Summary
Despite the importance of the discharge frequency in neuronal communication, little is known about the firing rate patterns of cortical populations. Using large-scale recordings from multiple layers of the entorhinal-hippocampal loop, we found that firing rates of principal neurons showed a lognormal-like distribution in all brain states. Mean and peak rates within place fields of hippocampal neurons were also strongly skewed. Importantly, firing rates of the same neurons showed reliable correlations in different brain states, testing situations and across familiar and novel environments. The fraction of neurons participating in population oscillations displayed a lognormal pattern. Such skewed rates of individual neurons may be brought about by skewed distribution of synaptic weights, supported by our observation of a lognormal distribution of the efficacy of spike transfer from principal neurons to interneurons. The persistent skewed distribution of firing rates implies that a preconfigured highly active minority dominates information transmission in cortical networks.
Introduction
The dominant communication across neurons occurs via spikes. Yet, despite the central role of spiking activity in transmitting information, only limited data is available about the firing rates of unbiased neuronal populations in intact networks (Hromadka et al., 2008; O'Connor et al., 2010). It is generally assumed that cortical principal cells fire sparsely, involving only a small percentage of spiking neurons in most situations while the majority of the population remains silent (Levy and Baxter, 1996; Wolfe et al., 2010). The term ‘sparse coding’ refers to a model in which a small fraction of neurons is engaged in any situation, as opposed to a dense population code in which firing rate fluctuations of individual members represents the input (Olhausen and Field, 1997). In this postulated high signal-to-noise ratio scheme, slow firing neurons contribute largely unwanted noise, viewed as an inevitable consequence of brain organization (Lisman, 1997; Shadlen and Newsome, 1998) rather than information. One possible solution for sparse coding is that a small fraction of cortical neurons is active under diverse circumstances but the active subset varies flexibly across brain states and situations. Alternatively, a largely heterogeneous group of neurons in each cortical layer may form a ‘skeleton’ network in which firing rates are largely determined by the intrinsic properties of individual neurons and/or their distinct wiring. Under the latter scenario, largely the same fraction of cells would be active under all conditions and the information carried by their spikes can be represented by the deviation of their firing rate or timing from an overall default pattern. Whether cortical networks deploy an egalitarian or an inequitable solution can be learned only from a quantitative knowledge of the firing rate distributions of unbiased populations in different conditions (Barth and Poulet, 2012).
Firing patterns in the hippocampus and entorhinal cortex (EC) are affected by a variety of factors. Place cells of the hippocampus fire clusters of spikes in their typically singular place fields (O'Keefe and Nadel, 1978), while grid cells in the EC have multiple fields that form a periodic triangular array, or grid (Hafting et al., 2005). However, little is known about the mechanisms that control the firing rate distribution in the population. A main reason for this caveat is that definitions of the various spatial and non-spatial features are typically based on an experimenter-determined threshold and, as a consequence, neurons with low rates are often excluded because, viewed one by one, they typically do not yield statistically reliable behavioral correlations (Krupic et al., 2012). Thus, it has remained unclear whether the ‘representative’ examples reflect the mean behavior of a homogeneous neuronal population or taken from the high end of a heterogeneous distribution. Addressing this question is essential for understanding the nature of communication across neuron populations (Hromadka et al., 2008; Barth and Poulet, 2012; Song et al., 2005; Ikegaya et al., 2012; Yassin et al., 2010). Using a large database of physiologically characterized neurons (Mizuseki et al., 2009; Mizuseki et al., 2011; Mizuseki et al., 2012; Pastalkova et al., 2008; Diba et al., 2008), we examined the firing rate distributions of principal cells and putative interneurons in the main layers of the entorhinal cortex and hippocampal subregions in the waking and sleeping rat. The analyses revealed a strongly skewed distribution of neuronal firing rates in all subregions that remained largely similar across different brain states and testing environments.
Results
Analyses have been performed on a database of 7,327 neurons in the entorhinal cortex (EC) and hippocampus of 11 rats. Local field potentials (LFP) and unit firing were recorded by multiple-shank silicon probes (Mizuseki et al., 2009; Fujisawa et al., 2008) from the hippocampal CA1 and CA3 pyramidal layers and dentate gyrus. In 4 animals, recordings were made simultaneously in CA1 and in multiple layers of the medial EC. Histological localization of the electrodes, criteria for clustering of single units and separation of principal neurons and interneurons in these animals have been described in detail previously (Mizuseki et al., 2009; Diba et al., 2008). Recordings were carried out while the animal ran on various maze configurations (Mizuseki et al., 2009; Mizuseki et al., 2011; Mizuseki et al., 2012; Pastalkova et al., 2008; Diba et al., 2008) and theta periods from all maze behaviors were lumped together as RUN. Periods without theta were concatenated as immobility or consummatory behaviors (IMM). Additional recordings were carried out during sleep, including several epochs of REM sleep and slow wave sleep (SWS) in the animal’s home cage (Mizuseki et al., 2011; Mizuseki et al., 2012).
Long-tail, skewed distribution of firing rates of principal cells and interneurons
Both hippocampal and EC neurons showed characteristic population firing patterns during different brain states (Figure 1A; Csicsvari et al., 1999) but the directions of rate changes were different in the two regions. While the overall rates of hippocampal principal neurons were lowest during REM sleep, EC neurons fired maximally during REM (Figure S1). Comparison of individual principal neurons revealed a three orders of magnitude range of the mean firing rates from 0.001 Hz to 10 Hz (Figure 1). The firing rate distribution of CA1 pyramidal cells strongly deviated from Gaussian and showed an excellent fit to lognormal pattern during SWS. The distributions of log firing rate during other brain states were asymmetric with long and heavy tails toward lower frequencies (Figure 1B). The right tail also extended significantly toward higher rates during RUN (proportion of >2 Hz neurons is largest during RUN; RUN = 12.3%, REM = 6.0 %, IMM = 7.1%, SWS = 5.7%; chi-square test, P < 0.00001).
Figure 1. Lognormal firing rate distribution of principal cells.
(A) LFP and spiking activity of CA1 and EC neurons. Dots above the CA1 LFP during SWS represent ripple oscillations. Note trains of spikes followed by long silence periods in the waking rat and strong population synchrony during ripples. Colored ticks, principal neurons. Gray and black ticks (i), interneurons.
(B) Distribution of firing rates of individual CA1 pyramidal cells in different brain states. Note log × axis. Distribution during RUN extends to both left and right relative to SWS. Dots, data; Lines, lognormal fit.
(C) Lorenz plots of the distribution of firing rates. Inset: Illustration of the Gini coefficient. Gini coefficient is determined by dividing A (the area between the line of equity and the Lorenz curve) by the areas marked with A and B.
(D) Gini coefficients in different hippocampal regions and EC layers in different brain states (mean ± S.E.M). Brackets indicate significant differences (p<0.05; ANOVA, followed by Tukey’s test).
(B) to (D) Same color codes for brain states are used.
See also Figure S1.
Firing rate inequality among individual neurons can be quantitatively described by Lorenz statistics (O'Connor et al., 2010; Ikegaya et al., 2012). The Lorenz curve of the firing rate distribution characterizes the cumulative spike share of individual neurons of the population (Figure 1C). In this display, the diagonal (x = y) indicates that all neurons have the same firing rate. The magnitude of the deviation from equality is quantified by the Gini coefficient (Figure 1C, inset); the higher the coefficient, the more unequal the share of number of spikes (Ikegaya et al., 2012). For example, during RUN, 70.4 percent of the recorded CA1 pyramidal cells had a mean rate <1 Hz, whereas a small fraction of strongly active pyramidal cells (13.3%) contributed to 50% of all spikes. Remarkably, the brain state dependence of firing rate inequality was similar for the principal cells in all regions of the hippocampus and all layers of the EC, namely Gini coefficients were higher during awake (RUN and IMM) than during sleep (REM and SWS) (Figure 1D). The firing rate distributions in all regions and layers across behaviors are shown in Figure S1. In summary, firing rates of hippocampal and EC principal neurons displayed strongly skewed firing rate distributions, with the widest frequency range of individual neurons in the waking animal.
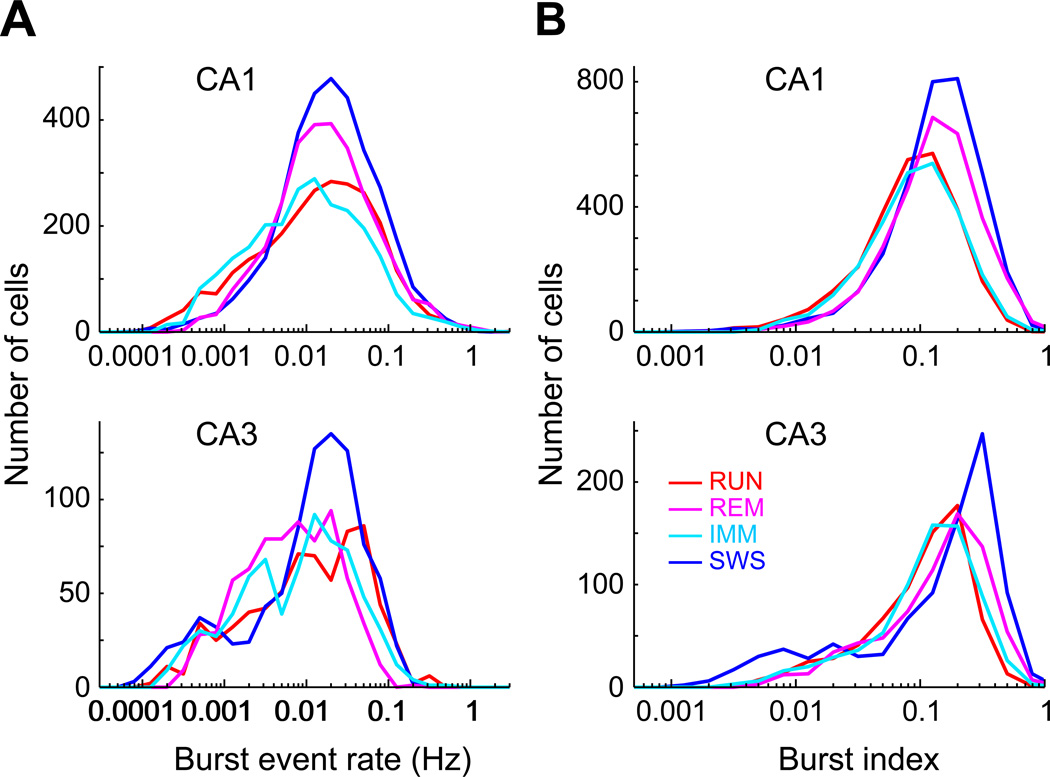
Skewed distribution of spike bursts of principal cells
In addition to single spikes, pyramidal neurons also fire complex spike bursts. It has been suggested that generation of bursts is enhanced under special conditions (Harris et al., 2001), and that bursts play a distinct role in plasticity (Magee and Johnston, 1997; Thomas et al., 1998; Pike et al., 1999, Harris et al., 2001) and computation (Lisman, 1997; Thomson, 2000), therefore revealing burst event rate of unbiased neuronal populations is essential for understanding their role in circuit operations. Burst is defined here as a series of three or more spikes with less than 8 ms interspike intervals (Harris et al., 2001; Mizuseki et al., 2012). Burst event rate of individual neurons also showed a lognormal-like distribution (Figure 2A), as did the distribution of burst index, defined as a fraction of spikes within bursts to all spikes (Mizuseki at al., 2012, Figure 2B). The burst index was highest during SWS for all EC and hippocampal principal cells (Figure S2A; P < 0.05 for all brain regions but the DG; Kruskal-Wallis ANOVA, followed by Tukey’s honestly significant difference test). A small fraction of CA1 (RUN = 0.4%, REM = 3.1%, IMM = 0.5%, SWS = 2.9%) and CA3 (RUN = 0.8%, REM = 2.1%, IMM = 0.9%, SWS = 4.1%) pyramidal cells were ‘super-bursters’ since >50% of the spikes they emitted were classified as bursts. The burst index was weakly but significantly correlated with firing rate (P < 0.011; Figure S2 B and C). Essentially identical results were obtained using <6 ms or <10 ms interspike intervals for the identification of burst events.
Figure 2. Spike bursts of principal cells in different brain states.
(A) Distribution of burst event rate of individual CA1 and CA3 pyramidal cells in different brain states (3 spikes or more at ≤8 ms intervals).
(B) Distribution of burst index (number of spikes in bursts divided by number of all spikes) of individual CA1 and CA3 pyramidal cells in different brain states.
See also Figure S2.
Skewed distribution of hippocampal place cell firing
The spontaneous and evoked firing patterns of cortical neurons are often dramatically different, although these respective patterns may be related to each other. Since place-related firing can be conceived of as a specific, environment-driven activity (O'Keefe and Nadel, 1978), we examined place-related activity of pyramidal cells at increasing levels of criteria. Place fields on the open field were first defined as a continuous region of at least 225 cm2 (9 bins), in which the firing rate was above 10% (or 20%) of the peak firing rate. Restricted subsets of these groups included only place fields with spatial coherence (Muller and Kubie, 1989) greater than 0.7 (Hafting et al., 2008; Mizuseki et al., 2009). The spatial coherence of each firing field was defined as the correlation of the firing rate in each bin of the firing field with the firing rate in its neighboring bins to measure local smoothness of firing rate in space (Muller and Kubie, 1989). These two steps of restrictions created four subgroups of place cells with the highest (20% and 0.7 spatial coherence) to lowest (10% and no spatial coherence restriction) quality of place fields. Irrespective how we defined place fields, mean within-field firing rates of CA1 and CA3 pyramidal cells showed skewed distribution (Figures 3A and S3). Only 4.6% of CA1 pyramidal cells (8.4% CA3) sustained ≥10 Hz within-field rates, whereas the majority (89.6% CA1; 71.4% CA3) fired below the mean theta frequency (~8 Hz). Within-place field peak firing rates also displayed a skewed rate distribution (Figures 3B and S3). Within-field and peak rates were strongly correlated (CA1, R = 0.85; CA3, R = 0.78; Ps < 0.00001) as were the within-field versus session mean (CA1, R = 0.66; CA3, R = 0.50; Ps < 0.00001) and peak rate versus session mean (CA1, R = 0.66; CA3, R = 0.48; Ps < 0.00001) rates. Skewed distribution of firing rate is, therefore, a fundamental feature of hippocampal neurons.
Figure 3. Distribution of firing rates in place cells.
(A) Distribution of mean within-field firing rates of CA1 and CA3 pyramidal cells on the square maze.
(B) Distribution of peak firing rates in field.
R’s in panels, correlation coefficients between session (RUN) firing rate and mean within-field rate or peak rate. Place fields were defined by >10% peak firing rate and spatial coherence > 0.7.
See also Figure S3.
Since firing rates may be related to several features of place cells, our next analysis examined the relationship between log rate and place features on the square maze. Cell firing stability, defined as the pixel-by-pixel correlation of firing rates in the first and second parts of a session, showed significant and sigmoid-like relationship with log firing rate (Figure 4A; CA1 = 0.62; CA3, R = 0.75, Ps < 0.00001). Similarly, spatial coherence of firing also displayed a sigmoid-like relationship with log firing rate (Figure 4B; CA1, R = 0.71; CA3, R = 0.82; Ps < 0.00001). Another frequently used index, ‘information rate’ (bits per second) of spiking activity (Skaggs et al., 1993), a quantity that defines the relationship between spikes and the rat’s position on the maze, was also strongly correlated with log firing rate (Figure 4C; CA1, R = 0.73; CA3, R = 0.76; Ps < 0.00001). Figure 4 contains both place cells (using the 10% and 0.7 spatial coherence definition; black circles) and other neurons (gray circles). As expected, very few neurons with low firing rates met the criteria of place cell definition. On the other hand, not all fast neurons were classified as place cells either. The correlation between log rate of place cells and information rate was also significant (CA1, R = 0.77; CA3, R = 0.76; Ps < 0.00001). The correlation between log rate of bona fide place cells and stability of CA1 place cells was weaker but still significant (r = 0.16, P < 0.001).
Figure 4. Firing rates are correlated with spike behavioral measures.
(A) Relationship between log firing rate and stability index (pixel-by-pixel correlation of rate between the first and second half of a session in the square maze).
(B) Relationship between log firing rate and spatial coherence.
(C) Relationship between log firing rate and information rate.
Black circles, place cells (10%, 0.7 coherence criteria). Gray circles, other neurons. Color lines indicate median values for original and downsampled rates. Only medians are shown for downsampled rates.
See also Figure S4.
Fast firing neurons may acquire their advantage for spatial representation simply from their high rates. To test this possibility, we downsampled neuronal rates so that the reduced rate was equal to 0.05 Hz to 1 Hz (Figure 4). Re-computing the analyses described above with the downsampled rates dramatically reduced stability, spatial coherence and information rate measures to or below the levels of the neurons which naturally fired at such frequencies (Figure 4). The magnitude of the loss from downsampling was proportional to the original frequency. Thus, fast discharging neurons gain their efficacy from the high frequency of spikes they emit.
Firing rate was also correlated with the number of place fields (CA1, R = 0.22; CA3, R = 0.39; Ps < 0.00001), place field size (CA1, R = 0.41; P < 0.00001; CA3, R = 0.25; P < 0.001) and phase precession (O'Keefe and Recce, 1993) slope (CA1, R = 0.11; P < 0.05; Figure S4), adding further support for the computational advantage of high rates of activity.
Skewed distribution of population synchrony
Another major network pattern in the EC-hippocampus is the self-organized sharp wave ripple (SPW-R; 140–180 Hz; Buzsaki et al., 1992; Csicsvari et al., 2000). We used 3 indexes to examine the SPW-R-related firing patterns for each neuron during SWS or IMM. First, the ‘proportion of spikes during SPW-Rs’, defined as the number of spikes during SPW-Rs divided by the number of all the spikes in that session. The second is the ‘proportion of SPWs in which neuron fired’, calculated as the fraction of SPW-Rs in which the neuron fired at least once. The third index is ‘mean number of spikes per SPW-Rs’. The number of spikes within SPW-Rs was divided by the total number of SPW-Rs in that session. Each of these indexes showed a lognormal-like distribution during both IMM and SWS (Figure S5). A small fraction (SWS = 1.4 %, IMM = 1.5 %) of pyramidal cells dominated by participating in 50% of SPW-R events, whereas half of all neurons fired in <10.6 % (SWS; IMM < 9.1 %) of SPW-Rs. The proportion of spikes during SPW-Rs was negatively correlated with the overall spike rate (SWS R = −0.12; IMM R = −0.56; Ps < 0.00001), indicating that slowly firing neurons emitted action potentials dominantly during SPW-R events when the overall excitability of the EC-hippocampal networks is high (Csicsvari et al., 2000). The proportion of SPWs in which neuron fired and the mean number of spikes per SPW-Rs were positively correlated with firing rates in both pyramidal cells and interneurons (Rs > 0.70; Ps < 0.00001). These results were essentially the same when the threshold for SPW-R selection was decreased from of >7 standard deviation (SD) amplitude to >6, >5, >4 or >3 SD above the mean.
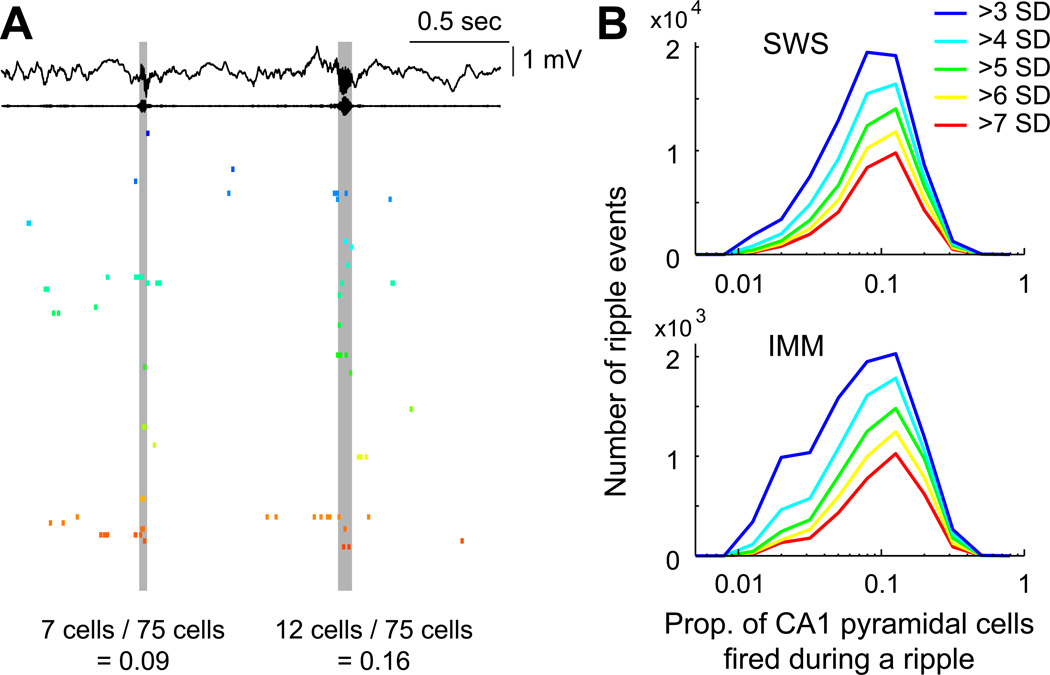
Since SPW-Rs are self-organized events, they provided us the opportunity to examine how neurons with lognormal-like firing rate statistics interact with each other at the population level. To this end, the fraction of principal neurons firing at least one spike in each SPW-Rs (Figure 5) was computed, using 5 different groups of SPW-R magnitude (thresholds of >7, >6, >5, >4 or >3 SD above the mean). Only sessions with ≥50 simultaneously recorded CA1 pyramidal cells were included in these analyses (15 sessions from 5 animals). Independent of the applied threshold, the magnitude of SPW-R synchrony displayed a skewed distribution during both SWS and IMM (Figure 5B). Population synchrony of neurons was strongly correlated with the peak power (for >3 SD events, SWS R = 0.45, IMM R = 0.49; Ps < 0.00001) or the mean power of the SPW-Rs (SWS R = 0.41, IMM R = 0.49; Ps < 0.00001).
Figure 5. Skewed distribution of the magnitude of population synchrony during ripples.
(A) Wide-band and ripple band (140–230 Hz) filtered LFP (top) and spiking activity of simultaneously recorded 75 CA1 pyramidal cells. Two ripple events with relatively low (0.09) and high (0.16) fraction of neurons firing synchronously during ripple.
(B) Distribution of synchrony of CA1 pyramidal cells’ firing during ripples using various ripple detection thresholds (from small, >3 SD to large, >7 SD) during SWS and IMM.
See also Figure S5.
To examine the distribution of population synchrony outside SPW-Rs, we have plotted the proportion of principal neurons of CA1 and CA3 pyramidal cells which fired together in 10, 20, 50, 100 or 200 msec time bins. Similar to the analysis of synchrony during SPW-R events, the magnitude of population synchrony showed lognormal-like distribution in all brain states (data not shown). These findings demonstrate that the magnitude of neuronal synchrony during self-organized SPW-R and theta oscillations also follows lognormal statistics.
Firing rate correlations across brain states and testing conditions
The small fraction of highly active neurons can vary from state to state or the rate distribution may reflect a relative ‘fixed’ property of neurons (Barth and Poulet, 2012). To differentiate between these possibilities, we first compared the firing rates across different brain states. Firing rates of individual principal cells were robustly correlated between SWS and REM (Figure 6A; RSWS-REM, CA1 = 0.78, CA3 = 0.82, DG = 0.67, EC2 = 0.89, EC3 = 0.87, EC5 = 0.67 ; Ps < 0.00001, t-test; Csicsvari et al., 1999; Hirase et al., 2001) as well as across other states in all regions (RSWS-RUN, CA1 = 0.57; CA3 = 0.65, DG = 0.74, EC2 = 0.23, EC3 = 0.28, EC5 = 0.33; RSWS-IMM, CA1 = 0.58, CA3 = 0.66, DG = 0.82, EC2 = 0.23, EC3 = 0.33, EC5 = 0.37,; RREM-RUN, CA1 = 0.49, CA3 = 0.60, DG = 0.82, EC2 = 0.30, EC3 = 0.37, EC5 = 0.32; RREM-IMM, CA1 = 0.50, CA3 = 0.57, DG = 0.82, EC2 = 0.26, EC3 = 0.39, EC5 = 0.31, RRUN-IMM, CA1 = 0.90, CA3 = 0.89, DG = 0.88, EC2 = 0.94, EC3 = 0.94, EC5 = 0.89; Ps < 0.00001). In a second comparison, we tested rate changes of CA1 and CA3 pyramidal cells while the animal successively performed in two different familiar mazes in the same room (Figures 6B and S6; n = 6 rats; ‘rate remapping’; Leutgeb et al., 2005) or running in opposite directions on a linear-track maze (Figures 6D and S6, ‘global remapping’; Leutgeb et al., 2005). Again, we found that log firing rates of the neurons across the different testing situations were robustly conserved (CA1, RMaze A-Maze B = 0.65; CA3, RMaze A-Maze B = 0.59, Ps < 0.00001) and between two directions of linear maze runs (CA1, RLeft-Right = 0.62; CA3, RLeft-Right = 0.51, Ps < 0.00001). Even the correlation between linear firing rates was significant (CA1, RMaze A-Maze B = 0.59, CA3, RMaze A-Maze B = 0.26, Ps < 0.00001; CA1, RLeft-Right = 0.48, CA3, RLeft-Right = 0.20, Ps < 0.00001). In addition to single spikes, the burst index of neurons was also preserved across two different mazes (Figure 6C; CA1, RMaze A-Maze B = 0.63; CA3, RMaze A-Maze B = 0.57; Ps < 0.00001). Finally, we compared log firing rates between familiar and novel environments. Log firing rates during RUN in familiar and novel mazes were significantly correlated (Figure 6F; CA1, Rfamiliar-novel = 0.68, P < 0.00001; linear rate Rfamiliar-novel = 0.32, P < 0.002, 4 sessions). Comparison between RUN and SWS both before and after the behavioral performance in the familiar maze (Figure 6E) also yielded significant log rate correlations (CA1, RSWS before-RUN = 0.55, RSWS after-RUN = 0.53; CA3, RSWS before-RUN = 0.59, RSWS after-RUN = 0.61; Ps < 0.00001). Similarly, log firing rates were highly correlated between RUN and pre-RUN SWS or post-RUN SWS when exploration took place in a novel environment (Figure 6G, 10 sessions from 4 animals, CA1 RSWS before-RUN = 0.46, RSWS after-RUN = 0.52, Ps < 0.00001; CA3 RSWS before-RUN = 0.59, RSWS after-RUN = 0.65; Ps < 0.0005). The largest variability across brain states occurred in the slow firing population, since many of the slow firing neurons during SWS became silent during RUN (Thompson and Best, 1989). The significant rate correlations across states, testing environments and especially between SWS before the novel experience and exploration of novel environment imply that firing rate of individual neuron is relatively “fixed”.
Figure 6. Preserved firing rates of principal neurons across brain states and distinct environments.
(A) Comparison of firing rates of the same neurons during SWS and REM in different hippocampal regions and EC layers.
(B) Comparison of firing rates of the same neurons in different mazes.
(C) Comparison of burst index in different mazes.
(D) Comparison of firing rates during left and right runs on the linear track (‘global remapping’).
(E) Firing rate comparison between RUN in familiar maze and SWS in the home cage either before or after the maze session.
(F) Comparison of firing rate of the same neurons in familiar and novel mazes.
(G) Comparison between firing rates during exploration of a novel maze (RUN) and SWS in the home cage either before or after the maze session.
(A) to (G), each dot represents a single principal neuron. R-values are correlation coefficients of log firing rates. All correlations were significant (p < 0.00001).
See also Figure S6
Spike transmission between pyramidal cells and interneurons
Several computational models suggest that the lognormal distribution of synaptic strengths observed between neurons in vitro (Song et al., 2005) should give rise to skewed distributions of firing rates in vivo (Ikegaya et al., 2012; Koulakov et al., 2009; Roxin et al., 2011). Unfortunately, to date, no method exists for the direct measurement of synaptic strengths in the behaving animal. An indirect estimation is quantifying the spike transmission probability between neuron pairs under the assumption that the magnitude of spike transmission, as measured in short time spike cross-correlations, is proportional to the synaptic weight between them (Figure 7A; Experimental Procedures; Mizuseki et al., 2009; Fujisawa et al., 2008; Marshall et al., 2002; Bartho et al., 2004; Maurer et al., 2006; Dupret et al., 2013). Of the 32,406 principal neuron-interneuron cell pairs examined, 2,163 showed significant peaks within < 4 ms (CA1 = 1,183 of 17,234; CA 3 = 368 of 6,412; EC = 612 of 8,760 cell pairs). The magnitude of the peak (i.e., the excess numbers of postsynaptic spikes divided by the number of presynaptic spikes, reflecting the efficacy of spike transmission probability) of the cross-correlogram was taken as a proxy for the synaptic strength between the neurons (Figure 7A).
Figure 7. Spike transmission probability distributions in different brain states.
(A) Monosynaptic drive of a putative interneuron by a pyramidal cell. Left: Superimposed filtered waveforms (800 Hz – 5 kHz) of a pyramidal cell (pyr) and an interneuron (int) triggered by a spiking of pyramidal cell. The two neurons were recorded from different silicon probe shanks. Right: Three example cross-correlograms showing short-latency, putative monosynaptic interactions between pyramidal-interneuron pairs (recorded from two different electrodes). The first example corresponds to the left filtered waveforms. Dashed red lines indicate 0.1% and 99.9% global confidence intervals estimated by spike jittering on a uniform interval of [±5,5] ms (Fujisawa et al., 2008), blue, mean. Note different magnitude probability scales. Bottom row: shuffling corrected histograms of the same neuron pairs.
(B) Distribution of spike transmission probability values (note log scale) between CA1 pyramidal cells and putative interneurons in different brain states. Circled numbers indicate the probability values shown in panel A.
(C) Comparison of spike transmission probability between RUN and SWS. Note larger values during RUN. Each dot represents single cell pair.
(D) Spike transmission probability between principal cells and putative interneurons in the CA1, CA3 regions and entorhinal cortex (neuron pairs from EC layers were combined) in different brain states. Median, lower and upper quartiles are shown.
Brackets indicate significant differences (P < 0.05, Kruskal-Wallis ANOVA, followed by Tukey’s honestly significant difference test).
See also Figure S7.
Spike transmission efficacy showed a large variability across pairs, with the majority of pairs weakly coupled and a small minority strongly coupled, as quantified by the lognormal-like distribution of efficacy values (Figures 7B and S7A). Spike transmission efficacy was brain state-dependent with strongest efficacy during RUN and REM and weakest during SWS in CA1 (Figures 7C and D, S7B, P < 0.05, Kruskal-Wallis ANOVA, followed by Tukey’s honestly significant difference test). Overall, these findings support the hypothesis that synaptic weight distribution is strongly skewed in the cortex (Song et al., 2005; Sayer et al., 1990; Mitra et al., 2012).
Discussion
We have found that the distribution of firing rates, burst discharges and spike transmission probability between principal cells and interneurons have an approximately lognormal distribution in the EC and hippocampus. In all regions and layers examined, firing rate distribution was skewed in all brain states. Firing rates and bursts were correlated across brain states, between familiar environments and even between familiar and novel environments. The magnitude of population synchrony during ripple events, reflecting the network-level cooperation of many individual neurons, was also strongly skewed. These findings suggest that a preconfigured skewed firing rate distribution of the population is a robust and important aspect of cortical computation.
A preserved minority of neurons is active in all brain states and environments
Hippocampal neurons in different environments typically ‘re-map’, that is, the firing rate population vectors of each situations are unique (Leutgeb et al., 2005; Muller and Kubie, 1987; Samsonovich and McNaughton, 1997). Against this background, the highly conserved firing rates across brain states and testing situations are surprising but not exclusive. Our findings thus indicate that such environment-induced changes are superimposed on relatively stable discharge rates of individual neurons.
An unexpected finding of our experiments is that a minority of highly active and bursting neurons is responsible for nearly half of the spikes in any time window. This highly active subgroup may be responsible for a reliable propagation of activity in multiple layers of the feed-forward EC-hippocampal network (Mizuseki et al., 2009). Equally important is our observation that the remaining half of the action potentials in a given time window is contributed by a very large fraction of slow-discharging neurons. The activity of this majority may be critical for optimal performance. In addition, the slowly firing majority may serve to provide excitation to interneurons and thereby secure a sufficient level of inhibition to counter the effects of the fast firing minority. Such balance mechanism may be essential to maintain a self-organized, sustained activity in large-scale networks. In support of this hypothesis, skewed distribution of synaptic weights has been shown to be critical in stabilizing neuronal circuits in tissue cultures (Mitra et al., 2012), and elimination of either a large fraction of weakly coupled and slow firing neurons or a minority very active cells was sufficient to abort ongoing network activity in computer models (Ikegaya et al., 2012; Izhikevich et al., 2004). In the present experiments, brain state and environmental influences affected mostly slow firing neurons, in line with previous observations (Dragoi et al., 2003). Sleep may be a critical state to differentially affect slow firing neurons and weak synaptic connections, while preserving the strongly firing minority ‘core’ (Tononi and Cirelli, 2006; Grosmark et al., 2012). It remains for future research to identify the conditions which may convert slowly discharging neurons to highly active ones and vice versa (Hirase et al., 2001; Mankin et al., 2012; Ziv et al., 2013).
Skewed distribution of spike transmission probability
How does lognormal pattern of firing rates arise? A suggested possibility is that many highly efficient synapses converge on a minority of cells (Barth and Poulet, 2012; Koulakov et al., 2009; Loewenstein et al., 2011). Indeed, the distribution of synaptic strengths between cortical cells is strongly skewed (Song et al., 2005; Ikegaya et al., 2012; Sayer et al., 1990; Feldmeyer et al., 2002; Arellano et al., 2007). Such large variation of synaptic efficacy can be structurally related to the lognormal distribution of dendritic spine sizes of cortical neurons (Loewenstein et al., 2011). Our findings of the skewed distribution of spike transmission probability between pyramidal cells and interneurons provide further support to this idea. A second possibility is a disproportional distribution of excitatory and inhibitory inputs to neurons (Yassin et al., 2010). Additional source of rate variability may be the skewed distribution of membrane conductances across neurons (Narayanan and Johnston, 2012). The lognormal-like distribution of burst rates supports this latter hypothesis since burst occurrence depends largely on intrinsic properties of neurons (Harris et al., 2001; Jarsky et al., 2008). In light of previous observations (Yassin et al., 2010), our finding suggests that rate distribution of principal neurons may also indicate how neurons are embedded in cortical circuits. Targeted recordings in vivo in fosGFP transgenic mice showed that neurons which had previously expressed c-fos, therefore labeled by GFP, fired faster than non-labeled neurons in layer 2/3 of primary sensory cortex. Importantly, the highly active fosGFP+ neurons were connected to each other more frequently than fosGFP− neurons (Yassin et al., 2010), suggesting that the high firing and strongly bursting minority may form special highly active subnetworks.
Conclusions
The skewed distribution of discharge rate of cortical neurons has important consequences for the interpretation of neuronal interactions. First, lumped models of cortical operations using neurons with ‘representative’ activity cannot adequately describe network functions because there is no physiologically meaningful ‘mean’ or ‘typical’ rate for cortical neurons. Second, hypotheses about sparse and energy efficient coding mechanisms should be supported by recordings from large populations of neurons because mean rates of a small group of cells cannot describe the true population behavior of neurons or predict the mechanisms that bring about the activation of their targets (Mizuseki et al., 2009). Third, comparisons of mean rate distributions across testing conditions should not use statistics that require Gaussian distribution as a precondition. Fourth, high firing rate neurons are important because they carry more information. Reducing spike rates of fast firing neurons lead to a loss of their advantage in spatial coherence, stability and spatial information rate, indicating that the source of their gain is their high frequency firing. The highly active minority and the slowly firing majority may be wired differently or possess highly distinct intrinsic biophysical properties (Barth and Poulet, 2012; Dragoi and Tonegawa, 2011; Lee et al., 2012). An important goal for future research is to explore the physiological mechanisms that underlie the continuum of the lognormal rule of synaptic efficacy and firing rate distributions and identify their gene expression, molecular, morphological and circuit properties.
EXPERIMENTAL PROCEDURES
Animals and surgery
Eleven male Long-Evans rats (250–400 g) were implanted with a 4- or 8-shank silicon probe in the right dorsal hippocampus under isoflurane anesthesia (1–1.5%) and recorded from dorsal CA1 pyramidal layers. In four of the rats, another 4-shank silicon probe was also implanted in the right dorsocaudal medial entorhinal cortex (Mizuseki et al., 2009). The silicon probes were attached to micromanipulators and moved slowly to the target. Each shank had 8 recording sites (160 µm2 each site; 1–3 MΩ impedance) and inter-shank distance was 200 µm. Recordings sites were staggered to provide a two-dimensional arrangement (20 µm vertical separation). The EC probe was positioned so that the different shanks recorded from different layers (Mizuseki et al., 2009). At the end of the physiological recordings, a small anodal DC current (2–5 µA, 10 sec) was applied to recording sites one or two days prior to sacrificing the animals. The rat was deeply anesthetized and perfused with 10% formalin solution. The position of the electrodes was confirmed histologically and reported previously in detail (Mizuseki et al., 2009; Diba and Buzsaki, 2008). Two stainless steel screws inserted above the cerebellum were used as indifferent and ground electrodes during recordings. All protocols were approved by the Institutional Animal Care and Use Committee of Rutgers University and New York University.
Behavioral testing
After recovery from surgery (~1 week), physiological signals were recorded during six different types of active waking behaviors. (A) On the elevated linear track (250 cm × 7 cm) the animal was required to run back and forth for 30 µl water reward on both ends (Mizuseki et al., 2009). (B) In the open field task the rats chased randomly dispersed drops of water or pieces of Froot Loops (~25 mg, Kellogg’s) on an elevated square platform (180 cm × 180 cm, or 120 cm × 120 cm) (Mizuseki et al., 2009). (C) The rewarded wheel-running task and (D) the alternation task in the T-maze (100 cm × 120 cm) with wheel running delay were described previously (Mizuseki et al., 2009; Pastalkova et al., 2008). (E) The task on an elevated plus maze (100 cm × 100 cm) and (F) zigzag maze (100 cm × 200 cm) with 11 corridors were described previously (Mizuseki et al., 2009; Mizuseki et al., 2012). Theta periods from all maze behaviors were lumped together as ‘RUN’. Recordings were also carried out during sleep, typically both before and after tasks, in the animal’s home cage. The maze was regarded as “novel” when the animal performed the task on it for the first time, and “familiar” after at least 3 testing sessions.
Data collection and cell type classification
Detailed information about the recording system and spike sorting has been described (Mizuseki et al., 2009; Diba and Buzsaki, 2008; Csicsvari et al., 1999). Briefly, signals were amplified (1,000×), bandpass-filtered (1 Hz to 5 kHz) and acquired continuously at 20 kHz (DataMax system; RC Electronics) or 32 kHz (NeuraLynx, MT) at 16-bit resolution. After recording, the signals were down-sampled to 1,250 Hz for the local field potential (LFP) analysis. Positive polarity is up in all illustrations. To maximize the detection of very slowly discharging (‘silent’) neurons (Thompson and Best, 1989), clustering was performed on concatenated files of several behavioral and sleep sessions recorded at the same electrode position on the same day. Spike sorting was performed automatically, using KlustaKwik (http://klustakwik.sourceforge.net), followed by manual adjustment of the clusters (Klusters software package, http://klusters.sourceforge.net). After spike sorting, we plotted the spike features of units as a function of time, and the units and sessions with signs of significant drift over the period of recording were discarded. Within the remaining data, only units with clear refractory periods and well-defined cluster boundaries were included in the analyses (Harris et al., 2000; Harris et al., 2001).
Classification of pyramidal cells and interneurons of hippocampal and entorhinal cortical neurons was described previously (Mizuseki et al., 2009). A total of 3541 (CA1), 962 (CA3), 66 (DG), 491 (EC2), 576 (EC3) and 559 (EC5) principal neurons and 468 (CA1), 216 (CA3), 52 (DG), 85 (EC2), 217 (EC3) and 94 (EC5) interneurons were identified and used for analyses. The tip of the probe either moved spontaneously or was moved by the experimenter between recording days. However, we cannot exclude the possibility that some neurons recorded in different days were identical because spikes recorded on each day were clustered separately.
Detection of brain states
Theta periods during task performance (RUN), immobility (IMM), REM epochs (REM) and slow wave sleep (SWS) were detected using the ratio of the power in theta band (6–10 Hz) to delta band (1–4 Hz) of LFP, followed by manual adjustment with the aid of visual inspection of whitened power spectra and the raw traces (Mizuseki et al., 2011; Mizuseki et al., 2012). The manual adjustment was necessary to remove falsely detected short segments of data and epochs containing movement artifacts. REM periods were cross-validated with experimenter notes taken while observing theta activity on-line in sleep session and verifying that the rat was sleeping. The total length of recording was 57.4 ± 36.1 min for RUN, 20.8 ± 13.2 min for REM, 40.0 ± 23.2 min for IMM, 114.7 ± 59.5 min for SWS (mean ± SD).
Spiking activity during sharp wave ripples
To detect ripple events, LFP in CA1 pyramidal layer during non-theta periods was band-pass filtered (140–230Hz), and the power (root mean square) was calculated in 17 ms time windows. A ripple epoch was defined as a period during which ripple power was continuously greater than mean + 3S.D. and peak of power in the period were greater than mean + 7 S.D. Results were similar when we used 5 different thresholds (mean + 7, 6, 5, 4 or 3 S.D. for peak of power and mean + 3, 2.5, 2, 1.8 or1.5 S.D for the start and end of the epochs, respectively). Events shorter than 15 ms were discarded. Mean rates of detected ripple events (number of events per minute) were 16.1 ± 3.4, 20.3 ± 4.1, 25.5 ± 4.8, 32.5 ± 5.6 and 43.8 ± 6.9 during SWS and 4.7 ± 3.5, 6.6 ± 4.2, 9.5 ± 4.9, 14.6 ± 6.2, 24.5 ± 10.9 during IMM (mean + 7, 6, 5, 4 or 3 S.D. for peak power threshold).
To quantify the spiking activity of individual CA1 neurons during SPW-Rs, the following three measurements were used for SWS and IMM separately. First, ‘proportion of spikes during SWP-Rs’ was defined as the number of spikes during SPW-Rs divided by the number of all the spikes. Second, ‘proportion of SPW-Rs in which neuron fired’ was defined as the number of SPW-Rs in which the neuron fired at least once, divided by the total number of SPW-Rs. Third, to measure ‘mean number of spikes per SPW-Rs’, the number of spikes within SPW-Rs was divided by the total number of SPW-Rs.
To quantify the magnitude of CA1 pyramidal neurons synchrony during SPW-Rs, only sessions with > 50 simultaneously recorded CA1pyramidal neurons were used. To calculate the proportion of CA1 pyramidal cells fired at least once during a ripple, the number of neurons fired during a ripple epoch was divided by the number of simultaneously recorded neurons during the session.
To quantify the magnitude of CA1 (or CA3) pyramidal neurons synchrony in each brain state, only sessions with > 40 simultaneously recorded CA1 (or CA3) pyramidal neurons were used. To calculate the proportion of CA1 (or CA3) pyramidal cells fired at least once in 10, 20, 50, 100 or 200 ms bins, the number of neurons fired in each time bin was divided by the number of simultaneously recorded neurons during the session.
Bursts and firing rate
A burst event is defined as a series of three or more spikes with less than 8 ms inter-spike intervals. A burst index was defined as the ratio of spikes in bursts to all spikes. To calculate burst event rate, number of burst events was divided by the recording time for each brain state. To fit the firing rate to lognormal distribution, the maximum likelihood method was used.
Spatial tuning of spiking activity
The data recorded on the open field (180 cm × 180 cm or 120 cm × 120 cm) and linear track (250 cm) were used for the analysis of spatial tuning of spiking activity. Only the data during theta epochs were used. Position of the animal was estimated by recording LEDs on the head stage at 30 Hz. For the linear track, the positions were projected onto the track axis. The position and spiking data were sorted into 5 cm × 5 cm (open field) or 5 cm (linear track) bins, generating the raw maps of spike number and occupancy. A raw rate map was constructed by dividing a raw spike map by a raw occupancy map, and used to compute spatial coherence. Gaussian kernel (S.D. = 5 cm) was applied for both raw maps of spike and occupancy, then a smoothed rate map was constructed by dividing the smoothed spike map by the smoothed occupancy map. Peak and mean firing rate in the place field, number of place fields, stability and spatial information rate were computed from the smoothed rate map.
A place field was defined as a contiguous region of at least 225 cm2 (9 bins) for the open field and 15 cm (3 bins) for the linear track where the firing rate was above 10 % of the peak rate in the maze and the peak firing rate of the area was > 2 Hz, and special coherence of the region was > 0.7. Using a threshold of 20 % of the peak rate and omitting the special coherence criteria gave similar results (Figure S3).
Spatial coherence (Muller and Kubie, 1989) was defined as the correlation between a list of firing rates in each pixel and a corresponding list of firing rates averaged over the adjacent pixels of each pixel, and measures the local smoothness of firing rate in space (Muller and Kubie, 1989; Hafting et al., 2008; Mizuseki et al., 2009) (8 adjacent pixels for the open fields, 2 adjacent pixels for the linear track). Place map stability was defined by the pixel-by-pixel correlation coefficient between the firing rate maps of the first and second halves of the recording session.
For the linear track, spatial representation (rate map, spatial coherence and phase precession) was analyzed for each direction separately. Area at 0–25 cm (starting point) was excluded from the analysis to exclude the effect of behavioral variability. To quantify the degree of phase precession, place fields were identified on the linear track using >2 Hz peak firing rate, >10 % peak firing rate and >0.7 spatial coherence as criteria (Hafting et al., 2008; Mizuseki et al., 2009). Place fields with fewer than 50 spikes and fields that included the turning position of the track were discarded (Hafting et al., 2008; Mizuseki et al., 2009). The theta phases of spikes were displayed as a function of the distance from the start of the place field, and the theta phase-position correlation was determined by parametrically rotating the phase by the position matrix for each place field. Phase rows were shifted by 1° steps from 0° to 360°. For each rotation, a linear regression curve was fitted. The slope of the regression line at the phase rotation that gave the largest explained variance R2 was used as the degree of phase precession (degree per cm). In some cases, this objective and automatic method gives a spurious positive slope value even when visual inspection suggest negative slope (Hafting et al., 2008; Mizuseki et al., 2009).
The spatial information rate (bits per second) (Skaggs et al., 1993) was calculated according to the following formulae:
Where i = 1, … , N represents pixel identification number, pi is the probability of occupancy of pixel i, λi is the mean firing rate of pixel i, λ is the overall mean firing rate of the cell on the maze.
Probability of spike transmission
First, principal cell-interneuron pairs with putative monosynaptic excitation were identified by cross-correlogram analysis (Fujisawa et al., 2008) using all spikes recorded during all brain states. Although the validity of this assumption should be strengthened with rigorous experiments, the available evidence, using intracellular recordings and optogenetic activation of principal cells is in support of this hypothesis (Marshall et al., 2002; Quilichini et al., 2010; Stark et al., 2012). Cross-correlation histograms were normalized by dividing the spike count in each bin by the number of reference spikes, yielding probability of referred neurons’s spikes given the reference event. Monosynaptic excitation between cell pairs were detected by a non-parametric significance test based on jittering of spike trains as described previously in detail (Fujisawa et al., 2008). Briefly, for each cell pair, each spike from each neuron in the original data set was randomly and independently jittered on a uniform interval of [−5,+5] ms, to form a surrogate data set. The process was repeated independently 1,000 times to form 1,000 such surrogate data sets. Then, the cross-correlograms were constructed for surrogate data sets as a function of latency across the interval [−20, +20] ms (Fujisawa et al., 2008). Global bands at acceptance level 99.9% were constructed for the cross-correlogram from the maximum and minimum of each jitter surrogate cross-correlogram across the interval [−20, +20] ms. The short latency peak bin(s) in the original cross-correlogram were determined to be statistically significant (at P < 0.001) when the probabilities in the bin(s) in the cross-correlogram were atypical with respect to the global bands anywhere at the latency [1,4] ms. For cell pairs recorded from the same electrode, the 0–1 ms bin was not considered, because our clustering program cannot resolve superimposed spikes. After using the jittering method, all the cell pairs identified as monosynaptically connected pairs were visually inspected, and spurious cell pairs (e.g., an erroneously identified pair caused by potential contamination of spikes between units of the pair recorded from the same silicon probe shank) were not used from the further analysis.
To estimate spike transmission probability for each brain state, only cell pairs in which both neurons fired more than 20 times and the number of events in the interval [−20, +20] ms in the original cross-correlogram was larger than 100 in that brain state were used. For each cell pairs with significant monosynaptic excitation, the same jitter method above was applied 1,000 times and mean cross-correlogram of surrogate data sets was calculated for each brain states. The excess of probabilities in the original cross-correlogram over mean of surrogated cross-correlogram in the short latency peak bin(s) was taken as a probability of monosynaptic transmission.
Data analysis was carried out by custom-written MATLAB-based software.
Supplementary Material
Highlights.
Firing rates of neurons in the hippocampal region show lognormal-like distribution.
Discharge rates of single cells are correlated across brain states and environments.
Rate distributions may be supported by lognormal distribution of spike transfer strength.
Preconfigured highly active minority neurons may dominate information transmission.
ACKNOWLEDGMENTS
We thank K. Diba and E. Pastalkova for sharing their data, and Kenneth Harris, Christof Koch, Jeff Magee, Alex Reyes, Eric Schomburg and Richard Tsien for comments. Supported by National Institutes of Health (NS034994; MH54671), National Science Foundation, the J.D. McDonnell Foundation, Uehara Memorial Foundation (K.M.), Astellas Foundation for Research on Metabolic Disorders (K.M.) and the Japan Society of Promotion for Sciences (K.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arellano JI, Benavides-Piccione R, Defelipe J, Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci. 2007;1:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AL, Poulet JF. Experimental evidence for sparse firing in the neocortex. Trends Neurosci. 2012;35:345–355. doi: 10.1016/j.tins.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J. Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. J. Neurosci. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Mamiya A, Buzsaki G. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron. 2000;28:585–594. doi: 10.1016/s0896-6273(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Diba K, Buzsaki G. Hippocampal network dynamics constrain the time lag between pyramidal cells across modified environments. J. Neurosci. 2008;28:13448–13456. doi: 10.1523/JNEUROSCI.3824-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Harris KD, Buzsaki G. Place representation within hippocampal networks is modified by long-term potentiation. Neuron. 2003;39:843–853. doi: 10.1016/s0896-6273(03)00465-3. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature. 2011;469:397–401. doi: 10.1038/nature09633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, O'Neill J, Csicsvari J. Dynamic Reconfiguration of Hippocampal Interneuron Circuits during Spatial Learning. Neuron. 2013;78:166–180. doi: 10.1016/j.neuron.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Lubke J, Silver RA, Sakmann B. Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column. J. Physiol. 2002;538:803–822. doi: 10.1113/jphysiol.2001.012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S, Amarasingham A, Harrison MT, Buzsaki G. Behavior-dependent short-term assembly dynamics in the medial prefrontal cortex. Nat. Neurosci. 2008;11:823–833. doi: 10.1038/nn.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsaki G. REM sleep reorganizes hippocampal excitability. Neuron. 2012;75:1001–1007. doi: 10.1016/j.neuron.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Bonnevie T, Moser MB, Moser EI. Hippocampus-independent phase precession in entorhinal grid cells. Nature. 2008;453:1248–1252. doi: 10.1038/nature06957. [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J. Neurophysiol. 2000;84:401–414. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
- Harris KD, Hirase H, Leinekugel X, Henze DA, Buzsaki G. Temporal interaction between single spikes and complex spike bursts in hippocampal pyramidal cells. Neuron. 2001;32:141–149. doi: 10.1016/s0896-6273(01)00447-0. [DOI] [PubMed] [Google Scholar]
- Hirase H, Leinekugel X, Czurko A, Csicsvari J, Buzsaki G. Firing rates of hippocampal neurons are preserved during subsequent sleep episodes and modified by novel awake experience. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9386–9390. doi: 10.1073/pnas.161274398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromadka T, Deweese MR, Zador AM. Sparse representation of sounds in the unanesthetized auditory cortex. PLoS. Biol. 2008;6:e16. doi: 10.1371/journal.pbio.0060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Sasaki T, Ishikawa D, Honma N, Tao K, Takahashi N, Minamisawa G, Ujita S, Matsuki N. Interpyramid Spike Transmission Stabilizes the Sparseness of Recurrent Network Activity. Cereb. Cortex. 2012 doi: 10.1093/cercor/bhs006. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM, Gally JA, Edelman GM. Spike-timing dynamics of neuronal groups. Cereb. Cortex. 2004;14:933–944. doi: 10.1093/cercor/bhh053. [DOI] [PubMed] [Google Scholar]
- Jarsky T, Mady R, Kennedy B, Spruston N. Distribution of bursting neurons in the CA1 region and the subiculum of the rat hippocampus. J. Comp Neurol. 2008;506:535–547. doi: 10.1002/cne.21564. [DOI] [PubMed] [Google Scholar]
- Koulakov AA, Hromadka T, Zador AM. Correlated connectivity and the distribution of firing rates in the neocortex. J. Neurosci. 2009;29:3685–3694. doi: 10.1523/JNEUROSCI.4500-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupic J, Burgess N, O'Keefe J. Neural representations of location composed of spatially periodic bands. Science. 2012;337:853–857. doi: 10.1126/science.1222403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Lin BJ, Lee AK. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science. 2012;337:849–853. doi: 10.1126/science.1221489. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Levy WB, Baxter RA. Energy efficient neural codes. Neural Comput. 1996;8:531–543. doi: 10.1162/neco.1996.8.3.531. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Loewenstein Y, Kuras A, Rumpel S. Multiplicative dynamics underlie the emergence of the log-normal distribution of spine sizes in the neocortex in vivo. J. Neurosci. 2011;31:9481–9488. doi: 10.1523/JNEUROSCI.6130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, Leutgeb JK. Neuronal code for extended time in the hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2012;109:19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Henze DA, Hirase H, Leinekugel X, Dragoi G, Buzsaki G. Hippocampal pyramidal cell-interneuron spike transmission is frequency dependent and responsible for place modulation of interneuron discharge. J. Neurosci. 2002;22:RC197. doi: 10.1523/JNEUROSCI.22-02-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. Phase precession in hippocampal interneurons showing strong functional coupling to individual pyramidal cells. J. Neurosci. 2006;26:13485–13492. doi: 10.1523/JNEUROSCI.2882-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A, Mitra SS, Tsien RW. Heterogeneous reallocation of presynaptic efficacy in recurrent excitatory circuits adapting to inactivity. Nat. Neurosci. 2012;15:250–257. doi: 10.1038/nn.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Sirota A, Pastalkova E, Buzsaki G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron. 2009;64:267–280. doi: 10.1016/j.neuron.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Diba K, Pastalkova E, Buzsaki G. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat. Neurosci. 2011;14:1174–1181. doi: 10.1038/nn.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K, Royer S, Diba K, Buzsaki G. Activity dynamics and behavioral correlates of CA3 and CA1 hippocampal pyramidal neurons. Hippocampus. 2012;22:1659–1680. doi: 10.1002/hipo.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J. Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The firing of hippocampal place cells predicts the future position of freely moving rats. J. Neurosci. 1989;9:4101–4110. doi: 10.1523/JNEUROSCI.09-12-04101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. Functional maps within a single neuron. J. Neurophysiol. 2012;108:2343–2351. doi: 10.1152/jn.00530.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron. 2010;67:1048–1061. doi: 10.1016/j.neuron.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Olshausen BA, Field DJ. Sparse coding with an overcomplete basis set: a strategy employed by V1? Vision Res. 1997;37:3311–3325. doi: 10.1016/s0042-6989(97)00169-7. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Oxford University Press; 1978. [Google Scholar]
- O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike FG, Meredith RM, Olding AW, Paulsen O. Rapid report: postsynaptic bursting is essential for 'Hebbian' induction of associative long-term potentiation at excitatory synapses in rat hippocampus. J. Physiol. 1999;518(Pt 2):571–576. doi: 10.1111/j.1469-7793.1999.0571p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilichini P, Sirota A, Buzsaki G. Intrinsic circuit organization and theta-gamma oscillation dynamics in the entorhinal cortex of the rat. J. Neurosci. 2010;30:11128–11142. doi: 10.1523/JNEUROSCI.1327-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxin A, Brunel N, Hansel D, Mongillo G, van VC. On the distribution of firing rates in networks of cortical neurons. J. Neurosci. 2011;31:16217–16226. doi: 10.1523/JNEUROSCI.1677-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonovich A, McNaughton BL. Path integration and cognitive mapping in a continuous attractor neural network model. J. Neurosci. 1997;17:5900–5920. doi: 10.1523/JNEUROSCI.17-15-05900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer RJ, Friedlander MJ, Redman SJ. The time course and amplitude of EPSPs evoked at synapses between pairs of CA3/CA1 neurons in the hippocampal slice. J. Neurosci. 1990;10:826–836. doi: 10.1523/JNEUROSCI.10-03-00826.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci. 1998;18:3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Gothard KM, Markus EJ. An information-theoretic approach to deciphering the hippocampal code. In: Hanson SJ, Cowan JD, Giles CL, editors. Advances in Neural Information Processing Systems. vol. 5. Morgan Kaufmann; 1993. pp. 1030–1037. [Google Scholar]
- Song S, Sjostrom PJ, Reigl M, Nelson S, Chklovskii DB. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS. Biol. 2005;3:e68. doi: 10.1371/journal.pbio.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Koos T, Buzsaki G. Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. J. Neurophysiol. 2012;108:349–363. doi: 10.1152/jn.00153.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Watabe AM, Moody TD, Makhinson M, O'Dell TJ. Postsynaptic complex spike bursting enables the induction of LTP by theta frequency synaptic stimulation. J. Neurosci. 1998;18:7118–7126. doi: 10.1523/JNEUROSCI.18-18-07118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, Best PJ. Place cells and silent cells in the hippocampus of freely-behaving rats. J. Neurosci. 1989;9:2382–2390. doi: 10.1523/JNEUROSCI.09-07-02382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Facilitation, augmentation and potentiation at central synapses. Trends Neurosci. 2000;23:305–312. doi: 10.1016/s0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Houweling AR, Brecht M. Sparse and powerful cortical spikes. Curr. Opin. Neurobiol. 2010;20:306–312. doi: 10.1016/j.conb.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Yassin L, Benedetti BL, Jouhanneau JS, Wen JA, Poulet JF, Barth AL. An embedded subnetwork of highly active neurons in the neocortex. Neuron. 2010;68:1043–1050. doi: 10.1016/j.neuron.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Burns LD, Cocker ED, Hamel EO, Ghosh KK, Kitch LJ, El GA, Schnitzer MJ. Long-term dynamics of CA1 hippocampal place codes. Nat. Neurosci. 2013;16:264–266. doi: 10.1038/nn.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.