Abstract
Objective
To evaluate the relationship of hip radiographic osteoarthritis (ROA) and MR findings of cartilage lesions, labral tears, bone marrow edema like lesions (BMEL) and subchondral cysts with self-reported and physical function.
Design
Eighty five subjects were classified as controls (n= 55, KL 0, 1) or having mild-moderate ROA (n = 30, KL 2, 3). T2-weighted MR images at 3-Tesla were graded for presence of cartilage lesions, labral tears, BMELs and subchondral cysts. Posterior wall sign, cross-over sign, center-edge angle and alpha angle were also recorded. Function was assessed using Hip Osteoarthritis Outcome Score (HOOS), Timed-Up and Go (TUG) test and Y-Balance Test (YBT). Analysis compared function between subjects with and without ROA and those with and without femoral or acetabular cartilage lesions, adjusted for age. Non-parametric correlations were used to assess the relationship between radiographic scores, MR scores and function.
Results
Subjects with acetabular cartilage lesions had worse HOOS (Difference = 5–10%, P = 0.036–0.004), but not TUG or YBT, scores. Acetabular cartilage lesions, BMELs and subchondral cysts were associated with worse HOOS scores (ρ= 0.23–0.37, P = 0.041–0.001). Differences in function between subjects with and without ROA or femoral cartilage lesions were not significant. Other radiologic findings were not associated with function.
Conclusions
Acetabular cartilage defects, but not femoral cartilage defects or ROA, were associated with greater self-reported pain and disability. BMELs and subchondral cysts were related to greater hip related self-reported pain and disability. None of the radiographic or MR features were related to physical function.
Keywords: BMEL, subchondral cyst, Kellgren-Lawrence, Balance, Y-Balance Test, Labral tears
INTRODUCTION
One in four individuals is at risk of developing symptomatic hip osteoarthritis (OA) in their lifetime. [1] People with hip OA have significant disability which impacts their quality of life with a large number eventually requiring total hip arthroplasty. [2, 3] Clinical diagnosis of hip OA is made using a combination of symptoms and radiographic findings characteristic of osteoarthritis. [4, 5] Although radiographs are inexpensive and easily available, they only allow gross visualization of changes in bone and joint space. [6] They also entail exposure to ionizing radiation and offer poor reproducibility for characterizing OA related degeneration. [7, 8] Finally, radiographs are insensitive to the earliest changes associated with OA [6] or to differences between the acetabular and the femoral cartilage. MRI offers greater soft-tissue contrast and allows direct visualization of cartilage, labrum, bone marrow edema like lesions (BMEL), subchondral cysts and other soft-tissue pathologies. [9, 10] Hence, there is a need to compare the association of radiographic and MR descriptors of disease severity with measures of pain and disability for hip OA.
MR imaging for OA related tissue degeneration is more established for knee [6, 11] and there is substantial literature investigating the relationship of radiographic and MR findings with patient pain, symptoms and function in populations with knee OA. [12, 13] Relatively fewer studies have investigated MR imaging for hip OA. [10, 11, 14, 15] The hip joint is functionally and structurally very different compared to the knee and it has been more challenging to develop clinical MR imaging which allows adequate visualization of hip structures due to the shape and location of the hip joint. [16] Hence, even though hip OA is associated with significant loss of function, there is not enough information on the relationships of radiographic and MR findings of hip OA with pain, disability and physical performance. [10, 17, 18] It is also unknown if cartilage defects in the femur and the acetabulum have different relationships with functional deficits. Furthermore, there have been reports of abnormal MR findings like cartilage lesions, labral tears and cam-type lesions being present in asymptomatic individuals which also necessitate understanding the clinical relevance of these imaging findings. [19–21]
Cartilage thinning and defects are the primary characteristic of the osteoarthritis disease process and radiographs are thought to provide an indirect measure of cartilage loss. Since MR imaging provides a direct visualization of femoral and acetabular cartilage, it needs to be seen if cartilage defects in these areas are associated with functional deficits. Hence, the primary purpose of this study was to evaluate the associations of radiographic OA (ROA) and MR evidence of femoral and acetabular cartilage lesions with self-reported pain and disability, and physical function in adults with mild-moderate hip ROA. Secondary purpose was to evaluate the association of MR evidence of BMELs, subchondral cysts, labral tears and features of developmental dysplasia of the hip (DDH) and femoroacetabular impingement (FAI) with self-reported pain and disability, and physical function in adults with mild-moderate hip ROA.
MATERIALS AND METHODS
Subjects
Subjects were recruited from the community using flyers and advertisements. The inclusion criteria for ROA (n = 30) patients (+ROA) were Kellgren-Lawrence (KL) grade of 2 or 3 at the hip on weight-bearing anterior-posterior radiographs.[22] The side with greater KL grade was selected as the “index hip”. The control (n= 55) subjects (-ROA) had a radiographic KL grade of 0 or 1 at both hips, and were without history of diagnosed OA or previous hip injuries. Exclusion criteria for all subjects were a any contra-indications to MR imaging, KL grade of 4, a total joint replacement of any lower extremity joint, previous hip trauma, pain at any other lower extremity joint, radiographic evidence of any knee or ankle joint OA, systemic inflammatory arthritis or any other spine or lower extremity condition that would affect their ability to complete the functional tests. All subjects signed a written informed consent approved by the University of California, San Francisco Committee on Human Research.
MRI acquisitions
All imaging was performed with a 3-Tesla MR scanner (GE MR750, GE Healthcare, Waukesha, WI, USA) and an 8 -channel cardiac coil (GE Healthcare, Waukesha, WI, USA). Patient positioning aids were used to immobilize and support patients, and ensure a consistent, reproducible, and comfortable hip positioning during scanning. Patients were positioned supine with their feet taped together, their knees supported by cushions to prevent movement. The imaging protocol and parameters are shown in Table 1.
Table 1.
MR sequence parameters.
| Sequence | Parameters |
|---|---|
| Coronal Fast Spin Echo – T2 weighted Fat Suppressed | TR/TE = 2496/60, Echo Train Length = 16, Matrix = 288 × 224, # of slices = 16, Field of View = 20, Slice Thickness = 4, Bandwidth = 50.0, Acquisition Time = 4 min 40 sec |
| Sagittal Fast Spin Echo – T2 weighted Fat Suppressed | TR/TE = 3678/60, Echo Train Length = 16, Matrix = 288 × 224, # of slices = 24, Field of View = 14, Slice Thickness = 4, Bandwidth = 50.0, Acquisition Time = 4 min |
| Axial Fast Spin Echo – T2 weighted Fat Suppressed | TR/TE = 2800/60, Matrix = 288 × 224, # of slices = 18, Field of View = 18, Slice Thickness =3, Bandwidth = 50.0, Acquisition Time = 3 min 50 sec |
Image analysis
Clinical MR grading for features of hip OA
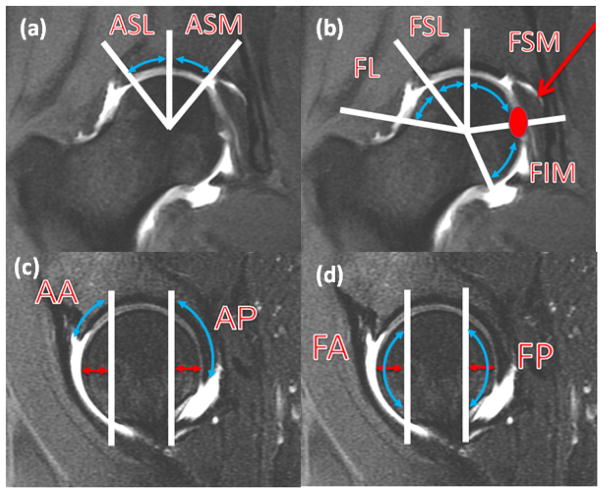
Experienced board-certified musculoskeletal radiologists (TML, LN, SL) performed the clinical grading for each subject on the coronal and sagittal MR studies. The features scored included cartilage defects, labral tears, bone marrow edema like lesions (BMEL) and subchondral cysts. For cartilage lesions, BMEL and subchondral cysts, the femoral and acetabular segments were divided into six subregions (4 femoral, 2 acetabular) on the coronal studies and 4 subregions (2 femoral, 2 acetabular) on the sagittal studies, for a total of 10 subregions (Fig 1). The mid portion of the femoral head was defined on the sagittal images and subdivided into four subregions on the coronal images, from lateral to medial (Fig 1a,b). The landmark for division was lateral acetabular rim for lateral and superolateral, a vertical line from center of femoral head for superolateral and supermedial, and ligamentum teres for supermedial and inferior subregions. On the sagittal MR study, the anterior subregion represented the anterior 1 cm of the femoral head and the posterior subregion represented the posterior 1 cm of the femoral head (Fig 1c,d). The division was based on a simplified version of the geographic zone method described by Ilizaliturri Jr. et al. for hip arthroscopy which showed superior inter-observer reproducibility compared to the clock-face method. [23] Cartilage defects were graded as 0 (no defect), 1 (partial thickness) and 2 (full thickness). BMEL were graded as 0(absent), 1 (< or= 0.5 cm), 2 (0.5–1.5 cm) and 3 (> or = 1.5 cm). Subchondral cysts were graded as 0 (absent), 1 (< or = 0.5 cm) and 2 (> 0.5 cm). The labrum was graded on the sagittal images in the anterosuperior region, coronal images in the superolateral regions and on the axial images in the anterior and posterior regions. Labral tears were graded as 0 (normal or normal variant), 1 (fraying or signal abnormality), 2 (simple tear), 3 (labor-cartilage sepeartion), 4 (complex tear) and 5 (maceration). Total scores were calculated for cartilage lesions (femoral and acetabular), labral tears, BMELs and subchondral cysts. Consensus readings were performed in case of a disagreement.
Figure 1.
Subregions of articular cartilage. for femur on coronal (a) and sagittal (c) images. Subregions for acetabulum on coronal (b) and sagittal (d) images. (a) coronal MR image demonstrating acetabular superolateral (ASL) and superomedial (ASM) subregions divided by vertical line extending from femoral head center.(b) coronal MR image demonstrating femoral lateral (FL), superolateral (FSL) and superomedial (FSM) and inferior (FIM) subregions divided by line extending from femoral head center, to the lateral acetabular rim, to straight vertical direction and to the ligamentum teres attachment. (c) sagittal MR image demonstrates acetabular anterior (AA) and posterior (AP) subregion, demarcated by vertical line 1 cm from the most anterior and posterior aspect of the femoral head. (d) sagittal MR image demonstrates femoral anterior (FA) and posterior (FP) subregion, demarcated by vertical line 1 cm from the most anterior and posterior aspect of the femoral head.
Intra and inter-rater reliability for the measures was established on a subset of 30 subjects by 2 radiologists. The intra- and inter-reader reliability per observation was rated with Cohen Kappa values and percent agreements. Linear weighted kappa was used for features with point-scale greater than two. Intra-reader kappa values were between 0.70 – 0.79 (cartilage = 0.70, BMEL = 0.79, cyst = 0.78, labrum = 0.73) and percent agreement was between 74%–98% (cartilage = 85%, BMEL = 96%, cyst =98%, labrum = 74%). Inter-rater kappa values were between 0.55 – 0.71 (cartilage = 0.57, BMEL = 0.55, cyst = 0.71, labrum = 0.65) and percent agreement was between 66%–97% (cartilage = 78%, BMEL = 91%, cyst =97%, labrum = 66%).
Clinical assessment of features of DDH and FAI
Presence or absence of the posterior wall sign and the cross-over sign, and the center-edge angle were recorded from the radiographs.[24] Additionally, alpha angle [25] was measured on the oblique axial MR images.
Self report function
Self-reported function was assessed using Hip disability and Osteoarthritis Outcome Score (HOOS).[26] The HOOS covers 5 separate dimensions of hip function: Pain, Symptoms, Activities of Daily Living (ADL), Sport and Recreation Function (Sport), and Hip-Related Quality of Life (QOL). All dimensions are scored from 0 to 4, and then scores are transformed to a percentage score of 0 to 100, with 0 representing extreme hip problems and 100 representing no hip problems. The HOOS has been shown to be a valid, reliable, and responsive measure of overall hip joint function in people with OA. [26] For this study HOOS subscales of Pain, Symptoms and ADL were used in the analyses.
Physical Function Tests
Two tests were used.
Timed-up and Go Test
The TUG requires a subject to rise from a chair, walk 3 m, turn and come back to sit down. Participants were instructed to walk as quickly as they felt safe and comfortable. A stopwatch was be used to measure the time to complete the TUG within the nearest one hundredth of a second. In a recent review TUG has been shown to be one of the 2 tests with best measurement properties among the sit to stand tests for people with hip or knee OA. [27]
Y-Balance Test (YBT)
YBT is a modification of the valid and reliable Star Excursion Balance Test (SEBT) which eliminates the redundancy in the directions of SEBT and overcomes some of its limitations [28]. The test was devised after Plisky et al [29] incorporated the anterior (A), postero-medial (PM) and postero-lateral (PL) directions of the SEBT. The YBT requires the participant to stand barefeet on an elevated central plastic footplate 1 in (2.54 cm) off the ground and push a rectangular reach indicator block with the foot along a 1.5-m length of plastic tubing in each of the 3 directions. The reach distance is recorded in half centimeters as farthest distance the participant is able to push the reach indicator. The YBT takes less time to complete, has a standard protocol and high inter-rater (0.99–1.00) and intra-rater reliability (0.85–0.91).[30] Three repetitions were performed for each of these 3 directions on both extremities and the greatest reach distance recorded. The difference in the greatest reach distance from each direction between right and left extremities was used to calculate a symmetry score. For this study the reach distance from PM and PL directions were used in the analyses which have been shown to have the greatest agreement with SEBT. [28]
Statistical Analysis
Primary analyses were to compare self –report and physical function measures between +ROA and –ROA groups (adjusted for age) and those without (grade = 0) and with (grade >0) cartilage lesions in the femur and in the acetabulum (adjusted for age) using ANOVA. Additionally, non-parametric Spearman’s ρ correlations were used to assess the relationship between KL scores, cartilage scores (femoral and acetabular), labral scores, BMEL scores and subchondral cyst scores; and between these scores and functional metrics (HOOS, TUG, YBT).
Exploratory analyses were performed to compare function (HOOS, TUG, YBT) with subjects stratified those with (grade >0) and without (grade = 0) BMELs, those with (grade > 3) and without (grade <= 3) complex labral tears and with (grade >0) and without (grade =0) subchondral cysts, adjusting for age, using ANOVA. Finally, Pearson’s correlations were used to evaluate the relationships of center-edge angle and alpha angle with function (HOOS, TUG, YBT); and non-parametric Spearman’s ρ correlation was used to assess their relationship with other imaging scores (KL, cartilage, labral, BMEL, subchondral cyst scores).
RESULTS
Subjects
A total of 180 people were screened for this study and 95 met the eligibility criteria. Out of them 10 were excluded due to various reasons (changed their mind = 7, missed appointment = 1, unable to complete testing = 1, claustrophobia = 1).
Age, BMI and gender distribution are shown in Table 2. The +ROA subjects were older (P < 0.001) compared to −ROA. Similarly, subjects with femoral cartilage lesions (P = 0.008) and those with acetabular cartilage lesions (P = 0.001) were older than subjects without cartilage lesions.
Table 2.
Group characteristics for age, BMI and gender distribution
| Control (n = 55) | Osteoarthritis (n = 30) | P | No-FemCL (n = 33) | FemCL (n = 52) | P | No- AceCL (n = 51) | AceCL (n = 34) | P | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years)* | 43.3 (13.6) | 53.9 (11.2) | <0.001 | 42.0 (13.2) | 50.1 (13.3) | 0.008 | 43.1 (13.8) | 52.7 (11.7) | 0.001 |
| BMI (kg/m2)* | 23.8 (3.3) | 23.8 (3.3) | 0.954 | 23.6 (3.9) | 24.0 (2.9) | 0.615 | 23.5 (3.5) | 24.4 (2.9) | 0.234 |
| Male: Female** | 27:28 | 17:13 | 0.504 | 17:16 | 27:25 | 0.971 | 25:26 | 19:15 | 0.535 |
FemCL = cartilage lesion in the femur
AceCL = cartilage lesion in acetabulum
P value from independent samples T-test
P value from Chi-square test
Function
The HOOS, TUG and YBT scores are shown in Table 3. The HOOS scores for all subscales were no different between +ROA and −ROA groups (P > 0.05), and those with and without femoral cartilage lesions (P > 0.05). Subjects with acetabular cartilage lesions had worse scores (P < 0.05) on all HOOS subscales. The differences in TUG and YBT scores were not significant for any of the comparisons (P > 0.05).
Table 3.
Mean (95 % Confidence Intervals) for self-report measures and physical function tests across groups.
| Control | Osteoarthritis | P* | No-FemCL | FemCL | P* | No- AceCL | AceCL | P* | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HOOS | Symptoms | 90.0 (85.8, 94.2) | 87.6 (81.6, 93.6) | 0.709 | 92.3 (88.0, 96.6) | 87.3 (82.5, 92.1) | 0.265 | 93.6 (90.6,96.5) | 83.0 (76.4, 89.7) | 0.004 |
| Pain | 92.0 (88.0, 96.0) | 89.2 (83.0, 95.5) | 0.406 | 92.2 (87.0, 97.5) | 90.3 (85.8, 94.7) | 0.549 | 93.7 (90.2, 97.2) | 87.2 (80.9, 93.5) | 0.036 | |
| ADL | 94.9 (91.7, 98.1) | 93.0 (88.0, 97.4) | 0.527 | 95.1 (91.2, 98.9) | 93.7 (90.1, 97.3) | 0.678 | 96.5 (94.0, 98.9) | 91.0 (85.7, 96.3) | 0.037 | |
| TUG (sec) | 6.3 (5.9, 6.7) | 6.4 (6.0, 6.7) | 0.387 | 6.4 (5.9, 6.9) | 6.3 (6.0, 6.6) | 0.232 | 6.3 (5.9,6.6) | 6.4 (6.0, 6.8) | 0.671 | |
| YBT (cm) | Posteromedial | 3.8 (2.9,4.7) | 3.5 (2.3, 4.7) | 0.460 | 3.2 (2.0, 4.4) | 4.0 (3.1, 4.9) | 0.421 | 3.4 (2.5, 4.3) | 4.1 (2.9, 5.3) | 0.432 |
| Posterolateral | 4.6 (3.5, 5.8) | 5.5 (3.7, 7.3) | 0.927 | 4.1 (3.0, 5.3) | 5.5 (4.1, 6.9) | 0.387 | 4.8 (3.6, 5.9) | 5.2 (3.4,7.0) | 0.747 | |
FemCL = cartilage lesion in the femur
AceCL = cartilage lesion in acetabulum
P values adjusted for age.
In the exploratory analyses, no significant differences were seen (P > 0.05) on comparing the HOOS data between subjects with and without labral tears. Subjects with BMEL had worse Symptom (P = 0.003) but not Pain (P = 0.268) or ADL (P = 0.453) scores, compared to subjects without BMELs. Subjects with subchondral cysts had worse scores on all subscales - Symptom (P = 0.001), Pain (P = 0.006), ADL (P = 0.019), compared to those without cysts. No significant differences (P > 0.05) were seen in TUG of YBT scores for any of these comparisons.
Relationships
Results from non-parametric tests are shown in Table 4. KL score was not significantly related with any functional measure. Greater total score of femoral cartilage lesions had a close to significance negative association with HOOS Symptom and ADL scores but not with Pain score. Total acetabular cartilage score was negatively related with all HOOS scores. Total BMEL and subchondral cysts scores were also related to worse scores on all HOOS subscales. Greater severity of labral tears was not associated with any HOOS score. None of the MR measures showed a significant association with any of the physical performance test scores in this cohort.
Table 4.
Non-parametric Spearman’s ρ correlations and associated P values between KL score, MR findings and functional measures.
| HOOS | Y Balance Test | |||||
|---|---|---|---|---|---|---|
| Timed Up and Go Test | Symptoms | Pain | ADL | Posteromoedial | Posterolateral | |
| KL Score | 0.15 (0.216) | −0.17 (0.147) | −0.12 (0.307) | −0.12 (0.287) | 0.07 (0.58) | 0.05 (0.704) |
| Total Femoral Cartilage Score | 0.04 (0.741) | −0.22 (0.058) | −0.17 (0.146) | −0.2 (0.055) | 0.14 (0.247) | 0.06 (0.626) |
| Total Acetabular Cartilage Score | 0.11 (0.328) | −0.34 (0.003) | −0.25 (0.026) | −0.32 (0.004) | 0.16 (0.179) | −0.01 (0.970) |
| Total Labral Score | −0.05 (0.658) | −0.18 (0.126) | −0.10 (0.378) | −0.12 (0.316) | 0.10 (0.437) | 0.10 (0.443) |
| Total BMEL Score | 0.06 (0.595) | −0.26 (0.021) | −0.29 (0.010) | −0.23 (0.041) | −0.008 (0.949) | 0.21 (0.084) |
| Total Subchondral Cyst Score | 0.15 (0.199) | −0.35 (0.001) | −0.37 (0.001) | −0.31 (0.006) | 0.11 (0.360) | 0.11 (0.38) |
KL = Kellgren- Lawrence grade; BMEL = Bone Marrow Edema Lesion; HOOS = Hip osteoarthritis outcome score; ADL = Activities of Daily Living
Exploratory analyses showed that the center-edge angle and alpha angle were not associated with any of the functional measures (P > 0.05).
Prevalence of pathologies on MR
There were associations between worsening KL score and increasing number and severity of femoral cartilage defects (ρ = 0.338, P = 0.002), acetabular cartilage defects (ρ = 0.347, P = 0.001), subchondral cysts (ρ = 0.303, P = 0.005) labral tears (ρ = 0.405, P <0.001) and a trend for BMELs (ρ = 0.192, P = 0.079). There was a greater prevalence of acetabular cartilage lesions and subchondral cysts in subjects with hip OA (Table 5). Subjects with cartilage lesions in the femur or acetabulum had higher prevalence of BMELs and subchondral cysts. Furthermore, subjects with cartilage lesions in the acetabulum had greater prevalence of labral tears.
Table 5.
Prevalence of different pathologies in controls and hip OA, and different cartilage lesion groups. P values are from chi-square tests.
| Control | Osteoarthritis | P | No-FemCL | FemCL | P | No- AceCL | AceCL | P | |
|---|---|---|---|---|---|---|---|---|---|
| Cartilage Lesion Femur (%) | 54.6 | 73.3 | 0.089 | NA | NA | NA | 37.3 | 97.1 | <0.001 |
| Cartilage Lesion Acetabulum (%) | 30.9 | 56.7 | 0.021 | 3.0 | 63.5 | <0.001 | NA | NA | NA |
| Complex Labral Tear or maceration (%) | 40.0 | 53.3 | 0.237 | 33.3 | 51.9 | 0.093 | 35.3 | 58.8 | 0.033 |
| BMEL (%) | 10.9 | 23.3 | 0.128 | 0 | 25 | 0.002 | 2.0 | 35.3 | <0.001 |
| Subchondral Cyst (%) | 9.1 | 26.7 | 0.031 | 3.0 | 23.1 | 0.012 | 3.9 | 32.4 | <0.001 |
FemCL = cartilage lesion in the femur
AceCL = cartilage lesion in acetabulum
The prevalence of the subjects with a positive cross-over sign (overall prevalence = 20%) or a positive posterior wall sign (overall prevalence = 39%) were not different between +ROA and –ROA groups, or those with and without femoral or acetabular cartilage lesions. Additionally, the center-edge angle (overall = 31.8±8.6°) did not have a significant relationship with KL grade, or with cartilage, labral, BMEL and cyst scores (P > 0.05). However, alpha angle (overall = 56.9±14.2°) had a positive association with KL grade (ρ = 0.27, P = 0.012), total femoral cartilage score (ρ = 0.38, P < 0.001), total acetabular cartilage score (ρ = 0.45, P < 0.001)total BMEL score (ρ = 0.23, P = 0.034) and close to significance association with total cyst score (ρ = 0.20, P = 0.071) but not with total labral score (ρ = 0.17, P = 0.117).
DISCUSSION
The aim of this study was to investigate the association of hip ROA, femoral and acetabular cartilage lesions with self-reported and physical function. We found that individuals with acetabular cartilage defects reported worse pain and disability compared to those without acetabular cartilage defects. Such differences were not seen between +ROA and –ROA groups, or between those with and without femoral cartilage lesions. Additionally the results show that even complex labral tears are not associated with functional deficits where as acetabular cartilage lesions, BMELs and subchondral cysts are associated with worse self-report disability. Radiologic features of DDH and FAI were also not found to be associated with function in this cohort. These results highlight the importance of MR imaging to guide diagnosis and prognosis for people with hip related disability, and highlight the strengths of MR imaging over radiography for investigating the osteoarthritis disease process at the hip. The findings also enhance the understanding of clinical significance of degeneration of different soft-tissues of the hip in the osteoarthritis disease process.
We did not observe worse disability in individuals with ROA or an association of ROA with function. Earlier reports on the relationship of ROA and function have been inconclusive [31], similar to the results of much more extensive evaluations of association of radiographic knee OA and symptoms. [31, 32] This is further complicated by the differences in radiographic definitions of hip OA and the reproducibility of the radiographic measures. [4, 22, 33] MR imaging could therefore provide an alternative to radiographic imaging since it allows a more detailed characterization of the OA disease process and in the current study was found to be significantly related to self-reported functional impairments.
Worse self-reported disability in individuals with acetabular cartilage lesions and association of acetabular cartilage lesions with HOOS scores, suggests that lesions in the acetabular cartilage may hold greater clinical significance than those in the femur. The reason for this is not clear from this cross-sectional analysis. Overall the prevalence of femoral cartilage lesions (61%) was greater than acetabular cartilage lesions (40%) but all of the acetabular cartilage defects were in the anterior and superior acetabular regions. Using arthroscopy, Nepple et al. reported majority of acetabular cartilage defects to be present in the anterior and superior regions. [34] Finite element modeling work has shown higher contact stresses in the anterior and superior regions than the posterior regions. [35] Further investigation in our cohort shows worse self-reported disability (results not shown) in people with femoral cartilage defects in the superior and anterior regions compared to those without femoral cartilage lesions in these subregions. Hence, it is possible that cartilage defects in the anterior and superior regions of both femur and acetabulum have greater clinical significance. Operative treatment of cartilage defects [36] in this population may reduce pain and improve function. Conservative to prevent further cartilage damage could also be considered for people with cartilage defects. Future studies in larger samples would be needed to confirm this. The results highlight the strength of MR imaging at being able to differentiate acetabular from femoral cartilage defects which would not be possible from conventional radiography.
We did not observe any association between complex labral tears or macerated labrum and disability. We also found a high proportion of our subjects (~ 88%) to have at least simple labral tear and upto 45% to have a complex labral tear or a macerated labrum. Recent studies have reported similar prevalence of 70–86% in asymptomatic population. [37] [38] Roemer et al. also did not find an association between any grade of labral tears and self-reported function in their study. [10] The labral score did show positive correlation with radiographic OA, consistent with recent literature suggesting labral tear may contribute to early OA. [39] MRI is known to offer limited sensitivity towards detecting labral lesions at the hip with arthroscopic evaluation being the gold standard. [40] However, recently optimized non-contrast hip MRI has shown favorable results. [41] The ability to visualize the labrum in our study was enhanced by the use an optimized non-contrast hip MRI protocol, using a small field of view on a 3-Tesla scanner. Surgical treatment is often recommended for labral tears [42] and future work should investigate the presence of other abnormalities which may be related to patient symptoms.
Exploratory analysis in the study showed worse hip related symptoms in subjects with BMELs, and worse self-reported pain, symptoms and function in subjects with subchondral cysts. Furthermore, worse BMEL and cyst scores were associated with worse self-reported disability in our cohort, similar to what has been found in knee OA. [12, 13] We also found higher KL grade associated with worse femoral and cartilage scores, labral scores and subchondraly cysts scores even in this cohort of individuals with mild-moderate hip OA, similar to the findings of Roemer et al.[10] Hence, radiographs may have some utility towards providing indirect evidence of tissue pathologies characteristic of OA. Considering the small number of subjects with BMELs and cysts in the study, replication of these results in larger samples is warranted.
Measures of physical performance did not show any significant relationships with MR or radiographic findings in this report. Our cohort was relatively high functioning with mean HOOS scores being > 80% across all subscales. The discordance between the relationship of hip degeneration with self-reported vs. physical function suggests that there may be an interaction of structural, socio-economic and psychological factors which affects the magnitude of disability. [18, 43, 44] Another possibility could be that the performance based tests used here are not sensitive enough to evaluate physical function for this population. Using 3-D motion analysis techniques, it has been shown the people with mild-moderate hip OA walk with reduced joint excursion and reduced hip flexion moment in late stance. [45] Motion discontinuity (MD) in the sagittal plane has been proposed as a biomarker of hip OA since it is associated with presence and severity of hip OA. [46] It is possible that a more objective evaluation of functional movement patterns using biomechanical techniques may be more sensitive than the functional tests used in this study. Future work is needed to evaluate the association of hip MR abnormalities and biomechanical descriptors of hip movement patterns.
In this cohort of individuals with mild-moderate hip OA, we did not observe an association between center-edge or alpha angle and hip function. The prevalence of subjects with a positive posterior wall or cross-over sign was not different between +ROA and −ROA groups, or those with and without femoral or acetabular cartilage lesions. These features are common descriptors of DDH and FAI, both of which are known risk factors for development of hip OA. [47, 48] Considering the positive association of alpha angle with ROA and cartilage scores, by excluding individuals with KL = 4, it is possible that those with more severe cam-type FAI may have been excluded. Also, we excluded individuals with previous hip surgeries which would have excluded symptomatic individuals with DDH and FAI who underwent surgery, perhaps biasing our sample. Findings of DDH and FAI are not uncommon in asymptomatic individuals [21, 49] and not all these individuals progress to hip OA. [49, 50] Bony morphology, soft-tissue morphology, amount and types of physical activity) and other unknown factors are likely related to the individuals with a FAI or DDH being symptomatic or progressing to OA. [50]
This study has limitations which need to be taken into consideration while interpreting the results. Radiographic OA was defined only using KL grade since it is the most common definition of OA. Other definitions including minimum joint space width measures may lead to different findings. Also the sample size and inclusion of only individuals with mild-moderate radiographic hip OA limit the generalization of the findings. Further analysis related to the location of the different MR features including cartilage defects, BMELs, subchondral cysts and labral and functional outcomes was not done due to the small sample size. Future studies would be needed to evaluate the effect of the location of cartilage lesions etc. with pain and disability in these individuals. Also, longitudinal studies would be needed to study the effect of various tissue pathologies on symptomatic progression of hip OA.
To conclude we found that individuals with acetabular cartilage defects had greater self-reported disability compared to those without, and acetabular cartilage defects were associated with worse self-reported disability. Such differences and associations were not observed with ROA or femoral cartilage lesions. Additionally, presence of BMELs and subchondral cysts was related to greater hip related pain and disability. None of the radiographic or MR features of OA, DDH or FAI were related to physical function in this cohort. MRI may serve as a useful tool towards evaluating the effect of osteoarthritis disease process on the structure of the hip joint.
Acknowledgments
The authors thank Melissa Guan for help in recruiting and consenting patients for the study. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number NIH –NIAMS P50 AR060752. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
AUTHOR CONTRIBTUTIONS
Conception and Design: Kumar, Link, Majumdar, Souza
Acquisition of data: Kumar, Wyatt
Analysis and Interpretation of Data: Kumar, Wyatt, Lee, Nardo, Link, Majumdar, Souza
Drafting of article or revising it critically for important intellectual content: Kumar, Wyatt, Lee, Nardo, Link, Majumdar, Souza
Final approval of the version of the article to be published: Kumar, Wyatt, Lee, Nardo, Link, Majumdar, Souza
Authors Kumar (Deepak.kumar@ucsf.edu) and Souza (Richard.rosuza@ucsf.edu) take full responsibility for the integrity of this work as a whole.
CONFLICT OF INTEREST : No conflict of interest for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy LB, Helmick CG, Schwartz TA, Renner JB, Tudor G, Koch GG, et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage. 2010;18:1372–9. doi: 10.1016/j.joca.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salaffi F, Carotti M, Stancati A, Grassi W. Health-related quality of life in older adults with symptomatic hip and knee osteoarthritis: a comparison with matched healthy controls. Aging Clin Exp Res. 2005;17:255–63. doi: 10.1007/BF03324607. [DOI] [PubMed] [Google Scholar]
- 3.Katz JN, Losina E, Barrett J, Phillips CB, Mahomed NN, Lew RA, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83-A:1622–9. doi: 10.2106/00004623-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsen S, Sonne-Holm S, Soballe K, Gebuhr P, Lund B. Radiographic case definitions and prevalence of osteoarthrosis of the hip: a survey of 4 151 subjects in the Osteoarthritis Substudy of the Copenhagen City Heart Study. Acta Orthop Scand. 2004;75:713–20. doi: 10.1080/00016470410004085. [DOI] [PubMed] [Google Scholar]
- 5.Altman RD. Criteria for classification of clinical osteoarthritis. J Rheumatol Suppl. 1991;27:10–2. [PubMed] [Google Scholar]
- 6.Hunter DJ, Guermazi A. Imaging techniques in osteoarthritis. PM R. 2012;4:S68–74. doi: 10.1016/j.pmrj.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Spector TD, Cooper C. Radiographic assessment of osteoarthritis in population studies: whither Kellgren and Lawrence? Osteoarthritis Cartilage. 1993;1:203–6. doi: 10.1016/s1063-4584(05)80325-5. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Gunther KP, Brenner H. Reliability of radiographic grading of osteoarthritis of the hip and knee. Scand J Rheumatol. 1997;26:155–65. doi: 10.3109/03009749709065675. [DOI] [PubMed] [Google Scholar]
- 9.Hunter DJ, Arden N, Conaghan PG, Eckstein F, Gold G, Grainger A, et al. Definition of osteoarthritis on MRI: results of a Delphi exercise. Osteoarthritis Cartilage. 2011;19:963–9. doi: 10.1016/j.joca.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roemer FW, Hunter DJ, Winterstein A, Li L, Kim YJ, Cibere J, et al. Hip Osteoarthritis MRI Scoring System (HOAMS): reliability and associations with radiographic and clinical findings. Osteoarthritis Cartilage. 2011;19:946–62. doi: 10.1016/j.joca.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Guermazi A, Roemer FW, Haugen IK, Crema MD, Hayashi D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat Rev Rheumatol. 2012 doi: 10.1038/nrrheum.2012.223. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review Ann Rheum Dis. 2011;70:60–7. doi: 10.1136/ard.2010.131904. [DOI] [PubMed] [Google Scholar]
- 13.Menashe L, Hirko K, Losina E, Kloppenburg M, Zhang W, Li L, et al. The diagnostic performance of MRI in osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2011;20:13–21. doi: 10.1016/j.joca.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gold SL, Burge AJ, Potter HG. MRI of hip cartilage: joint morphology, structure, and composition. Clin Orthop Relat Res. 2012;470:3321–31. doi: 10.1007/s11999-012-2403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mamisch TC, Zilkens C, Siebenrock KA, Bittersohl B, Kim YJ, Werlen S. MRI of hip osteoarthritis and implications for surgery. Magn Reson Imaging Clin N Am. 2009;18:111–20. doi: 10.1016/j.mric.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Potter HG, Schachar J. High resolution noncontrast MRI of the hip. J Magn Reson Imaging. 31:268–78. doi: 10.1002/jmri.22025. [DOI] [PubMed] [Google Scholar]
- 17.Arokoski MH, Haara M, Helminen HJ, Arokoski JP. Physical function in men with and without hip osteoarthritis. Arch Phys Med Rehabil. 2004;85:574–81. doi: 10.1016/j.apmr.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Juhakoski R, Tenhonen S, Anttonen T, Kauppinen T, Arokoski JP. Factors affecting self-reported pain and physical function in patients with hip osteoarthritis. Arch Phys Med Rehabil. 2008;89:1066–73. doi: 10.1016/j.apmr.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Register B, Pennock AT, Ho CP, Strickland CD, Lawand A, Philippon MJ. Prevalence of abnormal hip findings in asymptomatic participants: a prospective, blinded study. Am J Sports Med. 40:2720–4. doi: 10.1177/0363546512462124. [DOI] [PubMed] [Google Scholar]
- 20.Duthon VB, Charbonnier C, Kolo FC, Magnenat-Thalmann N, Becker CD, Bouvet C, et al. Correlation of Clinical and Magnetic Resonance Imaging Findings in Hips of Elite Female Ballet Dancers. Arthroscopy. 2013 doi: 10.1016/j.arthro.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Jung KA, Restrepo C, Hellman M, AbdelSalam H, Morrison W, Parvizi J. The prevalence of cam-type femoroacetabular deformity in asymptomatic adults. J Bone Joint Surg Br. 2011;93:1303–7. doi: 10.1302/0301-620X.93B10.26433. [DOI] [PubMed] [Google Scholar]
- 22.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilizaliturri VM, Jr, Byrd JW, Sampson TG, Guanche CA, Philippon MJ, Kelly BT, et al. A geographic zone method to describe intra-articular pathology in hip arthroscopy: cadaveric study and preliminary report. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2008;24:534–9. doi: 10.1016/j.arthro.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis--what the radiologist should know. AJR Am J Roentgenol. 2007;188:1540–52. doi: 10.2214/AJR.06.0921. [DOI] [PubMed] [Google Scholar]
- 25.Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84:556–60. doi: 10.1302/0301-620x.84b4.12014. [DOI] [PubMed] [Google Scholar]
- 26.Nilsdotter AK, Lohmander LS, Klassbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;4:10. doi: 10.1186/1471-2474-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobson F, Hinman RS, Hall M, Terwee CB, Roos EM, Bennell KL. Measurement properties of performance-based measures to assess physical function in hip and knee osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2012;20:1548–62. doi: 10.1016/j.joca.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Coughlan GF, Fullam K, Delahunt E, Gissane C, Caulfield BM. A comparison between performance on selected directions of the star excursion balance test and the y balance test. J Athl Train. 2012;47:366–71. doi: 10.4085/1062-6050-47.4.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plisky PJ, Rauh MJ, Kaminski TW, Underwood FB. Star Excursion Balance Test as a predictor of lower extremity injury in high school basketball players. J Orthop Sports Phys Ther. 2006;36:911–9. doi: 10.2519/jospt.2006.2244. [DOI] [PubMed] [Google Scholar]
- 30.Plisky PJ, Gorman PP, Butler RJ, Kiesel KB, Underwood FB, Elkins B. The reliability of an instrumented device for measuring components of the star excursion balance test. N Am J Sports Phys Ther. 2009;4:92–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Kinds MB, Welsing PM, Vignon EP, Bijlsma JW, Viergever MA, Marijnissen AC, et al. A systematic review of the association between radiographic and clinical osteoarthritis of hip and knee. Osteoarthritis Cartilage. 2011;19:768–78. doi: 10.1016/j.joca.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Dieppe PA, Cushnaghan J, Shepstone L. The Bristol ‘OA500’ study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997;5:87–97. doi: 10.1016/s1063-4584(97)80002-7. [DOI] [PubMed] [Google Scholar]
- 33.Lanyon P, Muir K, Doherty S, Doherty M. Age and sex differences in hip joint space among asymptomatic subjects without structural change: implications for epidemiologic studies. Arthritis Rheum. 2003;48:1041–6. doi: 10.1002/art.10886. [DOI] [PubMed] [Google Scholar]
- 34.Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39:296–303. doi: 10.1177/0363546510384787. [DOI] [PubMed] [Google Scholar]
- 35.Harris MD, Anderson AE, Henak CR, Ellis BJ, Peters CL, Weiss JA. Finite element prediction of cartilage contact stresses in normal human hips. J Orthop Res. 2011;30:1133–9. doi: 10.1002/jor.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5:244–53. doi: 10.1007/s12178-012-9134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz MR, Campbell SE, Fajardo RS, Kadrmas WR. Identification of acetabular labral pathological changes in asymptomatic volunteers using optimized, noncontrast 1. 5-T magnetic resonance imaging. The American journal of sports medicine. 2012;40:1337–41. doi: 10.1177/0363546512439991. [DOI] [PubMed] [Google Scholar]
- 38.Register B, Pennock AT, Ho CP, Strickland CD, Lawand A, Philippon MJ. Prevalence of abnormal hip findings in asymptomatic participants: a prospective, blinded study. The American journal of sports medicine. 2012;40:2720–4. doi: 10.1177/0363546512462124. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy JC, Noble PC, Schuck MR, Wright J, Lee J. The watershed labral lesion: its relationship to early arthritis of the hip. The Journal of arthroplasty. 2001;16:81–7. doi: 10.1054/arth.2001.28370. [DOI] [PubMed] [Google Scholar]
- 40.Smith TO, Hilton G, Toms AP, Donell ST, Hing CB. The diagnostic accuracy of acetabular labral tears using magnetic resonance imaging and magnetic resonance arthrography: a meta-analysis. Eur Radiol. 2011;21:863–74. doi: 10.1007/s00330-010-1956-7. [DOI] [PubMed] [Google Scholar]
- 41.Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2005;21:385–93. doi: 10.1016/j.arthro.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 42.Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2:105–17. doi: 10.1007/s12178-009-9052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Baar ME, Dekker J, Lemmens JA, Oostendorp RA, Bijlsma JW. Pain and disability in patients with osteoarthritis of hip or knee: the relationship with articular, kinesiological, and psychological characteristics. J Rheumatol. 1998;25:125–33. [PubMed] [Google Scholar]
- 44.Knight JB, Callahan LF, Luong ML, Shreffler J, Schoster B, Renner JB, et al. The association of disability and pain with individual and community socioeconomic status in people with hip osteoarthritis. Open Rheumatol J. 2011;5:51–8. doi: 10.2174/1874312901105010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eitzen I, Fernandes L, Nordsletten L, Risberg MA. Sagittal plane gait characteristics in hip osteoarthritis patients with mild to moderate symptoms compared to healthy controls: a cross-sectional study. BMC Musculoskelet Disord. 2012;13:258. doi: 10.1186/1471-2474-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foucher KC, Schlink BR, Shakoor N, Wimmer MA. Sagittal plane hip motion reversals during walking are associated with disease severity and poorer function in subjects with hip osteoarthritis. J Biomech. 2012;45:1360–5. doi: 10.1016/j.jbiomech.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87:1012–8. doi: 10.1302/0301-620X.87B7.15203. [DOI] [PubMed] [Google Scholar]
- 48.Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003:112–20. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 49.Hartofilakidis G, Bardakos NV, Babis GC, Georgiades G. An examination of the association between different morphotypes of femoroacetabular impingement in asymptomatic subjects and the development of osteoarthritis of the hip. J Bone Joint Surg Br. 93:580–6. doi: 10.1302/0301-620X.93B5.25236. [DOI] [PubMed] [Google Scholar]
- 50.Bardakos NV, Villar RN. Predictors of progression of osteoarthritis in femoroacetabular impingement: a radiological study with a minimum of ten years follow-up. J Bone Joint Surg Br. 2009;91:162–9. doi: 10.1302/0301-620X.91B2.21137. [DOI] [PubMed] [Google Scholar]