Abstract
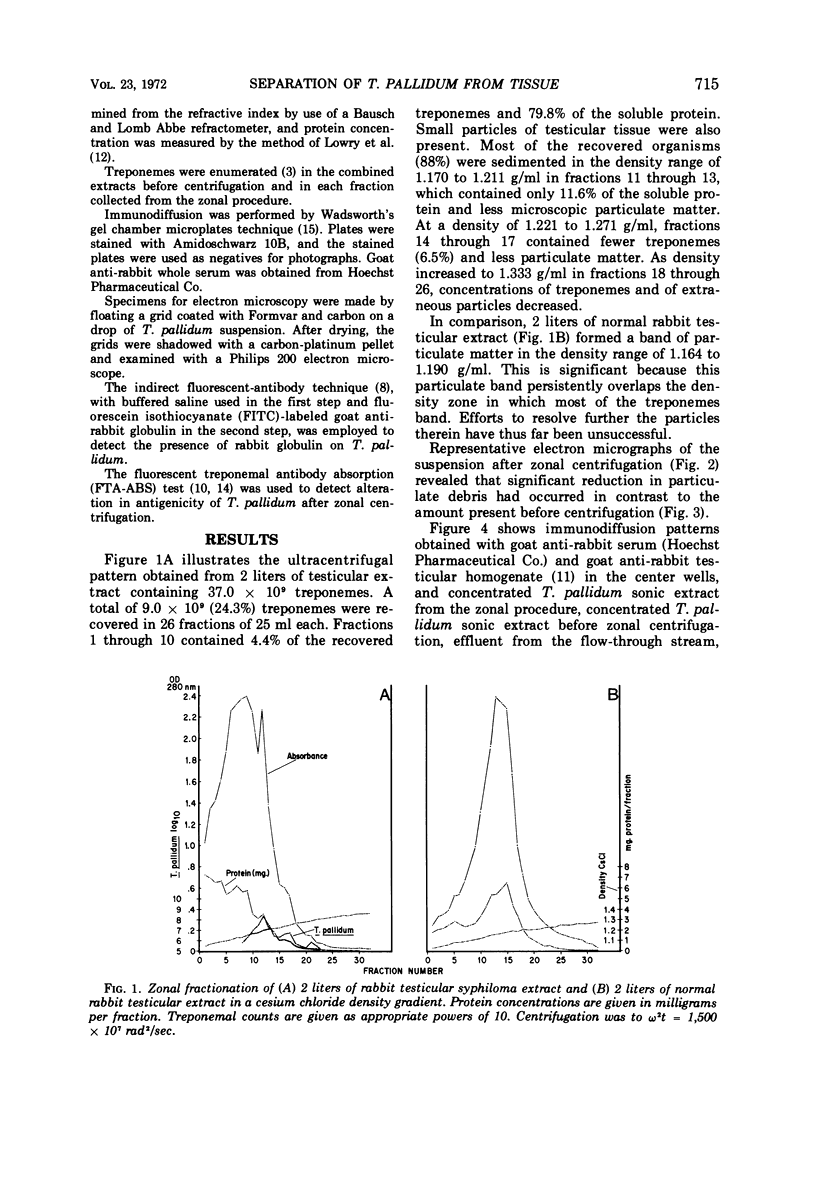
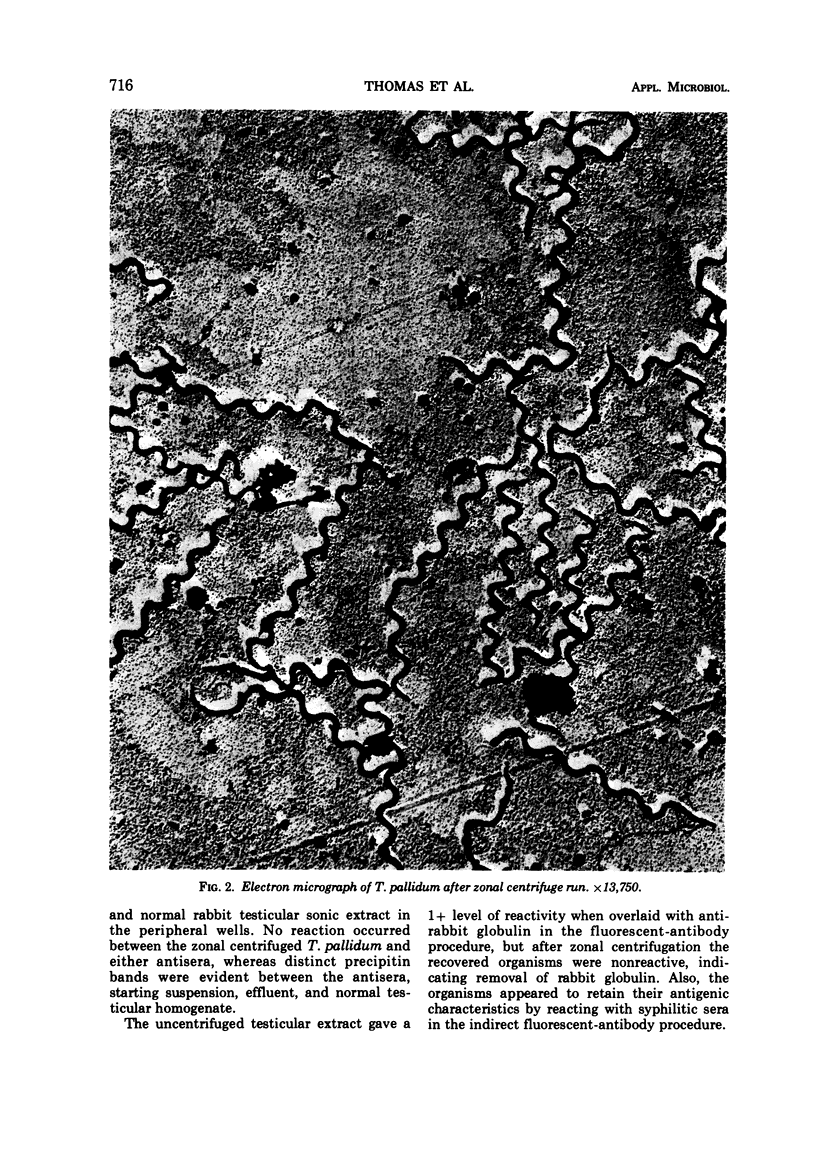
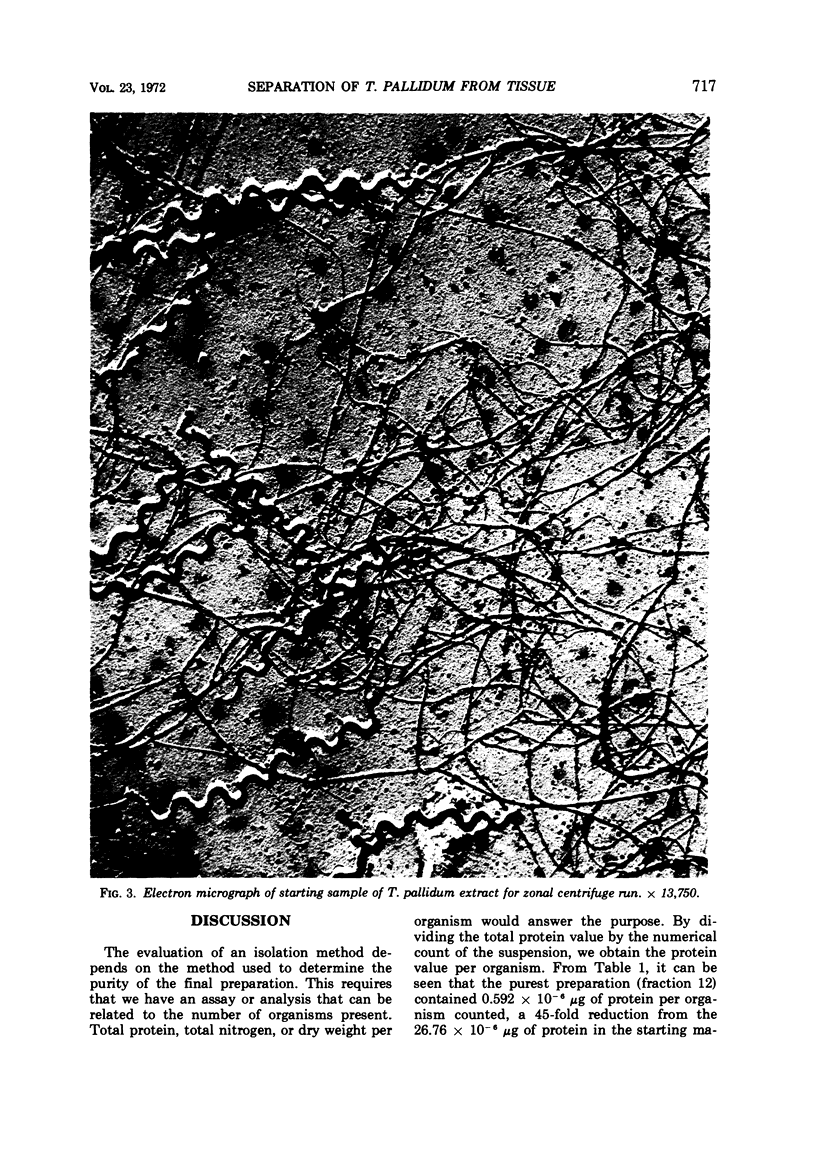
The zonal ultracentrifuge was used for separation of Treponema pallidum from large volumes of rabbit testicular syphiloma extracts by continuous-flow centrifugation in a cesium chloride density gradient. The gradient was linear with radius from a density of 1.05 to 1.36 g/ml. Operating speeds were 15,000 rev/min for the continuous-flow phase and 25,000 rev/min for a 30-min banding period. A total of 9 × 109 (24.3%) treponemes were recovered from the original extract. Of the treponemes recovered, 88% formed a band at a density of 1.170 to 1.211 g/ml. Within the limits of present methods of assay, these fractions were relatively free from testicular particulates and protein when compared with treponemes recovered after differential centrifugation. Observations of the isolated fractions by dark-field and electron microscopy indicated a lack of gross morphological damage to T. pallidum. Their antigenic characteristics were also retained, as evidenced by their ability to react with syphilitic sera in the indirect fluorescent-antibody procedure.
Full text
PDF
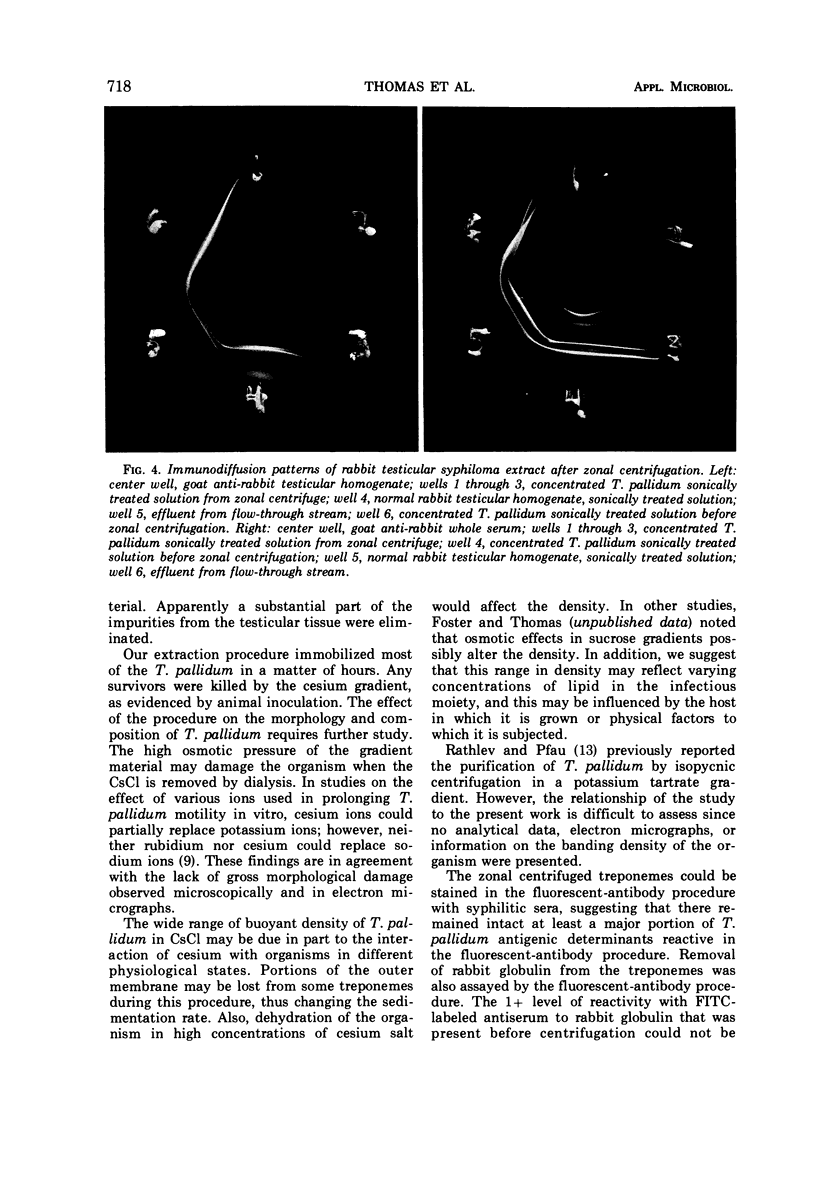
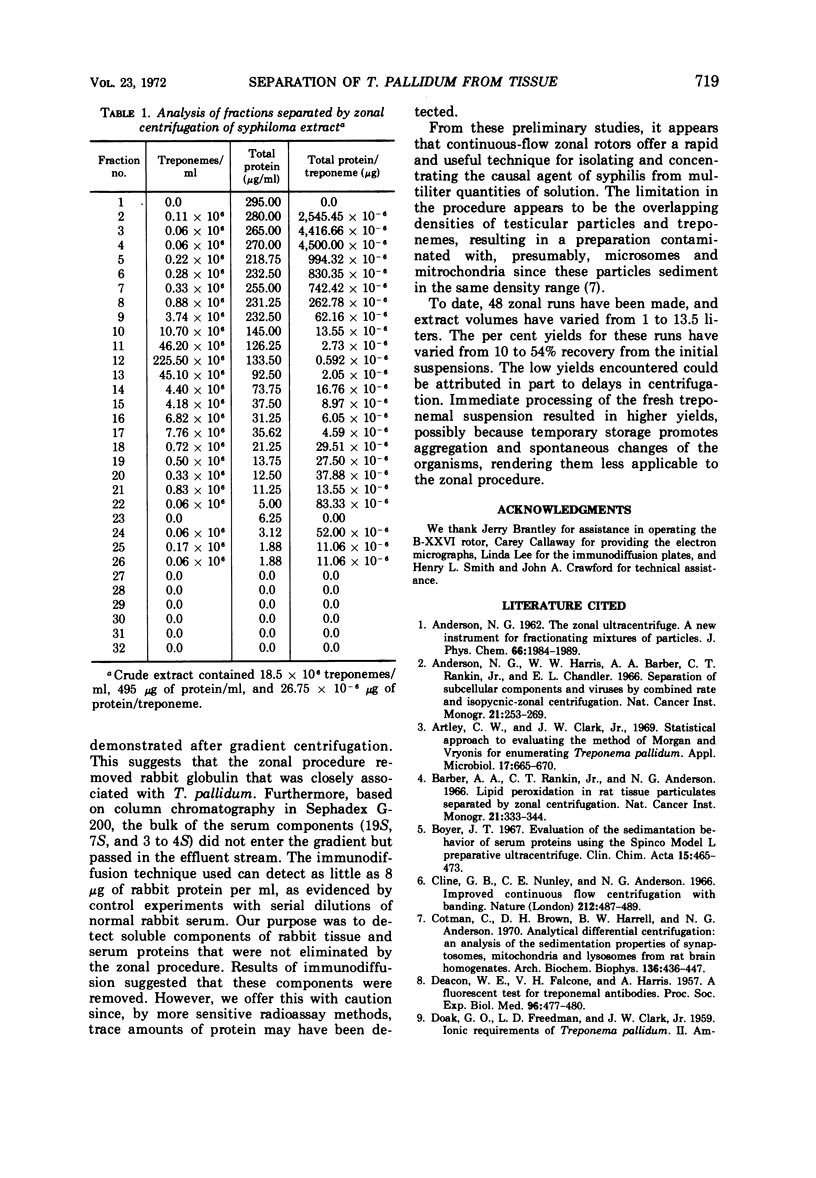

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Harris W. W., Barber A. A., Rankin C. T., Jr, Candler E. L. Separation of subcellular components and viruses by combined rate- and isopycnic-zonal centrifugation. Natl Cancer Inst Monogr. 1966 Jun;21:253–283. [PubMed] [Google Scholar]
- Artley C. W., Clark J. W., Jr Statistical approach to evaluating the method of Morgan and Vryonis for enumerating Treponema pallidum. Appl Microbiol. 1969 May;17(5):665–670. doi: 10.1128/am.17.5.665-670.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A. A., Rankin C. T., Jr, Anderson N. G. Lipid peroxidation in rat tissue particulates separated by zonal centrifugation. Natl Cancer Inst Monogr. 1966 Jun;21:333–344. [PubMed] [Google Scholar]
- Boyer J. T. Evaluation of the sedimentation behavior of serum proteins using the spinco model-L preparative ultracentrifuge. Clin Chim Acta. 1967 Mar;15(3):465–473. doi: 10.1016/0009-8981(67)90011-3. [DOI] [PubMed] [Google Scholar]
- Cline G. B., Nunley C. E., Anderson N. G. Improved continuous flow centrifugation with banding. Nature. 1966 Oct 29;212(5061):487–489. doi: 10.1038/212487a0. [DOI] [PubMed] [Google Scholar]
- Cotman C., Brown D. H., Harrell B. W., Anderson N. G. Analytical differential centrifugation: an analysis of the sedimentation properties of synaptosomes, mitochondria and lysosomes from rat brain homogenates. Arch Biochem Biophys. 1970 Feb;136(2):436–447. doi: 10.1016/0003-9861(70)90215-8. [DOI] [PubMed] [Google Scholar]
- DEACON W. E., FALCONE V. H., HARRIS A. A fluorescent test for treponemal antibodies. Proc Soc Exp Biol Med. 1957 Nov;96(2):477–480. doi: 10.3181/00379727-96-23512. [DOI] [PubMed] [Google Scholar]
- HUNTER E. F., DEACON W. E., MEYER P. E. AN IMPROVED FTA TEST FOR SYPHILIS, THE ABSORPTION PROCEDURE (FTA-ABS). Public Health Rep. 1964 May;79:410–412. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- RATHLEV T., PFAU C. J. PURIFICATION OF THE PATHOGENIC TREPONEMA PALLIDUM BY DENSITY GRADIENT CENTRIFUGATION. Scand J Clin Lab Invest. 1965;17:130–134. doi: 10.1080/00365516509077298. [DOI] [PubMed] [Google Scholar]