Abstract
Objective
The purpose of this study was to evaluate the impact of prior antipsychotic exposure (PAE) on safety and tolerability outcomes in pediatric subjects receiving aripiprazole treatment.
Methods
This study was a post-hoc analysis of pooled data from two 8-week, double-blind, randomized, placebo-controlled studies evaluating aripiprazole for the treatment of irritability in pediatric subjects with autistic disorder, aged 6–17 years. Subjects were stratified by PAE; adverse events (AEs), and changes in weight, and metabolic measures were evaluated. For subjects receiving aripiprazole, regardless of PAE, baseline weight, age, gender, and symptom severity were evaluated in a regression model predicting body weight change.
Results
Of 316 randomized subjects, 259 (82.0%) were antipsychotic naïve (AN) and 57 (18.0%) had a PAE. Aripiprazole-treated AN subjects were more likely than PAE subjects to report somnolence (11.9% vs. 2.8%), sedation (22.7% vs. 11.1%), or fatigue (17.0% vs. 13.9%). Rates of extrapyramidal disorder and drooling, but not akathisia or tremor, were marginally higher in AN subjects. Overall, 10.8% of aripiprazole-treated AN subjects had at least one AE leading to discontinuation compared with 8.3% of aripiprazole-treated PAE subjects. AN subjects receiving aripiprazole had a larger change in weight from baseline to endpoint compared with those receiving placebo (1.9 vs. 0.7 kg; treatment difference 1.2 kg, 95% CI: 0.5, 1.9) than PAE subjects receiving aripiprazole compared with subjects receiving placebo (0.4 vs. –0.4 kg; treatment difference 0.9 kg, 95% CI: –0.6, 2.4). Regression analysis identified that younger subjects with higher baseline weight z-score were at highest risk for weight gain. There were no significant changes in metabolic measures compared with placebo in either group.
Conclusions
Weight gain was more pronounced in AN subjects and more likely to occur in younger subjects with a higher baseline weight z-score. AN subjects were more likely to experience AEs related to somnolence. However, based on discontinuations rates from AEs, overall tolerability was good for both AN and PAE groups.
Clinical trial registration
Study of aripiprazole in the treatment of children and adolescents with autistic disorder. Registry: www.clinicaltrials.gov. Identifiers: NCT00332241 and NCT00337571.
Introduction
Autistic disorder is characterized by impaired social interaction, abnormal language development, and repetitive and restricted patterns of behavior. Children with autistic disorder display broad differences in abilities and needs, but accompanying maladaptive behaviors, such as self-injurious behavior, aggression, and tantrums are common and frequently severe enough to limit their educational and developmental progress (RUPP 2005). A variety of treatments, including medication and educational interventions, have been employed in the management of these behaviors, and evidence has shown that atypical antipsychotics may be a reasonable option for some subjects (Myers and Johnson 2007; Owen et al. 2009). However, all antipsychotics have a potential impact on body composition and metabolic parameters, with significant but varying amounts of weight gain and wide-ranging metabolic changes associated with each antipsychotic, especially in antipsychotic-naïve (AN) pediatric subjects (Correll et al. 2009).
Aripiprazole is an atypical antipsychotic that has been approved by the United States Food and Drug Administration for the treatment of pediatric subjects (6–17 years) with irritability associated with autistic disorder. Two prior clinical trials have shown that aripiprazole is efficacious and well tolerated in pediatric subjects (Marcus et al. 2009; Owen et al. 2009). The data from these trials showed a greater increase in weight and a greater incidence of clinically significant weight gain (≥7% increase from baseline) in subjects receiving aripiprazole compared with those receiving placebo. However, these studies did not assess the effects of prior antipsychotic exposure (PAE) on weight gain or on other adverse events (AEs). Therefore, the current post-hoc analysis pooled data from these two 8-week, double-blind, randomized, placebo-controlled, parallel-group studies to evaluate the impact of PAE on AEs, discontinuations from AEs, weight gain, and metabolic changes, as well as to assess the potential predictive factors for weight gain.
Methods
Study design and subject inclusion
A detailed description of the methods for the two 8-week, randomized, double-blind, placebo-controlled trials in subjects aged 6–17 years with irritability associated with autistic disorder has been presented previously (Marcus et al. 2009; Owen et al. 2009). Both trials evaluated the efficacy of aripiprazole versus placebo using the caregiver-rated Aberrant Behavior Checklist irritability (ABC-I) (Aman and Singh 1986) subscale score as the primary outcome measure. The primary difference between the two trials was that one was flexibly dosed (2–15 mg/day; target dose of 5, 10, or 15 mg/day) and the other had a fixed-dose schedule. Subjects met Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR) criteria for autistic disorder, and demonstrated behaviors such as irritability, tantrums, aggression, self-injurious behavior, or a combination of these symptoms. Diagnosis was confirmed by the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994) using modified rater-training criteria. Subjects also were required to have a Clinical Global Impressions–Severity of Illness (CGI-S) scale (Guy 1976) score of ≥4 and an ABC-I subscale score of ≥18 at screening and baseline.
Statistical analyses
For the purposes of this post-hoc analysis, the data from the flexibly dosed trial (Owen et al. 2009) was combined and pooled with the data from the fixed-dose trial (Marcus et al. 2009). Subjects were stratified according to whether or not they had a PAE, defined as having received any antipsychotic during the screening phase, which could last up to 6 weeks, or within 2 weeks prior to screening. Subjects without antipsychotic exposure in this time-frame were classified as AN. Changes in weight and fasting metabolic measures from baseline to endpoint were evaluated compared with placebo using an analysis of covariance (ANCOVA) model with treatment and protocol as main effects and baseline assessment as covariate. Descriptive statistics were used to report AEs and discontinuations from AEs. AEs occurring in ≥5% of subjects in the pooled aripiprazole group and discontinuations from AEs occurring in ≥2% of subjects were also evaluated.
Predictive factors
Potential predictive factors for weight gain were assessed, regardless of PAE, using a linear regression model, with body weight change from baseline as the response variable. The factors included baseline weight, weight z-score, body mass index (BMI), BMI z-score, age, age category (either 6–12 or 13–17 years old), sex, ABC-I (Aman and Singh 1986) subscale score at baseline, and the CGI-S (Guy 1976) scale score at baseline. Significant factors identified were then evaluated simultaneously via a separate regression model, with body weight change from baseline as the response variable.
Results
Of 316 randomized subjects, 259 (82.0%) were AN, and 57 (18.0%) had a PAE. Baseline demographic characteristics were similar for AN and PAE subjects regardless of treatment assignment. Altogether, AN or PAE subjects receiving aripiprazole or placebo treatment had a mean age ranging from 9.4 to 10.0 years, 77.2–79.2% were between the ages of 6 and 12 years, and 87.3–96.5% were male. Mean baseline ABC-I scores ranged from 28.4 to 31.0. Mean baseline CGI-S scores for placebo and aripiprazole, respectively, were 4.8–4.9 for AN and 5.2–5.3 for PAE subjects.
AEs occurring in ≥5% of subjects in the pooled aripiprazole group are summarized in Table 1. In general, sedation, somnolence, and fatigue were much more common in AN subjects, and although extrapyramidal disorder, drooling, and salivary hypersecretion occurred more frequently in AN subjects, akathisia and tremor did not. Although vomiting also appeared more common in AN subjects, placebo-subtracted rates were similar between AN and PAE subjects.
Table 1.
Incidence of Treatment-Emergent Adverse Events Reported in ≥5% of Subjects Receiving Aripiprazole Treatment by Prior Antipsychotic Exposure, Safety Sample
| |
Antipsychotic naïve (n=256) |
Prior antipsychotic exposure (n=57) |
||
|---|---|---|---|---|
| Placebo (n=80) | Aripiprazole (n=176) | Placebo (n=21) | Aripiprazole (n=36) | |
| Akathisia | 4 (5.0) | 1 (0.6) | 0 | 2 (5.6) |
| Constipation | 3 (3.8) | 9 (5.1) | 1 (4.8) | 2 (5.6) |
| Cough | 4 (5.0) | 9 (5.1) | 0 | 4 (11.1) |
| Decreased appetite | 1 (1.3) | 13 (7.4) | 1 (4.8) | 1 (2.8) |
| Diarrhea | 7 (8.8) | 14 (8.0) | 2 (9.5) | 2 (5.6) |
| Drooling | 0 | 17 (9.7) | 0 | 2 (5.6) |
| Dry mouth | 0 | 1 (0.6) | 0 | 2 (5.6) |
| Extrapyramidal disorder | 0 | 12 (6.8) | 0 | 1 (2.8) |
| Fatigue | 2 (2.5) | 30 (17.0) | 0 | 5 (13.9) |
| Gastroenteritis, viral | 2 (2.5) | 4 (2.3) | 0 | 2 (5.6) |
| Headache | 7 (8.8) | 13 (7.4) | 3 (14.3) | 3 (8.3) |
| Increased appetite | 5 (6.3) | 23 (13.1) | 2 (9.5) | 4 (11.1) |
| Insomnia | 6 (7.5) | 6 (3.4) | 4 (19.0) | 5 (13.9) |
| Lethargy | 0 | 10 (5.7) | 0 | 0 |
| Nasal congestion | 0 | 7 (4.0) | 2 (9.5) | 2 (5.6) |
| Nasopharyngitis | 5 (6.3) | 13 (7.4) | 0 | 5 (13.9) |
| Pyrexia | 1 (1.3) | 18 (10.2) | 0 | 1 (2.8) |
| Restlessness | 3 (3.8) | 3 (1.7) | 0 | 2 (5.6) |
| Rhinorrhea | 2 (2.5) | 5 (2.8) | 0 | 3 (8.3) |
| Salivary hypersecretion | 1 (1.3) | 12 (6.8) | 0 | 0 |
| Sedation | 4 (5.0) | 40 (22.7) | 0 | 4 (11.1) |
| Somnolence | 3 (3.8) | 21 (11.9) | 1 (4.8) | 1 (2.8) |
| Tremor | 0 | 17 (9.7) | 0 | 4 (11.1) |
| Vomiting | 6 (7.5) | 27 (15.3) | 0 | 2 (5.6) |
AEs leading to discontinuation in aripiprazole-treated subjects occurred in 10.8% of AN and 8.3% of PAE subjects compared with 7.5% of AN and 4.8% of PAE placebo-treated subjects, leading to placebo-subtracted rates of 3.3% for AN and 3.5% for PAE subjects. AEs leading to discontinuation at an incidence of ≥2% in AN subjects receiving aripiprazole were: sedation (3.4%; n=6/176), drooling and tremor (2.3% each; n=4/176); for AN subjects who received placebo, only mania was observed at a frequency >2% (2.5%; n=2/80). For PAE subjects, AEs leading to discontinuation at a ≥2% incidence in the aripiprazole treatment group were akathisia, extrapyramidal disorder, sedation, tachycardia, weight increase, increased appetite, and hypertension (2.8% each; n=1/36); for the placebo group, convulsion was reported in one subject (4.8%), and it led to discontinuation.
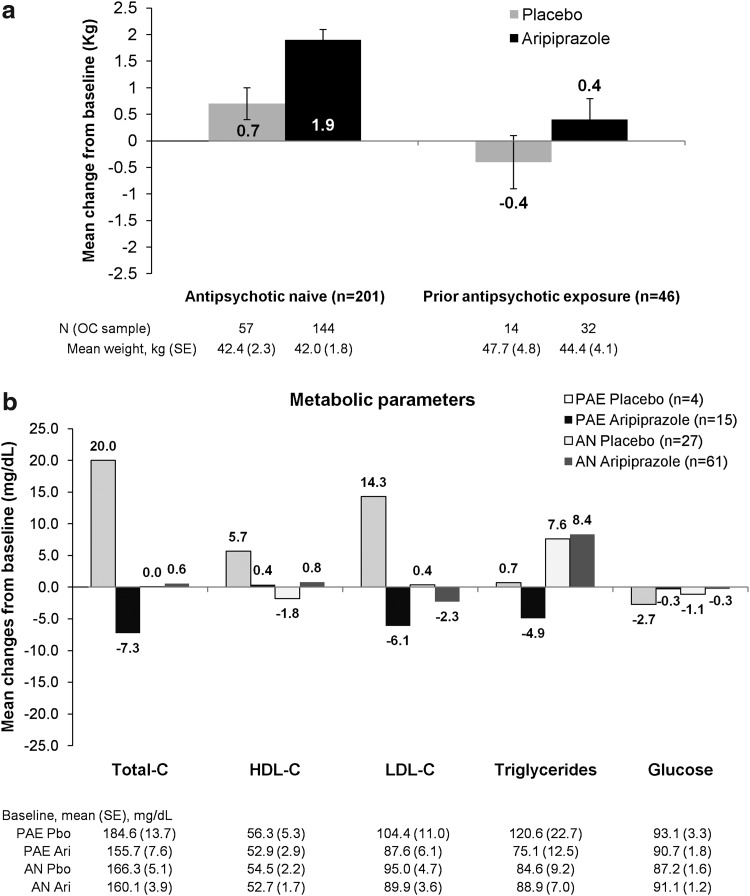
Aripiprazole-treated subjects gained more weight than those receiving placebo, regardless of prior exposure. In AN subjects, the treatment difference (aripiprazole–placebo) was 1.2 kg, 95% CI: 0.5, 1.9. In PAE subjects, the treatment difference (aripiprazole–placebo) was 0.9 kg, 95% CI: −0.6, 2.4 (Fig. 1a). In AN subjects, the incidence of clinically significant weight gain (≥7% increase from baseline) was significantly greater in subjects receiving aripiprazole compared with those receiving placebo (relative risk [RR]: 4.6; 95% CI: 1.8, 12.1). For PAE subjects receiving aripiprazole, three subjects (n=32; 9.4%) reported clinically significant weight gain compared with no subjects receiving placebo. Changes in fasting metabolic parameters from baseline to endpoint in aripiprazole-treated subjects (Fig. 1b) compared with placebo-treated subjects were small (or negative), and none were statistically significant (95% CIs of the difference all included zero).
FIG. 1.
Mean changes in weight and fasting metabolic parameters (mg/dL±SE) from baseline to endpoint (Week 8, OC), by prior exposure to antipsychotics. AN, antipsychotic naïve; Ari, aripiprazole; C, cholesterol; PAE, prior antipsychotic exposure; Pbo, placebo; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OC, observed cases; SE, standard error.
In the initial regression model evaluating potential predictive factors for weight gain, baseline weight z-score, baseline BMI z-score, age, and age category (6–12 years, 13–17 years) were statistically significant factors for the aripiprazole-treated group (p<0.05). When the latter factors were evaluated simultaneously in a separate regression model, baseline weight z-score and age category were identified as potential predictors of weight gain. When the mean change from baseline to endpoint in body weight was evaluated based on age group and baseline weight z-score, 6–12-year-olds with a baseline weight z-score ≥2 gained an average (±standard error) 2.4±2.8 kg (n=28) compared with 1.8±4.1 kg (n=8) in 13–17 year olds with the same weight z-score cutoff. For 6–12-year-olds with a baseline weight z-score of 0–1, the mean weight change from baseline was 1.8±1.7 kg (n=41) compared with −1.2±3.2 (n=8) in 13–17 year olds with a similar baseline weight z-score.
Discussion
Data from this post-hoc analysis of pediatric subjects with irritability associated with autistic disorder demonstrated that AN subjects receiving aripiprazole have a different AE profile in terms of weight gain and somnolence-related AEs compared with subjects with a PAE. AN subjects receiving aripiprazole had greater mean changes in weight and were more likely to experience clinically significant weight gain (≥7% weight gain from baseline) than placebo subjects. PAE subjects receiving aripiprazole also had greater mean changes in weight compared with placebo subjects, but to a smaller degree. A regression model analysis, independent of prior exposure, identified baseline weight z-score and age as potential predictors of weight gain. Despite the small number of subjects in each group and the substantial variance in weight changes, there was a trend indicating that younger subjects with a higher baseline weight z-score gained the most weight across z-score and age categories. Overall, changes in fasting metabolic parameters from baseline to endpoint for AN subjects were <5 mg/dL, except for triglycerides, regardless of treatment. PAE subjects receiving aripiprazole showed decreases in total cholesterol, low-density lipoprotein cholesterol, triglycerides, and glucose, and a small increase in high-density lipoprotein cholesterol. Regarding AEs, it is clear that AN subjects had the highest risk of a new-onset event related to somnolence, sedation, or fatigue. The relationship to PAE and extrapyramidal symptoms, however, did not appear to be as strong.
Prior evidence has shown that AN children and adolescents treated with atypical antipsychotics can exhibit clinically significant weight gain (Correll et al. 2009). The current analysis, specific to subjects with autistic disorder, showed that AN subjects receiving aripiprazole exhibited greater weight gain compared with placebo than those with a PAE. However, the weight gain appears to plateau over time for subjects with irritability associated with autistic disorder receiving long-term aripiprazole treatment (Marcus et al. 2011). The finding that heavier children had the greatest increase in weight with antipsychotic exposure may reflect an underlying predisposition to weight gain, perhaps genetic, regardless of etiology (antipsychotic induced or otherwise). Interestingly, this finding in children is different from that seen in adults, in which subjects with lower BMI had the greatest weight gain. A possible and simple explanation for this may be related to pubertal status – pre-pubertal patients are physiologically “set up” to take on weight, and this may be amplified in those heavier patients who are genetically predisposed. In the current analysis, AN subjects also had a higher risk for somnolence-related AEs. Therefore, clinicians who treat children and adolescents with antipsychotics should always be mindful of the potential for weight gain and somnolence-related AEs, among other AEs, as they are more likely to occur in AN patients.
Limitations
The findings reported here should be considered in light of potential limitations, such as the post-hoc nature of the analysis, and that not all subjects classified as AN were entirely AN throughout their lifetime. However, to capture potentially “new to treatment” AEs, subjects with exposures that had occurred at least 2 weeks prior to screening, but not since, were included. In addition, results should be interpreted with caution as the number of subjects in the PAE group was small. Finally, an 8-week trial does not provide insights into longer-term treatment.
Conclusions
AN subjects receiving aripiprazole for the treatment of irritability associated with autistic disorder showed greater risk for weight gain and somnolence-related AEs than subjects receiving placebo. Changes in metabolic parameters in AN subjects receiving aripiprazole treatment were small and similar to those in subjects receiving placebo. Clinicians who treat children and adolescents with antipsychotics should be aware of the potential for metabolic disturbances associated with treatment, and should regularly monitor physical health.
Clinical Significance
Atypical antipsychotics are used for the management of irritability associated with autistic disorder. However, all antipsychotics have a potential impact on body composition and metabolic parameters, with significant but varying amounts of weight gain and wide-ranging metabolic changes associated with each antipsychotic, especially in antipsychotic-AN pediatric subjects. The findings presented here showed that AN subjects receiving aripiprazole for the treatment of irritability associated with autistic disorder showed greater risk for weight gain and somnolence-related AEs than subjects receiving placebo. However, changes in metabolic parameters in AN subjects receiving aripiprazole treatment were small and similar to those in subjects receiving placebo. Nevertheless, clinicians who treat children and adolescents with antipsychotics should be aware of the potential for metabolic disturbances associated with treatment, and should regularly monitor physical health.
Disclosures
George Manos, Sabrina Marler, and Ronald Marcus are employees of Bristol-Myers Squibb. Raymond Mankoski is a former employee of Bristol-Myers Squibb and is currently employed with Genzyme. Gwen Stockton and Robert McQuade are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. Robert A Forbes is a former employee of Otsuka Pharmaceutical Development & Commercialization, Inc. and is currently employed with Genentech.
This study was supported by Bristol-Myers Squibb (Princeton, NJ) and Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan). Editorial support for the preparation of this manuscript was provided by Ogilvy Healthworld Medical Education.
Acknowledgments
The authors thank the participants and their families for their involvement in this study.
References
- Aman MG. Singh NN. Aberrant Behavior Checklist: Manual. East Aurora, NY: Slosson Educational Publications; 1986. [Google Scholar]
- Correll CU. Manu P. Olshanskiy V. Napolitano B. Kane JM. Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. Rockville, MD: National Institute of Mental Health; 1976. Clinical Global Impressions (CGI). ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare Publication (ADM) 76-338; pp. 218–222. [Google Scholar]
- Lord C. Rutter M. Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Marcus RN. Owen R. Kamen L. Manos G. McQuade RD. Carson WH. Aman MG. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:1110–1119. doi: 10.1097/CHI.0b013e3181b76658. [DOI] [PubMed] [Google Scholar]
- Marcus RN. Owen R. Manos G. Mankoski R. Kamen L. McQuade RD. Carson WH. Corey-Lisle PK. Aman MG. Aripiprazole in the treatment of irritability in pediatric patients (aged 6–17 years) with autistic disorder: Results from a 52-week, open-label study. J Child Adolesc Psychopharmacol. 2011;21:229–236. doi: 10.1089/cap.2009.0121. [DOI] [PubMed] [Google Scholar]
- Myers SM. Johnson CP. Management of children with autism spectrum disorders. Pediatrics. 2007;120:1162–1182. doi: 10.1542/peds.2007-2362. [DOI] [PubMed] [Google Scholar]
- Owen R. Sikich L. Marcus RN. Corey-Lisle P. Manos G. McQuade RD. Carson WH. Findling RL. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics. 2009;124:1533–1540. doi: 10.1542/peds.2008-3782. [DOI] [PubMed] [Google Scholar]
- RUPP: Research Units on Pediatric Psychopharmacology Autism Network. Risperidone treatment of autistic disorder: Longer-term benefits and blinded discontinuation after 6 months. Am J Psychiatry. 2005;162:1361–1369. doi: 10.1176/appi.ajp.162.7.1361. [DOI] [PubMed] [Google Scholar]