolph KS. Muscle stabilization strategies in people with medial knee osteoarthritis: the effect of instability. J Orthop Res. 2008;26:1180–1185. doi: 10.1002/jor.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubley-Kozey C, Deluzio K, Dunbar M. Muscle co-activation patterns during walking in those with severe knee osteoarthritis. Clin Biomech (Bristol, Avon) 2008;23:71–80. doi: 10.1016/j.clinbiomech.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Heinlein B, Kutzner I, Graichen F, Bender A, Rohlmann A, Halder AM, et al. ESB Clinical Biomechanics Award 2008: Complete data of total knee replacement loading for level walking and stair climbing measured in vivo with a follow-up of 6–10 months. Clin Biomech (Bristol, Avon) 2009;24:315–326. doi: 10.1016/j.clinbiomech.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 19.D'Lima DD, Patil S, Steklov N, Slamin JE, Colwell CW., Jr The Chitranjan Ranawat Award: in vivo knee forces after total knee arthroplasty. Clin Orthop Relat Res. 2005;440:45–49. doi: 10.1097/01.blo.0000186559.62942.8c. [DOI] [PubMed] [Google Scholar]
- 20.Benedetti MG, Catani F, Bilotta TW, Marcacci M, Mariani E, Giannini S. Muscle activation pattern and gait biomechanics after total knee replacement. Clin Biomech (Bristol, Avon) 2003;18:871–876. doi: 10.1016/s0268-0033(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 21.Venema DM, Karst GM. Individuals With Total Knee Arthroplasty Demonstrate Altered Anticipatory Postural Adjustments Compared With Healthy Control Subjects. J Geriatr Phys Ther. 2012 doi: 10.1519/JPT.0b013e3182353ee4. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida Y, Mizner RL, Ramsey DK, Snyder-Mackler L. Examining outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time. Clin Biomech (Bristol, Avon) 2008;23:320–328. doi: 10.1016/j.clinbiomech.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson FC, Pandy MG. Static and dynamic optimization solutions for gait are practically equivalent. J Biomech. 2001;34:153–161. doi: 10.1016/s0021-9290(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 24.Taylor WR, Heller MO, Bergmann G, Duda GN. Tibio-femoral loading during human gait and stair climbing. J Orthop Res. 2004;22:625–632. doi: 10.1016/j.orthres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Shelburne KB, Torry MR, Pandy MG. Contributions of muscles, ligaments, and the ground-reaction force to tibiofemoral joint loading during normal gait. J Orthop Res. 2006;24:1983–1990. doi: 10.1002/jor.20255. [DOI] [PubMed] [Google Scholar]
- 26.Hurwitz DE, Sumner DR, Andriacchi TP, Sugar DA. Dynamic knee loads during gait predict proximal tibial bone distribution. J Biomech. 1998;31:423–430. doi: 10.1016/s0021-9290(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt LC, Rudolph KS. Influences on knee movement strategies during walking in persons with medial knee osteoarthritis. Arthritis Rheum. 2007;57:1018–1026. doi: 10.1002/art.22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchanan TS, Lloyd DG, Manal K, Besier TF. Neuromusculoskeletal modeling: estimation of muscle forces and joint moments and movements from measurements of neural command. J Appl Biomech. 2004;20:367–395. doi: 10.1123/jab.20.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 2009;42:2294–2300. doi: 10.1016/j.jbiomech.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Kumar D, Rudolph K, Manal K. An EMG-Driven Modeling Approach to Muscle Force and Joint Load Estimations: Case Study in Knee Osteoarthritis. Journal of Orthopedic Research. 2011 doi: 10.1002/jor.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis Classification of osteoarthritis of the knee Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 32.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 33.Liang MH, Larson MG, Cullen KE, Schwartz JA. Comparative measurement efficiency and sensitivity of five health status instruments for arthritis research. Arthritis Rheum. 1985;28:542–547. doi: 10.1002/art.1780280513. [DOI] [PubMed] [Google Scholar]
- 34.Lequesne M. Quantitative measurements of joint space during progression of osteoarthritis:“chondrometry”. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995. pp. 427–444. [Google Scholar]
- 35.Hsu RW, Himeno S, Coventry MB, Chao EY. Normal axial alignment of the lower extremity and load-bearing distribution at the knee. Clin Orthop Relat Res. 1990:215–227. [PubMed] [Google Scholar]
- 36.Moore TM, Meyers MH, Harvey JP., Jr Collateral ligament laxity of the knee. Long-term comparison between plateau fractures and normal. J Bone Joint Surg Am. 1976;58:594–598. [PubMed] [Google Scholar]
- 37.Delagi EIJ, Perotto A. Anatomic guide for the electromyographer, the limbs. Springfield, IL: 1981. [Google Scholar]
- 38.Lloyd DG, Besier TF. An EMG-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J Biomech. 2003;36:765–776. doi: 10.1016/s0021-9290(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 39.Delp SL, Loan JP. A graphics-based software system to develop and analyze models of musculoskeletal structures. Comput Biol Med. 1995;25:21–34. doi: 10.1016/0010-4825(95)98882-e. [DOI] [PubMed] [Google Scholar]
- 40.Goffe WL, Ferrier GD, Rogers J. Global optimization of stastical functions with simulated annealing. J Econometrics. 1994;60:65–99. [Google Scholar]
- 41.D'Lima DD, Patil S, Steklov N, Slamin JE, Colwell CW., Jr Tibial forces measured in vivo after total knee arthroplasty. J Arthroplasty. 2006;21:255–262. doi: 10.1016/j.arth.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Kutzner I, Heinlein M, Bender A, Graichen F, Halder A, Beier A, et al. Medio-lateral Force Distribution in the Knee Joint during Level Walking. 55th Annual Meeting of the Orthopaedic Research Society; New Orleans, LA, USA. 2010. [Google Scholar]
- 43.Kutzner I, Heinlein B, Graichen F, Bender A, Rohlmann A, Halder A, et al. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J Biomech. 2010;43:2164–2173. doi: 10.1016/j.jbiomech.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 44.Shelburne KB, Torry MR, Pandy MG. Muscle, ligament, and joint-contact forces at the knee during walking. Med Sci Sports Exerc. 2005;37:1948–1956. doi: 10.1249/01.mss.0000180404.86078.ff. [DOI] [PubMed] [Google Scholar]
- 45.Mundermann A, Dyrby CO, D'Lima DD, Colwell CW, Jr, Andriacchi TP. In vivo knee loading characteristics during activities of daily living as measured by an instrumented total knee replacement. J Orthop Res. 2008;26:1167–1172. doi: 10.1002/jor.20655. [DOI] [PubMed] [Google Scholar]
- 46.Zhao D, Banks SA, D'Lima DD, Colwell CW, Jr, Fregly BJ. In vivo medial and lateral tibial loads during dynamic and high flexion activities. J Orthop Res. 2007;25:593–602. doi: 10.1002/jor.20362. [DOI] [PubMed] [Google Scholar]
- 47.Chang A, Hayes K, Dunlop D, Hurwitz D, Song J, Cahue S, et al. Thrust during ambulation and the progression of knee osteoarthritis. Arthritis Rheum. 2004;50:3897–3903. doi: 10.1002/art.20657. [DOI] [PubMed] [Google Scholar]
- 48.Fregly BJ, D'Lima DD, Colwell CW., Jr Effective gait patterns for offloading the medial compartment of the knee. J Orthop Res. 2009;27:1016–1021. doi: 10.1002/jor.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo M, Axe MJ, Manal K. The influence of foot progression angle on the knee adduction moment during walking and stair climbing in pain free individuals with knee osteoarthritis. Gait Posture. 2007;26:436–441. doi: 10.1016/j.gaitpost.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Chang A, Hayes K, Dunlop D, Song J, Hurwitz D, Cahue S, et al. Hip abduction moment and protection against medial tibiofemoral osteoarthritis progression. Arthritis Rheum. 2005;52:3515–3519. doi: 10.1002/art.21406. [DOI] [PubMed] [Google Scholar]