Abstract
Objective
The purpose of this study was to investigate associations between body weight and illness characteristics, including weight gain and therapeutic efficacy, in adolescents with schizophrenia.
Methods
Adolescents ages 13–17 years (n=107) with American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) schizophrenia enrolled in a 6 week, double-blind, placebo-controlled trial comparing olanzapine and placebo. Therapeutic response was assessed by the Brief Psychiatric Rating Scale for Children (BPRS-C). Secondary outcomes included the Clinical Global Impressions-Severity (CGI-S) scale and Positive and Negative Syndrome Scale (PANSS). Obesity was defined as sex-/age-adjusted body mass index (BMI)≥95th percentile. Linear regression was used to analyze the relationship between weight gain and psychiatric symptom improvement; logistic regression was conducted to identify predictors of baseline obesity.
Results
Weight gain was significantly correlated with greater BPRS-C reduction among olanzapine-treated subjects (r=−0.31, p<0.01), whereas a trend was observed among placebo-treated subjects (r=−0.31, p=0.08). However, this relationship became nonsignificant when analyses were controlled for duration of olanzapine treatment (p=0.12), and a treatment by weight gain interaction did not emerge in a repeated-measures mixed model analysis that included time in the study (t=1.27, p=0.21). Additionally, weight gain ≥7% was not significantly associated with response or remission. Among 17 adolescents (16%) with obesity at study entry, obesity was not significantly associated with endpoint BPRS-C illness severity. However, girls (p=0.03), individuals hospitalized within the past year (p=0.02), and those with less severe overall (p=0.03) and negative symptoms (p=0.003) according to the CGI-S and PANSS negative subscale, respectively, were more likely to be obese at baseline.
Conclusion
Baseline obesity was associated with lower illness severity, which could be mediated by greater treatment adherence, leading to more weight gain. Olanzapine-related weight gain was not independently associated with symptomatic outcome when controlling for treatment duration. Additional studies are needed to extend these findings to other disorders and medications.
Introduction
The prevalence of early-onset schizophrenia occurring prior to 18 years of age is ∼0.01–0.5% (Masi and Liboni 2011). When compared with adult-onset cases, schizophrenia in adolescence is associated with more severe symptoms (Frazier et al. 2007), higher rates of psychiatric comorbidity (Ross et al. 2006), and a poorer prognosis (Gillberg et al. 1993; Werry et al. 1994; McClellan et al. 1999). Among second-generation antipsychotics (SGAs) used to treat adolescent schizophrenia, published randomized, controlled trials have established efficacy in acute symptom management for olanzapine (Kryzhanovskaya et al. 2009a), risperidone (Haas et al. 2009), aripiprazole (Findling et al. 2008), paliperidone (Singh et al. 2011), and quetiapine (Findling et al. 2012). However, safety concerns have arisen related to SGA use in youth with mental illness, including the development of weight gain, hyperglycemia, and dyslipidemia (Theisen et al. 2001; Correll 2008).
Some studies suggest that adolescents are more susceptible than adults to developing adverse events from SGA administration, including weight gain and extrapyramidal symptoms (Keepers et al. 1983; Krishnamoorthy and King 1998; Theisen et al. 2001; Ratzoni et al. 2002; Correll 2008; Goldstein et al. 2008; Kumra et al. 2008; Correll et al. 2009). From 1993 to 2002 there was an approximate sixfold increase in the number of office-based visits by children and adolescents that included prescription of antipsychotic medications, therefore an increased risk for adverse events in this population is concerning (Olfson et al. 2006).
Although obesity is associated with several adverse health consequences, some reports have identified an association between antipsychotic-induced weight gain and improved therapeutic outcomes in adults with schizophrenia (Leadbetter et al. 1992; Czobor et al. 2002; Ascher-Svanum et al. 2005b; Bai et al. 2006). On the other hand, the independence from confounds, such as adherence and treatment duration, and the clinical significance of such a relationship has also been questioned (Correll et al. 2011; De Hert et al. 2011; Hermes et al. 2011). However, little information is available about whether a relationship between weight gain and clinical efficacy exists in youth with schizophrenia. Using data from a double-blind trial comparing olanzapine and placebo, we sought to examine for the first time whether a relationship exists between weight gain and treatment outcome in adolescent schizophrenia. It was hypothesized that a significant relationship would be found between weight gain and response to treatment with olanzapine but not placebo, even when taking into account the length of study participation. We also investigated whether baseline obesity was associated with various illness characteristics, including baseline symptom severity.
Method
Data were derived from an acute (6 week), randomized, double-blind study comparing olanzapine and placebo in adolescent schizophrenia (Kryzhanovskaya et al. 2009a). This industry-sponsored study was conducted as part of a randomized, multisite, multinational controlled trial as a requisite for receiving United States Food and Drug Administration (FDA) approval in a population with adolescent schizophrenia. Olanzapine is indicated by the FDA for the treatment of schizophrenia in adults and adolescents ages 13–17, whereas olanzapine is not currently indicated by the European Medicines Agency for use in the treatment of children and adolescents. Participants were ages 13–17 and diagnosed with schizophrenia according to American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) and confirmed by the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime (Kaufman et al. 1997). Prior to beginning the acute study phase, participants entered a 2–14 day washout period to taper off all psychotropic medications.
Participants
Subjects were both inpatients and outpatients recruited from the United States and Russia. Participants were required to have a total score of ≥35 or higher on the anchored version of the Brief Psychiatric Rating Scale for Children (BPRS-C) (Overall and Pfefferbaum 1982), with a score of ≥3 or higher on at least one of the following BPRS-C items at enrollment and randomization: hallucinations, delusions, or peculiar fantasies. Participants meeting DSM-IV-TR criteria for substance dependence within the last 30 days or those with a current comorbid psychiatric or developmental disorder were excluded from participation. Eligible participants were randomly assigned in a 2:1 ratio to either olanzapine (2.5–20.0 mg/day) or placebo at night. The dose was titrated to at least 10.0 mg/day by the 3rd week. Thereafter, investigators were instructed to increase the dose of study medication to the highest tolerated dose, provided there were no tolerability concerns. Study visits occurred at baseline, day 3.5, and weeks 1, 2, 3, 4, 5, and 6. Data on medication adherence were not available. Additional details of the parent study have been previously published (Kryzhanovskaya et al. 2009a).
The present post-hoc analyses included 107 subjects randomized to the olanzapine (n=72) or placebo (n=35) arms who had a baseline and at least one postbaseline assessment of body weight and of BPRS-C. The primary efficacy measure was the mean change from baseline-to-endpoint in the BPRS-C total score. Body weight and symptom severity according to the BPRS-C were used to investigate the link between weight gain and therapeutic response. Level of psychopathology was also measured by the Positive and Negative Syndrome Scale (PANSS) (Kay et al. 1987) and the Overt Aggression Scale (OAS) (Yudofsky et al. 1986). Response was defined as a ≥30% reduction in the BPRS-C total score from baseline to endpoint and a Clinical Global Impressions-Severity (CGI-S) score of ≤3 at the last measurement. Applying the definition of Andreasen et al. (2005) cross-sectionally, remission was defined as a score ≤3 on seven BPRS-C variables (items 4, 7, 8, 11, 12, 15, and 16) at the end of the double-blind phase. Sex- and age-adjusted body mass index (BMI) z-scores were used to define overweight/obesity (Krebs et al. 2007) and were calculated using a Web-based program (http://www.kidsnutrition.org/bodycomp/bmiz2.html).
Statistical analysis
Pearson product moment correlations were used to examine the association between weight change and BPRS-C total score change for each treatment group. The relationship between weight gain and overall improvement in psychiatric symptom severity was investigated by separate linear regression models for the olanzapine and placebo groups. Percentage change in symptom severity over the 6 week study period as measured by the BPRS-C total score, was the dependent variable. Independent variables included age, gender, race, age at illness onset, change in BMI z-score, and the baseline values for BMI z-score, blood pressure, high density lipoprotein cholesterol (HDL), low density lipoprotein cholesterol (LDL), BPRS-C total score, and CGI-S score. The effect of adding treatment duration to models evaluating percentage change in BPRS-C total score was also assessed. Additionally, a single mixed effects repeated measures regression model with change in BPRS-C from baseline at each time point as outcome was examined (Fitzmaurice et al. 2004). Predictors included treatment, time, weight change, and both a treatment by time interaction and a treatment by weight change interaction.
Potential clinical variables associated with obesity status were assessed using Fisher's exact test for categorical measures and t tests for continuous measures. A logistic regression analysis was also conducted to identify variables predictive of obesity at study entry. Candidate variables included country of origin, race, gender, lifetime and current psychotic features, psychiatric hospitalization within the past year, atypical antipsychotic use prior to study entry, age of onset, and baseline scores on the CGI-S, OAS, PANSS and BPRS-C. Statistical significance was set at α=0.05. Statistical analyses were performed using SAS 9.2.
Results
A total of 107 participants entered the double-blind trial and were randomized to olanzapine (n=72) or placebo (n=35). Postbaseline assessments of body weight and symptom change were not available in one subject randomized to placebo. The completion rate for this 6 week trial was 68.1% (n=49) and 42.9% (n=15) in the olanzapine and placebo groups, respectively (p=0.02).
Associations between obesity and clinical characteristics
At baseline, 16% of the sample (n=17) met criteria for obesity according to a BMI percentile≥95%. Clinical illness characteristics and their association with obesity at baseline are reported in Table 1. As the trial enrolled participants from both the United States and Russia, overweight and obesity status were compared by country of enrollment. A significantly higher percentage of United States adolescents were overweight (≥85th percentile) or obese (≥95th percentile) as compared with Russian adolescents (44% [n=25] vs. 20% [n=10], p<0.01 and 23% [n=13] vs. 8% [n=4], p=0.04, respectively).
Table 1.
Relationship Between Clinical Characteristics and Obesity at Baseline
| |
Obese (≥95th percentile for BMI) |
|
||
|---|---|---|---|---|
| Variable | No | Yes | % | pa |
| Therapy | ||||
| Olanzapine (n=72) | 62 | 10 | 13.9 | |
| Placebo (n=35) | 28 | 7 | 20 | 0.41 |
| Race | ||||
| White (n=77) | 67 | 10 | 13 | |
| Non-white (n=30) | 23 | 7 | 23.3 | 0.24 |
| Gender | ||||
| Female (n=32) | 23 | 9 | 28.1 | |
| Male (n=75) | 67 | 8 | 10.7 | 0.04 |
| Psychiatric hospitalization (previous year) | ||||
| No (n=47) | 43 | 4 | 8.5 | |
| Yes (n=60) | 47 | 13 | 21.7 | 0.11 |
| Psychotic features (current) | ||||
| No (n=80) | 69 | 11 | 13.8 | |
| Yes (n=27) | 21 | 6 | 22.2 | 0.36 |
| Psychotic features (lifetime) | ||||
| No (n=79) | 68 | 11 | 13.9 | |
| Yes (n=28) | 22 | 6 | 21.4 | 0.38 |
| Mean (SD) | Mean (SD) | t | p | Effect sizeb | |
|---|---|---|---|---|---|
| n=70 | n=15 | ||||
| Previous episodes | 2.41 (3.81) | 2.53 (2.13) | −0.17 | 0.87 | 0.03 |
| n=90 | n=17 | ||||
| Onset age | 12.73 (3.15) | 13.29 (2.69) | −0.69 | 0.49 | 0.18 |
| CGI Severity | 4.92 (0.74) | 4.59 (0.62) | 1.75 | 0.08 | 0.46 |
| OAS total score | 1.91 (2.52) | 2.94 (3.78) | −1.08 | 0.29 | 0.38 |
| PANSS total score | 96.38 (14.11) | 89.33 (12.46) | 1.77 | 0.08 | 1.31 |
| Positive total | 22.73 (5.04) | 22.65 (4.08) | 0.07 | 0.95 | 0.02 |
| Negative total | 25.64 (5.37) | 20.59 (6.25) | 3.47 | <.01 | 0.91 |
| BPRS-C Total Score | 50.63 (9.06) | 47.94 (11.62) | 1.07 | 0.29 | 0.29 |
Fisher's exact test.
Cohen's d effect size.
BMI, body mass index; BPRS-C, Brief Psychiatric Rating Scale for Children; CGI, Clinical Global Impressions Scale; OAS, Overt Aggression Scale; PANSS, Positive and Negative Syndrome Scale.
A logistic regression found female gender (p=0.01), a psychiatric hospitalization within the past year (p=0.04), lower symptom severity as measured by the CGI-S (p=0.02), and less severe negative symptoms according to the PANSS to be significantly associated with obesity status at study entry (Table 2). There was no significant relationship between the CGI-S score or PANSS negative subscale score and having a psychiatric hospitalization within the past year.
Table 2.
Logistic Regression Analysis: Odds of Obesity at Baseline
| |
|
|
|
Odds ratio (OR) estimates |
||
|---|---|---|---|---|---|---|
| Effect | Estimate | SE | p | OR | 95% LCL | 95% UCL |
| Gender (Male) | −1.567 | 0.614 | 0.011 | 0.209 | 0.063 | 0.695 |
| Psychiatric hospitalizationa | 1.395 | 0.674 | 0.038 | 4.034 | 1.078 | 15.101 |
| CGI Severity | −1.181 | 0.501 | 0.018 | 0.307 | 0.115 | 0.819 |
| PANSS Negative Subscale | −0.175 | 0.059 | 0.003 | 0.839 | 0.747 | 0.943 |
Previous year
CGI, Clinical Global Impressions Scale; LCL, lower control limit; PANSS, Positive and Negative Syndrome Scale; UCL, upper control limit.
Associations between weight gain and clinical response
Baseline body weight and BMI percentiles were 67.0±13.3 kg or 70th percentile for the olanzapine group and 68.9±16.9 kg or 70th percentile for the placebo groups (p=0.47). Olanzapine-treated subjects gained 4.3±3.3 kg, whereas placebo-treated subjects gained 0.1±2.8 kg (p<0.001). Significantly more olanzapine-treated versus placebo-treated subjects gained ≥7% of their body weight at any time during treatment (45.8% [n=33] versus 14.7% [n=5]; p=0.002). The overall improvement at study endpoint, as measured by the total score on the BPRS-C, was significantly greater for olanzapine- than for placebo-treated subjects (p=0.003).
The percentage decrease in BPRS-C scores was marginally greater among participants who experienced clinically significant weight gain in both the olanzapine and placebo groups. Among olanzapine-treated participants, BPRS-C scores decreased by 45.6% for those with a clinically significant weight increase as compared with a 31.9% reduction in those without a clinically significant weight increase (p=0.04). In the placebo group, the BPRS-C total score decreased by 34.1% among participants experiencing a clinically significant weight increase, whereas a 16.8% decrease occurred among participants without significant weight gain (p=0.36). In addition, no significant differences occurred in the likelihood of achieving categorical response or remission in association with clinically significant weight gain for either the olanzapine or placebo groups (Table 3).
Table 3.
Symptom Improvement Among Olanzapine- and Placebo-Treated Patients with Clinically Significant Weight Gain
|
Outcome |
≥7% weight gain |
<7% weight gain |
|
|
|---|---|---|---|---|
| Olanzapine | (n=33) % | (n=39) % | OR | 95% CI |
| Responsea | 67 | 54 | 1.71 | (0.66–4.47) |
| Remissionb | 61 | 56 | 1.19 | (0.46–3.05) |
| Early responsec | 76 | 56 | 2.42 | (0.87–6.68) |
| Placebo | (n=5) % | (n=29) % | ||
|---|---|---|---|---|
| Responsea | 60 | 45 | 3.33 | (0.47–23.54) |
| Remissionb | 60 | 38 | 2.45 | (0.35–17.08) |
| Early responsec | 80 | 71 | 5.67 | (0.56–57.23) |
≥30% Improvement in BPRS-C (LOCF) at endpoint
≤3 on items 4, 7, 8, 11, 12, 15, and 16 of BPRS-C at endpoint
≥20% Improvement in BPRS-C at week 2
BPRS-C, Brief Psychiatric Rating Scale for Children; LOCF, last observation carried forward.
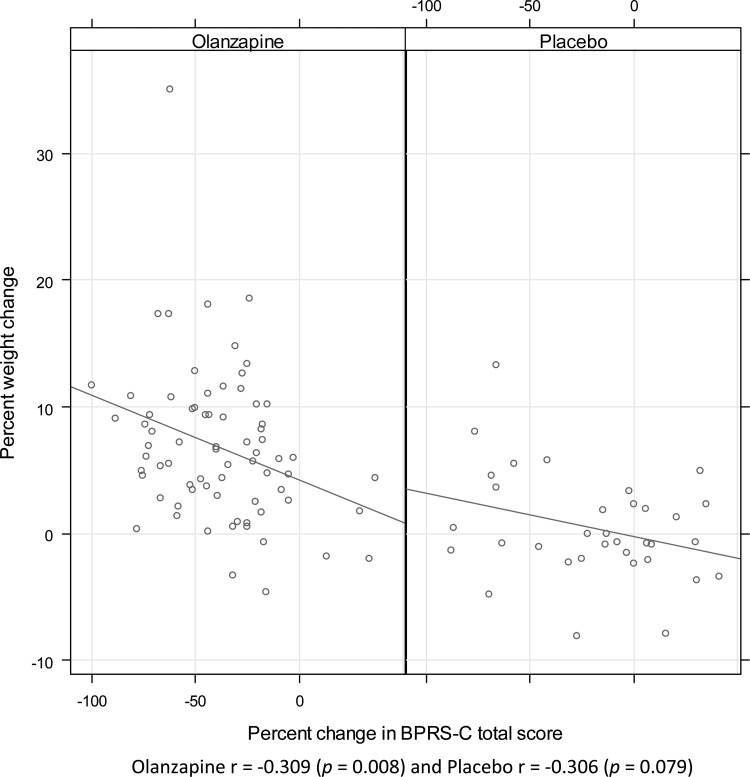
Figure 1 demonstrates that greater percent weight gain was significantly correlated with symptom improvement among olanzapine-treated subjects (r=−0.31, p<0.01, 95% CI=[−0.50, −0.08]), whereas a trend was observed for subjects receiving placebo (r=−0.31, p=0.08, CI=[−0.58, 0.04]). A significant correlation also occurred between change in BMI z-score and symptom improvement with olanzapine treatment (r=−0.29, p=0.015, CI=[−0.48, −0.06]) but did not reach statistical significance with placebo (r=−0.33, p=0.06, CI=[−0.59, 0.02]), although the magnitude of the correlations was similar.
FIG. 1.
Correlations between percent weight change and percent change in Brief Psychiatric Rating Scale for Children (BPRS-C) total score from baseline to study endpoint (Week 6; last observation carried forward). Olanzapine r=−0.309 (p=0.008) and placebo r=−0.306 (p=0.079)
Separate linear regression models for the olanzapine and placebo groups assessed for clinical factors that could predict percent change in BPRS-C score at study endpoint. In the final model for olanzapine-treated subjects, the only variable significantly associated with treatment response was change in BMI z-score (p=0.01). However, change in BMI z-score was strongly correlated with treatment duration. When time on treatment was entered into the model, change in BMI z-score was no longer significant (p=0.12). Among placebo-treated subjects, treatment duration (p<0.001) and baseline diastolic blood pressure (p=0.01) were the only variables predictive of symptom improvement. Although statistically significant, the effect of diastolic blood pressure was small and lacked clinical significance (regression coefficient=1.4±0.54). Finally, a mixed effects repeated measures regression model analysis of response profiles with change in BPRS-C from baseline at each time point as the dependent variable was examined. Included in the model were the treatment by time interaction, weight change, and the overall interaction between treatment and weight change. The interaction between treatment and time was significant at week 3 (t=−2.45, p=0.02) and at week 5 (t=−2.26, p=0.03), favoring greater decreases from baseline to end of treatment in the olanzapine group. There was no significant interaction between treatment and weight change (t=1.27, p=0.21) during the randomized treatment phase.
Olanzapine dose and weight gain
There was no significant correlation between the modal dose of olanzapine per subject (overall mean: 12.6±4.6 mg) and percentage weight gain (r=−0.008, p=0.95). When prescribed in the range of 2.5–20 mg/day, the results suggest that higher doses of olanzapine were not associated with greater weight gain than lower doses.
Discussion
To our knowledge, this is the first published study evaluating the relationship between weight gain and therapeutic outcome on olanzapine treatment in a sample of adolescents with any psychiatric condition. A significant inverse correlation was observed between percent change in body weight and percent change in BPRS-C severity among those receiving olanzapine, whereas a trend was observed among placebo-treated subjects. However, this relationship became nonsignificant when analyses were controlled for duration of olanzapine treatment, which was both significantly related to weight gain and symptomatic improvement. In addition, time was modeled directly as a repeated-measures variable using a mixed effects repeated measures regression approach that included change in weight as a time-varying covariate and change in the BPRS-C severity score as the dependent variable. The findings suggest that adolescents who improve with olanzapine may be more likely to remain on treatment and secondarily experience greater weight gain, although it remains possible that adolescents who gain weight are more likely to improve and therefore remain on treatment. However, the lack of a significant interaction effect by treatment group between weight gain and outcome as well as the lack of significant differences in the likelihood of achieving categorical response or remission in association with clinically significant weight gain makes the latter assumption less likely.
We also explored whether obesity at baseline was associated with certain demographic and clinical characteristics. Adolescents with obesity were more likely to be female and have experienced a psychiatric hospitalization within the past year. They also presented with less severe negative symptoms and overall illness acuity according to the PANSS negative subscore and CGI total score, respectively.
Although to our knowledge this is the first study to report on the association between weight gain and therapeutic efficacy in a placebo-controlled sample of adolescents with schizophrenia, an association was identified between weight gain and improved clinical response to antipsychotics more than four decades ago (Singh et al. 1970). Among 187 olanzapine-treated adults with schizophrenia, a moderately large correlation was observed between treatment efficacy and corresponding weight change (r=−0.43) (Ascher-Svanum et al. 2005b). A much larger study involving 1337 olanzapine- or haloperidol-treated patients with schizophrenia spectrum disorders also found that weight gain was associated with improvement in the core symptoms of schizophrenia (Ascher-Svanum et al. 2005a). However, all three of these studies were limited by a 6 week study duration. This may represent an important limitation, as a prior study involving olanzapine identified treatment duration as the key variable mediating the relationship between symptom reduction and weight gain in a 6 month study (Hennen et al. 2004).
In contrast to the short-term acute trials, the relationship between weight gain and therapeutic response in schizophrenia has also been evaluated over 18 months in a post-hoc analysis of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study (Hermes et al. 2011). A statistically significant association occurred between change in PANSS total score and change in BMI, with no significant differences among the various antipsychotic treatments. However, despite achieving statistical separation, the relationship was determined to be too small to be clinically meaningful, as change in BMI accounted for ≤3% of the variance in PANSS change scores (Hermes et al. 2011). Consistent with CATIE, the present analysis found the correlation between weight change and symptom improvement to approach significance among placebo-treated participants, but the absolute change in body weight was clinically insignificant.
Additional synthesis of existing studies in adult samples suggests that a potential or, at least, statistical association between weight gain and greater therapeutic response is not observed across all antipsychotic medications. In a study of clozapine, the percentage change in weight from baseline significantly predicted BPRS total and positive symptoms subscale improvement at 6 weeks and 6 months (Meltzer et al. 2002). Czobor and colleagues (2002) found that greater therapeutic responses to olanzapine and clozapine were associated with greater weight gain, but this association was not observed with risperidone or haloperidol. Another study comparing olanzapine with haloperidol found the correlation between weight gain and therapeutic response to be numerically larger for olanzapine (−0.24) than for haloperidol (−0.10), although both analyses were statistically significant (Ascher-Svanum et al. 2005a). However, weight gain was not correlated with response in two open trials of clozapine (Umbricht et al. 1994; Hummer et al. 1995) and a controlled trial of clozapine and haloperidol (Bustillo et al. 1996). In two large studies comparing olanzapine with haloperidol and risperidone, patients with better clinical outcomes gained significantly more weight than those with poorer clinical outcomes, but the effect size was relatively modest (Basson et al. 2001). A complicating factor in evaluating the results from these studies is that adherence was rarely assessed in any detail and that treatment duration was often not considered as a confounding factor, although both these variables are associated with greater weight gain and therapeutic efficacy.
Although the exact mechanisms remain unclear, olanzapine may lead to weight gain through histamine H1 receptor binding, serotonin 2A receptor antagonism, and inverse agonist effects at serotonin 2C receptors (Rauser et al. 2001; Roerig et al. 2011). Dopamine D1 and D2 receptor antagonism through their impact on the reward system may also play a role, as it has recently been shown there is an increased anticipatory reward responsivity toward food reward in the inferior frontal cortex, striatum, and anterior cingulate cortex during olanzapine treatment (Mathews et al. 2012).
In the context of existing studies, it can be assumed that weight gain is neither sufficient nor necessary to produce an antipsychotic treatment response. Atypical antipsychotics characterized by more benign weight and cardiometabolic profiles are often found to have few efficacy differences in comparison with weight-promoting antipsychotics when evaluating their long-term effectiveness in adult patients with schizophrenia (Johnsen and Jørgensen 2008). Moreover, the strategy of switching to a medication with a lower liability for weight gain can result in significant loss of body weight without an accompanying increase in efficacy failures or substantial worsening of symptoms (Stroup et al. 2011). In the Treatment of Early-Onset Schizophrenia study, the atypical antipsychotics olanzapine and risperidone were similar in efficacy when compared with the conventional antipsychotic molindone; however, both atypical antipsychotics were associated with greater degrees of weight gain (Sikich et al. 2008).
Sustained weight gain often leads to medical complications and difficulties with long-term treatment adherence (Ross et al. 2003). Among children and adolescents in the United States, ∼17% are ≥the 95th percentile for BMI (Ogden et al. 2010). The 23% rate of obesity among United States adolescents with schizophrenia in the present sample is already higher than the rate of obesity in the general population and would be expected to increase even further after treatment with olanzapine. In a pooled safety analysis from four clinical trials of adolescents 13–17 years old, olanzapine treatment for up to 32 weeks was associated with significant increases in body weight (Kryzhanovskaya et al. 2009b). The magnitude of weight gain was greater in adolescents than in adults, averaging a mean of 7.4 kg versus 3.2 kg, respectively. These findings, although unadjusted for expected growth in the pediatric sample, suggest a need for greater emphasis on weight and cardiometabolic monitoring among youth and emphasize the importance of balancing efficacy with tolerability.
Relationship between schizophrenia and obesity
Little is known about how obesity and cardiometabolic illnesses affect treatment response to antipsychotics. No association was identified between the metabolic syndrome and symptom severity or neurocognitive dysfunction in CATIE (Meyer et al. 2005). In youth with bipolar disorder, obesity was independently associated with younger age, non-white race, a history of psychiatric hospitalization, and treatment with medications from ≥2 classes associated with weight gain (Goldstein et al. 2008). A lifetime history of physical abuse and substance use disorder were associated with nearly a threefold increased prevalence of overweight and obesity, suggesting increased psychiatric burden may be related to body weight status.
In the present report, obesity at study entry was associated with being female, having a psychiatric hospitalization within the past year, and lower overall and negative symptom severity as measured by the CGI-S and PANSS negative subscore, respectively. The finding of lower symptom severity among obese youth with schizophrenia runs counter to our initial hypothesis, as greater symptom severity has been linked to obesity in adults with bipolar disorder (Thompson et al. 2006). Additionally, studies of adult and pediatric patients with mood disorders have found obesity to be associated with higher rates of attempted suicide, poorer response to medication treatments, earlier rates of relapse, and overall increased psychiatric burden (Fagiolini et al. 2003, 2005; Thompson et al. 2006; Kloiber et al. 2007; Goldstein et al. 2008; Kemp et al. 2010). It is possible that the pathophysiological underpinnings of obesity may overlap with those of schizophrenia, affecting changes in neurotransmitters and cytokines relevant to psychopathology. Alternatively, the study inclusion and exclusion criteria, as well as differences in prior medication exposure, may account for the lower severity scores in this sample, although atypical antipsychotic treatment status prior to enrollment was not found to predict baseline obesity. Information about treatment adherence and symptom reduction prior to baseline and randomization was not available. It is possible that participants with baseline obesity had potentially been more adherent to antipsychotic treatment and gained more weight, leading to greater symptom reduction and less illness severity than participants without obesity at baseline. However, future studies of baseline characteristics should assess the nature, effects, and adherence to treatments prior to study baseline more carefully.
Limitations
These findings should be considered in the context of several limitations. The analyses were conducted post-hoc, as the trial was not designed to examine the role of obesity and weight change in relationship to symptom improvement. The sample size was moderate, and the study may not have been powered to detect significant associations in the exploratory outcomes. With regard to generalizability, the strict inclusion and exclusion criteria may limit application of the findings to the larger population of patients with early onset schizophrenia accompanied by comorbid substance dependence or other psychiatric disorders. Also, neither medication adherence nor dietary intake was systematically assessed. Outcomes were only evaluated during the acute phase of illness and may not generalize to longer treatment periods. This is notable, as some studies suggest that weight gain may not plateau until several months after initiating olanzapine (Ross et al 2003; Maloney and Sikich 2010).
As the analyses were restricted to olanzapine, additional studies are needed to determine if these relationships are replicated with other antipsychotics. Future studies of interest in adolescent populations might assess sensitivity to weight gain as a predictor of long-term adherence and whether genetic polymorphisms contribute to severe antipsychotic-induced weight gain. Also, the association between obesity and treatment outcomes warrants further study in other pediatric populations, such as those with bipolar disorder.
Conclusion
To our knowledge, this is the first study to examine the association between acute weight gain and symptom improvement in adolescents with schizophrenia treated with an antipsychotic in a randomized, placebo-controlled study. Greater weight gain during the first 6 weeks of olanzapine treatment, and to a lesser extent treatment with placebo, was found to be an indicator of improved therapeutic efficacy. However, this relationship was eliminated when duration in the study was included as a covariate in the analysis. Additionally, a treatment by weight gain interaction did not emerge in a repeated measures mixed model analysis that controlled for time in the study, suggesting that the association between weight gain and symptom improvement might reflect the longer administration of a weight-promoting medication. Participants with obesity at study entry had a greater number of psychiatric hospitalizations within the past year and were less severely ill on some, but not all, measures of symptom severity. Additional studies are needed to determine if similar relationships exist in adolescents with bipolar disorder and during treatment with other antipsychotic drugs. Given the high rate of clinically significant weight gain observed in this trial, clinicians must closely balance improvement in efficacy with the potential for long-term adverse cardiometabolic side effects.
Clinical Significance
In adults with schizophrenia, some reports suggest an association between antipsychotic-induced weight gain and improved therapeutic outcomes. Using data from a double-blind trial comparing olanzapine and placebo, we examined whether a relationship exists between weight gain and treatment outcome in adolescent schizophrenia. A significant inverse correlation was observed between percent change in body weight and percent change in BPRS-C severity among those receiving olanzapine, whereas a trend was observed among placebo-treated subjects. However, this relationship became nonsignificant when analyses were controlled for duration of olanzapine treatment, which was both significantly related to weight gain and symptomatic improvement. Additionally, when time was modeled directly using a mixed effects repeated measures regression approach, no significant interaction effect was observed by treatment group between weight gain and outcome. These findings suggest that adolescents who improve with olanzapine may be more likely to remain on treatment and secondarily experience greater weight gain. Future studies assessing the relationship between weight gain and improved outcome should assess adherence and treatment duration as potential confounding factors, as both of these variables may be associated with greater weight gain and therapeutic efficacy with antipsychotic treatment.
Acknowledgment
The authors acknowledge Michael Case for his assistance in association with this manuscript.
Disclosures
Drs. Chang, Correll, DelBello, Kemp, and Tohen received no financial support from Eli Lilly and Company or its subsidiaries in connection with the development of this manuscript. Drs. Ganocy and Findling received financial support in the form of a research grant from Eli Lilly and Company. Dr. Chang has acted as a consultant for GlaxoSmithKline, Lilly, Bristol-Myers Squibb, and Merck in the past 2 years. He has received research funding from GlaxoSmithKline and Merck. Dr. Correll has been a consultant and/or advisor to or has received honoraria from: Actelion, Alexza, American Academy of Child and Adolescent Psychiatry, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Gerson Lehman Group, IntraCellular Therapies, Ortho-cNeill/Janssen/J&J, MedAvante, Medscape, Merck, Novartis, Otsuka, Pfizer, Sunovion, Takeda, and Teva. He has received grant support from Bristol-Myers Squibb, Feinstein Institute for Medical Research, Janssen/J&J, National Institute of Mental Health (NIMH), National Alliance for Research in Schizophrenia and Depression (NARSAD), and Otsuka. Dr. DelBello is a consultant and/or advisor to or receives honoraria from Bristol-Myers Squibb, Merck, Schering-Plough, Pfizer and receives research support from AstraZeneca, Eli Lilly, GlaxoSmithKline, Johnson and Johnson, Janssen, Merck, Novartis, Otsuka, and Pfizer. Dr. Findling receives or has received research support from, acted as a consultant for, received royalties from, and/or served on a speaker's bureau for Abbott, Addrenex, Alexza, American Psychiatric Press, AstraZeneca, Biovail, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson & Johnson, KemPharm, Lilly, Lundbeck, Merck, National Institutes of Health, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Physicians' Post-Graduate Press, Rhodes Pharmaceuticals, Roche, Sage, Sanofi-Aventis, Schering-Plough, Seaside Therapeutics, Sepracore, Shionogi, Shire, Solvay, Stanley Medical Research Institute, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, WebMD and Wyeth. Dr. Ganocy has received research support from AstraZeneca and Eli Lilly. Dr. Kemp has acted as a consultant and/or served on a speaker's bureau for Astra-Zeneca, Bristol-Myers Squibb, Janssen, Pfizer, Corcept and Teva. Dr. Kemp's spouse has been a minor shareholder of Abbott and Sanofi. Dr. Tohen was an Eli Lilly employee when the study was conducted. Dr. Tohen was formerly employed by Lilly (to 2008). The original study was conducted while he was still a full-time employee. He has received honoraria or consulted for AstraZeneca, BristolMyersSquibb, Forest, Glaxo-SmithKline, Eli Lilly, Johnson & Johnson, Merck, Otzuka, Sepracor, Roche, Lundbeck, Elan, and Wyeth Corporations; his spouse is a current employee and minor stockholder at Eli Lilly.
References
- American Psychiatric Association. Text Revision. 4th. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Andreasen NC. Carpenter WT., Jr. Kane JM. Lasser RA. Marder SR. Weinberger DR. Remission in schizophrenia: Proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- Ascher-Svanum H. Stensland M. Zhao Z. Kinon BJ. Acute weight gain, gender, therapeutic response to antipsychotics in the treatment of patients with schizophrenia. BMC Psychiatry. 2005a;5:3. doi: 10.1186/1471-244X-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher-Svanum H. Stensland M. Zhao Z. Kinon BJ. Weight gain as a prognostic indicator of therapeutic improvement during acute treatment of schizophrenia with placebo or active antipsychotic. J Psychopharmacol. 2005b;19(Suppl 6):110–117. doi: 10.1177/0269881105058978. [DOI] [PubMed] [Google Scholar]
- Bai YM. Lin CC. Chen JY. Lin CY. Su TP. Chou P. Association of initial antipsychotic response to clozapine and long-term weight gain. Am J Psychiatry. 2006;163:1276–1279. doi: 10.1176/ajp.2006.163.7.1276. [DOI] [PubMed] [Google Scholar]
- Basson BR. Kinon BJ. Taylor CC. Szymanski KA. Gilmore JA. Tollefson GD. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. J Clin Psychiatry. 2001;62:231–238. doi: 10.4088/jcp.v62n0404. [DOI] [PubMed] [Google Scholar]
- Bustillo JR. Buchanan RW. Irish D. Breier A. Differential effect of clozapine on weight: A controlled study. Am J Psychiatry. 1996;153:817–819. doi: 10.1176/ajp.153.6.817. [DOI] [PubMed] [Google Scholar]
- Correll CU. Assessing and maximizing the safety and tolerability of antipsychotics used in the treatment of children and adolescents. J Clin Psychiatry. 2008;69(Suppl 4):26–36. [PubMed] [Google Scholar]
- Correll CU. Lencz T. Malhotra AK. Antipsychotic drugs and obesity. Trends Mol Med. 2011;17:97–107. doi: 10.1016/j.molmed.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU. Manu P. Olshanskiy V. Napolitano B. Kane JM. Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czobor P. Volavka J. Sheitman B. Lindenmayer JP. Citrome L. McEvoy J. Cooper TB. Chakos M. Lieberman JA. Antipsychotic-induced weight gain and therapeutic response: A differential association. J Clin Psychopharmacol. 2002;22:244–251. doi: 10.1097/00004714-200206000-00003. [DOI] [PubMed] [Google Scholar]
- De Hert M. Detraux J. van Winkel R. Yu W. Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8:114–126. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- Fagiolini A. Frank E. Scott JA. Turkin S. Kupfer DJ. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005;7:424–430. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- Fagiolini A. Kupfer DJ. Houck PR. Novick DM. Frank E. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry. 2003;160:112–117. doi: 10.1176/appi.ajp.160.1.112. [DOI] [PubMed] [Google Scholar]
- Findling RL. McKenna K. Earley WR. Stankowski J. Pathak S. Efficacy and safety of quetiapine in adolescents with schizophrenia: a 6-week, double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol. 2012;22:327–342. doi: 10.1089/cap.2011.0092. [DOI] [PubMed] [Google Scholar]
- Findling RL. Robb A. Nyilas M. Forbes RA. Jin N. Ivanova S. Marcus R. McQuade RD. Iwamoto T. Carson WH. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165:1432–1441. doi: 10.1176/appi.ajp.2008.07061035. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM. Laird NM. Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- Frazier JA. McClellan J. Findling RL. Vitiello B. Anderson R. Zablotsky B. Williams E. McNamara NK. Jackson JA. Ritz L. Hlastala SA. Pierson L. Varley JA. Puglia M. Maloney AE. Ambler D. Hunt–Harrison T. Hamer RM. Noyes N. Lieberman JA. Sikich L. Treatment of early-onset schizophrenia spectrum disorders (TEOSS): Demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2007;46:979–988. doi: 10.1097/chi.0b013e31807083fd. [DOI] [PubMed] [Google Scholar]
- Gillberg IC. Hellgren L. Gillberg C. Psychotic disorders diagnosed in adolescence. Outcome at age 30 years. J Child Psychol Psychiatry. 1993;34:1173–1185. doi: 10.1111/j.1469-7610.1993.tb01781.x. [DOI] [PubMed] [Google Scholar]
- Goldstein BI. Birmaher B. Axelson DA. Goldstein TR. Esposito–Smythers C. Strober MA. Hunt J. Leonard H. Gill MK. Iyengar S. Grimm C. Yang M. Ryan ND. Keller MB. Preliminary findings regarding overweight and obesity in pediatric bipolar disorder. J Clin Psychiatry. 2008;69:1953–1959. doi: 10.4088/jcp.v69n1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. Unis AS. Armenteros J. Copenhaver MD. Quiroz JA. Kushner SF. A 6-week, randomized, double-blind, placebo-controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia. J Child Adolesc Psychopharmacol. 2009;19:611–621. doi: 10.1089/cap.2008.0144. [DOI] [PubMed] [Google Scholar]
- Hennen J. Perlis RH. Sachs G. Tohen M. Baldessarini RJ. Weight gain during treatment of bipolar I patients with olanzapine. J Clin Psychiatry. 2004;65:1679–1687. doi: 10.4088/jcp.v65n1214. [DOI] [PubMed] [Google Scholar]
- Hermes E. Nasrallah H. Davis V. Meyer J. McEvoy J. Goff D. Davis S. Stroup TS. Swartz M. Lieberman J. Rosenheck R. The association between weight change and symptom reduction in the CATIE schizophrenia trial. Schizophr Res. 2011;128:166–170. doi: 10.1016/j.schres.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummer M. Kemmler G. Kurtz M. Kurtzthaler I. Oberbauer H. Fleischhacker WW. Weight gain induced by clozapine. Eur Neuropsychopharmacol. 1995;5:437–440. doi: 10.1016/0924-977x(95)00012-e. [DOI] [PubMed] [Google Scholar]
- Johnsen E. Jørgensen HA. Effectiveness of second generation antipsychotics: A systematic review of randomized trials. BMC Psychiatry. 2008;8:31. doi: 10.1186/1471-244X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kay SR. Fiszbein A. Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keepers GA. Clappison VJ. Casey DE. Initial anticholinergic prophylaxis for neuroleptic-induced extrapyramidal syndromes. Arch Gen Psychiatry. 1983;40:1113–1117. doi: 10.1001/archpsyc.1983.01790090075012. [DOI] [PubMed] [Google Scholar]
- Kemp DE. Gao K. Chan PK. Ganocy SJ. Findling RL. Calabrese JR. Medical comorbidity in bipolar disorder: relationship between illnesses of the endocrine/metabolic system and treatment outcome. Bipolar Disord. 2010;12:404–413. doi: 10.1111/j.1399-5618.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloiber S. Ising M. Reppermund S. Horstmann S. Dose T. Majer M. Zihl J. Pfister H. Unschuld PG. Holsboer F. Lucae S. Overweight and obesity affect treatment response in major depression. Biol Psychiatry. 2007;62:321–326. doi: 10.1016/j.biopsych.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Krebs NF. Himes JH. Jacobson D. Nicklas TA. Guilday P. Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S193–228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy J. King BH. Open-label olanzapine treatment in five preadolescent children. J Child Adolesc Psychopharmacol. 1998;8:107–13. doi: 10.1089/cap.1998.8.107. [DOI] [PubMed] [Google Scholar]
- Kryzhanovskaya L. Schulz SC. McDougle C. Frazier J. Dittmann R. Robertson–Plouch C. Bauer T. Xu W. Wang W. Carlson J. Tohen M. Olanzapine versus placebo in adolescents with schizophrenia: a 6-week, randomized, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009a;48:60–70. doi: 10.1097/CHI.0b013e3181900404. [DOI] [PubMed] [Google Scholar]
- Kryzhanovskaya LA. Robertson–Plouch CK. Xu W. Carlson JL. Merida KM. Dittmann RW. The safety of olanzapine in adolescents with schizophrenia or bipolar I disorder: a pooled analysis of 4 clinical trials. J Clin Psychiatry. 2009b;70:247–58. doi: 10.4088/jcp.08m03538. [DOI] [PubMed] [Google Scholar]
- Kumra S. Oberstar JV. Sikich L. Findling RL. McClellan JM. Vinogradov S. Charles Schulz S. Efficacy and tolerability of second-generation antipsychotics in children and adolescents with schizophrenia. Schizophr Bull. 2008;34:60–71. doi: 10.1093/schbul/sbm109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter R. Shutty M. Pavalonis D. Vieweg V. Higgins P. Downs M. Clozapine-induced weight gain: prevalence and clinical relevance. Am J Psychiatry. 1992;149:68–72. doi: 10.1176/ajp.149.1.68. [DOI] [PubMed] [Google Scholar]
- Maloney AE. Sikich L. Olanzapine approved for the acute treatment of schizophrenia or manic/mixed episodes associated with bipolar I disorder in adolescent patients. Neuropsychiatr Dis Treat. 2010;6:749–66. doi: 10.2147/NDT.S6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi G. Liboni F. Management of schizophrenia in children and adolescents: focus on pharmacotherapy. Drugs. 2011;71:179–208. doi: 10.2165/11585350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Mathews J. Newcomer JW. Mathews JR. Fales CL. Pierce KJ. Akers BK. Marcu I. Barch DM. Neural correlates of weight gain with olanzapine. Arch Gen Psychiatry. 2012;69:1226–1237. doi: 10.1001/archgenpsychiatry.2012.934. [DOI] [PubMed] [Google Scholar]
- McClellan J. McCurry C. Snell J. DuBose A. Early-onset psychotic disorders: Course and outcome over a 2-year period. J Am Acad Child Adolesc Psychiatry. 1999;38:1380–1388. doi: 10.1097/00004583-199911000-00012. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Perry E. Jayathilake K. Clozapine-induced weight gain predicts improvement in psychopathology. Schizophr Res. 2002;59:19–27. doi: 10.1016/s0920-9964(01)00326-7. [DOI] [PubMed] [Google Scholar]
- Meyer JM. Nasrallah HA. McEvoy JP. Goff DC. Davis SM. Chakos M. Patel JK. Keefe RS. Stroup TS. Lieberman JA. The Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE) Schizophrenia Trial: Clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res. 2005;80:9–18. doi: 10.1016/j.schres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Ogden CL. Carroll MD. Curtin LR. Lamb MM. Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- Olfson M. Blanco C. Liu L. Moreno C. Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63:679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- Overall JE. Pfefferbaum B. The Brief Psychiatric Rating Scale for Children. Psychopharmacol Bull. 1982;18:10–16. [PubMed] [Google Scholar]
- Ratzoni G. Gothelf D. Brand–Gothelf A. Reidman J. Kikinzon L. Gal G. Phillip M. Apter A. Weizman R. Weight gain associated with olanzapine and risperidone in adolescent patients: a comparative prospective study. J Am Acad Child Adolesc Psychiatry. 2002;41:337–343. doi: 10.1097/00004583-200203000-00014. [DOI] [PubMed] [Google Scholar]
- Rauser L. Savage JE. Meltzer HY. Roth BL. Inverse agonist actions of typical and atypical antipsychotic drugs at the human 5-hydroxytryptamine (2C) receptor. J Pharmacol Exp Ther. 2001;299:83–89. [PubMed] [Google Scholar]
- Roerig JL. Steffen KJ. Mitchell JE. Atypical antipsychotic-induced weight gain: Insights into mechanisms of action. CNS Drugs. 2011;25:1035–1059. doi: 10.2165/11596300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ross RG. Heinlein S. Tregellas H. High rates of comorbidity are found in childhood-onset schizophrenia. Schizophr Res. 2006;88:90–95. doi: 10.1016/j.schres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Ross RG. Novins D. Farley GK. Adler LE. A 1-year open-label trial of olanzapine in school-age children with schizophrenia. J Child Adolesc Psychopharmacol. 2003;13:301–309. doi: 10.1089/104454603322572633. [DOI] [PubMed] [Google Scholar]
- Sikich L. Frazier JA. McClellan J. Findling RL. Vitiello B. Ritz L. Ambler D. Puglia M. Maloney AE. Michael E. De Jong S. Slifka K. Noyes N. Hlastala S. Pierson L. McNamara NK. Delporto–Bedoya D. Anderson R. Hamer RM. Lieberman JA. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizo-affective disorder: Findings from the treatment of early-onset schizophrenia spectrum disorders (TEOSS) study. Am J Psychiatry. 2008;165:1420–1431. doi: 10.1176/appi.ajp.2008.08050756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J. Robb A. Vijapurkar U. Nuamah I. Hough D. A randomized, double-blind study of paliperidone extended-release in treatment of acute schizophrenia in adolescents. Biol Psychiatry. 2011;70:1179–1187. doi: 10.1016/j.biopsych.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Singh MM. De Dios LV. Klein NS. Weight as a correlate of clinical response to psychotropic drugs. Psychosomatics. 1970;11:562–570. doi: 10.1016/s0033-3182(70)71577-6. [DOI] [PubMed] [Google Scholar]
- Stroup TS. McEvoy JP. Ring KD. Hamer RH. Lavange LM. Swartz MS. Rosenheck RA. Perkins DO. Nussbaum AM. Lieberman JA the Schizophrenia Trials Network. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: Comparison of Antipsychotics for Metabolic Problems (CAMP) Am J Psychiatry. 2011;168:947–956. doi: 10.1176/appi.ajp.2011.10111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theisen FM. Linden A. Geller F. Schäfer H. Martin M. Remschmidt H. Hebebrand J. Prevalence of obesity in adolescent and young adult patients with and without schizophrenia and in relationship to antipsychotic medication. J Psychiatr Res. 2001;35:339–345. doi: 10.1016/s0022-3956(01)00041-3. [DOI] [PubMed] [Google Scholar]
- Thompson WK. Kupfer DJ. Fagiolini A. Scott JA. Frank E. Prevalence and clinical correlates of medical comorbidities in patients with bipolar I disorder: Analysis of acute phase data from a randomized controlled trial. J Clin Psychiatry. 2006;67:783–788. doi: 10.4088/jcp.v67n0512. [DOI] [PubMed] [Google Scholar]
- Umbricht DS. Pollack S. Kane JM. Clozapine and weight gain. J Clin Psychiatry. 1994;55(Suppl B):157–160. [PubMed] [Google Scholar]
- Werry JS. McClellan JM. Andrews LK. Ham M. Clinical features and outcome of child and adolescent schizophrenia. Schizophr Bull. 1994;20:619–630. doi: 10.1093/schbul/20.4.619. [DOI] [PubMed] [Google Scholar]
- Yudofsky SC. Silver JM. Jackson W. Endicott J. Williams D. The Overt Aggression Scale for the objective rating of verbal and physical aggression. Am J Psychiatry. 1986;143:35–3n9. doi: 10.1176/ajp.143.1.35. [DOI] [PubMed] [Google Scholar]