Abstract
Phytate is the primary storage form of phosphate in plants. Monogastric animals like poultry, pigs and fishes have very low or no phytase activities in their digestive tracts therefore, are incapable to efficiently utilize phytate phosphorus from the feed. Phytase from microbial sources are supplemented to feedstuff of these to increase the uptake of phytate phosphorus. In the present work efforts were made to isolate and characterize proficient phytase producing fungi from soil. Phytase producing fungi were isolated using phytate specific medium. Fungal isolates were selected according to their higher phytase activities. These isolates were further characterized and identified by morphological and microscopic analysis and confirmed by amplification of 18S rRNA gene, using specific primers. This gene was subsequently sequenced and phylogenetic affiliations were assigned. Fungal isolates were identified as various species of Aspergillus. Phytases from these fungi could be utilized as a feed additive in poultry and swine industries.
Keywords: phytase, phytate, fungi, 18S rRNA gene, Aspergillus
Introduction
In many plants, phytic acid (myo-inositol 1,2,3,4,5,6 hexakisphosphate) is one of the main storage forms of phosphate, accounting for 60–80% of total phosphorous (P) in grains, legumes and oilseeds (Sharma and Gole, 1978; Wang et al., 2007). Cereal grains and oilseed meals are major ingredients of animal feed. The amount of phosphorus in cereal grains and oilseed meals would meet the requirement of optimal growth of animals if all the phosphorus from phytate were available to animals. Phytate phosphorus is not available to monogastric animals (such as poultry, piggery and fish) due to lack of adequeate levels of phytase enzyme in their gastrointestinal tracts (Brinch-Pedersen et al., 2002; Singh and Satayanarayana, 2010). Phosphate supplementation is required for optimal growth of animals. Phytase catalyzes the dephosphorylation of phytate to inositol and orthophosphate (Wodzinski and Ullah, 1996; Hamada et al., 2005). Moreover, in the regions with intense animal production, a large amount of undigested and excreted phosphate (phytate) contributes significantly to the environmental pollution. Furthermore, phytic acid is also considered as an anti-nutritional factor, since it causes mineral deficiency due to efficient chelation of metal ions (Fe3+, Zn2+, Ca2+, Mg2+, K+) and forming complexes with proteins, thus affecting their digestion (Urbano et al., 2000). Microbial phytase produced by fermentation as a feed additive is widely used to manage the nutritional and environmental problems caused by phytate.
Commercial production of phytase as feed additives is mostly focused on fungi and yeasts, as they are the most prolific extracellular producers of this enzyme (Farhat et al., 2008). Most of the naturally occurring phytases having high thermostability and a broad pH range were identified from fungi (Simon and Igbasan, 2002). Due to immense industrial and environmental implication of phytases there is an ongoing interest in isolation of new fungal strain producing phytase and optimization of this enzyme. In the present work efforts were made for isolation, morphological and molecular characterization of proficient phytase producing fungal strains.
Materials and Methods
Sample collection
Samples were collected in sterile polythene bags from agricultural land, vermicompost and food based sources such as poultry and pig wastes, and fishery pond located at Jawaharlal Nehru Agricultural University Campus, Jabalpur, Madhya Pradesh, India (23°17′ N; 72°98′ E).
Isolation of phytase producing fungi
Soil samples (0.2 g) were suspended in 10 mL of 0.9% saline solution and kept on incubator shaker with 150 rpm for 2 h. Soil suspension (1 mL) was inoculated into 100 mL of phytate specific medium (PSM) containing 1.5% glucose, 0.5% (NH4)2SO4, 0.05% KCl, 0.01% MgSO4.7H2O, 0.01% NaCl, 0.01% CaCl2.2H2O, 0.001% FeSO4.7H20, 0.001% MnSO4.H20, pH 6.5 with 0.5% sodium phytate (Hosseinkhani et al., 2009). Medium was sterilized by autoclaving (15 psi, 121 °C, 20 min), with the exception of sodium phytate, which was sterilized by membrane filtration (Millipore, 0.45 μm) and added aseptically to cooled autoclaved media. The samples were kept in incubator shaker with shaking at 150 rpm at 30 °C for 10 days. Fungal cultures were re-inoculated in fresh PSM medium to ensure enrichment of phytase producing fungi and incubated at 30 °C, 165 rpm for 7 days.
Fungal cultures obtained through the enrichment process were inoculated in PSM agar medium containing calcium phytate (0.5%) as sole source of phosphorus. Plates were kept for incubation at 30 °C for 5 days. After incubation zone of clearing around the fungal growth on PSM agar plates were observed. The zone of clearing around the fungal growth is indicator of phytase production. The samples which showed clear zone were considered as positive samples. Counterstaining for confirmation of phytase activity was performed for the positive isolates according to the method of Bae et al. (1999).
Phytase enzyme activity assay
Isolates that produced clear zones on screening medium were tested for phytase production in PSM broth with sodium phytate. A spore suspension of 1 × 107/mL was inoculated in 100 mL of PSM medium in a 500 mL Erlenmeyer flask and incubated at 30 °C with 200 rpm shaking for 7 days. Cultures (2 mL) were centrifuged and the supernatant was used for phytase activity assays. The enzyme activity was estimated according to the method described by Engelen et al. (1994). The incubation mixture (2 mL) contained 1 mL of the culture filtrate, 1mL of substrate solution [10 mM sodium phytate as substrate and 0.2 M sodium acetate buffer (pH 5.5)] and incubated at 37 °C for 1 h. The reaction was terminated by the addition of 1mL of the colour developing reagent [250 mL of ammonium hepta-molybdate solution (10% of ammonium molybdate in 0.25% ammonia solution), 250 mL of ammonium vanadate solution (2.35 g of ammonium vanadate in 1 L of 2% (v/v) nitric acid solution), and 165 mL of 65% nitric acid, finally diluted to 1L with water]. The absorbance was measured at 415 nm. Distilled water was used as a blank. One unit of phytase activity was defined as 1 μmol of phosphate produced per min per mL of culture filtrate under the assay condition (pH 5.5, temperature 37 °C and substrate concentration, sodium phytate [C6H6O24P6Na12] at 0.0051 mol/L). Standard curve was prepared using potassium dihydrogen phosphate (KH2PO4) in the range 0–1000 μmol.
pH and temperature optima of crude phytase enzyme
To determine the pH optimum curve, the enzyme was incubated with sodium phytate prepared in 0.2 M Sodium acetate buffer, pH 3.0, 3.6, 4.2, 4.8, 5.5; 0.2 M citrate buffer, pH 6.0 and 6.6 and 0.2 M Tris, pH 7.0, 7.5 and 8.5 for 1 h at 37 °C and released phosphate ions were assayed. The temperature optimum was determined by incubating the enzyme with substrate, prepared in 0.2 M sodium acetate pH 5.5 at different temperatures for 1 h and the phytase activity was assayed.
Morphological characterization
The morphological identification of isolates (DD1–DD3; IG1–IG5) was conducted using four different types of media viz. Czapek dox agar (CDA), Czapek yeast agar (CYA), Czapek yeast 20% sucrose agar (CY2S) and Malt yeast agar (MYA). Creatine sucrose agar (CREA) was used to study acid or base production by fungi (Samson et al., 2007). All media were incubated at 28 °C for 7 days. Colonies on each medium were compared for their diameters, overall colors, colors of conidia, reverse colors, texture, zonation and sporulation. All the isolates were also subjected to microscopic analysis for their characterization and identification.
Identification of fungi using 18S rRNA gene analysis
Fungal mycelium or spores were cultured on potato dextrose agar medium (Himedia, India). The plates were incubated at 30 °C for 2 to 3 days. The fungal mycelium was used for DNA isolation. DNA was extracted using method described by Hunt et al. (2004).
PCR amplification of 18S rRNA gene
PCR amplification of fungal small-subunit rDNA (18S rRNA gene) was carried out using the primer set EF4 and EF3 (Smit et al., 1999). The EF4 and EF3 primers amplified a 1.5-kb section of the 18S rRNA gene. Primer sequences were as follows: EF4 (5′-GGAAGGG[G/A]TGTATTTATTAG-3′) and EF3 (5′-TCCTCTAAATGACCAAGTTTG-3′). PCR amplification was performed in a 25 μL reaction containing 2.5 U of Taq DNA polymerase (Sigma), a 10 X dilution of the manufacturer’s buffer (Sigma), 200 μM concentrations of each deoxynucleoside triphosphate (dNTPs), and 20 pM of primers EF4 and EF3 and 50 ng of genomic DNA. The reaction conditions were as follows: initial denaturation at 94 °C for 4 min, 40 amplification cycles of denaturation at 94 °C for 1 min, annealing at 48 °C for 1 min and primer extension at 72 °C for 3 min; followed by a final extension at 72 °C for 10 min. PCR amplifications were carried out using a Thermo-Hybaid PCR thermal cycler (Thermo Fisher Scientific USA). Aliquots of the PCR products (5 μL) were analyzed in 1% (w/v) agarose gels (Sigma, USA) by horizontal gel electrophoresis. DNAs were visualized by UV excitation after staining with ethidium bromide (0.5 mg/L). The PCR product was purified using Bangalore Genie PCR purification kit following the manufacturer’s instruction. The 18S rRNA nucleotide sequence was determined by PCR-direct sequencing done by Chromous Biotech Pvt. Ltd., Bangalore, India.
Phylogenetic analysis of the 18S rRNA gene sequences was performed with CLC DNA workbench version 6. The phylogenetic trees were inferred using the neighbour-joining method (Saitau and Nei, 1987) and bootstrap analyses were performed. The evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura et al., 2004).
Results and Discussion
Isolation of phytase producing fungi from soil
Twenty-five soil samples were collected from different locations for the isolation of phytase producing fungi. Out of these only fourteen samples showed fungal growth in phytate specific screening medium containing 1% sodium phytate. The fungal cultures obtained from these soil samples were re-inoculated in PSM broth for enrichment of these phytate utilizing cultures. For further confirmation of their phytase producing trait, fungal cultures were inoculated on PSM agar medium containing 0.5% calcium phytate. Eight fungal isolates produced zone of clearing on PSM agar medium. Counterstaining was also performed to visualize the zone of clearing. These fungal isolates were designated as IG 1, IG 2, IG 3, IG 4, IG 5, DD 1, DD 2 and DD 3. Counterstaining approach was carried out to overcome the selection of false positive isolates for phytase production on PSM medium (Fredrikson et al., 2002; Chadha et al., 2004). The formation of clear zone is attributed to the production of various acids (acetic acid, maleic acid, etc.), which lowers the pH of the medium and hence increase the solubility of calcium phytate (Bae et al., 1999).
Phytase activity and its optimization
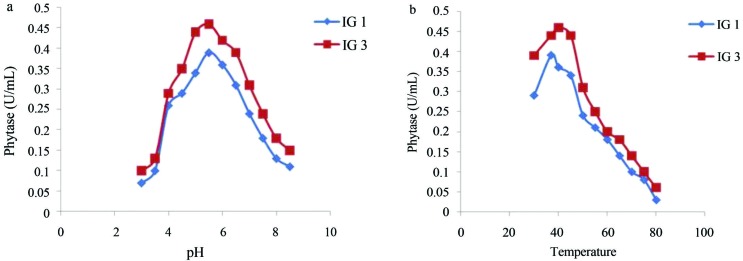
Phytase activity was determined by measuring the amount of liberated inorganic phosphate and its reaction with colour reagent. It was carried out for fungal isolates irrespective of clear zone formation on PSM medium. It was found that fungal isolates which did not form clear zone on PSM, showed negligible phytase activities in liquid medium. Isolate IG 3 and IG 1 showed highest phytase activities which were 0.46 U mL−1 and 0.39 U mL−1, respectively. Enzyme activities as well as size of zone of clearance for different isolates are shown in Table 1. Optimization of phytase activity for isolate IG 1 and IG 3 were carried out to determine their optimum pH and temperature. Phytase activity for isolates IG 1 and IG 3 was optimum at pH 5.5 as depicted from Figure 1a and optimum temperature was 37 °C and 40 °C respectively as shown in Figure 1b. Phytase often has a low-pH optimum range (pH 4.5–6.0) with a rapid drop in activity at pH value above 6.0 (Marlida et al., 2010). It is needed to be determined that phytase activity for the fungal isolates is extracellular or cell-associated.
Table 1.
Phytase activities for different fungal isolates.
| S No. | Isolates | Enzyme activity (U mL−1) mean ± SD | Size of clear zone (mm) mean ± SD | Source of isolation (Soil) |
|---|---|---|---|---|
| 1 | IG 1 | 0.39 ± 0.007 | 28 ± 0.2 | Agricultural land |
| 2 | IG 2 | 0.22 ± 0.002 | 19 ± 0.1 | Vermicompost |
| 3 | IG 3 | 0.46 ± 0.005 | 32 ± 0.2 | Poultry waste |
| 4 | IG 4 | 0.18 ± 0.002 | 12 ± 0.3 | Pig waste |
| 5 | IG 5 | 0.20 ± 0.004 | 17 ± 0.1 | Poultry waste |
| 6 | DD 1 | 0.32 ± 0.005 | 22 ± 0.6 | Fish pond |
| 7 | DD 2 | 0.26 ± 0.006 | 19 ± 0.2 | Agricultural land |
| 8 | DD 3 | 0.21 ± 0.002 | 14 ± 0.3 | Poultry waste |
Figure 1.
Phytase activities for isolates IG 1 (A. niger) and IG 3 (A. awamori) at different pH (a) and temperature (b).
Morphological and microscopic characterization of fungi
Fungal isolates were grown in different growth medium (CDA, CYA, MYA and CY2S) and colonies on each medium were recorded for their diameters, overall colors, colors of conidia, reverse colors, texture, zonation and sporulation as shown in Table 2. Isolates IG 5, DD2 showed similar results to IG 1 and similarly, IG 3 and DD1 showed comparable results on different culture media so only a single representatives from them were taken into consideration and shown in Table 2. On the basis of morphological characters and growth pattern on different media, isolates IG 1, IG 5, DD 2 were suggested to be Aspergillus niger (Sharma and Pandey, 2010). Likewise, isolates IG 2 and IG 4 were reported to be A. fumigatus and A. terreus, respectively whereas isolates DD1 and IG 3 showed similarity to A. awamori (McClenny, 2005; Zain et al., 2009; Perrone et al., 2011). Growth abilities of isolates were tested on CREA medium. CREA is the semi-selective media useful for classification of various fungal cultures (Samson et al., 2007). On CREA, characteristics of colonial growth, production of acid (turning of the medium from purple to yellow) can be used as diagnostic features. Isolate IG 1, IG 3, IG 4, IG 5 and DD 1, DD 2 showed moderate growth and good acid having production resulting in large yellowish halo around the colonies on CREA medium. Similar results for A. niger were reported by Samson et al. (2007), showing this as a characteristic feature for distinguishing biseriate species (A. niger, A. awamori and A. terreus) from uniseriate species. IG 2 showed poor growth and limited acid production on CREA medium as characteristic feature of uniseriate species.
Table 2.
Morphological colony characteristics and sporulation pattern of fungal isolates on different culture media.
| Isolate | Media | Colony diam (mm) | Colony characters | Zonation | Sporulation | Reaction on CREA | Identified as | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Texture | Surface colour | Reverse colour | ||||||||
| IG 1 | CDA | 47.33 ± 3.05 | Velvety | White with dusty yellow sporulating area | White | Slightly Radially furrowed | Moderate | Moderate growth and good acid production (large yellow halo around colony) | Aspergillus niger | Sharma and Pandey, 2010 |
| CYA | 47.00 ± 1.00 | Powdery | White periphery with black spores | Yellowish | Radially furrowed | Heavy | ||||
| MYA | 59.00 ± 3.60 | Powdery | White periphery with black spores | Cream | Radially furrowed at the centre | Moderate | ||||
| CY2S | 71.00 ± 2.65 | Powdery | White periphery with black spores | Cream | Heavily wrinkled | Heavy | ||||
|
| ||||||||||
| IG 2 | CDA | 34.33 ± 1.15 | Velvety | White with grayish green concentric sporulating rings | Cream | Concentric zones | Poor | Poor growth and low acid production | A. fumigatus | McClenny, 2005 |
| CYA | 52.00 ± 1.73 | Powdery | Cream periphery grayish green sporulating area | Yellowish | Radially furrowed | Heavy | ||||
| MYA | 47.33 ± 2.08 | Powdery | Cream with green periphery and grayish green sporulating area | Yellowish | Radially furrowed | Moderate | ||||
| CY2S | 73.66 ± 3.21 | Powdery | Cream periphery grayish green sporulating area | Cream | Wrinkled and radially furrowed | Heavy | ||||
|
| ||||||||||
| IG 3 | CDA | 55.66 ± 0.58 | Velvety | White with brownish sporulating area | White | irregularly furrowed | Moderate | Moderate growth and good acid production (large yellow halo around colony) | A. awamori | Perrone et al., 2011 |
| CYA | 43.33 ± 1.15 | Powdery | White periphery with dark brown sporulating area | Cream | Heavily radially furrowed | Heavy | ||||
| MYA | 57.66 ± 2.08 | Velvety | White periphery with dark brown sporulating area | Cream | Heavily radially furrowed | Moderate | ||||
| CY2S | 67.33 ± 1.53 | Velvety | White periphery with dark brown sporulating area | Cream | Wrinkled | Moderate | ||||
|
| ||||||||||
| IG 4 | CDA | 45.33 ± 1.15 | Floccose | Green periphery with green sporulating area | Yellow | Radially furrowed | Poor | Moderate growth and good acid production (large yellow halo around colony) | A. terreus | Zain et al., 2009 |
| CYA | 44.33 ± 1.53 | Velvety | White with dark green sporulating area | Light orange | Radially furrowed | Poor | ||||
| MYA | 55.66 ± 2.31 | Powdery | Green periphery with dark green sporulating area | Peach | Radially furrowed | Moderate | ||||
| CY2S | 75.00 ± 1.00 | Powdery | Green periphery with dark green sporulating area | Light orange | Irregularly furrowed | Heavy | ||||
Microscopic characterization for definitive identification of the isolates was carried out. In case of isolate IG 2 the microscopic analysis showed that the mycelium was wide, septate and hyaline with acute angle branching. Conidial head was uniseriate and columnar. Isolates IG 1, IG 5 and DD 2, also showed wide, septate and hyaline mycelium with acute angle branching but their conidial head was biseriate and radiate and conidia were attached in chains. Similar results were reported for A. fumigatus and A. niger, respectively by McClenney (2005), thus isolates IG1, IG 5, DD2 could be predicted as A. niger and IG 2 as A. fumigatus. In microscopic analysis of IG 4, mycelium was found to be wide, septate and conidiophores were long, columnar and hyaline. Conidial head were globose to slightly elliptical and biseriate. Additionally, hyaline accessory conidia were also visible on the hyphae. A. terreus is the only member of the genus Aspergillus that produces such structures (Balajee, 2009). The Isolates IG 3 and DD1 showed mycelial characters similar to those of isolates IG 1, IG 5 and DD 2 but conidia showed some ornamentation and were distinctly rough, hence these two isolates could be subspecies of A. niger.
Molecular characterization of fungi
The PCR amplification of 18S rRNA gene was done by using gene specific primers. An amplification product of 1.5kb was obtained for all the isolates as shown in Figure 2. The nuclear small subunit ribosomal DNA (18S rDNA) was selected for characterization and identification of fungi firstly, because established universal fungal primers are available based on the conserved regions of 18S rDNA, making it possible to obtain the PCR products from most of the fungi. Secondly, the large numbers of 18S rDNA sequences are available in GenBank which makes similarity searches convenient. Several workers have also reported characterization of fungi based on 18S rRNA gene sequence analysis (Smit et al., 1999; Borneman and Hartin, 2000).
Figure 2.
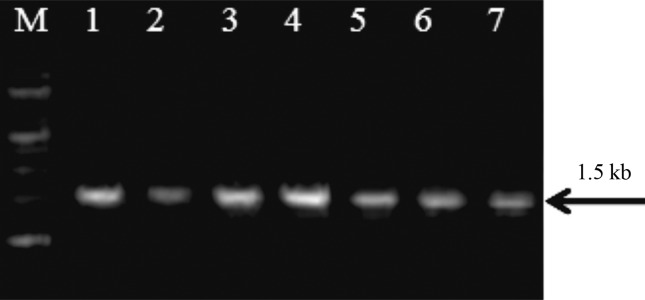
PCR amplification of 18S rRNA gene for fungal isolates Lane M: 1 kb DNA ladder; Lane 1–7: fungal isolates IG 1–IG 5, DD1–DD2.
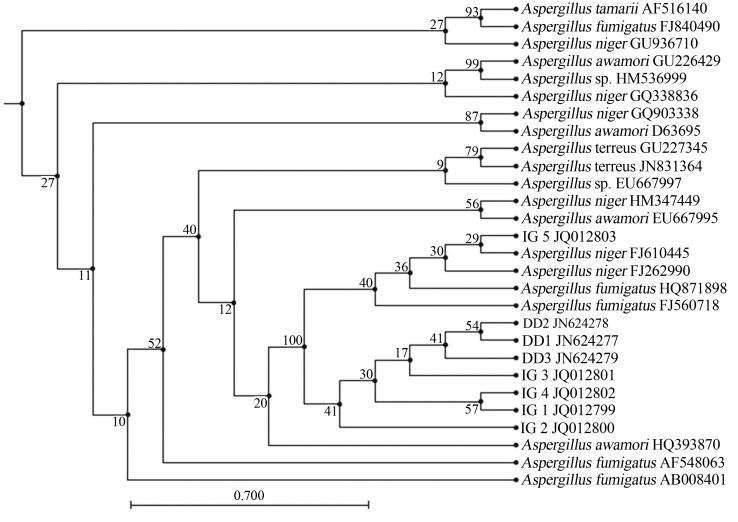
On the basis of 18S rRNA gene similarity isolates IG 1, IG 5 and DD 2 showed 99% sequence homology with Aspergillus niger (FJ262990) (Yang et al., 2012), IG 2 and DD3 showed 98% sequence homology with Aspergillus fumigatus (AF648063) (Wu et al., 2003). The 18S rRNA partial sequence placed the isolates IG 3 and DD1 within Aspergillus genera with 99% sequence homology with Aspergillus awamori (HQ393870) (Srinivasan et al., 2012). Similarly, isolate IG 4 showed maximum sequence similarity with Aspergillus terreus (JN831364) (Yin et al., 2012). Partial 18S rRNA sequences of all the isolates were submitted to NCBI Genbank under the following accession numbers: IG 1, JQ012799; IG 2, JQ012800; IG 3, JQ012801; IG 4, JQ012802, IG 5, JQ012803; DD1, JN624277; DD2, JN624278 and DD3, JN624279. Phylogenetic relationship of the fungal isolates with other fungi is shown in Figure 3.
Figure 3.
Phylogenetic tree showing the relationships of the isolates to closely related fungi. The numbers at branching points refer to bootstrap values, based on 100 replicates.
A. niger is well known for its phytase activity. Phytase activity from A. niger has been extensively studied and reported by several workers (Vats and Banerjee, 2005; Gunashree and Venkateswaran, 2009; Soni et al., 2010). Similarly, phytase activity from Aspergillus fumigatus has previously been reported (Mullaney et al., 2000; Rodriguez et al., 2000). Isolate IG 3 showed close similarity with A. awamori and has considerably high phytase activity. A. awamori and A. terreus are less extensively studied for phytase activity. To best of our knowledge this is the second report of phytase production from A. awamori and A. terreus. Report of phytase production from A. awamori and A. terreus with further isolation and characterization of phy gene from these Aspergillus strains will add new information to the database of phy genes.
Conclusion
Our finding suggests that phytase production by A. niger strain IG 1 and A. awamori strain IG 3 have significant values which can be exploited for industrial production of phytase. Moreover, this enzyme can be used in the animal feed industry for improving the nutritional status of feed. The native fungal communities of soil which degrade phytate from manures and soil with subsequent release of orthophosphate making it available to plants, thus enhance the benefits of manure-derived fertilizer and in combating environmental pollution due to phytate. Additionally, phy gene from these strains could be used to develop transgenic plants (maize, sorghum and oat) which would in turn utilized as animal feed.
Acknowledgments
IG is grateful to Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi for financial assistance.
References
- Bae HD, Yanke LJ, Cheng KJ, Selinger LB. A novel staining method for detecting phytase activity. J Microbiol Methods. 1999;39:17–22. doi: 10.1016/s0167-7012(99)00096-2. [DOI] [PubMed] [Google Scholar]
- Balajee SA. Aspergillus terreus complex. Med Mycol. 2009;47:S42–S46. doi: 10.1080/13693780802562092. [DOI] [PubMed] [Google Scholar]
- Borneman J, Hartin RJ. PCR primers that amplify fungal rRNA genes from environmental samples. Appl Environ Microbiol. 2000;66:4356–4360. doi: 10.1128/aem.66.10.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinch-Pedersen H, Sorensen LD, Holm PB. Engineering crop plants: Getting a handle on phosphate. Trends Plant Sci. 2002;7:118–125. doi: 10.1016/s1360-1385(01)02222-1. [DOI] [PubMed] [Google Scholar]
- Chadha BS, Gulati H, Minhas M, Saini HS, Singh N. Phytase production by the thermophilic fungus Rhizomucor pusillus. World J Microbiol Biotechnol. 2004;20:105–109. [Google Scholar]
- Engelen AJ, van der Heeft FC, Randsdorp PH, Smit EL. Simple and rapid determination of phytase activity. J AOAC Int. 1994;77:760–764. [PubMed] [Google Scholar]
- Farhat A, Chouayekh H, Farhat MB, Bouchaala K, Bejar S. Gene cloning and characterization of a thermostable phytase from Bacillus subtilis US417 and assessment of its potential as a feed additive in comparison with a commercial enzyme. Mol Biotechnol. 2008;40:127–135. doi: 10.1007/s12033-008-9068-1. [DOI] [PubMed] [Google Scholar]
- Fredrikson M, Andlid T, Haikara A, Sandberg AS. Phytate degradation by micro-organisms in synthetic media and pea flour. J Appl Microbiol. 2002;93:197–204. doi: 10.1046/j.1365-2672.2002.01676.x. [DOI] [PubMed] [Google Scholar]
- Gunashree BS, Venkateswaran G. Screening of asporogenic mutants of phytase-producing Aspergillus niger CFR 335 strain. Microb Ecol Health Dis. 2009;21:57–63. [Google Scholar]
- Hamada A, Yamaguchi K, Ohnishi N, Harada M, Nikumaru S, Honda H. High-level production of yeast (Schwanniomyces occidentalis) phytase in transgenic rice plants by a combination of signal sequence and codon modification of the phytase gene. Plant Biotechnol J. 2005;3:43–55. doi: 10.1111/j.1467-7652.2004.00098.x. [DOI] [PubMed] [Google Scholar]
- Hosseinkhani B, Emtiazi G, Nahvi I. Analysis of phytase producing bacteria (Pseudomonas sp.) from poultry faeces and optimization of this enzyme production. Afr J Biotechnol. 2009;8:4229–4232. [Google Scholar]
- Hunt J, Boddy L, Randerson PF, Rogers HJ. An evaluation of 18S rDNA approaches for the study of fungal diversity in grassland soils. Microb Ecol. 2004;47:385–395. doi: 10.1007/s00248-003-2018-3. [DOI] [PubMed] [Google Scholar]
- Marlida Y, Delfita R, Adnadi P, Ciptaan G. Isolation, characterization and production of phytase from endophytic fungus its application for feed. Pak J Nutr. 2010;9:471–474. [Google Scholar]
- McClenny N. Laboratory detection and identification of Aspergillus species by microscopic observation and culture: The traditional approach. Med Mycol Suppl. 2005;43:S125–S128. doi: 10.1080/13693780500052222. [DOI] [PubMed] [Google Scholar]
- Mullaney EJ, Daly CB, Sethumadhavan K, Rodriquez E, Lei XG, Ullah AH. Phytase activity in Aspergillus fumigatus isolates. Biochem Biophys Res Commun. 2000;275:759–763. doi: 10.1006/bbrc.2000.3234. [DOI] [PubMed] [Google Scholar]
- Perrone G, Stea G, Epifani F, Varga J, Frisvad JC, Samson RA. Aspergillus niger contains the cryptic phylogenetic species A. awamori. Fungal Biol. 2011;115:1138–1150. doi: 10.1016/j.funbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Mullaney EJ, Lei XG. Expression of the Aspergillus fumigatus phytase gene in Pichia pastoris and characterization of the recombinant enzyme. Biochem Biophys Res Commun. 2000;268:373–378. doi: 10.1006/bbrc.2000.2121. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Samson RA, Noonim P, Meijer M, Houbraken J, Frisvad JC, Varga J. Diagnostic tools to identify black aspergilli. Stud Mycol. 2007;59:129–145. doi: 10.3114/sim.2007.59.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma CB, Gole M. Myo-inositol hexaphos-phytate as a potential inhibitor of amylases of different origin. Phytochemistry. 1978;17:203–204. [Google Scholar]
- Sharma G, Pandey RR. Influence of culture media on growth, colony character and sporulation of fungi isolated from decaying vegetable wastes. J Yeast Fungal Res. 2010;1:157–164. [Google Scholar]
- Simon O, Igbasan F. In vitro properties of phytases from various microbial origins. Int J Food Sci Technol. 2002;37:813–822. doi: 10.1080/17450390009381958. [DOI] [PubMed] [Google Scholar]
- Singh B, Satyanarayana T. Application of phytases of thermophilic mould Sporotrichum thrmophile: A review. J Sci Ind Res. 2010;69:411–414. [Google Scholar]
- Smit E, Leeflang P, Glandorf B, van Elsas JD, Wernars K. Analysis of fungal diversity in the wheat rhizosphere by sequencing of cloned PCR-amplified genes encoding 18S rRNA and temperature gradient gel electrophoresis. Appl Environ Microbiol. 1999;65:2614–2621. doi: 10.1128/aem.65.6.2614-2621.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni SK, Magdum A, Khire JM. Purification and characterization of two distinct acidic phytases with broad pH stability from Aspergillus niger NCIM 563. World J Microbiol Biotechnol. 2010;26:2009–2018. doi: 10.1007/s11274-010-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Alagawadi AR, Yandigeri MS, Meena KK, Saxena AK. Characterization of phosphate-solubilizing microorganisms from salt-affected soils of India and their effect on growth of sorghum plants Sorghum bicolor (L.) Moench. Ann Microbiol. 2012;62:93–105. [Google Scholar]
- Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano G, Lopez-Jurado M, Aranda P, Vidal-Valverde C, Tenorio E, Porres J. The role of phytic acid in legumes: Antinutrient or beneficial function? J Physiol Biochem. 2000;56:283–294. doi: 10.1007/BF03179796. [DOI] [PubMed] [Google Scholar]
- Vats P, Banerjee U. Biochemical characterization of extracellular phytase (myoinositol hexakisphosphate phosphohydrolase) from a hyper-producing strain of Aspergillus niger van Teighem. J Ind Microbiol Biotechnol. 2005;32:141–147. doi: 10.1007/s10295-005-0214-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao X, Su Q, Wu W, An L. Expression of heat stable phytase from Aspergillus fumigatus in tobacco (Nicotiana tabacum L. cv. NC89) Ind J Biochem Biophys. 2007;44:26–30. [PubMed] [Google Scholar]
- Wodzinski RJ, Ullah AH. Phytase. Adv Appl Microbiol. 1996;42:263–302. doi: 10.1016/s0065-2164(08)70375-7. [DOI] [PubMed] [Google Scholar]
- Wu Z, Tsumura Y, Blomquist G, Wang XR. 18S rRNA gene variation among common airborne fungi, and development of specific oligonucleotide probes for the detection of fungal isolates. Appl Environ Microbiol. 2003;69:5389–5397. doi: 10.1128/AEM.69.9.5389-5397.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Sun JY, Guo JL, Weng XY. Identification and proteomic analysis of a novel gossypol-degrading fungal strain. J Sci Food Agri. 2012;92:943–951. doi: 10.1002/jsfa.4675. [DOI] [PubMed] [Google Scholar]
- Yin Y, Gao Q, Zhang F, Li Z. Medium optimization for the high yield production of single (+)-terrein by Aspergillus terreus strain PF26 derived from marine sponge Phakellia fusca. Process Biochem. 2012;47:887–891. [Google Scholar]
- Zain ME, Razak AA, El-Sheikh HH, Soliman HG, Khalil AM. Influence of growth medium on diagnostic characters of Aspergillus and Penicillium species. Afr J Microbiol Res. 2009;3:280–286. [Google Scholar]