Abstract
Purpose
To evaluate the role of corneal hysteresis (CH) as a risk factor for the rate of visual field progression in a cohort of glaucoma patients followed prospectively over time.
Design
Prospective observational cohort study.
Participants
The study group included 114 eyes of 68 glaucoma patients followed for an average of 4.0 ± 1.1 years. Visual fields were obtained with standard automated perimetry. Included eyes had a median number of 7 (range: 5 to 12) tests during follow-up.
Methods
CH measurements were acquired at baseline using the Ocular Response Analyzer (Reichert Instruments, Depew, NY, USA). Evaluation of rates of visual field change during follow-up was performed using the Visual Field Index (VFI). Linear mixed models were used to investigate the relationship between rates of visual field loss and baseline CH, baseline intraocular pressure (IOP) and central corneal thickness (CCT), while adjusting for potentially confounding factors. An interaction term between IOP and CH was included in the model to investigate whether the effect of IOP on rates of progression depended on the level of CH.
Main Outcome Measures
Effects of CH, IOP and CCT on rates of VFI loss over time.
Results
CH had a significant effect on rates of visual field progression over time. In the univariable model including only CH as a predictive factor along with time and their interaction, each 1mmHg lower CH was associated with 0.25%/year faster rate of VFI decline over time (P<0.001). The multivariable model showed that the effect of IOP on rates of progression depended on CH. Eyes with high IOP and low CH were at increased risk for having fast rates of disease progression. CH explained a larger proportion of the variation in slopes of VFI change than CCT (17.4% versus 5.2%, respectively).
Conclusion
CH measurements were significantly associated with risk of glaucoma progression. Eyes with lower CH had faster rates of visual field loss than those with higher CH. The prospective longitudinal design of this study supports the role of CH as an important factor to be considered in the assessment of the risk of progression in glaucoma patients.
INTRODUCTION
Detection of progression and assessment of rates of change is a cornerstone of glaucoma management. The disease is characterized by progressive optic nerve changes associated with functional losses over time, but the rate of deterioration can be markedly variable among patients.1–3 Assessment of rates of disease progression, along with considerations about life expectancy and treatment side effects, can help clinicians customize aggressiveness of therapy, in order to increase the chances that patients will avoid functional impairment during lifetime.4
Identification of baseline risk factors associated with rates of disease progression may help clinicians identify those patients at high risk for having rapid deterioration which could lead to functional impairment. High intraocular pressure (IOP) has been regarded as the main risk factor for the disease and a positive relationship between IOP levels and glaucoma progression has been shown in numerous clinical trials.5–9 More recently, attention has also been directed towards other potential risk factors such as thin central corneal thickness (CCT). Results from the Ocular Hypertension Treatment Study10 and Early Manifest Glaucoma Trial5 suggest that eyes with thin corneas are at increased risk for development and progression of glaucoma. Although the relationship between CCT and risk of disease progression may be due to an induced tonometric artifact, there has also been speculation that CCT could be an independent risk factor for the disease.11, 12 This could be related to an association between corneal thickness and structural properties of the sclera at the level of the optic nerve head, although such relationship has not been supported by histologic studies.13, 14
Besides thickness, other corneal biomechanical properties have been suggested as potential risk factors for glaucoma, such as corneal hysteresis (CH). CH is a measure of the viscoelastic damping of the cornea which can be estimated by analyzing corneal responses to deformation induced by an air pulse. Such procedure is incorporated into the commercially available Ocular Response Analyzer (ORA, Reichert Instruments, Depew, NY, USA). The ability of the cornea to resist deformation might reflect the constitution of its extracellular matrix (ECM) which could be hypothesized to be related to the ECM composition of posterior ocular tissues related to glaucomatous damage, such as the lamina cribrosa and peripapillary sclera. An eye with a more deformable cornea could potentially have an optic disc that is more susceptible to IOP damage. Congdon et al15 showed that CH measurements were associated with increased risk of glaucoma progression, as determined by retrospective medical chart review. In another retrospective study, De Moraes and colleagues16 reported an association between lower CH values and faster glaucomatous progression. However, in both studies, CH measurements were not acquired at baseline, but rather at some time during follow-up or after progression had occurred. Therefore, it is not possible to determine from these studies whether the reported relationship between lower CH and disease progression actually reflects a cause or an effect. It is possible, for example, that lower CH values could actually be the result of progressive glaucomatous damage or hypotensive treatment, rather than being a true risk factor for disease progression.17
The purpose of this study was to evaluate the role of CH as a risk factor for progression in a cohort of glaucoma patients followed prospectively over time. Subjects had CH acquired at baseline and were longitudinally followed in order to determine the relationship between CH and rates of disease progression, after adjusting for confounding variables.
METHODS
This was an observational cohort study. Participants from this study were included in a prospective longitudinal study designed to evaluate optic nerve structure and visual function in glaucoma (DIGS – Diagnostic Innovations in Glaucoma Study) conducted at the Hamilton Glaucoma Center, University of California, San Diego. Participants in the DIGS were longitudinally evaluated according to a pre-established protocol that included regular follow-up visits in which patients underwent clinical examination and several other imaging and functional tests. All the data were entered in a computer database. All participants from the DIGS study who met the inclusion criteria described below were enrolled in the current study. Informed consent was obtained from all participants. The University of California San Diego Human Subjects Committee approved all protocols and the methods described adhered to the tenets of the Declaration of Helsinki.
Subjects were followed at 6-month intervals. At each visit during follow-up, subjects underwent a comprehensive ophthalmologic examination including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement using Goldmann applanation tonometry (GAT), gonioscopy, dilated fundoscopic examination, stereoscopic optic disc photography, and automated perimetry using Swedish Interactive Threshold Algorithm (SITA Standard 24 –2). All patients also had central corneal thickness (CCT) measurements obtained by a trained technician using ultrasound pachymetry (Pachette GDH 500, DGH Technology, Inc., Philadelphia, Pennsylvania, USA). Axial length measurements were acquired with IOLMaster (Carl-Zeiss Meditec, Dublin, CA). Only subjects with open angles on gonioscopy were included. Subjects were excluded if they presented best-corrected visual acuity less than 20/40, spherical refraction outside ± 5.0 diopters and/or cylinder correction outside 3.0 diopters, or any other ocular or systemic disease that could affect the optic nerve or the visual field.
The study included 114 eyes from 68 patients diagnosed with glaucoma, as determined on the baseline visit. Eyes were classified as glaucomatous if they had repeatable abnormal visual field test results on the baseline visits and/or a glaucomatous appearing optic disc based on masked stereophotograph assessment. An abnormal visual field was defined as a pattern standard deviation (PSD) outside of the 95% normal confidence limits, or a Glaucoma Hemifield Test result outside normal limits. Signs of glaucomatous damage to the optic nerve were considered diffuse or localized neuroretinal rim loss, excavation and retinal nerve fiber layer defects.
Standard Automated Perimetry
All visual fields were evaluated by the University of California, San Diego (UCSD) Visual Field Assessment Center (VisFACT).18 A minimum of 5 reliable SAP tests during a minimum of 2 years of follow-up were required for inclusion in the study. Visual fields with more than 33% fixation losses or false-negative errors, or more than 15% false-positive errors were excluded. The only exception was the inclusion of visual fields with false-negative errors of more than 33% when the field showed advanced disease. Visual fields were further reviewed for the following artifacts: lid and rim artifacts, fatigue effects, inappropriate fixation, evidence that the visual field results were due to a disease other than glaucoma (such as homonymous hemianopia), and inattention.18 The VisFACT requested repeats of unreliable visual field test results, and these were obtained whenever possible.
Evaluation of rates of visual field change during follow-up was performed using the Visual Field Index (VFI) provided by the Humphrey perimeter (Carl-Zeiss Meditec, Inc., Dublin, CA).19 The VFI is currently the standard method used to evaluate rates of visual field progression incorporated on the Humphrey Guided Progression Analysis (GPA) software. Evaluation of rates of functional loss in glaucoma eyes with the VFI has been suggested to be less susceptible than mean deviation to the effects of cataract or diffuse media opacities.19 The VFI can range from 100% (normal visual field) to 0% (perimetrically blind field).
Corneal Hysteresis Measurement
CH measurements were acquired at baseline using the ORA. Subjects underwent testing with the ORA by a trained technician. Three measurements were obtained for each eye and the average of the measurements per eye was considered for analysis. The ORA determines corneal biomechanical properties using an applied force-displacement relationship. Details of its operation have been previously described.20 During an ORA measurement, a precisely metered air pulse is delivered to the eye, causing the cornea to move inward, past a first applanation and move into a slight concavity. Milliseconds after the first applanation, the air pump generating the air pulse is shut down and the pressure applied to the eye decreases in an inverse-time, symmetrical fashion. As the pressure decreases, the cornea passes through a second applanated state while returning from concavity to its normal convex curvature. The two applanations take place within approximately 20 milliseconds, a time sufficiently short to ensure that ocular pulse effects or eye position do not change during the measurement process. An electro-optical collimation detector system monitors the corneal curvature in the central 3.0 mm diameter throughout the 20 millisecond measurement period, based on the reflection of light from the cornea. When the cornea is flat (applanated), the reflection of light is maximal, generating a peak. A filtered version of the detector signal defines two precise applanation times corresponding to two well-defined peaks produced by inward and outward applanation events. Two corresponding pressures of an internal air supply plenum are determined from the applanation times derived from the detector applanation peaks. These 2 pressures are defined as the intersection of a vertical line drawn through the peaks of the applanation curve with the plenum pressure curve. The two applanation pressures are different primarily because of the biomechanical properties of the cornea. The difference between the two applanation pressures is the CH, measured in mmHg, and is related to the viscous damping property of the cornea.
The device provides a waveform score to reflect the quality of measurements. Only measurements associated with a waveform score greater than 5 were considered for inclusion.
Data Analysis
The evaluation of the effect of CH measurements on rates of visual field progression was performed using random-coefficient models. These models are a type of linear mixed model that involves both random intercepts and random slopes and that takes into account the clustered structure of the data. The details on the use of these models for evaluation of rates of change in glaucoma and to model longitudinal processes can be found elsewhere.3, 9, 21 In linear mixed models, the average evolution of the outcome variable (visual field measurements) is described using a linear function of time and random intercepts and random slopes introduce subject- and eye-specific deviations from this average evolution. The model can account for the fact that different eyes can have different rates of visual field loss over time, while also accommodating correlations between both eyes of the same individual.21, 22 Interaction terms between time and putative predictors (e.g., CH) can be included in the model to test whether there is a significant effect of the putative predictor on changes of the outcome variable over time. Several different predictors were investigated in this study, including baseline age, race, baseline CH, baseline GAT IOP, CCT and axial length. We initially constructed univariable models containing only one putative predictor along with its interaction with time. Subsequently, more complex models containing multiple predictors and interactions were constructed to evaluate the effect of certain predictors while adjusting for potentially confounding factors. The models had the following general form:
Yij = β1 + β2tij + β3*predictor[k] + β4*predictor[k]*tij + b1i + b2itij + eij, j = 1,…,ni
Yij corresponds to the visual field measurement Y for the ith eye at the jth measurement occasion. β1 and β2 correspond to the fixed effects regression parameters for intercept and slope, respectively. β3 corresponds to the effect of the kth predictor on the baseline (intercept) visual field measurement and β4 corresponds to the effect of the kth predictor on the changes of visual field measurements over time. The coefficients b1i and b2i correspond to the random effects for intercept and slope for each eye. The β’s are assumed to be the same for all individuals and have population-averaged interpretations, whereas the bi’s correspond to eye-specific regression parameters and indicate how the conditional mean response for an individual eye deviates from the population average. An unstructured covariance between random effects was assumed, allowing for correlation between intercepts and slopes of change. This is important as rates of visual field loss may depend on disease severity. The evaluation of the impact of different predictors in explaining variation in the linear mixed models was performed using the method described by Xu.23
Estimates of rates of change for individual eyes were obtained by best linear unbiased prediction (BLUP) from the mixed models. BLUPs have many advantages over ordinary least square (OLS) estimates. OLS estimates can be very imprecise in eyes with large intra-individual variability.24, 25 Individual OLS estimates (i.e., individual regression lines) also do not take into account the information provided by the whole population, whereas BLUPs are shrinkage estimates that take into account the results obtained by evaluating the whole sample of eyes giving less weight to estimates obtained from eyes with few measurement occasions and/or large intra-individual variability (that is, more “noise”).26 In eyes with large number of measurements over time, BLUP and OLS estimates give similar results. We have previously used BLUPs to estimate individual rates of change measured by different instruments in glaucoma.3, 25, 27
Statistical Analyses were performed using STATA v. 12.0 (StataCorp, College Station, Texas). The alpha level (type I error) was set at 0.05.
RESULTS
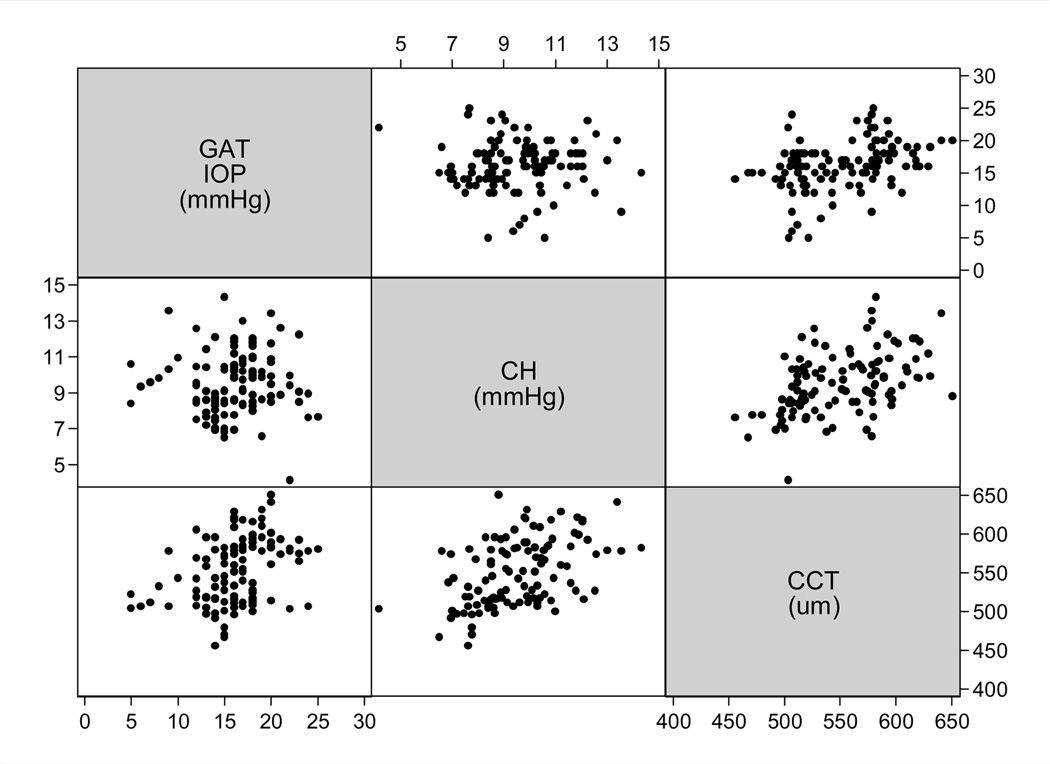
The study included 114 eyes of 68 glaucoma patients followed for an average of 4.0 ± 1.1 years (range: 2.0 to 6.5 years). Included eyes had a median number of 7 (range: 5 to 12) visual field tests during follow-up. Table 1 shows baseline clinical and demographic information for the eyes included in the study. Figure 1 shows a scatterplot matrix illustrating the relationship between GAT IOP, CCT and CH. GAT IOP was significantly influenced by CCT (r = 0.36; P<0.001) but not by CH (r = 0.01; P = 0.93). There was a statistically significant relationship between CCT and CH (r = 0.48; P<0.001).
Table 1.
Clinical and demographic characteristics for eyes/subjects included in the study.
| Parameter | |
|---|---|
| Age (years) | 68.3 ± 9.8 |
| Gender, female (%) | 40 (57%) |
| Race | |
| Caucasian, n (%) | 52 (74) |
| African-American, n (%) | 18 (26) |
| Baseline Mean Deviation (dB) | −2.45 ± 3.22 |
| Baseline VFI (%) | 94 ± 9 |
| Baseline PSD (dB) | 3.32 ± 2.84 |
| Baseline Corneal hysteresis mmHg) | 9.5 ± 1.7 |
| Baseline GAT IOP (mmHg) | 16.1 ± 3.8 |
| CCT (µm) | 551 ± 43 |
| Axial length (mm) | 24.1 ± 1.2 |
Values are given in mean ± standard deviation, unless otherwise noted. VFI - visual field index; PSD - pattern standard deviation, CCT - central corneal thickness; GAT - Goldmann applanation tonometry; IOP - intraocular pressure.
Figure 1.
Scatterplot matrix illustrating the relationship between corneal hysteresis, corneal thickness and Goldmann applanation tonometry (GAT) measurements. IOP = intraocular pressure; CH = corneal hysteresis
CH had a significant effect on rates of visual field progression over time. In the univariable model including only hysteresis as a predictive factor along with time and their interaction, each 1mmHg lower CH was associated with 0.25%/year faster rate of VFI decline over time (P<0.001). Figure 2 illustrates the relationship between VFI values over time and CH. Table 2 shows the effect of each putative predictive factor on the rates of VFI loss over time according to the univariable models. Baseline GAT IOP was also significantly associated with rates of VFI change over time, with each 1mmHg higher baseline GAT IOP associated with 0.11%/year faster rate of VFI loss. In the univariable model, CCT had only a borderline association with rates of change, although it significantly influenced baseline VFI measurements. The associations between baseline age, race, and axial length with rates of VFI change were not statistically significant.
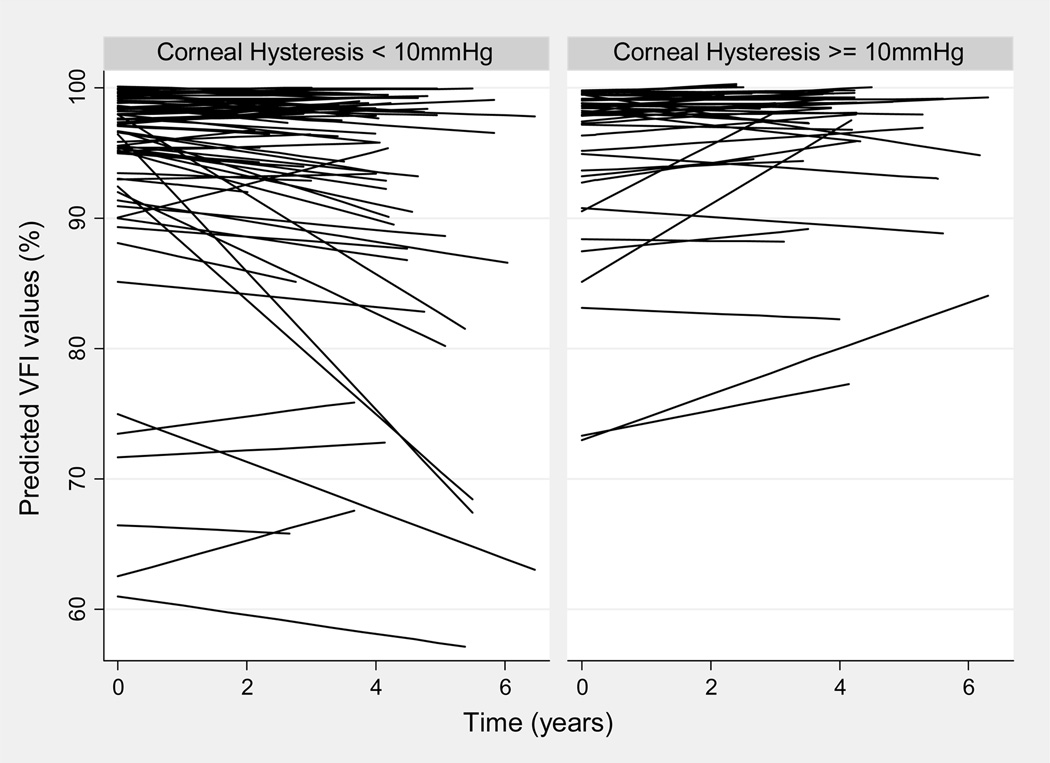
Figure 2.
Relationship between Visual Field Index (VFI) values over time and corneal hysteresis measurements as predicted by the linear mixed model. For illustrative purposes, eyes were divided into those with corneal hysteresis lower than 10mmHg and greater than or equal to 10mmHg. The plot shows that eyes with lower corneal hysteresis values tended to show worse declines of VFI values over time.
Table 2.
Results of univariable models assessing the effect of each putative predictive factor on Visual Field Index measurements at baseline and over time.
| Parameter | Effect on baseline (intercept) | Effect on change over time | ||
|---|---|---|---|---|
| β3 (SE) | P | β4 (SE) | P | |
| Baseline Age (per 1 year older) | −0.05 (0.08) | 0.52 | −0.006 (0.014) | 0.65 |
| Race (African American) | −1.19 (1.56) | 0.44 | −0.32 (0.29) | 0.27 |
| Baseline Corneal Hysteresis (per 1mmHg lower) | −0.16 (0.46) | 0.73 | −0.25 (0.07) | <0.001 |
| Baseline IOP (per 1mmHg higher) | 1.10 (0.18) | <0.001 | −0.11 (0.03) | 0.001 |
| Central cornel thickness (per 100µm thinner) | −5.91 (1.74) | 0.001 | −0.57 (0.30) | 0.057 |
| Axial length (per 1mm longer) | 1.26 (0.63) | 0.046 | −0.10 (0.11) | 0.36 |
The coefficient β3 corresponds to the effect of each predictive factor on the baseline visual field index (VFI) measurements. Negative values correspond to lower VFI measurements at baseline. Coefficient β4 corresponds to the effect on change over time. Negative values correspond to faster VFI decline over time. Refer to the equation presented on the methods. SE - standard error; IOP - intraocular pressure.
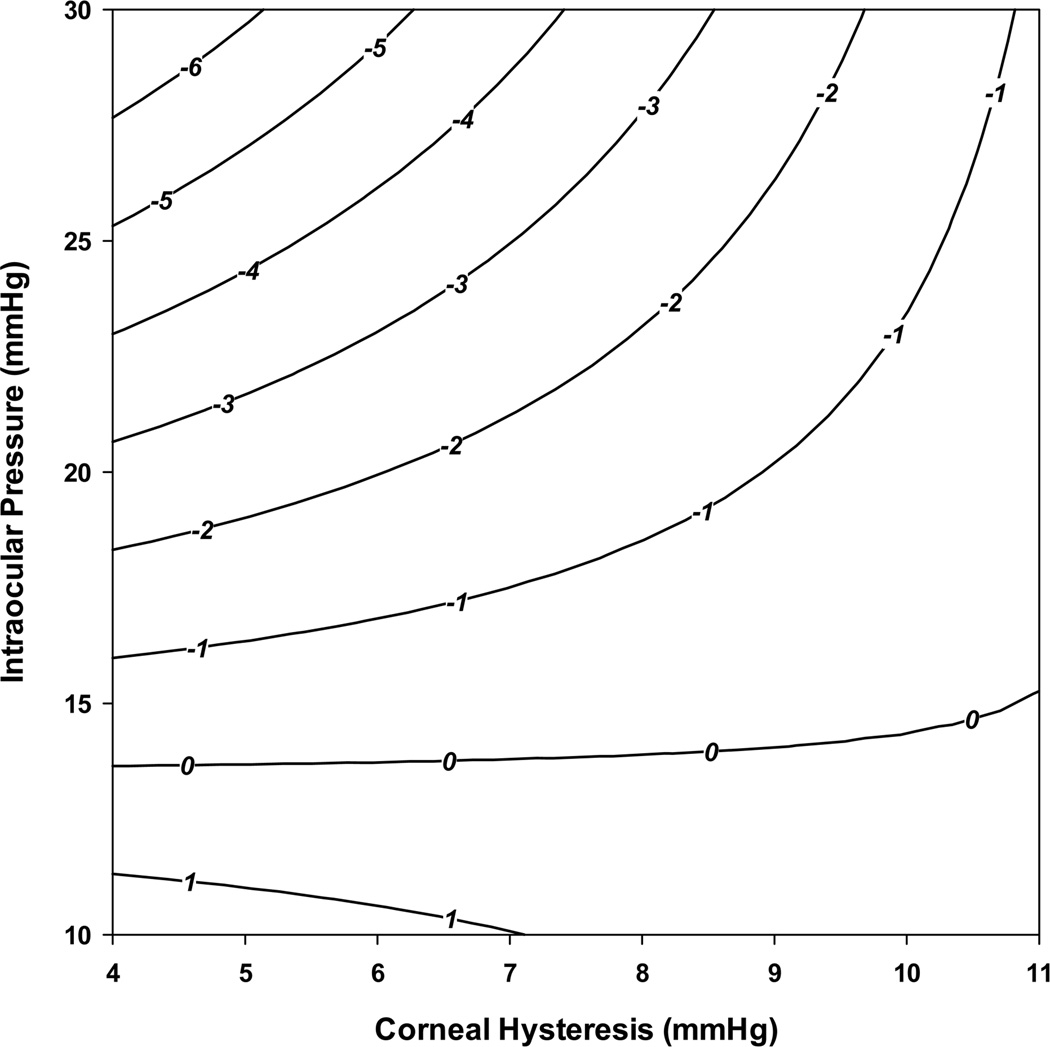
Table 3 shows the results of the multivariable model investigating the effects of baseline CH on rates of VFI change over time, adjusting for the effect of age, race, axial length, baseline GAT IOP, and CCT. A third-order interaction term between CH, GAT IOP and time was also included in order to investigate whether the effect of GAT IOP on the rate of visual field loss was different according to different levels of CH. The interaction term was statistically significant (β= −0.054; P = 0.001) as well as a combined Wald test for the effect of CH on the rate of visual field loss (P <0.001). This result indicates that in eyes with lower CH, IOP had a significantly larger impact on rates of visual field loss compared to eyes with higher CH. For example, in eyes with CH of 5mmHg, each 1mmHg higher IOP was associated with 0.38%/year faster rate of VFI loss. For eyes with CH of 10mmHg, each 1mmHg higher IOP was associated with 0.11%/year faster rate of VFI loss. Similarly, the impact of CH on rates of visual field loss depended on IOP levels. For eyes with baseline IOP of 15mHg, each 1mmHg lower CH was associated with only 0.10%/year faster rate of VFI decline, whereas for IOP of 30mmHg, each 1mmHg lower CH was associated with 0.89%/year faster rate of VFI loss. These results indicate that a combination of high IOP and low CH will be particularly detrimental in terms of rates of VFI loss. For example, considering average values of other variables, an eye with baseline IOP of 30mmHg and CH of 5mmHg would have an estimated rate of VFI change of −6.1%/year. In contrast, an eye with baseline IOP of 15mmHg and CH of 10mmHg would have an estimated rate of change of essentially zero. Figure 3 illustrates the relationship between CH, IOP and predicted rates of VFI change over time. Corneal thickness was also statistically significantly associated with rates of VFI loss in the multivariable model. Each 100µm thinner cornea was associated with 0.76%/year faster rate of VFI loss. The multivariable model explained 49% of the variation in the slopes of VFI change. CH explained 17.4% of the variation, whereas CCT explained only 5.2%.
Table 3.
Results of multivariable linear mixed effects model investigating the effect of corneal hysteresis on Visual Field Index (VFI) decline over time, adjusting for potentially confounding factors.
| Parameter | β (SE) | P |
|---|---|---|
| Intercept (%) | 94.9 (0.74) | <0.001 |
| Time (%/year) | 0.20 (0.13) | 0.11 |
| Baseline Age (per 1 year older) | −0.07 (0.07) | 0.35 |
| Baseline Age × time | 0.012 (0.012) | 0.33 |
| Race (African American) | −1.11 (1.43) | 0.44 |
| Race × time | 0.15 (0.26) | 0.57 |
| Baseline Corneal hysteresis (per 1 mmHg lower) | 0.27 (0.46) | 0.56 |
| Baseline Corneal hysteresis × time | −0.16 (0.07) | 0.03 |
| Baseline intraocular pressure (per 1 mmHg higher) | 0.97 (0.19) | <0.001 |
| Baseline intraocular pressure × time | −0.13 (0.03) | <0.001 |
| Baseline intraocular pressure × Baseline corneal hysteresis × time |
−0.054 (0.017) | 0.001 |
| Central corneal thickness (per 100 µm thinner) | −2.7 (2.0) | 0.180 |
| Central corneal thickness × time | −0.78 (0.34) | 0.022 |
| Axial length (per 1 mm longer) | 0.34 (0.58) | 0.55 |
| Axial length × time | −0.07 (0.10) | 0.47 |
The interaction terms with time correspond to the effect of the predictive factor on rate of VFI change over time. Negative values correspond to faster rate of VFI decline over time. Variables were centered at their mean values. SE - standard error.
Figure 3.
Contour plot illustrating the relationship between predicted rates of Visual Field Index (in %/year) change, intraocular pressure and corneal hysteresis measurements.
DISCUSSION
In the current study, we demonstrated that CH was significantly associated with the rate of visual field loss in a cohort of glaucoma patients followed over time. Eyes with lower baseline hysteresis tended to progress significantly faster than those with higher hysteresis values. Such relationship was present even in a multivariable model adjusting for other factors known to potentially affect rates of glaucoma progression. To our knowledge, this is the first prospective longitudinal study to evaluate the role of CH as a risk factor for glaucoma progression. Our findings suggest that evaluation of CH may add significant information to the assessment of the risk of disease progression and prediction of rates of change in the disease.
In the univariable model, each 1mmHg lower CH was associated with 0.25%/year faster rate of VFI decline over time (P<0.001). However, analysis based on the multivariable model showed that the relationship between CH and progression was more complex. We found a significant interaction between IOP and CH, suggesting that the effect of IOP on rates of glaucoma progression was dependent on the CH levels. For eyes with lower CH, the impact of IOP was significantly larger than in eyes with higher CH levels. For example, in eyes with CH of 5mmHg, each 1mmHg higher IOP was associated with 0.38%/year faster rate of VFI loss, whereas the same change in IOP was associated with only 0.11%/year faster rate of VFI loss for eyes with CH of 10mmHg. Although such numbers may appear deceptively small, they actually translate into large differences in the amount of field loss if the differences in IOP are maintained over long-term. The combination of low CH and high IOP was particularly detrimental, as shown on Figure 3. For example, considering average values of other variables, an eye with baseline IOP of 30mmHg and CH of 5mmHg had an estimated rate of VFI change of −6.1%/year, which would translate into an approximate decrease of 30% in VFI values over a 5-year period. Our findings that CH could act as a modifier of the effect of IOP is in agreement with previous studies suggesting that hysteresis could be related to the susceptibility of the optic disc to damage induced by IOP.28
Hysteresis is a physical property related to the ability of connective tissues to dampen pressure changes. Such property is related to the extracellular matrix constituents of the cornea, which may be related to those of the posterior ocular tissues. This has led to speculations that CH could be related to the viscoelastic properties of the posterior segment of the eye, in special of the lamina cribrosa and peripapillary sclera. The lamina and peripapillary sclera are the main load-bearing structures of the optic nerve head and computational models have shown that scleral stiffness and collagen fiber organization dictate the IOP-induced deformation by the optic nerve head.29 There is evidence from theoretical and experimental models to suggest that a stiffer sclera may be protective against the development of glaucoma.30, 31 Monkey eyes with stiff or thick sclera seem to be less prone to biomechanical changes in response to chronic IOP elevation.30 In addition, a clinical study by Wells et al28 found that CH was associated with deformation of the optic nerve surface during transient elevations of IOP. Our finding are compatible with this hypothesis by demonstrating that the effect of IOP on glaucoma progression depended on CH values.
Cross-sectional studies have shown that glaucoma patients have lower CH values than healthy subjects.15, 32, 33 In addition, glaucoma patients with asymmetric disease in both eyes have lower CH values in the eye with more severe damage.34 In a retrospective medical chart review, Congdon et al15 found that CH measurements, but not CCT, were associated with past history of progression. In another retrospective study, De Moraes and colleagues16 reported an association between lower CH values and faster glaucomatous progression. However, in both studies, CH measurements were not acquired at baseline, but rather at variable points during follow-up. Therefore, these studies could not clarify whether lower CH was a consequence of, or a predisposition towards, the development or progression of glaucoma. In fact, it is possible that the ocular structures may change their biomechanical properties as a result of the increase in IOP.35 By collecting baseline information on CH and evaluating the patients in a longitudinal prospective design, we avoided the main confounding factor limiting previous investigations. Our findings suggest that CH is a factor related to glaucoma progression, rather than just an epiphenomenon resulting from the disease process.
Corneal thickness was also a significant risk factor for progression in our study. Eyes with thinner corneas had faster rates of decline of VFI values compared to those with thicker corneas in the multivariable model. This is in agreement with results from previous longitudinal studies supporting the role of CCT as a predictive factor for both development as well as progression of glaucoma.5, 10, 36, 37 However, a comparison between the effects of CH and CCT revealed that while CH explained 17.4% of the variation in the rates of progression, CCT explained only 5.2%. This finding is in agreement with the previous study by De Moraes et al16 suggesting that CH may be a more important risk factor related to rates of glaucomatous visual field loss. It is important to note that as GAT IOP measurements are ultimately dependent on CCT, it is not possible to establish the role of CCT as a true independent risk factor for glaucoma beyond what can be achieved by statistical adjustment in a multivariable model.12 Contrary to CCT, we did not find GAT IOP to be influenced by CH in the present study, a finding similar to other investigations.38 However, other studies have described a weak but significant relationship between GAT IOP and CH and this could confound the determination of the independent role of CH as a risk factor for progression.39, 40,42 It should be noted, however, that even though part of the predictive effect of CH might be explained by its effect on GAT measurements, CH would still be a valid and relevant predictive factor, as shown by the model presented in our study. Further studies should clarify the role of CH as a true independent risk factor for glaucoma progression by evaluating its role in multivariable models including cornealindependent IOP measurements. In addition, although our findings suggest that CH may be a more important risk factor than CCT, it will be important to perform external validation of predictive models that include CH, before this parameter can be fully incorporated into clinical practice.
Our study has limitations. We assumed a linear rate of visual field loss over time. Several studies have suggested that functional changes do not follow a linear course over the natural history of the disease,41, 42 which might be related to the logarithmic scaling (decibel) of visual field sensitivity data. Nevertheless, the assumption of linear change is probably a reasonable one for short and medium follow-up periods, as performed in clinical practice. We used the VFI to assess rates of visual field progression as it is the current standard method used in the Humphrey Field Analyzer. However, limitations of the VFI in assessing visual field progression in early disease have been described.43 When we performed analyses using mean deviation instead of VFI, similar results were obtained in univariable and multivariable models.
In conclusion, CH measurements were significantly associated with risk of glaucoma progression. Eyes with lower hysteresis had faster rates of visual field loss than those with higher hysteresis. The prospective longitudinal design of this study supports the role of CH as an important factor to be considered in the assessment of the risk of progression in glaucoma patients.
Acknowledgments
Supported in part by NIH/NEI grants EY021818 (FAM), EY11008 (LMZ), EY14267 LMZ), CAPES grant BEX 1066/11-0, an unrestricted grant from Research to Prevent Blindness (New York, NY), grant for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck and Santen. Financial Disclosures: Research support from Reichert, Inc. (FAM), Carl-Zeiss Meditec (FAM, LMZ, RNW). Consultant to Carl-Zeiss Meditec, Inc. (RNW).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Collaborative Normal-Tension Glaucoma Study Group. Natural history of normal-tension glaucoma. Ophthalmology. 2001;108:247–253. doi: 10.1016/s0161-6420(00)00518-2. [DOI] [PubMed] [Google Scholar]
- 2.Heijl A, Bengtsson B, Hyman L, Leske MC. Early Manifest Glaucoma Trial Group. Natural history of open-angle glaucoma. Ophthalmology. 2009;116:2271–2276. doi: 10.1016/j.ophtha.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 3.Medeiros FA, Zangwill LM, Alencar LM, et al. Rates of progressive retinal nerve fiber layer loss in glaucoma measured by scanning laser polarimetry. Am J Ophthalmol. 2010;149:908–915. doi: 10.1016/j.ajo.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Medeiros FA, Susanna R, Jr, Singh K. Who should be treated? In: Weinreb RN, Liebmann J, editors. Medical Treatment of Glaucoma. Amsterdam, The Netherlands: Kugler Publ.; 2010. pp. 1–19. [Google Scholar]
- 5.Leske MC, Heijl A, Hyman L, et al. EMGT Group. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 6.European Glaucoma Prevention Study (EGPS) Group. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112:366–375. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 8.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 9.Medeiros FA, Alencar LM, Zangwill LM, et al. The relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009;116:1125–1133. doi: 10.1016/j.ophtha.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon MO, Beiser JA, Brandt JD, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 11.Brandt JD, Gordon MO, Gao F, et al. Ocular Hypertension Treatment Study Group. Adjusting intraocular pressure for central corneal thickness does not improve prediction models for primary open-angle glaucoma. Ophthalmology. 2012;119:437–442. doi: 10.1016/j.ophtha.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros FA, Weinreb RN. Is corneal thickness an independent risk factor for glaucoma? Ophthalmology. 2012;119:435–436. doi: 10.1016/j.ophtha.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonas JB, Hayreh SS, Tao Y. Central corneal thickness and thickness of the lamina cribrosa and peripapillary sclera in monkeys [letter] Arch Ophthalmol. 2009;127:1395–1396. doi: 10.1001/archophthalmol.2009.243. [DOI] [PubMed] [Google Scholar]
- 14.Jonas JB, Holbach L. Central corneal thickness and thickness of the lamina cribrosa in human eyes. Invest Ophthalmol Vis Sci. 2005;46:1275–1279. doi: 10.1167/iovs.04-0851. [DOI] [PubMed] [Google Scholar]
- 15.Congdon NG, Broman AT, Bandeen-Roche K, et al. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006;141:868–875. doi: 10.1016/j.ajo.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 16.De Moraes CV, Hill V, Tello C, et al. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma. 2012;21:209–213. doi: 10.1097/IJG.0b013e3182071b92. [DOI] [PubMed] [Google Scholar]
- 17.Brandt JD, Gordon MO, Beiser JA, et al. Ocular Hypertension Treatment Study Group. Changes in central corneal thickness over time: the Ocular Hypertension Treatment Study. Ophthalmology. 2008;115:1550–1556. doi: 10.1016/j.ophtha.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Racette L, Liebmann JM, Girkin CA, et al. ADAGES Group. African Descent and Glaucoma Evaluation Study (ADAGES): III. Ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010;128:551–559. doi: 10.1001/archophthalmol.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145:343–353. doi: 10.1016/j.ajo.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Luce DA. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J Cataract Refract Surg. 2005;31:156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 21.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 22.Laird NM, Donnelly C, Ware JH. Longitudinal studies with continuous responses. Stat Methods Med Res. 1992;1:225–247. doi: 10.1177/096228029200100302. [DOI] [PubMed] [Google Scholar]
- 23.Xu R. Measuring explained variation in linear mixed effects models. Stat Med. 2003;22:3527–3541. doi: 10.1002/sim.1572. [DOI] [PubMed] [Google Scholar]
- 24.Beckett LA, Tancredi DJ, Wilson RS. Multivariate longitudinal models for complex change processes. Stat Med. 2004;23:231–239. doi: 10.1002/sim.1712. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros FA, Zangwill LM, Weinreb RN. Improved prediction of rates of visual field loss in glaucoma using empirical Bayes estimates of slopes of change. J Glaucoma. 2012;21:147–154. doi: 10.1097/IJG.0b013e31820bd1fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson GK. That BLUP is a good thing: the estimation of random effects. Stat Sci. 1991;6:15–32. [Google Scholar]
- 27.Medeiros FA, Alencar LM, Sample PA, et al. The relationship between intraocular pressure reduction and rates of progressive visual field loss in eyes with optic disc hemorrhage. Ophthalmology. 2010;117:2061–2066. doi: 10.1016/j.ophtha.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Wells AP, Garway-Heath DF, Poostchi A, et al. Corneal hysteresis but not corneal thickness correlates with optic nerve surface compliance in glaucoma patients. Invest Ophthalmol Vis Sci. 2008;49:3262–3268. doi: 10.1167/iovs.07-1556. [DOI] [PubMed] [Google Scholar]
- 29.Sigal IA, Yang H, Roberts MD, et al. IOP-induced lamina cribrosa displacement and scleral canal expansion: an analysis of factor interactions using parameterized eye-specific models. Invest Ophthalmol Vis Sci. 2011;52:1896–1907. doi: 10.1167/iovs.10-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girard MJ, Suh JK, Bottlang M, et al. Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011;52:5656–5669. doi: 10.1167/iovs.10-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinhart MR, Cone FE, Nguyen C, et al. Mice with an induced mutation in collagen 8A2 develop larger eyes and are resistant to retinal ganglion cell damage in an experimental glaucoma model. [Accessed January 6, 2013];Mol Vis [serial online] 2012 18:1093–1106. Available at: http://www.molvis.org/molvis/v18/a116/ [PMC free article] [PubMed] [Google Scholar]
- 32.Abitbol O, Bouden J, Doan S, et al. Corneal hysteresis measured with the Ocular Response Analyzer in normal and glaucomatous eyes. Acta Ophthalmol. 2010;88:116–119. doi: 10.1111/j.1755-3768.2009.01554.x. [DOI] [PubMed] [Google Scholar]
- 33.Bochmann F, Ang GS, Azuara-Blanco A. Lower corneal hysteresis in glaucoma patients with acquired pit of the optic nerve (APON) Graefes Arch Clin Exp Ophthalmol. 2008;246:735–738. doi: 10.1007/s00417-007-0756-5. [DOI] [PubMed] [Google Scholar]
- 34.Anand A, De Moraes CG, Teng CC, et al. Corneal hysteresis and visual field asymmetry in open angle glaucoma. Invest Ophthalmol Vis Sci. 2010;51:6514–6518. doi: 10.1167/iovs.10-5580. [DOI] [PubMed] [Google Scholar]
- 35.Sun L, Shen M, Wang J et al. Recovery of corneal hysteresis after reduction of intraocular pressure in chronic primary angle-closure glaucoma. Am J Ophthalmol. 2009;147:1061–1066. doi: 10.1016/j.ajo.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Medeiros FA, Sample PA, Zangwill LM, et al. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136:805–813. doi: 10.1016/s0002-9394(03)00484-7. [DOI] [PubMed] [Google Scholar]
- 37.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123:1351–1360. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 38.Lau W, Pye DA. clinical description of Ocular Response Analyzer measurements. Invest Ophthalmol Vis Sci. 2011;52:2911–2916. doi: 10.1167/iovs.10-6763. [DOI] [PubMed] [Google Scholar]
- 39.Kaushik S, Pandav SS, Banger A, et al. Relationship between corneal biomechanical properties, central corneal thickness, and intraocular pressure across the spectrum of glaucoma. Am J Ophthalmol. 2012;153:840–849. doi: 10.1016/j.ajo.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 40.Mangouritsas G, Mourtzoukos S, Mantzounis A, Alexopoulos L. Comparison of Goldmann and Pascal tonometry in relation to corneal hysteresis and central corneal thickness in nonglaucomatous eyes. Clin Ophthalmol. 2011;5:1071–1077. doi: 10.2147/OPTH.S23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medeiros FA, Zangwill LM, Bowd C, et al. The structure and function relationship in glaucoma: implications for detection of progression and measurement of rates of change. Invest Ophthalmol Vis Sci. 2012;53:6939–6946. doi: 10.1167/iovs.12-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Artes PH, O'Leary N, Hutchison DM, et al. Properties of the Statpac visual field index. Invest Ophthalmol Vis Sci. 2011;52:4030–4038. doi: 10.1167/iovs.10-6905. [DOI] [PubMed] [Google Scholar]