Abstract
Background
Given the problems associated with the escalation in methamphetamine (METH) use, the identification of more effective treatment strategies is essential. Group II metabotropic glutamate receptors (mGluRs) have been suggested to be a novel therapeutic target for psychostimulant addiction. We sought to test the ability of the selective group II mGluR agonist LY379268 to reduce METH self-administration in rats.
Methods
Rats were trained to self-administer METH on a progressive ratio (PR) schedule. Animals were then switched to fixed ratio responding and given daily extended access (6 h/day) to METH self-administration for 14 days. Rats were then re-tested on the PR schedule. The effect of LY379268 on METH-reinforced PR responding was determined before and after 14 days of extended access. To test for non-specific effects, a separate group of animals received LY379268 prior to a sucrose pellet-reinforced PR schedule.
Results
Animals escalated their daily intake of METH during extended access. PR responding did not change as a function of extended access. LY379268 significantly attenuated METH reinforced responding, both before and after extended access. The degree of attenuation did not change as a function of extended access. LY379268 had no effect on sucrose pellet-reinforced responding at any dose.
Conclusions
LY379268 selectively reduced the motivation to self-administer METH. In contrast to data with other compounds, the sensitivity to the effects of LY379268 did not change following extended access to METH self-administration. Group II mGluR agonists, therefore, may represent a relatively new class of compounds for the development of pharmacotherapies for METH addiction.
Keywords: methamphetamine, LY369268, self-administration, extended-access
1. Introduction
Abuse of methamphetamine (METH) is a growing problem within the United States and worldwide. Reports suggest that over 300,000 Americans used METH within a single month (SAMSHA, 2008a), and the annual cost of METH abuse to society has been estimated to be more than $23 billion (Nicosia et al., 2005). Of particular concern are emergency room reports which show that 95% of all stimulant admissions involved METH (SAMHSA, 2008b).
Despite the prevalence of use and abuse, there are currently no effective pharmacotherapies for METH addiction. Systemic METH administration has been shown to increase extracellular levels of dopamine, norepinephrine and serotonin in the brain (Sora et al., 2009), similar to cocaine (Hurd and Ungerstedt, 1989; Florin et al., 1994). However, in contrast to cocaine, METH is categorized as a releaser, whereby it enters the pre-synaptic terminal and reverses both vesicular monoamine transporters 2 (VMAT2) and plasma membrane monoamine transporters (Kuczenski and Segal, 1995; Eshleman et al., 1999), resulting in a substantial efflux of neurotransmitter into the synapse. Given the difference in the mechanism of action, potential medications for cocaine and METH addiction may also have different efficacies. Preclinical studies investigating new addiction therapies for METH have focused on monoamine targets, based on the premise that blocking these effects may attenuate the behavioral consequences of chronic METH use (Orio et al., 2010; Wee et al., 2007; Higley et al. 2011; Takamatsu et al., 2006; Meyer et al., 2011; Graves and Napier, 2011).
An alternative strategy for medication development may be to focus on targets downstream of these effects, such as the glutamate system. It has been well established that METH administration results in increased glutamate concentrations in the striatum (Nash and Yamamoto, 1992; Stephan and Yamamoto, 1994; Mark et al., 2004), and that glutamate release may be involved in the neurotoxicity induced by high doses of METH (Mark et al., 2004). Studies have also shown a pivotal role for glutamate in the effects of other psychostimulants, such as cocaine. For example, stimulating ionotropic glutamate receptors (NMDA and AMPA) within the nucleus accumbens augmented the reinforcing effects of cocaine (Cornish et al., 1999) and blocking the prefrontal cortex-nucleus accumbens glutamatergic projections attenuated cocaine-primed reinstatement of cocaine-seeking (McFarland et al., 2003). Though there are many potential targets within the glutamate system for therapeutic development, the group II metabotropic glutamate receptors (mGluRs) have shown particular promise, given their role in reward processing and drug-seeking (Moussawi and Kalivas, 2010). This family is comprised of two Gi coupled receptor subtypes: metabotropic glutamate receptor 2 (mGluR2) and mGluR3. mGluR2 are primarily presynaptic and function as autoreceptors to decrease neurotransmitter release (Conn and Pin, 1997; Xi et al., 2002), while mGluR3 are more widely distributed across pre-and post-synaptic elements, and their functions are more varied (Schoepp, 2001).
(−)-2-Oxa-4-aminobicylco hexane-4,6-dicarboxylic acid (LY379268), displays more than 80-fold selectivity for the group II mGluRs over other metabotropic glutamate receptors (Monn et al., 1999), though it has a slightly higher affinity for the mGluR3 versus mGluR2 (EC50 = 2.69 vs 4.58 at human mGluR2 and mGluR3, respectively). This highly selective agonist has been used in a number of studies to attenuate drug self-administration and reinstatement of drug-seeking (Baptista et al., 2004; Bossert et al., 2005, 2006; Adewale et al., 2006; Bäckström and Hyytiä, 2005; Kufahl et al., 2013). For example, LY379268 has been shown to block the expression of locomotor sensitization by amphetamine (Kim and Vezina, 2002), attenuate enhanced self-administration of amphetamine after a sensitizing drug regimen (Kim et al., 2005) and reduce cue- and METH-induced reinstatement of METH-seeking (Kufahl et al., 2013). What has not been determined, however, is the effect of LY379268 on the motivation to continue to self-administer METH using the progressive ratio schedule.
Some of the key characteristics of addiction as defined by the DSM-IV are a compulsion to take drugs, a loss of control in limiting intake, and a gradual escalation in the amount of drug taken over time (DSM-IV, APA, 2000). These aspects of addiction have been modeled by preclinical intravenous self-administration paradigms which result in increased, or escalated, daily drug intake. These models serve as a behavioral framework with which the neurobiological mechanisms underlying excessive drug intake may be examined. Given extended access (6 h per day) to self-administration, daily intake has been shown to progressively increase (Ahmed and Koob, 1998; Kitamura et al., 2006; Schwendt et al., 2009; Reichel et al., 2011). Some of the behavioral and neurobiological changes that occur as a result of this extended access paradigm in animals are thought to mirror those alterations found in humans, for example deficits in memory (George et al., 2008; Reichel et al., 2012), impulsivity (Dally et al., 2007) and attention (Parsegian et al., 2011). In addition, several neuroadaptations occur following extended access that parallel those seen in addicts, such as lower levels of dopamine and dopamine transporters (Brennan et al., 2010; Schwendt et al., 2012). Importantly, METH users exhibit a faster rate of progression towards regular use as compared to cocaine users (Gonzalez-Castro et al., 2000), suggesting that recreational experimentation and use can rapidly escalate to abuse and dependence. Thus the extended access paradigm may be particularly relevant to METH abuse and addiction. An additional consideration is that extended access to METH has been shown to result in a shift in the sensitivity of compounds in their ability to reduce self-administration (Wee et al., 1997; Orio et al., 2010). This alteration in the ability of compounds to reduce the reinforcing effects of drugs may be due to neuroadaptations that occur during extended access, which may only be transient in short access animals (Hao et al., 2010). The aims of the present study, therefore, were to investigate the impact of LY379268 on the motivation to self-administer METH and determine whether the effect of LY379268 changed as a result of extended access.
2. Methods
2.1. Animals, Surgery, and Housing
All experiments were conducted using male, Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) weighing approximately 350 g at the beginning of the experiment. Animals were given a week to acclimate to the laboratory environment. Animals were then anesthetized using a combination of ketamine (100 mg/kg; i.p.) and xylazine (8 mg/kg; i.p.), and a chronic, indwelling Silastic cannula was implanted into the right jugular vein that exited through the back of the animal in the region of the scapulae. Ketoprofen (5 mg/kg; i.p.) was used as a post-operative analgesic, and animals were allowed to recover from surgery for a minimum of 3 days. Following surgery, animals were housed in 30 × 30 × 30-cm operant chambers under a reversed 12-h light/dark cycle (lights on at 1500 hours) with ad libitum food and water access. All procedures were conducted in concordance with the Wake Forest University Animal Care and Use Committee guidelines and the “Guidelines for the Care and Use of Laboratory Rats” (National Research Council, 1996).
2.2. Methamphetamine self-administration
An overview of the experimental design is shown in Table 1. The beginning of the self-administration session was indicated by the extension of the lever into the self-administration chamber, which began 6 hours into the dark cycle (0900 hours). A single response on the lever (fixed ratio (FR) 1) resulted in an infusion of methamphetamine (0.05 mg/kg) over a 2 sec period, which was paired with a 2 second LED illumination, and no time out. Criterion for acquisition and FR1 training was that animals self-administered the maximum number of infusions (20) available per session for 3 consecutive days. The sessions occurred 7 days/week, and lasted 6 hours, or until 20 infusions had been achieved. After acquisition criterion had been satisfied and animals had completed FR1 training, all rats were switched to a progressive ratio schedule (PR) (Richardson and Roberts, 1996), see below for details. Following the PR schedule, subjects (n=11) were then switched back to FR1 responding and placed into an extended access paradigm (Kitamura et al., 2006), which consisted of 14 days (7 days/week) of 6 hour access to METH self-administration on an FR1 schedule (0.05 mg/kg/inf, no time out, unlimited number of infusions).
Table 1. Experimental Timeline.
2.3. Progressive Ratio Responding
The progressive ratio (PR) schedule used in these experiments has been previously described in detail (Richardson and Roberts, 1996). Briefly, PR measures the relative reinforcing properties of a drug by assessing the point at which animals will no longer work to obtain a reward. The number of lever presses required for a rat to receive a single injection of METH increases along an exponential function after each infusion through the following progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, and 603. Breakpoints were defined as the number of infusions received, and average breakpoints were based on at least 3 days of stable responding, defined as no upward or downward trends (Liu et al., 2005). Number of infusions rather than total number of responses or final ratio values were used to increase homogeneity of variance. Following stable performance, prior to extended access, LY379268 (0.01, 0.1, 0.3, 1.0 mg/kg, i.p.) was administered 30 min prior to behavioral testing, in order to determine its effect on PR responding. Each rat received each LY379268 dose once and in a randomized order. Responding was allowed to recover to baseline levels for at least two consecutive sessions before the next dose was administered. Doses for LY379268 were chosen based on existing literature (Baptista et al., 2004; Peters and Kalivas, 2006). Following 14 days extended access, animals were switched back to PR responding and break points were re-established in order to determine whether performance had been influenced by extended access (Table 1). Once stable break points had been established the effect of 0.3 mg/kg LY379268 on PR responding was determined.
2.4. Sucrose pellet-reinforced responding
Experimentally naïve rats (n=8) were 22-hours food deprived and trained to administer sucrose pellets (45 mg, Research Diets, Inc., New Brunswick, NJ) on an FR1 schedule, which was gradually increased to FR3. Once lever training was complete, rats were switched to a PR schedule, which was maintained throughout drug testing. Each PR session lasted 30 minutes and was followed by an hour of ad libitum food. Water was available ad libitum throughout the experiment. LY379268 was administered 30 minutes prior to the start of sucrose self-administration (0.01, 0.1, 0.3, 1.0 mg/kg, i.p.). Each rat received each LY379268 dose once and in a randomized order. Rats were weighed before the start of each daily session and after the hour of ad libitum food access.
2.5. Drugs
(+)-Methamphetamine hydrochloride (NIDA Drug Supply Program, Research Triangle Park, NC) and LY379268 (Tocris Bioscience, Ellisville, MO) were dissolved in physiological saline (0.9% NaCl). LY379268 was administered (i.p.) in a 1 ml/kg volume. All drug doses are expressed as the weight of the salt.
2.6. Data analysis
Daily intake and intake (mg/kg) in the first hour were used as the main dependent measures for self-administration under extended access conditions, while break points were used as the dependent measure during the PR schedule (note that final ratio was used to plot the data for Figures 2 and 3). Changes in the daily intake and intake in the first hour over the 14 days of 6 h access were assessed by a one-way analysis of variance (ANOVA) with repeated measures (SPSS, SPSS Inc., NY). A paired Student's t-test was used to determine whether break points on the PR schedule changed as a result of 14 days extended access, and whether LY379268 (0.3 mg/kg) affected break points following extended access. A one-way ANOVA with repeated measures was performed to test whether LY379268 (0.01, 0.1, 0.3, 1.0 mg/kg) significantly affected break points for METH self-administration or sucrose reinforcement. Values of p < 0.05 were considered statistically significant and appropriate post-hoc analyses were used.
Fig. 2. Responding for METH on a progressive ratio schedule before (black bars) and after (grey bars) extended access to METH self-administration following saline and 0.3 mg/kg LY379268. * p < 0.05 compared to pre-extended access baseline (N=6), † p < 0.05 compared to post-extended access baseline (N=7).
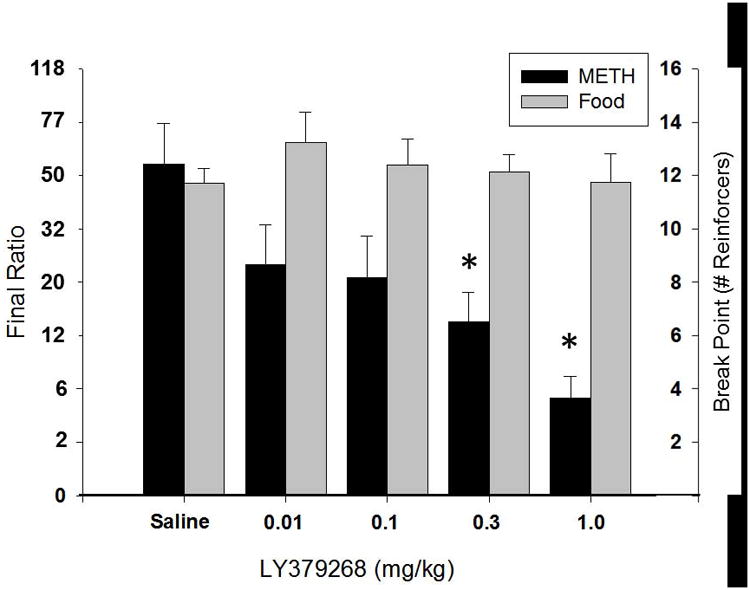
Fig. 3. Effect of LY379268 on progressive ratio responding for either METH (black bars) (N=6) or sucrose pellets (grey bars) (N=8). Pretreatment with LY379268 dose-dependently decreased progressive ratio responding for METH, but had no effect on responding for sucrose. * P < 0.05 compared saline responding.

3. Results
3.1. Effect of Extended Access Conditions on Self-Administration
A total of 20 animals initiated METH self-administration. 3 of these failed to acquire. The remaining animals completed self-administration training requirements within the first few days of lever exposure (mean ± S.E.M., 4.5 ± 0.6 days). Following acquisition of self-administration, animals (n=11) had unrestricted access to METH (0.05 mg/kg/infusion, FR1, no time out) for 6 h per day for 14 days. Daily intake progressively increased over the course of the study (first session: 3.5 ± 0.4 mg/kg versus final session: 6.5 ± 0.8 mg/kg; one-way repeated measures ANOVA, F(13,130) = 10.23, p < 0.001; Figure 1A). Post-hoc comparisons revealed that daily intake of METH significantly increased above first session intake from the seventh session onward (Tukey's test, p < 0.05). Intake during the first hour of each session also progressively increased over days (first session: 0.9 ± 0.1 mg/kg/h versus final session: 1.7 ± 0.2 mg/kg/h; one-way repeated measures ANOVA, F(13,130) = 6.215, p < 0.001; Figure 1B). Post-hoc comparisons revealed METH intake during the first hour was significantly higher on the ninth session and twelfth session onwards, compared to the first session (Tukey's test, p < 0.05).
Fig. 1. Progressive increases in the cumulative daily intake of METH (mg/kg) over 14 sessions (Panel A) and intake during the first hour of each session (Panel B). * p < 0.05 compared to intake on Day 1 (N=11).

3.2. Progressive ratio responding
Following acquisition of METH self-administration (see above), animals underwent training on a progressive ratio (PR) schedule. After stable rates of responding were achieved (mean ± S.E.M., 9.0 ± 1.8 days), the schedule changed and animals had unrestricted access to METH self-administration for 14 days (6 h per day FR1), following which PR responding was re-assessed. A paired Student's t-test revealed that there was no significant difference in break points before or after extended access to METH self-administration (Figure 2).
Following acquisition of METH self-administration, a separate group of animals (n=6) was trained on a PR schedule. Once PR responding was stable, LY379268 (0.01, 0.1, 0.3, 1.0 mg/kg i.p.) or saline was administered 30 min prior to behavioral testing. LY379268 significantly affected METH-reinforced responding (one-way repeated measures ANOVA, Chi-square = 19.93, 4 degrees of freedom, p < 0.001; Figure 3). Post-hoc analyses indicated that 0.3 and 1.0 mg/kg LY379268 significantly attenuated responding (Dunnett's test, p < 0.05) compared to vehicle pretreatment. LY379268 has been shown previously to reduce responding reinforced by food (Peters and Kalivas, 2006), suggesting that it may have non-specific effects. Therefore, in order to determine whether LY379268 had non-specific effects on responding, a separate group of animals (n=8) was trained to respond on a PR schedule reinforced by sucrose pellets. After stable PR responding was achieved, LY379268 (0.01, 0.1, 0.3, 1.0 mg/kg, i.p.) was administered 30 min prior to behavioral testing (Figure 3). Analyses failed to detect significant differences in responding following any dose of LY379268.
In order to determine whether 14 days of extended access resulted in a shift in the sensitivity to the effects of LY379268 on PR responding, a separate group of animals (n=7) was treated with LY379268 (0.3 mg/kg). This dose was chosen since it produced a robust effect on reducing PR responding and was on the ascending limb of the dose response curve. A paired Student's t-test revealed that LY379268 significantly reduced break points compared to vehicle (Figure 2, p < 0.05). To investigate whether there was a difference in the effect of 0.3 mg/kg LY379268 on PR responding before or after 14 days of extended access to METH self-administration, we conducted an unpaired Student's t-test, which revealed no significant difference between the two groups (Figure 2).
4. Discussion
The main findings from the current study were that: 1) extended access to METH resulted in increased daily intake, 2) responding on a PR schedule reinforced by METH did not change as a function of 14 days of extended access, 3) LY379268 dose-dependently reduced METH self-administration, 4) the ability of LY379268 to reduce METH self-administration was not affected by 14 days of extended access and 5) LY379268 failed to significantly affect responding on a PR schedule reinforced by sucrose pellets.
4.1. Methamphetamine Self-Administration
Extended access to self-administration for 6 h daily resulted in a gradual progressive increase in daily intake that was significantly different to the first day from day 7 onwards. This was accompanied by significant increases in the amount of METH self-administered in the first hour of the six hour session. These results are in agreement with a large body of literature regarding extended, or long, access to METH (Kitamura et al., 2006; Schwendt et al., 2009; Riechel et al., 2010), as well as other psychostimulants such as cocaine (Ahmed and Koob, 1998; Liu et al., 2005). In addition, we found that responding on the PR schedule, as measured by break points, was unaffected by extended access to METH, in agreement with previous studies with cocaine (Liu et al., 2005; Roberts et al., 2007; Quadros and Miczek, 2009), though other investigators have found an increase in PR responding (Wee et al., 1997; Orio et al., 2010). There are a number of possible explanations of these discrepant findings. For example, we have demonstrated previously that prior training history affects escalation of break points, wherein higher doses of cocaine experienced during training failed to increase break points following extended access (Liu et al., 2005). It is possible that animals self-administered relatively large amounts of METH during acquisition without reaching criterion for acquisition, thereby masking any potential differences in break points. Alternatively, it is possible that the continuous manner in which the sessions were run in the present study may have resulted in a blunting of alterations in break points. Animals in the current study self-administered 7 days/week in their home cage without any breaks. In contrast, many other studies conduct self-administration studies 5 days/week, allowing the animals to take ‘drug holidays’ over the weekend. It is possible that neuroadaptations occur during these breaks that may result in an increase in the motivational properties of the reinforcer.
4.2. Effects of LY379268 on Progressive Ratio Responding
LY379268 dose-dependently reduced METH-reinforced responding. These data are in agreement with other studies that have investigated the ability of LY379268 to attenuate psychostimulant-induced behavior. For example, Hao et al. (2010) reported that LY379268 dose-dependently reduced cocaine-reinforced PR responding, and Peters and Kalivas (2006) reported that LY379268 reduced cocaine-induced reinstatement of cocaine-seeking. In addition, a recent study by Olive and colleagues demonstrated that cue- and METH-induced reinstatement of METH seeking was dose-dependently reduced by LY379268 (Kufahl et al., 2013). Our data extends these findings by demonstrating that LY379268 also attenuates the motivation to continue to self-administer METH, as assessed via the PR schedule.
Our data also demonstrate that the sensitivity to the effects of LY379268 on PR responding did not change as a result of extended access to chronic METH self-administration. Though we only tested one dose of LY379268 (0.3 mg/kg), this dose produced a robust response on PR responding pre-escalation and was on the ascending limb on the dose response curve. Thus, we predicted that it would be the most likely to demonstrate whether a shift in sensitivity had occurred. The similar effects of this dose of LY379268 on PR responding, despite the history of extended access to METH self-administration, are in contrast to previous studies. For example, while Wee et al. (1997) reported a rightward shift in the dose-response function of METH on a PR schedule following aripiprazole treatment, this effect was more pronounced in animals with a history of extended access versus a limited history of METH self-administration. Similarly, Orio et al. (2010) found that the D3 receptor antagonist PG01037 significantly reduced METH-reinforced responding in long-access animals, while having no effect in animals following short-access to METH self-administration. A recent study by Kufahl et al. (2013) reported a shift in the sensitivity of rats to the effects of 0.3 mg/kg LY379268 on cue-induced reinstatement of METH-seeking, but not METH primed reinstatement after a history of escalated intake. A possible explanation underlying these contrasting findings is that group II mGluRs may have different roles in mediating the motivation to continue drug-seeking (PR schedule) versus to motivation to initiate drug-seeking (reinstatement/ relapse). Despite this, our data suggests that activation of group II mGluRs may be a viable target for reducing METH intake, even if the degree of METH exposure is relatively small.
Glutamate has been shown to play a role in the reinforcing effects of other psychostimulants, such as cocaine. Early studies found that glutamate release within the nucleus accumbens mediated cocaine-seeking behavior (Cornish et al., 1999). Further research revealed the source of this increased glutamate in the nucleus accumbens to be afferents from the prefrontal cortex (McFarland et al., 2003). Mark et al. (2004) showed that the effects of METH on dopamine within the striatum lead to similar increases in extracellular glutamate in prefrontal cortex-nucleus accumbens synapses. Specifically, increases in nigrostriatal dopamine by D1 activation increased inhibition within the substantia nigra, in turn removing inhibition along nigrothalamic pathways. This removal of inhibition resulted in an increase in thalamocortical and corticostriatal glutamate release (Mark et al., 2004). More recent studies have demonstrated that projections into the nucleus accumbens can co-release glutamate and dopamine (Stuber et al., 2010; Tecuapetla et al., 2010). Group II mGluRs act primarily as inhibitory autoreceptors within these pathways to regulate this release of glutamate and dopamine into the nucleus accumbens (Conn and Pin, 1997; Hu et al., 1999; Karasawa et al., 2006; Xi et al., 2002). Previous research has established that activation of presynaptic group II mGluR decreased glutamate release (Dietrich et al., 2002) and that both systemic treatment and direct microinjections of LY379268 into the nucleus accumbens inhibited cocaine-seeking in rats (Peters and Kalivas, 2006). Additional microdialysis studies have shown the ability of group II mGluR antagonists to increase, and group II agonists decrease, dopamine release in the nucleus accumbens (Karasawa et al., 2006). Thus, it is possible that group II mGluRs activation could attenuate the rise in glutamate and dopamine in the nucleus accumbens following METH self-administration, accounting for the reduction in reinforcing its effects.
Since the extended access paradigm has been shown to reproduce some of the key aspects of the addiction process, such as an increase in drug-taking over time (APA, 2000), it is possible that it induces similar neuroadaptations that may occur as a result of chronic drug exposure (Ahmed and Koob, 2005). One of the aims of the current study was to test whether the ability of LY379268 to reduce the reinforcing effects of METH altered as a consequence of extended access, because chronic drug exposure has been shown to decrease basal glutamate and dopamine levels. Moreover, extended access to cocaine has been shown to result in a functional upregulation of group II mGluRs in the extended amygdala and hippocampus in rats (Hao et al., 2010) and the density of group II mGluR in the striatum of non-human primates following chronic cocaine self-administration (Beveridge et al., 2011). If these effects also occur following METH, these data suggest that LY379268 may have different effects depending on the history of METH exposure. The finding that LY379268 was equally effective in attenuating the reinforcing effects of METH, regardless of whether the animals had undergone extended access, suggests that either METH-exposed rats are more sensitive to the effects of group II mGluRs compared with medications that targets other receptors, or that METH acts more quickly than other drugs of abuse in dysregulating glutamatergic circuitry within the brain.
One of the important findings of this study was that LY379268 failed to significantly affect PR responding reinforced by sucrose pellets at the same doses that affected METH responding, suggesting that the effect was specific for METH and that a general suppression of motor activity (Cartmell et al., 2000) or generalized reward responding had not occurred. In contrast, Kufahl et al. (2013) reported a significant attenuation of cue-elicited reinstatement of sucrose-seeking following 1 mg/kg LY379268 and Peters and Kalivas (2006) reported a similar effect following 3 mg/kg. As stated previously, these paradigms measure different aspects of drug-taking and seeking, as compared to the PR schedule. However, they certainly indicate the LY379268 is capable of affecting sucrose-reinforced behavior at higher doses and warrant caution in drawing conclusions concerning its potential efficacy in treating human drug addicts. Despite this, our findings, and those of others, clearly demonstrate a central role for group II mGluRs in drug reinforcement and suggest that they remain a promising target for the development of pharmacotherapies for METH addiction.
Acknowledgments
This work was supported by P50 DA06634, DA14030 (DCSR) and DA26590 (TJRB) from the National Institute on Drug Abuse. The authors wish to thank Hilary Smith for her help in the preparation of this manuscript.
Role of funding source: Funding for this study was provided by the National Institute of Drug Abuse (P50 DA06634, DA14030 (DCSR) and DA26590 (TJRB)). NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors: Authors Beveridge and Roberts designed the study and undertook the statistical analysis analyzed the results. Author Crawford performed the experiments and helped author Beveridge write the manuscript. All authors contributed to and have read the final manuscript.
Conflict of Interest: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group II metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology. 2005;180:473–490. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-R) APA; Washington, DC: 2000. [Google Scholar]
- Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Group II metabotropic glutamate receptors in the striatum of non-human primates: dysregulation following chronic cocaine self-administration. Neurosci Lett. 2011;496:15–19. doi: 10.1016/j.neulet.2011.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan KA, Colussi-Mas J, Carati C, Lea RA, Fitzmaurice PS, Schenk S. Methamphetamine self-administration and the effect of contingency on monoamine and metabolite tissue levels in the rat. Brain Res. 2010;1317:137–146. doi: 10.1016/j.brainres.2009.11.069. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. Tolerance to the motor impairment, but not to the reversal of PCP-induced motor activities by oral administration of the mGlu2/3 receptor agonist, LY379268. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:39–46. doi: 10.1007/s002109900151. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–1367. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Lääne K, Theobald DE, Peña Y, Bruce CC, Huszar AC, Wojcieszek M, Everitt BJ, Robbins TW. Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacology. 2007;32:1195–1206. doi: 10.1038/sj.npp.1301220. [DOI] [PubMed] [Google Scholar]
- Dietrich D, Kral T, Clusmann H, Friedl M, Schramm J. Presynaptic group II metabotropic glutamate receptors reduce stimulated and spontaneous transmitter release in human dentate gyrus. Neuropharmacology. 2002;42:297–305. doi: 10.1016/s0028-3908(01)00193-9. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Carmolli M, Cumbay M, Martens CR, Neve KA, Janowsky A. Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J Pharmacol Exp Ther. 1999;289:877–885. [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Castro F, Barrington EH, Walton MA, Rawson RA. Cocaine and methamphetamine: differential addiction rates. Psychol Addict Behav. 2000;14:390–396. [PubMed] [Google Scholar]
- Graves SM, Napier TC. Mirtazapine alters cue-associated methamphetamine seeking in rats. Biol Psychiatry. 2011;69:275–281. doi: 10.1016/j.biopsych.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley AE, Kiefer SW, Li X, Gaal J, Xi ZX, Gardner EL. Dopamine D(3) receptor antagonist SB-277011A inhibits methamphetamine self-administration and methamphetamine-induced reinstatement of drug-seeking in rats. Eur J Pharmacol. 2011;659:187–192. doi: 10.1016/j.ejphar.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–416. [PubMed] [Google Scholar]
- Karasawa J, Yoshimizu T, Chaki S. A metabotropic glutamate 2/3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell. Neurosci Lett. 2006;393:127–130. doi: 10.1016/j.neulet.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Kim JH, Austin JD, Tanabe L, Creekmore E, Vezina P. Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci. 2005;21:295–300. doi: 10.1111/j.1460-9568.2004.03822.x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Vezina P. The mGlu2/3 receptor agonist LY379268 blocks the expression of locomotor sensitization by amphetamine. Pharmacol Biochem Behav. 2002;73:333–337. doi: 10.1016/s0091-3057(02)00827-4. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C, Zautra N, Olive MF. Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology. 2013;66:290–301. doi: 10.1016/j.neuropharm.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology (Berl) 2005;179:644–651. doi: 10.1007/s00213-004-2089-y. [DOI] [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AC, Horton DB, Neugebauer NM, Wooters TE, Nickell JR, Dwoskin LP, Bardo MT. Tetrabenazine inhibition of monoamine uptake and methamphetamine behavioral effects: locomotor activity, drug discrimination and self-administration. Neuropharmacology. 2011;61:849–856. doi: 10.1016/j.neuropharm.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, Harkness AR, Grutsch JL, Jr, Wright RA, Johnson BG, Andis SL, Kingston A, Tomlinson R, Lewis R, Griffey KR, Tizzano JP, Schoepp DD. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Nicosia N, Liccardo Pacula R, Koilmer B, Lundberg R, Chiesa J. The Economic Cost of Methamphetamine Use in the United States. RAND (Drug Policy Research Center); 2005. [Google Scholar]
- Orio L, Wee S, Newman AH, Pulvirenti L, Koob GF. The dopamine D3 receptor partial agonist CJB090 and antagonist PG01037 decrease progressive ratio responding for methamphetamine in rats with extended-access. Addict Biol. 2010;15:312–323. doi: 10.1111/j.1369-1600.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology. 2012;223:371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA (Substance Abuse and Mental Health Services Administration) Drug Abuse Warning Network, 2006: National Estimates of Drug-Related Emergency Department Visits. Rockville, MD: 2008. [Google Scholar]
- SAMSHA. United States Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Office of Applied Studies. National Survey on Drug Use and Health; Ann Arbor, MI: 2008. [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331:555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Li B, Fumushima S, Fukui A, Arime Y, Kasahara Y, Tomita H, Ikeda K. Monoamine transporter as a target molecule for psychostimulants. Int Rev Neurobiol. 2009;85:29–33. doi: 10.1016/S0074-7742(09)85003-4. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu Y, Yamamoto H, Ogai Y, Hagino Y, Markou A, Ikeda K. Fluoxetine as a potential pharmacotherapy for methamphetamine dependence: studies in mice. Ann N Y Acad Sci. 2006;1074:295–302. doi: 10.1196/annals.1369.026. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF. Effect of aripiprazole, a partial dopamine D2 receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacology. 2007;32:2238–2247. doi: 10.1038/sj.npp.1301353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]


