Abstract
Tissue oxygen tension regulates differentiation of multiple types of stem cells. In the placenta, hypoxia has been associated with abnormal trophoblast differentiation and placental insufficiency syndromes of preeclampsia (PE) and intrauterine growth restriction (IUGR). Peroxisome proliferator-activated receptor-γ (PPARγ) is a ligand-activated transcription factor involved in many cellular processes, including differentiation. We have previously shown that PPARγ-null trophoblast stem (TS) cells show a defect in differentiation to labyrinthine trophoblast, instead differentiating preferentially to trophoblast giant cells (TGC). Since PPARγ is known to be regulated by hypoxia in adipose tissue, we hypothesized that there may be a link between oxygen tension, PPARγ expression, and trophoblast differentiation. We found that hypoxia reduced PPARγ expression by a mechanism independent of both hypoxia-inducible factor (HIF) and histone deacetylases (HDACs). In addition, PPARγ partially rescued hypoxia-induced inhibition of labyrinthine differentiation in wild-type TS cells but was not required for hypoxia-induced inhibition of TGC differentiation. Finally, we show that induction of labyrinthine trophoblast differentiation by HDAC inhibitor treatment is independent of both PPARγ and Gcm1. We propose a model with two pathways for labyrinthine trophoblast differentiation of TS cells, one of which is dependent on PPARγ and inhibited by hypoxia. Since hypoxia is associated with PE and IUGR, we propose that PPARγ may at least partially mediate hypoxia-induced placental insufficiency and as such may be a promising therapeutic target for these disorders.
Introduction
The placenta is a transient organ, which plays a pivotal role in embryonic and fetal development [1]. Trophoblast, the epithelial cells of the placenta, carry out the primary functions of this organ, including establishment of maternal blood flow to the feto-placental unit, as well as nutrient and gas exchange [1–3]. In the mouse, the former is the primary function of invasive trophoblast giant cells (TGC), while the latter is carried out by syncytiotrophoblast (STB) in the labyrinthine portion of the placenta [1–3]. Placental insufficiency syndromes, including intrauterine growth restriction (IUGR) and preeclampsia (PE), are characterized by placental hypoxia and dysfunction, at least partially secondary to abnormalities in trophoblast differentiation and function [4,5]. Peroxisome proliferator-activated receptor-γ (PPARγ), a transcription factor and member of the ligand-activated nuclear hormone receptor superfamily [6], is abundantly expressed in all trophoblast subtypes and required for proper placental function [6–8]. In the mouse, PPARγ-null embryos fail to progress past mid-gestation, and display abnormal placentas, including lack of formation of the placental labyrinth [7,8]. We previously derived trophoblast stem (TS) cells from both wild-type (WT) and PPARγ-null blastocysts and showed that null TS cells preferentially differentiate into TGC [9]. PPARγ-null TS cells differentiate poorly into STB, and in fact show significantly reduced levels of Gcm1, the master regulator of labyrinthine differentiation [9–11].
Hypoxia is known to promote self-renewal and inhibit differentiation of several different tissue-specific stem cells, including hematopoietic, neural, and mesenchymal stem cells [12–15]. Hypoxia is also known to inhibit differentiation of trophoblast in the human placenta [16,17]. In adipocytes, hypoxia inhibits expression of PPARγ2, the primary isoform in this tissue; this, in turn, limits differentiation of these cells into mature adipocytes [18]. Hypoxia-induced inhibition of PPARγ2 in adipocytes is mediated by the hypoxia-inducible factor (HIF) complex [18], which also plays a major role in placental development [19–21]. Both HIF1α, the subunit stabilized in hypoxia, and HIF1β/ARNT are expressed at high levels early during mouse and human placental development and are required for trophoblast differentiation [19–21]. Similar to PPARγ-null embryos, ARNT-null embryos die at midgestation due to placental abnormalities [19]. In vitro, however, ARNT-null TS cells appear to have the opposite phenotype, differentiating exclusively into labyrinthine/STB instead of TGC; this phenotype is thought to be due to involvement of HIF in epigenetic modification of the TS cell genome, as it can be recapitulated by inhibiting histone deacetylases (HDACs) [22].
We set out to test the hypothesis that hypoxia also downregulates PPARγ1, the isoform expressed in mouse TS cells, through action of the HIF complex, and whether this pathway is involved in hypoxia-induced inhibition of labyrinthine trophoblast differentiation.
Materials and Methods
TS cell culture, chemical treatment, viral transduction, and morphologic assessment
PPARγ+/+ and PPARγ−/− TS cells were derived from E3.5 blastocysts as previously described [9]. The two ARNT-null TS cell lines (TS4 and TS10) were also previously described [19]. All TS cells for this study were grown in feeder-free cultures, in 30% TS cell medium [23] 70% feeder-conditioned (72 h) TS medium, 25 μg/mL FGF4 (Sigma), and 1 μg/mL heparin (Sigma; TSMFH medium). TS cells grown to confluence in TSMFH medium were switched to TS medium to induce differentiation. For normoxia, all cells were cultured in a standard incubator at 37°C under 95% room air and 5% CO2. For hypoxia, cells were cultured in an XVIVO system (Biospherix). The design of individual differentiation experiments in normoxia versus hypoxia are demonstrated in Figure 1A. For all experiments in hypoxia, media was changed and cells were lysed (for either RNA or protein analysis) or fixed (for immunofluorescence) in the XVIVO work chamber, under the indicated oxygen tension. Where indicated, cells were treated with sodium butyrate (stock solution of 1.25 M in PBS; used at 1:500 at a final concentration of 2.5 mM), or cycloheximide (stock solution of 100 mg/mL in DMSO; used at 1:1,000 at a final concentration of 100 μg/mL); control experiments were carried out with the same dilution of the respective carrier alone. HA-tagged full-length murine PPARγ1-expressing adenovirus was generated and used as described previously [9]. For assessment of morphologic differentiation into TGC versus STB, cells were stained with Dapi, phalloidin, and anti-HA-tag antibody (Abcam) if required and assessed as previously described [9]. Briefly, TGC were identified by large single or double nuclei, each at least twice the size of an undifferentiated TS cell nucleus; STB were identified as cells with three or more nuclei, each of which were similar in size to an undifferentiated TS cell nucleus.
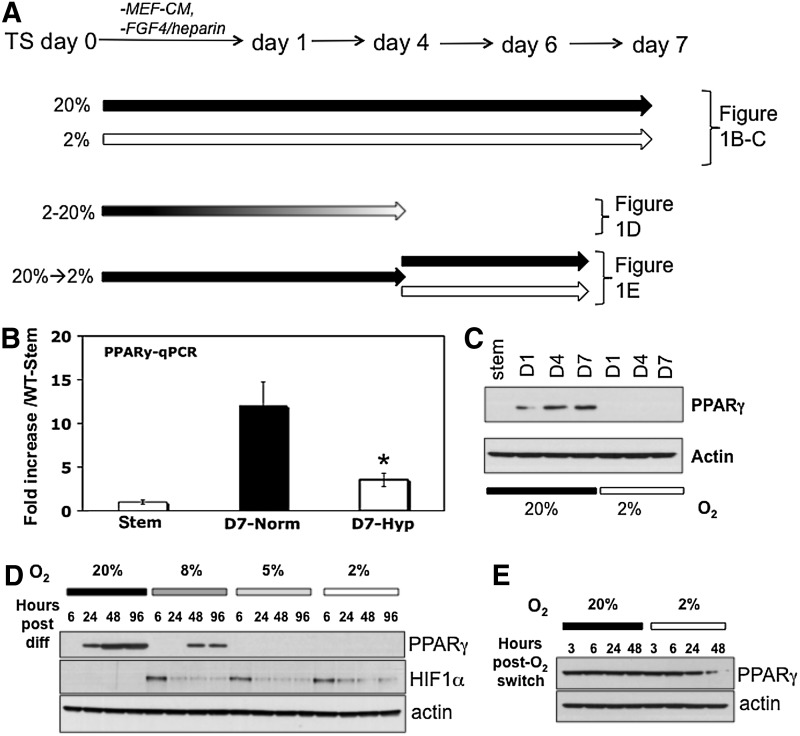
FIG. 1.
PPARγ expression is reduced in hypoxia. (A) Experimental design for (1B–1E). Block arrows indicate length of time in differentiation media. Different shades indicate various levels of oxygen tension. (B) PPARγ mRNA levels, measured by qPCR, in undifferentiated (stem) and differentiated (day 7) WT-TS cells under normoxia and hypoxia; * indicates statistically significant difference between levels in normoxia and hypoxia. (C) PPARγ protein levels assayed by western blot, in undifferentiated (stem) and differentiated (days 1, 4, and 7) WT-TS cells under normoxia and hypoxia. (D) PPARγ protein levels of WT-TS cells differentiated over a 4-day timecourse, in a range of oxygen tensions (20%, 8%, 5%, and 2%). (E) PPARγ protein levels in cells differentiated for 4 days in normoxia (20% oxygen), and then either switched to 2% oxygen, or maintained in normoxia, for the indicated number of hours. PPARγ, peroxisome proliferator-activated receptor-γ; TS, trophoblast stem; qPCR, quantitative polymerase chain reaction; WT, wild-type.
RNA isolation and quantitative reverse transcription–polymerase chain reaction
These protocols were previously described in detail [9]. Briefly, DNase-treated total RNA was isolated using NucleoSpin RNA II kit (Clontech), and cDNA was prepared from 1 μg RNA using iScript (Bio-Rad). Polymerase chain reaction (PCR) was performed using 625 nM of each primer and POWER SYBR Green PCR master mix (Applied Biosystems). Quantitative reverse transcription–PCR (qRT-PCR) was performed using a System 7300 instrument (Applied Biosystems) and a one-step program: 95°C, 10 min; 95°C, 30 s, 60°C, 1 min, for 40 cycles. All results were normalized against 18S rRNA. Relative mRNA expression levels, compared to 18S rRNA, were determined by the ΔΔCT method [24]. Fold change in normalized expression of individual genes in experimental samples was determined by comparison to expression in undifferentiated PPARγ+/+ cells. All primer pairs (Table 1) were checked for specificity using BLAST analysis and were checked by both agarose gel electrophoresis and thermal dissociation curves to ensure amplification of a single product with the appropriate size and melting temperature.
Table 1.
List of Primers Used for Quantitative Reverse Transcription–Polymerase Chain Reaction
| Gene abbreviation | Gene name | Primer sequence |
|---|---|---|
| Pl-I | Placental lactogen-1 | F 5′ TGGTGTCAAGCCTACTCCTTT 3′ |
| R 5′ CAGGGGAAGTGTTCTGTCTGT 3′ | ||
| Pl-II | Placental lactogen-2 | F 5′ CCAACGTGTGATTGTGGTGT 3′ |
| R 5′ TCTTCCGATGTTGTCTGGTG 3′ | ||
| synA | Syncytin-A | F 5′ CCCTTGTTCCTCTGCCTACTC 3′ |
| R 5′ TCATGGGTGTCTCTGTCCAA 3′ | ||
| Gcm1 | Glial cells missing-1 | F 5′ AACACCAACAACCACAACTCC 3′ |
| R 5′ CAGCTTTTCCTCTGCTGCTT 3′ | ||
| Tfeb | Transcription factor EB | F 5′ AACAAAGGCACCATCCTCAA 3′ |
| R 5′ CAGCTCGGCCATATTCACAC 3′ | ||
| Plf | Proliferin | F 5′ TGAGGAATGGTCGTTGCTTT 3′ |
| R 5′ TCTCATGGGGCTTTTGTCTC 3′ | ||
| PPARγ | Peroxisome proliferator-activated receptor-γ | F 5′ GACAGGAAAGACAACGGACAA 3′ |
| R 5′ AAACTGGCACCCTTGAAAAA 3′ | ||
| 18S | 18S ribosomal subunit | F 5′ CGCGGTTCTATTTTGTTGGT 3′ |
| R 5′ AACCTCCGACTTTCGTTCTTG 3′ |
Whole cell lysate and nuclear extract preparation and western blot
Whole cell lysates were prepared by scraping cells in boiled 1× Laemmli sample buffer (Bio-Rad), and passing the lysate through a 25G needle to sheer DNA and decrease viscosity. Nuclear extracts were prepared using the NE-PER kit (Pierce). Protein determination was done using a Coomassie-binding assay [25]. Thirty microgram of total protein was loaded in each lane for western blot. After transfer to PVDF, the membranes were blocked with 5% nonfat dried milk in TBS-Tween for 1 h at room temperature. Primary and secondary antibody incubations were similarly performed, with antibodies diluted in blocking buffer per the manufacturer's recommendation. Antibodies used included mouse anti-PPARγ monoclonal antibody (Santa Cruz), mouse anti-HIF1α (Abcam), mouse antiacetylated histone H4 (Abcam), mouse anti-TBP (Abcam), mouse antiactin monoclonal antibody (Abcam), and HRP-conjugated secondary antibodies (Jackson Immunochemicals). After treatment with ECL reagent (Pierce), membranes were exposed to autoradiography film (Kodak). Expression was quantified by densitometric scanning followed by normalizing expression to that of β-actin.
Statistical analysis
Unless otherwise stated, data presented are mean±standard deviation of three replicate wells of a single prep of cells; the results shown are representative of three to five separate experiments each performed on different days and using a different preparation of cells. Student's t-test was performed and a P-value of 0.05 was taken to indicate a statistically significant difference between the populations sampled.
Results
Hypoxia, PPARγ expression, and TS cell differentiation
TS cells were previously generated from both WT and PPARγ-null blastocysts [9]. We first differentiated WT-TS cells under various oxygen tensions to determine the effect of hypoxia on PPARγ expression (Fig. 1A). First, cells were cultured in either normoxia (20% oxygen) or hypoxia (2% oxygen) over a 7-day time-course. Two percent oxygen was chosen based on previous studies, which used a range of 1.5%–3% oxygen [19,20,22]. During the 7-day period, the cells were continuously kept under 20% or 2% oxygen, with media changed every other day under those same oxygen tensions. Both PPARγ RNA and protein were significantly reduced in hypoxia (Fig. 1B, C). In fact, PPARγ protein, normally induced at day 1 of differentiation (Fig. 1C) [9] was not detected in cells differentiated in hypoxia (Fig. 1C). We next asked whether this effect was seen at more physiologic oxygen tensions: PPARγ was in fact detectable, albeit at lower levels, at 8%, but was undetectable at 5% and 2% oxygen (Fig. 1D). HIF1α levels were highest at early timepoints (6 h) following switch to hypoxia, and lower with continuous hypoxia (Fig. 1D). Finally, we also tested whether hypoxia would decrease PPARγ expression, after it was induced by differentiation: we therefore, differentiated TS cells for 4 days in normoxia, and then either switched the cells to 2% oxygen, or continued differentiating in normoxia, for an additional 48 h. PPARγ expression was reduced after 24 h, further decreased after 48 h, in 2% oxygen (Fig. 1E). For the remaining experiments, hypoxia refers to 2% oxygen.
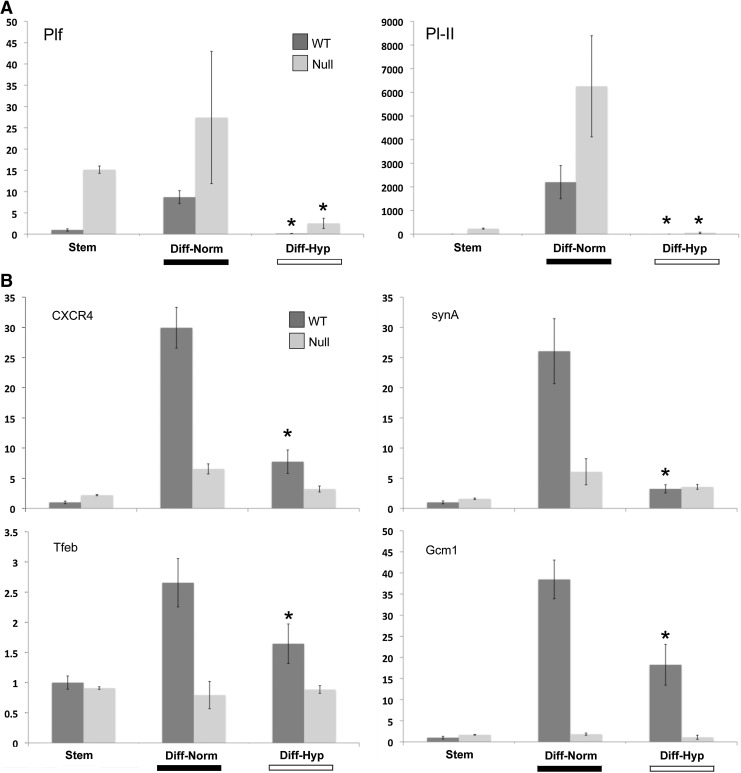
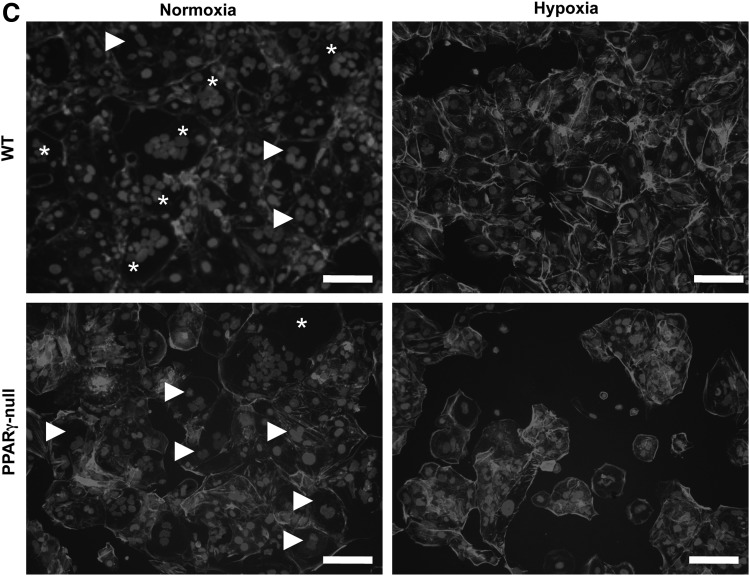
We next asked how differentiation is affected by oxygen tension. In WT-TS cells, hypoxia reduced markers of both TGC, such as Plf and Pl-II (Fig. 2A), and labyrinthine/STB, such as synA, Gcm1, Tfeb, and CXCR4 (Fig. 2B). PPARγ-null TS cells showed reduced markers of labyrinthine/STB markers when differentiated in normoxia, as previously reported (Fig. 2B) [9], and, similar to WT-TS cells, showed reduced TGC markers under hypoxic conditions (Fig. 2A). Morphologically, WT-TS cells differentiated in hypoxia showed mostly flattened, mononuclear cells, without the giant cells or multinucleated STB formed in normoxia (Fig. 2C). Differentiated PPARγ-null cells showed few STB even under normoxia; in hypoxia, they also comprised mainly of mononuclear cells, similar to differentiated WT-TS cells (Fig. 2C).
FIG. 2.
Hypoxia inhibits TS cell differentiation. WT and PPARγ-null TS cells were differentiated under normoxia or hypoxia (2% oxygen); qPCR was done for both giant cell markers (A) or labyrinthine/STB markers (B). Data are expressed as fold change over WT-Stem. * Indicates statistically significant difference between levels in normoxia and hypoxia for the indicated cell line. (C) Morphology of WT and PPARγ-null TS cells differentiated in either normoxia or hypoxia. Cells were stained with phalloidin and dapi. Arrowheads point to giant cells and * indicates multinucleated STB. Bar=100 μm. STB, syncytiotrophoblast.
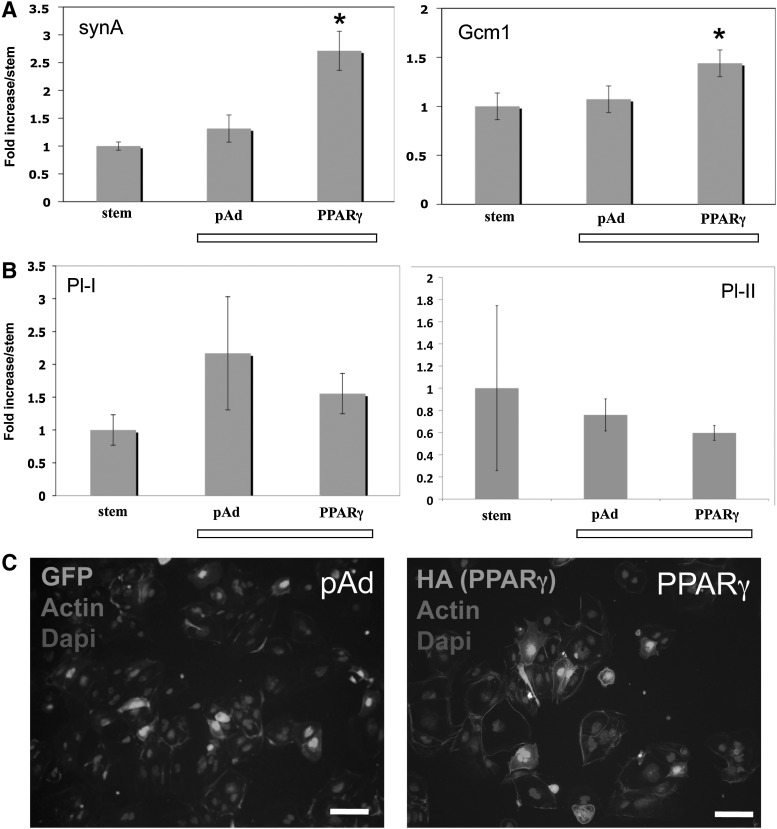
We have previously shown that overexpression of PPARγ in PPARγ-null TS cells rescued differentiation, specifically of the labyrinthine/STB lineage, based both on morphology and expression of lineage-specific markers [9]. We next asked whether forced expression of PPARγ in TS cells differentiated under hypoxia would enhance lineage-specific differentiation. We infected WT-TS cells with adenovirus expressing either GFP or HA-tagged full-length PPARγ1 [9], then differentiated the cells in hypoxia, and analyzed both lineage-specific markers by qPCR and cellular differentiation by morphology. Overexpression of PPARγ specifically induced labyrinthine/STB-specific markers (Fig. 3A), but did not induce giant cell markers (Fig. 3B) or alter formation of multinucleated STBs (Fig. 3C).
FIG. 3.
Reintroduction of PPARγ into differentiating WT-TS cells in hypoxia. qPCR was done for labyrinthine markers (A), and TGC markers (B). * Indicates statistically significant difference between levels in PPARγ-overexpressing cells versus pAd-GFP-expressing cells. (C) Morphology of WT-TS cells differentiating in hypoxia, overexpressing either GFP or PPARγ. Cells were stained with phalloidin and dapi; light gray shows infected cells, expressing either GFP (left) or HA-tagged PPARγ (right). Bar=100 μm. TGC, trophoblast giant cells.
Mechanism of hypoxia-induced downregulation of PPARγ
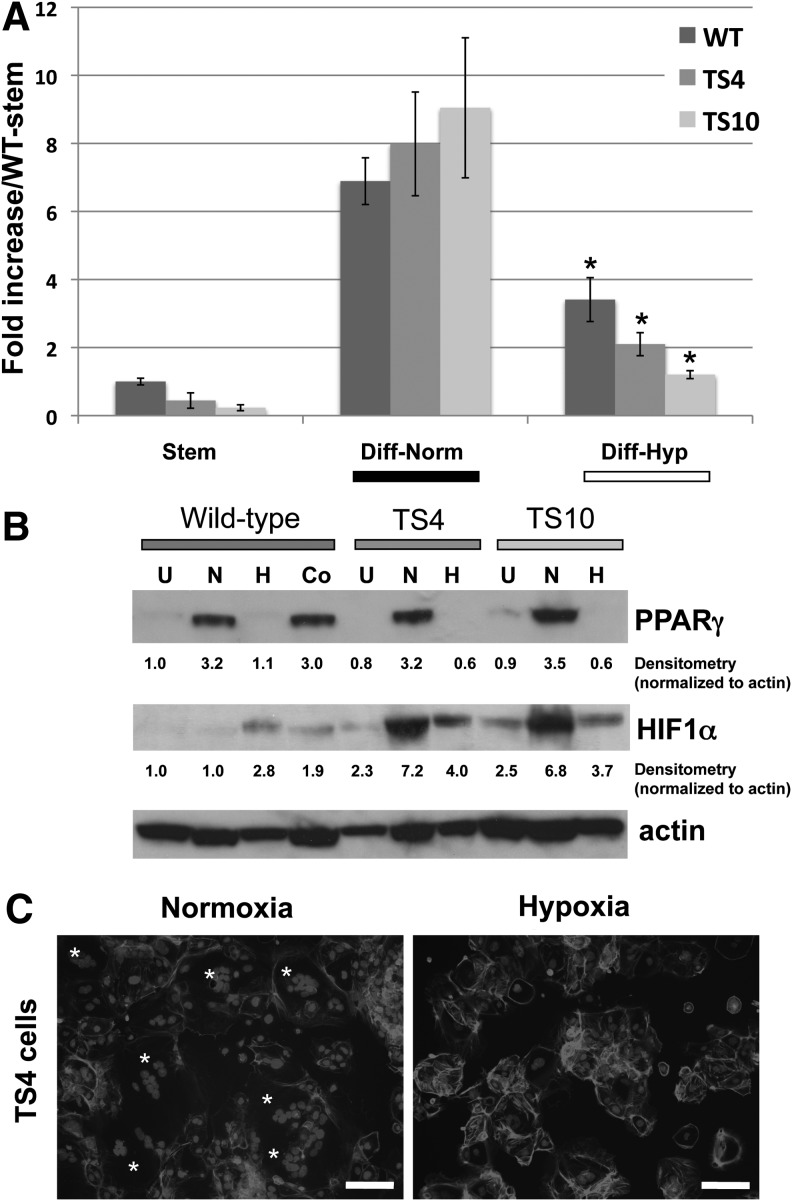
We next asked whether an intact HIF complex is required for hypoxia-induced downregulation of PPARγ and inhibition of STB formation. For this purpose, we differentiated WT and two HIF1β/ARNT-null TS cell lines (TS4 and TS10) [22] under normoxia or hypoxia, and measured PPARγ expression. Both PPARγ RNA and protein levels were decreased by hypoxia in both ARNT-null TS cell lines (Fig. 4A, B). Compared to undifferentiated TS cells, HIF1α protein levels were increased with hypoxic culture, but were even more greatly induced with differentiation in the ARNT-null TS cells (Fig. 4B). Morphologically, few STBs were detected in differentiated ARNT-null TS cells cultured in hypoxia (Fig. 4C).
FIG. 4.
Downregulation of PPARγ in hypoxia is HIF1β-independent. (A) PPARγ qPCR in WT or ARNT/HIF1β-null TS cells (TS4 and TS10), differentiated in either normoxia or hypoxia. * Indicates statistically significant difference between levels in normoxia and hypoxia for the indicated cell line. (B) PPARγ protein expression in WT or ARNT/HIF1β-null TS cells (TS4 and TS10), undifferentiated (U) or differentiated in normoxia (N), hypoxia (H), or in normoxia in the presence of cobalt chloride (Co). (C) Morphology of one of the ARNT-HIF1β-null TS cells (TS4), differentiated in normoxia or hypoxia. Cells were stained with phalloidin and dapi. * Indicates multinucleated STB. Bar=100 μm. HIF, hypoxia-inducible factor.
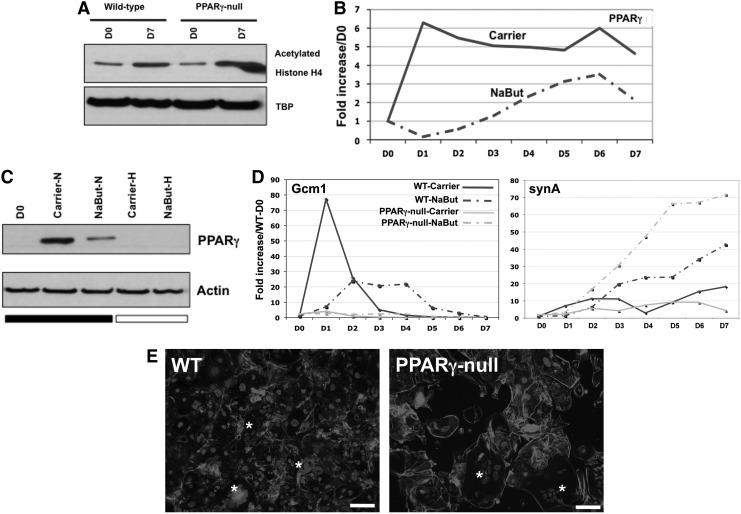
Hypoxia can also alter gene expression by HIF-independent mechanisms, including epigenetic modifications, such as changes in histone acetylation [26,27]. In fact, Maltepe et al. have shown that ARNT-null TS cells show increased histone acetylation and that inhibition of HDACs in WT-TS cells phenocopies the enhanced STB differentiation of ARNT-null TS cells [22]. We therefore, asked whether PPARγ-null TS cells, as phenotypic opposites of ARNT-null TS cells, show decreased histone acetylation, and whether HDAC inhibitors alter labyrinthine differentiation through induction of PPARγ. However, in contrast to these predictions, we found that PPARγ deficiency did not alter histone acetylation during differentiation (histone H4, Fig. 5A) and that sodium butyrate (NaBut), a known HDAC inhibitor, instead decreased both PPARγ mRNA and protein (Fig. 5B, C). We tried another HDAC inhibitor, namely Trichostatin A (TSA), but, even at low concentrations (1–10 μM), TSA was highly toxic to our TS cells (data not shown). We reasoned that HDAC inhibition may indeed bypass PPARγ and alter Gcm1 and synA levels directly; therefore, we evaluated Gcm1 and synA levels following NaBut treatment of both WT and PPARγ-null TS cells (Fig. 5D). We noted a reduced but more sustained expression of Gcm1 in WT cells but no alteration of expression in PPARγ-null TS cells; unexpectedly, we found that synA is induced following NaBut treatment, both in WT and PPARγ-null TS cells (Fig. 5D). In fact, both WT and PPARγ-null TS cells showed similar numbers of multinucleated STB with NaBut treatment (Fig. 5E).
FIG. 5.
Treatment with HDAC inhibitor induces STB formation, independent of PPARγ. (A) Nuclear lysates of undifferentiated and differentiated WT and PPARγ-null TS cells: histone acetylation increases during TS cell differentiation and is not altered by PPARγ deficiency. (B) PPARγ qPCR upon treatment with sodium butyrate (NaBut) or carrier during TS cell differentiation; representative results from one of four independent experiments are shown. (C) PPARγ western blot of whole cell lysates: TS cells were differentiated in normoxia or hypoxia, and treated with either NaBut or carrier alone. (D) Gcm1 and synA expression in WT and PPARγ-null TS cells differentiated in the presence of NaBut or carrier alone; representative results from one of four independent experiments are shown. (E) Cell morphology in WT and PPARγ-null TS cells differentiated for 7 days in the presence of NaBut. Cells were stained with phalloidin and dapi. * Indicates multinucleated STB. Bar=100 μm. HDAC, histone deacetylase.
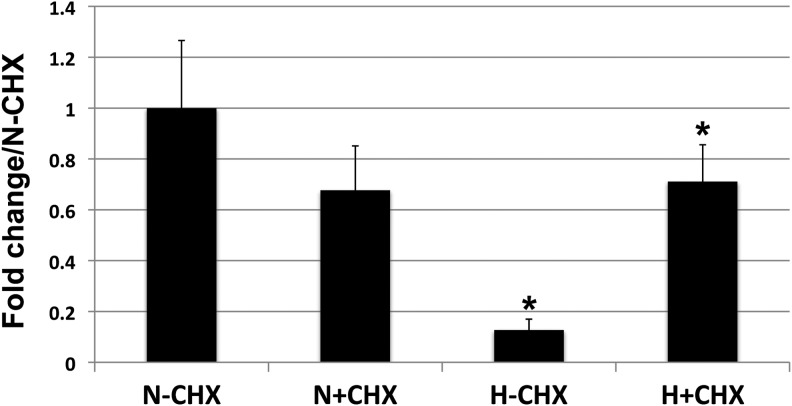
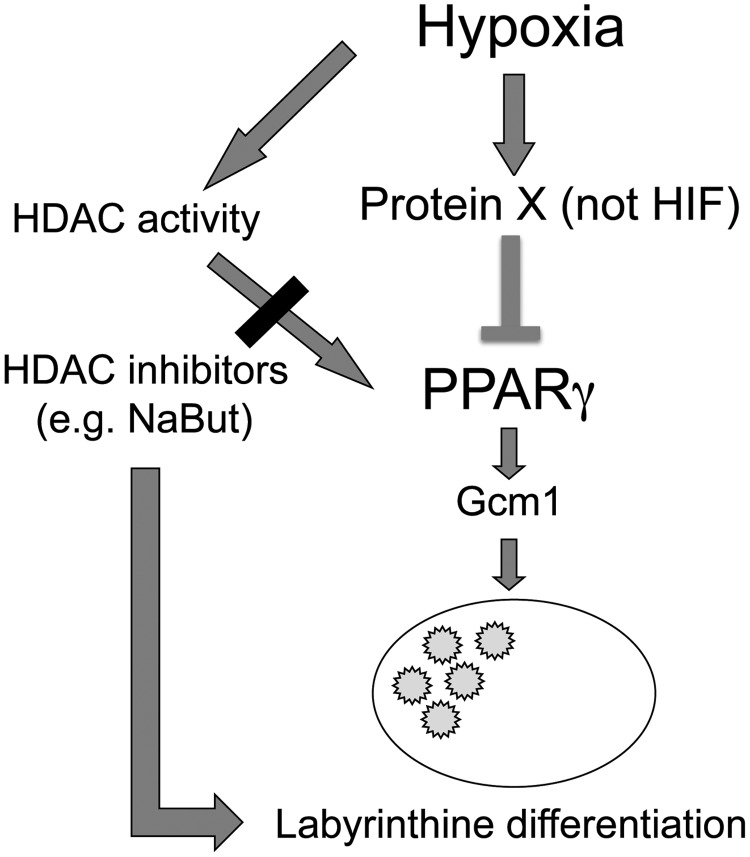
We next asked the broader question of whether protein synthesis is required for hypoxia-induced downregulation of PPARγ. We differentiated WT-TS cells in normoxia for 4 days, then either switched the cells to hypoxia, or continued differentiation in normoxia, for 48 h (similar to the experimental design in Fig. 1E), in the presence or absence of cycloheximide. In fact, treatment of hypoxic TS cells with cycloheximide restored PPARγ RNA levels to those in normoxia (Fig. 6). We propose a model in which labyrinthine/STB differentiation of TS cells can proceed through both a PPARγ-dependent and a PPARγ-independent pathway; the former is inhibited by hypoxia through a mechanism requiring new protein synthesis (Fig. 7).
FIG. 6.
Protein synthesis is required for hypoxia-induced downregulation of PPARγ. WT-TS Cells were differentiated in normoxia for 4 days to induce maximal expression of PPARγ. The cells were then subsequently switched to hypoxia (H; 2% oxygen) or continued in normoxia (N; 20% oxygen), in the presence or absence of cycloheximide (CHX), for a period of 48 h (Note: this is the same experimental design as in Fig. 1E). * Indicates statistically significant difference between levels in normoxia versus hypoxia without CHX (N-CHX and H-CHX), as well as between levels in hypoxia±CHX (H−CHX and H+CHX).
FIG. 7.
Proposed model for labyrinthine (STB) induction, through both PPARγ-dependent and -independent pathways.
Discussion
Hypoxia is a key player in both normal placental development and placenta-based pregnancy complications, such as PE and IUGR [4,5]. Hypoxia modulates gene expression through regulation of both transcription and translation: the former primarily occurs through the HIF complex, while the latter involves the ER stress (endoplasmic reticulum stress or unfolded protein response) and mTOR pathways [28]. HIF subunits, including HIF-1α, HIF2α, and ARNT/HIF1β are all expressed in TS cells; while ARNT is expressed constitutively, the expression of the HIFα subunits have been shown to be dependent both on oxygen tension and differentiation state of the TS cells [22]. The formation of an intact HIF complex is required for proper differentiation of TS cells in vitro, as well as normal placental development in vivo [20,22].
In this first report of hypoxia-induced downregulation of PPARγ expression in trophoblast, we found PPARγ mRNA to be significantly reduced, although additional regulation at the level of translation cannot be excluded. In a previous study of hypoxia-induced inhibition of adipocyte differentiation, PPARγ promoter was repressed through induction of Dec1/Stra13 downstream of HIF [18]. We therefore, focused on the HIF complex as the potential mediator of PPARγ downregulation in TS cells. Our data indicate that ARNT/HIF1β and an intact HIF complex are not required for hypoxia-induced downregulation of PPARγ mRNA expression in differentiating mouse TS cells. We also investigated Stra13 in TS cells, and found no effect on its expression with hypoxic culture (data not shown). Induction of HDAC activity was also ruled out as a mechanism, as treatment with an HDAC inhibitor mimicked rather than reversed the effects of hypoxia. Finally, cycloheximide blocked hypoxia-induced downregulation of PPARγ mRNA, implying that newly translated proteins are important for this response. One candidate for such a protein is Sirtuin-1 (Sirt-1), a protein deacetylase, known to repress PPARγ [29], and also to be induced in trophoblast under hypoxia [30]. We are currently investigating the role of Sirt-1 in regulation of PPARγ signaling in trophoblast.
The labyrinthine/STB lineage in the mouse placenta is regulated by Gcm1 and its downstream targets, the fusogenic syncytin genes [10,11,31,32]. We have previously shown that differentiated PPARγ-null TS cells show reduced levels of labyrinthine markers, including Gcm1 and synA [9]. This, combined with the failure to form labyrinth and midgestation lethality in both Gcm1 and PPARγ-null gestations, suggests that PPARγ may regulate labyrinthine differentiation through effects on Gcm1. We have shown that forced expression of PPARγ in null TS cells promotes both expression of labyrinthine markers, including Gcm1, and formation of multinucleated STB in vitro [9]. However, in the current study, forced expression of PPARγ in WT-TS cells under hypoxia induced only labyrinthine marker expression, without formation of multinucleated STB (Fig. 3). Similar dissociation between lineage-specific gene expression and morphologic differentiation has been noted with primary human cytotrophoblast cells, and is likely a consequence of in vitro culture [33]. Our results suggest that additional signaling, independent of PPARγ, is required to induce cell-cell fusion in hypoxia in vitro.
Placental insufficiency syndromes of PE and IUGR are associated with placental ischemia and oxidative stress [4]. While studies of these pregnancy complications have not shown consistent alteration of PPARγ expression [34,35], one feature of the severe form of PE, which is often accompanied by IUGR, is reduced levels of Gcm1 [36]. In fact, hypomorphic expression of Gcm1 in mouse placentae lead to defective STB differentiation and late gestational hypertension in the pregnant dams, a phenotype resembling PE [37]. We have previously shown that treatment of differentiating TS cells with the PPARγ agonist rosiglitazone can induce Gcm1, among other labyrinthine markers [9]. In addition, PPARγ antagonists have been shown to induce a PE-like syndrome in rats [38]. We therefore, propose that PPARγ may serve as a therapeutic target, and that in fact, PPARγ agonists may reverse some features of abnormal trophoblast differentiation associated with PE. However, PPARγ activation is also known to inhibit differentiation and function of invasive trophoblast, both in mouse [9] and human [39]; also prolonged treatment with such agonists has been shown to actually decrease the surface area of the placental labyrinth, as well as the spongiotrophoblast layer, and induce fetal growth restriction [40]. Therefore, targeting PPARγ in the PE placenta requires better understanding of its functions during each gestational period, elucidation of its upstream regulators, as well as further identification of its downstream targets in the placenta, including in trophoblast. Nevertheless, the established link between hypoxia and PPARγ in mouse TS cells can serve as a starting point for further investigation of the crosstalk between these two pathways in both animal models and diseased human placentae.
Acknowledgments
We would like to thank Dr. Mark Salata for technical assistance. This work was supported by grants R01HD040895 (D.S.M.) and R01HD071100 (M.M.P.) from the National Institute of Child Health and Human Development. V.T. was supported by a training grant from the California Institute for Regenerative Medicine, while conducting this work in the laboratory of M.M.P. at the University of California San Diego.
Author Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Huppertz B. Burton G. Cross JC. Kingdom JC. Placental morphology: from molecule to mother—a dedication to Peter Kaufmann—a review. Placenta. 2006;27(Suppl A):S3–S8. doi: 10.1016/j.placenta.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Red-Horse K. Zhou Y. Genbacev O. Prakobphol A. Foulk R. McMaster M. Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maltepe E. Bakardjiev AI. Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton GJ. Woods AW. Jauniaux E. Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMaster MT. Zhou Y. Fisher SJ. Abnormal placentation and the syndrome of preeclampsia. Semin Nephrol. 2004;24:540–547. doi: 10.1016/s0270-9295(04)00124-x. [DOI] [PubMed] [Google Scholar]
- 6.Barak Y. Sadovsky Y. Shalom-Barak T. PPAR signaling in placental development and function. PPAR Res. 2008;2008:142082. doi: 10.1155/2008/142082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barak Y. Nelson MC. Ong ES. Jones YZ. Ruiz-Lozano P. Chien KR. Koder A. Evans RM. PPARγ is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 8.Kubota N. Terauchi Y. Miki H. Tamemoto H. Yamauchi T. Komeda K. Satoh S. Nakano R. Ishii C, et al. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 9.Parast MM. Yu H. Ciric A. Salata MW. Davis V. Milstone DS. PPARγ regulates trophoblast proliferation and promotes labyrinthine trilineage differentiation. PLoS One. 2009;4:e8055.8. doi: 10.1371/journal.pone.0008055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anson-Cartwright L. Dawson K. Holmyard D. Fisher SJ. Lazzarini RA. Cross JC. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet. 2000;25:311–314. doi: 10.1038/77076. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber J. Riethmacher-Sonnenberg E. Riethmacher D. Tuerk EE. Enderich J. Bosl MR. Wegner M. Placental failure in mice lacking the mammalian homolog of glial cells missing, GCMa. Mol Cell Biol. 2000;20:2466–2474. doi: 10.1128/mcb.20.7.2466-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohyeldin A. Garzon-Muvdi T. Quinones-Hinojosa A. Oxygen in stem cell biology: a crintical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Suda T. Takubo K. Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 14.De Filippis L. Delia D. Hypoxia in the regulation of neural stem cells. Cell Mol Life Sci. 2011;68:2831–2844. doi: 10.1007/s00018-011-0723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das R. Jahr H. van Osch GJ. Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16:159–168. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 16.Genbacev O. Joslin R. Damsky CH. Polliotti BM. Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–550. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genbacev O. Zhou Y. Ludlow JW. Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 18.Yun Z. Maecker HL. Johnson RS. Giaccia AJ. Inhibition of PPARγ2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 19.Adelman DM. Gertsenstein M. Nagy A. Simon MC. Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowden Dahl KD. Fryer BH. Mack FA. Compernolle V. Maltepe E. Adelman DM. Carmeliet P. Simon MC. Hypoxia-inducible factors 1alpha and 2alpha regulate trophoblast differentiation. Mol Cell Biol. 2005;25:10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fryer BH. Simon MC. Hypoxia, HIF and the placenta. Cell Cycle. 2006;5:495–498. doi: 10.4161/cc.5.5.2497. [DOI] [PubMed] [Google Scholar]
- 22.Maltepe E. Krampitz GW. Okazaki KM. Red-Horse K. Mak W. Simon MC. Fisher SJ. Hypoxia-inducible factor-dependent histone deacetylase activity determines stem cell fate in the placenta. Development. 2005;132:3393–3403. doi: 10.1242/dev.01923. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka S. Kunath T. Hadjantonakis AK. Nagy A. Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Minamide LS. Bamburg JR. A filter paper dye-binding assay for quantitative determination of protein without interference from reducing agents or detergents. Anal Biochem. 1990;190:66–70. doi: 10.1016/0003-2697(90)90134-u. [DOI] [PubMed] [Google Scholar]
- 26.Kim MS. Kwon HJ. Lee YM. Baek JH. Jang JE. Lee SW. Moon EJ. Kim HS. Lee SK, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 27.Mie Lee Y. Kim SH. Kim HS. Jin Son M. Nakajima H. Jeong Kwon H. Kim KW. Inhibition of hypoxia-induced angiogenesis by FK228, a specific histone deacetylase inhibitor, via suppression of HIF-1alpha activity. Biochem Biophys Res Commun. 2003;300:241–246. doi: 10.1016/s0006-291x(02)02787-0. [DOI] [PubMed] [Google Scholar]
- 28.Van den Beucken T. Koritzinsky M. Wouters BG. Translational control of gene expression during hypoxia. Cancer Biol Ther. 2006;5:749–755. doi: 10.4161/cbt.5.7.2972. [DOI] [PubMed] [Google Scholar]
- 29.Picard F. Kurtev M. Chung N. Topark-Ngarm A. Senawong T. de Oliveira RM. Leid M. McBurney MW. Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPARγ. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen B. Nelson DM. Sadovsky Y. N-Myc down-regulated gene 1 modulates the response of term human trophoblasts to hypoxic injury. J Biol Chem. 2006;281:2764–2772. doi: 10.1074/jbc.M507330200. [DOI] [PubMed] [Google Scholar]
- 31.Schubert SW. Lamoureux N. Kilian K. Klein-Hitpass L. Hashemolhosseini S. Identification of integrin-alpha4, Rb1, and syncytin A as murine placental target genes of the transcription factor GCMa/Gcm1. J Biol Chem. 2008;283:5460–5465. doi: 10.1074/jbc.M710110200. [DOI] [PubMed] [Google Scholar]
- 32.Simmons DG. Natale DR. Begay V. Hughes M. Leutz A. Cross JC. Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development. 2008;135:2083–2091. doi: 10.1242/dev.020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kao LC. Caltabiano S. Wu S. Strauss JF. Kliman HJ. The human villous cytotrophoblast: interactions with extracellular matrix proteins, endocrine function, and cytoplasmic differentiation in the absence of syncytium formation. Dev Biol. 1988;130:693–702. doi: 10.1016/0012-1606(88)90361-2. [DOI] [PubMed] [Google Scholar]
- 34.Rodie VA. Young A. Jordan F. Sattar N. Greer IA. Freeman DJ. Human placental peroxisome proliferator-activated receptor delta and gamma expression in healthy pregnancy and in preeclampsia and intrauterine growth restriction. J Soc Gynecol Investig. 2005;12:320–329. doi: 10.1016/j.jsgi.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Holdsworth-Carson SJ. Lim R. Mitton A. Whitehead C. Rice GE. Permezel M. Lappas M. Peroxisome proliferator-activated receptors are altered in pathologies of the human placenta: gestational diabetes mellitus, intrauterine growth restriction and preeclampsia. Placenta. 2010;31:222–229. doi: 10.1016/j.placenta.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Drewlo S. Czikk M. Baczyk D. Lye S. Kingdom J. Glial cell missing-1 mediates over-expression of tissue inhibitor of metalloproteinase-4 in severe pre-eclamptic placental villi. Hum Reprod. 2011;26:1025–1034. doi: 10.1093/humrep/der053. [DOI] [PubMed] [Google Scholar]
- 37.Bainbridge SA. Minhas A. Whiteley KJ. Qu D. Sled JG. Kingdom JC. Adamson SL. Effects of reduced Gcm1 expression on trophoblast morphology, fetoplacental vascularity, and pregnancy outcomes in mice. Hypertension. 2012;59:732–739. doi: 10.1161/HYPERTENSIONAHA.111.183939. [DOI] [PubMed] [Google Scholar]
- 38.McCarthy FP. Drewlo S. English FA. Kingdom J. Johns EJ. Kenny LC. Walsh SK. Evidence implicating peroxisome proliferator-activator receptor-γ in the pathogenesis of preeclampsia. Hypertension. 2011;58:882–887. doi: 10.1161/HYPERTENSIONAHA.111.179440. [DOI] [PubMed] [Google Scholar]
- 39.Fournier T. Guibourdenche J. Handschuh K. Tsatsaris V. Rauwel B. Davrinche C. Evain-Brion D. PPARγ and human trophoblast differentiation. J Reprod Immunol. 2011;90:41–49. doi: 10.1016/j.jri.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Schaiff WT. Knapp FF., Jr Barak Y. Biron-Shental T. Nelson DM. Sadovsky Y. Ligand-activated peroxisome proliferator activated receptor gamma alters placental morphology and placental fatty acid uptake in mice. Endocrinology. 2007;148:3625–3634. doi: 10.1210/en.2007-0211. [DOI] [PubMed] [Google Scholar]