Abstract
Background
Access to healthcare, particularly effective primary and secondary preventive care, is critical for cancer survivors, in order to minimize the adverse sequelae of cancer and its treatment.
Purpose
The goal of the study was to evaluate the association between cancer survivorship and access to primary and preventive health care.
Methods
Cancer survivors (n=4960) and individuals without a cancer history (n=64,431) aged ≥18 years, from the 2008–2010 Medical Expenditure Panel Survey (MEPS), were evaluated. Multiple measures of access and preventive services use were compared. The association between cancer survivorship and access and preventive services was evaluated with multivariate logistic regression models, stratified by age group (18–64 years and ≥65 years), controlling for the effects of age, gender, race/ethnicity, education, marital status, and comorbidities. Data were analyzed in 2013.
Results
Cancer survivors aged ≥65 years had equivalent or greater access and preventive services use than individuals without a cancer history, in adjusted analyses. However, among those aged 18–64 years with private health insurance, cancer survivors were more likely than other individuals to have a usual source of care and to use preventive services, whereas uninsured or publicly insured cancer survivors were generally less likely to have a usual source of care and to use preventive services than were uninsured or publicly insured adults without a cancer history.
Conclusions
Although access and preventive care use in cancer survivors is generally equivalent or greater compared to that of other individuals, disparities for uninsured and publicly insured cancer survivors aged 18–64 years suggest that improvements in survivor care are needed.
Introduction
In 2008, there were approximately 12 million individuals with a history of cancer in the U.S.1 The prevalence of cancer survivorship is expected to increase dramatically in the future, due to the aging and growth of the U.S. population,1,2 and longer survival after diagnosis. However, cancer survivors have an increased risk of secondary cancers,3 may experience lasting or late effects of treatment,4 and are more likely to report worse health than individuals without a cancer history.5,6
Therefore, access to health care, particularly effective primary and secondary preventive care, is critical for cancer survivors, in order to minimize the adverse sequelae of cancer and its treatment. The 2005 IOM report, From Cancer Patient to Cancer Survivor: Lost in Transition, emphasized the importance of ensuring high-quality and ongoing preventive care for survivors.7 The Affordable Care Act will expand health insurance coverage to the previously uninsured and encourage use of preventive care.8
Having health insurance and a usual source of healthcare is consistently associated with greater preventive services use9 and diagnosis and treatment of chronic conditions.10 Even though newly diagnosed uninsured cancer patients may be medically eligible for Medicaid coverage during initial treatment in some states,11 longer-term cancer survivors may face barriers to maintaining insurance coverage and to care access.12 They may also forgo care because of cost.12,13
Findings from studies that compared preventive services use for cancer survivors and individuals without a cancer history are mixed.14–20 Some report less preventive services use by cancer survivors,15-17 whereas others report greater use.14,16,18 Most studies reporting less preventive services use were conducted in cohorts of elderly Medicare beneficiaries at similar times since diagnosis, using the linked SEER-Medicare data.15,17,21 Studies reporting similar or greater preventive services use were conducted in samples of prevalent survivors of all ages and cancer types, with all types of health insurance (including the uninsured), and a range of times since diagnosis.14,18–20 However, no prior population-based study has evaluated access to care or explored the role of insurance coverage in detail.
Considering these discrepant findings, more detailed comparisons of preventive service use in cancer survivors and individuals without a cancer history by age, insurance, and times since diagnosis are warranted. Age-related variation in preventive services use can reflect differential insurance coverage and access between older and younger populations. More than 95% of the population aged ≥65 years is enrolled in the Medicare program, whereas insurance for the younger population is more heterogeneous, and a substantial proportion is uninsured.22 Additionally, the elderly use more health care than younger populations.23 Differences in preventive service use between cancer survivors and other individuals also vary by age due to greater healthcare contact in elderly than younger populations without a cancer history.
In the current study, national data were used to evaluate access to care and preventive services use by adult cancer survivors compared to adults without a cancer history, separately for the elderly and non-elderly and within health insurance type. Access and preventive services use were also evaluated by time since diagnosis for cancer survivors. Findings will provide an important baseline for understanding access to care in cancer survivors prior to the scheduled implementation of the Affordable Care Act.
Methods
Sample
The sample was based on pooled data from 3 years (2008–2010) of the Medical Expenditure Panel Survey (MEPS) Household Component, which is nationally representative of the U.S. civilian non-institutionalized population. The MEPS is an ongoing survey of healthcare expenditures, insurance, utilization, and access to care. Data collection is initiated with a new sample (i.e., “panel”) each year that is followed for 2 years in five rounds of in-person interviews with a family member who typically responds for all family members in the household. Overlapping panels are combined for each MEPS annual file, and the combined average annual response rate for 2008–2010 was approximately 60%. More information about survey design and content is available from www.meps.ahrq.gov/mepsweb/.
Adult cancer survivors (n = 4960) were identified from a question about whether a doctor or other health professional had ever told them that they had cancer or a malignancy of any kind. Respondents were asked about the cancer type and age at diagnosis, for each diagnosis. The remaining 64,431 adults with no cancer history were the comparison group (125 individuals with missing data for this question were excluded). Individuals diagnosed with solely nonmelanoma skin cancer were not classified as cancer survivors.
Measures
Covariates: Sample characteristics used in the analysis were age, gender, race/ethnicity, marital status, education, and insurance type. Conditions other than cancer were ascertained with a series of questions about whether a doctor or other health professional ever told the person they had any MEPS priority conditions, including arthritis, asthma, hypertension, angina, coronary heart disease, stroke, diabetes, high cholesterol, heart attack, and emphysema. Conditions were categorized by the absolute number of priority conditions for each individual. Time since cancer diagnosis was calculated as the difference between age at first diagnosis and age at the interview (i.e., <2 years, 2–5 years, 6–10 years, and ≥11 years) for cancer survivors.5,6
Outcomes: Access to care was conceptualized as either perceived or realized based on an adaptation of Anderson and Aday's behavioral model of access to health care24 to reflect distinctions between perceptions about availability of care and actual utilization of healthcare services. Perceived access to care was measured in questions about a usual source of health care and ability to access, or delays in necessary medical care, dental care, or prescription medication. Realized access to care was measured by questions about preventive services use and cancer screening recommended by the U.S. Preventive Services Task Force25 and available in the MEPS, consistent with other studies.15-18 Preventive services included blood pressure and cholesterol evaluations within 2 years, and a dental check-up and an influenza vaccination within the past year.
Cancer screening was assessed among age- and gender-eligible women and men. Mammography within 2 years in women aged ≥40 years, Pap testing within 3 years in women aged ≥ 21 years who had not had their cervix removed, and home fecal occult blood testing within 1 year or endoscopy within 5 years among women and men aged ≥50 years were used to measure breast, cervical, and colorectal cancer screening, respectively. To ensure that these tests were for screening rather than surveillance for recurrence, test use was evaluated among only individuals without a specific cancer diagnosis, as has been done previously.18
Data Analysis
Descriptive statistics were calculated for cancer survivors and other individuals stratified by age group (18–64 years, ≥65 years), and distributions were compared with chi-square statistics. All estimates were weighted to account for the MEPS complex survey design and survey nonresponse using SUDAAN. The association between cancer survivorship and access to care was evaluated, stratified by age group and controlling for age, gender, race/ethnicity, educational attainment, marital status, and number of comorbid conditions, using multivariate logistic regression. Analyses were further stratified by insurance type and time since cancer diagnosis.
Wald statistics were used to test the significance of differences between cancer survivors and other individuals, the interaction between cancer survivorship and insurance type, and time since diagnosis among cancer survivors. Analyses did not consider multiple comparisons, but instead the discussion of results focuses on consistent patterns of findings across measures. Unadjusted bivariate estimates and adjusted predicted marginals are presented from the multivariate regression analyses. The predictive margins method26 directly standardizes the outcome of each group to the covariate distribution of the overall population. Standardized results can be compared like percentages. All tests of significance were two-sided. Because diagnosis dates for comorbid conditions in relation to cancer treatment(s) were not available, conditions could have preceded the cancer diagnosis and its treatment(s) or developed afterwards, potentially as a late or lasting effect of treatment. To understand the potential impact of either assumption about comorbidity, sensitivity analyses were conducted without controlling for comorbidities. The results of the two sets of analyses were similar, and adjusted estimates that control for comorbidities are presented.
Results
Cancer survivors were more likely to be older and have more comorbid conditions than individuals without a cancer history; these differences were more pronounced in the group aged 18–64 years than the group aged ≥65 years (Table 1). In both age groups, cancer survivors were more likely to have higher educational attainment and be non-Hispanic white than other individuals. In the younger group, cancer survivors were more likely to have either private or public insurance and less likely to be uninsured than individuals without a cancer history. In the older group, cancer survivors were more likely to have Medicare and private supplemental health insurance. The most common cancer diagnoses were breast and prostate (data not shown). In both age groups, most cancer survivors were diagnosed 6 or more years prior to the survey, with fewer cancer survivors diagnosed within 2 years or 2–5 years prior to the survey.
Table 1. Characteristics of cancer survivors and individuals without a cancer history, n (weighted %).
| Ages 18–64 years | Ages ≥65 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Survivors2 (n=2491) | Individuals without a Cancer History2 (n=55,972) | Cancer Survivors2 (n=2469) | Individuals without a Cancer History2 (n=8459) | |||||||
| p-value | p-value | |||||||||
| Age group (years) | <0.001 | |||||||||
| 18–44 | 677 | 24.5 | 34,347 | 59.8 | ||||||
| 45–49 | 296 | 11.3 | 6,391 | 11.2 | ||||||
| 50–54 | 405 | 16.3 | 6,104 | 11.5 | ||||||
| 55–59 | 507 | 21.1 | 5,136 | 9.6 | ||||||
| 60–64 | 606 | 26.8 | 3,994 | 7.9 | ||||||
| 65–69 | 598 | 23.4 | 2861 | 32.4 | <0.001 | |||||
| 70–74 | 533 | 21.9 | 1968 | 23.0 | ||||||
| 75–79 | 524 | 20.1 | 1524 | 18.3 | ||||||
| ≥80 | 814 | 34.6 | 2106 | 26.3 | ||||||
| Gender | <0.001 | <0.001 | ||||||||
| Male | 747 | 34.2 | 26,605 | 50.3 | 1,194 | 47.8 | 3483 | 41.8 | ||
| Female | 1,744 | 65.8 | 29,367 | 49.7 | 1,275 | 52.2 | 4976 | 58.2 | ||
| Education when first entered MEPSa | <0.001 | <0.001 | ||||||||
| Less than high school grad | 439 | 12.2 | 13,101 | 16.3 | 623 | 19.2 | 2724 | 23.9 | ||
| High school graduate | 780 | 30.0 | 17,026 | 29.1 | 798 | 34.7 | 2710 | 34.8 | ||
| Some college or more | 1,265 | 57.7 | 25,591 | 54.2 | 1,038 | 45.8 | 2959 | 40.8 | ||
| Race / ethnicity | <0.001 | <0.001 | ||||||||
| Non-Hispanic white only | 1,643 | 81.6 | 23,958 | 64.8 | 1,880 | 87.6 | 4877 | 76.5 | ||
| Non-Hispanic black only | 377 | 7.9 | 10,826 | 12.3 | 327 | 6.3 | 1573 | 9.4 | ||
| Hispanic | 348 | 6.8 | 15,751 | 15.7 | 154 | 3.6 | 1217 | 8.4 | ||
| Non-Hispanic other / multiple | 123 | 3.7 | 5,437 | 7.2 | 108 | 2.5 | 792 | 5.7 | ||
| Marital status | <0.001 | 0.820 | ||||||||
| Married | 1,400 | 61.1 | 28,684 | 52.9 | 1,328 | 54.5 | 4399 | 54.1 | ||
| Not married | 1,091 | 38.9 | 27,288 | 47.1 | 1,141 | 45.5 | 4060 | 45.9 | ||
| Health insurancea | <0.001 | |||||||||
| Aged <65 years, any private | 1,627 | 75.0 | 34,274 | 70.8 | ||||||
| Aged <65 years, public only | 544 | 14.8 | 7,931 | 10.1 | ||||||
| Aged <65 years, uninsured | 320 | 10.2 | 13,767 | 19.1 | ||||||
| Aged ≥65 years, Medicare only | 930 | 36.5 | 3213 | 38.6 | 0.002 | |||||
| Aged ≥65 years, Medicare and private | 1,220 | 54.0 | 3671 | 49.2 | ||||||
| Aged ≥65 years, Medicare and public | 296 | 8.5 | 1413 | 10.8 | ||||||
| Number of known MEPS priority conditions | <0.001 | <0.001 | ||||||||
| 0 | 642 | 26.5 | 30,375 | 52.8 | 139 | 5.9 | 739 | 8.3 | ||
| 1 | 598 | 25.3 | 12,788 | 23.8 | 327 | 13.5 | 1356 | 16.1 | ||
| 2 | 530 | 21.0 | 6,640 | 12.5 | 560 | 22.1 | 1939 | 23.9 | ||
| 3+ | 721 | 27.2 | 6,169 | 10.8 | 1,443 | 58.5 | 4425 | 51.7 | ||
Categories with missing data are not reported separately and will not add to 100%.
MEPS, Medical Expenditure Panel Survey
Perceived Access to Care
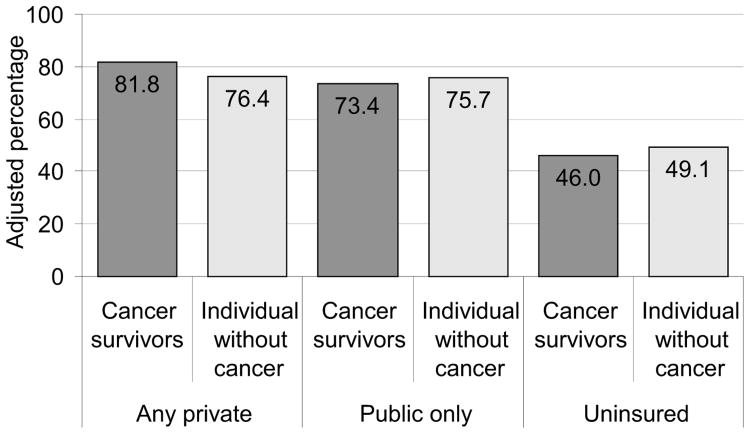
Cancer survivors aged 18–64 years were more likely to have a usual source of care than their counterparts without a cancer history, in adjusted analyses (Table 2). Having a usual source of care was not different for older groups. Associations between cancer survivorship and usual source of care varied by type of health insurance for the younger population, in adjusted analyses. Regardless of cancer history, the percentage with a usual source of care was highest among individuals with private insurance, lower for publicly insured, and lowest for the uninsured. Among the privately insured, cancer survivors were more likely than individuals without a cancer history to have a usual source of care, but among the publicly insured or uninsured, cancer survivors were less likely to have a usual source of care (Figure 1; p-value= 0.02 for interaction between insurance type and cancer history).
Table 2. Perceived Access to Care in Cancer Survivors and Individuals without a Cancer History.
| Ages 18–64 years | Ages ≥65 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Survivors (n = 2484) | Individuals without a Cancer History (n = 55,718) | Cancer Survivors (n = 2459) | Individuals without a Cancer History (n=8393) | |||||||
| %a | Adj %b | %a | Adj % b | p- valuec | %a | Adj %d | %a | Adj %d | p-valuec | |
| Usual source of healthcare | 0.007 | 0.265 | ||||||||
| Yes | 85.0 | 74.9 | 70.3 | 70.9 | 91.1 | 90.4 | 89.0 | 89.3 | ||
| No / missing | 15.0 | 25.1 | 29.7 | 29.1 | 8.9 | 9.6 | 11.0 | 10.7 | ||
| Among people with a usual source of health care, usual healthcare provider how difficult is it to get to usual healthcare provider | 0.044 | 0.187 | ||||||||
| Somewhat or very difficult | 6.3 | 5.9 | 4.5 | 4.6 | 5.4 | 5.3 | 5.9 | 5.9 | ||
| Not too difficult | 17.4 | 17.4 | 16.4 | 16.4 | 20.8 | 21.3 | 19.3 | 19.1 | ||
| Not at all difficult / missing | 76.3 | 76.8 | 79.0 | 79.0 | 73.8 | 73.4 | 74.8 | 74.9 | ||
| Unable to get or delayed any necessary medical care, dental care, or prescription medication | <0.001 | 0.053 | ||||||||
| Yes | 19.2 | 16.0 | 12.6 | 12.8 | 10.6 | 10.7 | 9.1 | 9.1 | ||
| No / missing | 80.8 | 84.0 | 87.4 | 87.2 | 89.4 | 89.3 | 90.9 | 90.9 | ||
| Among people who were unable to get or who delayed care/medication, problem not getting or delayed necessary medical care, dental care, or prescription medication | 0.316 | 0.002 | ||||||||
| A big problem | 66.2 | 63.0 | 58.5 | 58.7 | 40.4 | 40.4 | 52.6 | 52.6 | ||
| A small problem | 26.1 | 28.4 | 30.9 | 30.7 | 42.7 | 43.1 | 29.1 | 28.9 | ||
| Not a problem / missing | 7.8 | 8.6 | 10.6 | 10.5 | 16.9 | 16.5 | 18.3 | 18.5 | ||
Unadjusted weighted percentages
Predicted marginals from a logistic regression model with age (18–49 years, 50–54 years, 55–59 years, 60–64 years); number of comorbid conditions (0, 1, 2, 3+); marital status; educational attainment; race/ethnicity; and gender as covariates
p-value for Wald F from the logistic regression model
Predicted marginals from a logistic regression model with age (65–69 years, 70–74 years, 75–79 years, ≥80 years); number of comorbid conditions (0–1, 2, 3, 4+); marital status; educational attainment; race/ethnicity; and gender as covariates
Adj., adjusted
Figure 1. Usual source of health care by type of health insurance, ages 18–64 years.
Note: p-value=0.02 for interaction between health insurance type and cancer survivor status
Among the younger group, cancer survivors were more likely to be unable to get or to delay in getting necessary medical care compared to individuals without a cancer history (16.0% vs 12.8%, p<0.001). Being unable to get or delayed in getting necessary care was similar for cancer survivors and individuals without a cancer history with private health insurance (11.2% vs 9.5%), but worse for cancer survivors with public insurance (26.1% vs 19.5%) or who were uninsured (29.0% vs 22.1%), although the interaction for health insurance type and cancer history was not significant (p=0.341).
Regardless of cancer status, for the older group, those with Medicare and private insurance were most likely to have a usual source of care, and individuals with Medicare and public insurance were least likely to have a usual source. Within each type of insurance, the likelihood of having a usual source of health care was not different for survivors and individuals without a cancer history (Appendix A, available online at www.ajpmonline.org). Other aspects of perceived access also varied by type of insurance, but were not different for older cancer survivors and other individuals within insurance categories (data not shown).
Realized Access to Care
In the group aged 18–64 years, cancer survivors were more likely than other individuals to have had blood pressure or cholesterol evaluations, or influenza vaccinations in adjusted analyses (Table 3). Among age- and gender-eligible individuals, higher proportions of cancer survivors received cervical or colorectal cancer screening. In the group aged ≥65 years, the proportions with blood pressure and cholesterol evaluations were not different for cancer survivors and other individuals, but older cancer survivors were more likely to have received dental check-ups, influenza vaccinations, cervical or colorectal cancer screening.
Table 3. Realized access to care in cancer survivors and individuals without a cancer history.
| Ages 18–64 years | Ages ≥65 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Survivorsb (n=1697) | Individuals without a Cancer Historyb (n=37,813) | Cancer Survivorsb (n=1629) | Individuals without a Cancer Historyb (n=5560) | |||||||
| %a | Adj %b | %a | Adj %b | p-valuec | %a | Adj %d | %a | Adj %d | p-valuec | |
| Preventive services | ||||||||||
| Doctor checked blood pressure in last 2 years | 95.0 | 90.0 | 84.9 | 85.3 | <0.001 | 92.8 | 92.5 | 92.4 | 92.5 | 0.918 |
| Doctor checked cholesterol in last 2 years | 80.4 | 66.7 | 60.4 | 61.1 | <0.001 | 88.7 | 88.2 | 86.5 | 86.7 | 0.182 |
| Dental check-up at least once per year | 64.7 | 61.0 | 60.8 | 61.0 | 1.00 | 59.1 | 58.4 | 52.7 | 53.0 | <0.001 |
| Influenza vaccination in the last year | 45.9 | 34.4 | 29.5 | 30.1 | <0.001 | 71.8 | 69.7 | 65.4 | 66.1 | 0.010 |
| Cancer screening (among age- and gender-eligible individuals without the specific cancer) | ||||||||||
| Breast cancer screening (mammogram within 2 years) in women aged ≥40 years | 69.6 | 68.2 | 69.7 | 69.8 | 0.411 | 65.4 | 66.4 | 65.5 | 65.3 | 0.649 |
| Cervical cancer screening (Pap within 3 years) in women aged ≥21 years who had not had cervix removed | 87.2 | 88.1 | 84.2 | 84.1 | 0.011 | 62.6 | 63.6 | 58.8 | 58.6 | 0.048 |
| Colorectal cancer screening (Home FOBT within 1 year or endoscopy within 5 years) in men and women aged ≥50 years | 58.5 | 54.5 | 46.7 | 47.1 | <0.001 | 62.5 | 61.4 | 55.8 | 56.1 | <0.001 |
Unadjusted weighted percentages
Predicted marginals from a logistic regression model with age (18–49 years, 50–54 years, 55–59 years, 60–64 years); number of comorbid conditions (0, 1, 2, ≥3); marital status; educational attainment; race/ethnicity; and gender as covariates
p-value for Wald F from the logistic regression model
Predicted marginals from a logistic regression model with age (65–69 years, 70–74 years, 75–79 years, ≥80 years); number of comorbid conditions (0–1, 2, 3, ≥4); marital status; educational attainment; race/ethnicity; and gender as covariates
FOBT, fecal occult blood test
Associations between cancer survivorship and realized access to care varied by type of insurance in the population aged 18–65 years, in adjusted analyses. Among the privately insured, cancer survivors were generally more likely to receive blood pressure or cholesterol evaluations or dental check-ups than individuals without a cancer history, whereas among the publicly insured or the uninsured, cancer survivors were generally less likely to receive preventive services (Appendixes B and C, available online at www.ajpmonline.org). For example, having a recent dental check-up was more likely in cancer survivors than in individuals without a cancer history among those with private health insurance, (72.5% vs 69.3%) but less likely among those with public health insurance (36.5% vs 49.4%) or those who were uninsured (30.9% vs 36.5%, p<0.001 for interaction). Receipt of cancer screening among age-eligible adults was not different for cancer survivors and other individuals within insurance categories (data not shown).
In the population aged ≥65 years, preventive service use was highest for individuals with Medicare and supplemental private insurance, and lower for those with Medicare only or Medicare and public insurance, regardless of cancer history. In contrast to the patterns in the younger group, older cancer survivors consistently made equivalent or greater use of preventive services than individuals without a cancer history with the same insurance type (Appendixes D and E, available online at www.ajpmonline.org). Among cancer survivors in both age groups, preventive service use and cancer screening, varied little by time since diagnosis, in adjusted analyses (data not shown).
Discussion
Overall, cancer survivors of all ages were more likely to have access to care and equivalent or greater preventive services use across multiple measures compared to other individuals. Although these findings are encouraging, there is still room for improved care for cancer survivors, particularly because they have increased risk of developing other cancers and may experience late or lasting effects of treatment.3,4,27,28 For example, the proportion of cancer survivors aged 50–64 years and ≥65 years receiving colorectal cancer screening was 58.5% and 62.5%, respectively, whereas the 2020 Healthy People29 target is 70.5%. Ongoing monitoring is important for ensuring that all cancer survivors reach these important preventive health goals.
Striking effects of health insurance on access to care and preventive services use for cancer survivors and individuals without a cancer history were observed across multiple measures. The uninsured and publicly insured had diminished access to care and were less likely to use preventive services compared to the privately insured, as reported elsewhere.9 However, in the group aged 18–64 years, privately insured cancer survivors were more likely to have access to care and use preventive services than individuals without a cancer history, whereas publicly insured or uninsured cancer survivors were generally less likely to have access to care. As a result, disparities in access by type of insurance were greater for cancer survivors than for individuals without a cancer history.
Although some of the disparities by type of insurance are relatively modest, their impact may be large because approximately 40% of the 12 million cancer survivors are aged <65 years. Because this is one of the first studies to explore health insurance type and perceived and realized access to care for cancer survivors in detail, some of these findings are hypothesis-generating. For example, disparities by type of insurance coverage for younger cancer survivors and differences by age suggest that further exploration of the role of health insurance in accessing care, including ease of making timely appointments and having a network of providers, and the impact of delaying or being unable to get necessary care, will be important in efforts to improve survivorship care. These findings provide an important baseline for understanding the effects of health insurance expansions on cancer survivorship care.
Findings of equivalent or greater reported preventive services use for older survivors compared to older individuals without a cancer history, regardless of health insurance, while consistent with other studies conducted in samples of prevalent cancer survivors14,18,20 differs from most SEER-Medicare cohort studies, even when stratified by time since diagnosis. Prior studies using Medicare claims conducted in cohorts of cancer survivors aged ≥65 years with a single type of cancer and single time since diagnosis generally reported less use of preventive services by cancer survivors.15,17 One of the few exceptions reported higher use of preventive services in a population of long-term breast cancer survivors.16 Importantly, when the sample without cancer was restricted to those who had mammography in the same 2–year period as the breast cancer diagnosis (making past use of mammography similar in the two groups), apparent differences in the use of most other preventive services between survivors and controls were eliminated.16 Future work exploring the inter-relationship between time since diagnosis, usual source of care, type of insurance, and use of preventive services is needed, particularly for cancers with effective screening tests.
Limitations
Despite the strengths of a large nationally representative sample of adults of all ages and all types of insurance and the consistency of findings across multiple measures, this study has several limitations. Cancer survivors were identified by survey responses, and information about stage at diagnosis, treatment(s), recurrence, or other clinical characteristics was not available. Details about onset of comorbid conditions in relation to cancer treatment or the role of oncologists in usual care and care coordination that would have allowed a more sophisticated analysis of access to care were also not available. Perhaps more importantly, population-based surveys generally include only small numbers of newly diagnosed, rare cancers, or cancers associated with short survival, and mainly consist of long-term survivors of common adult cancers; often participating many years after their diagnosis. Evaluation of perceived and realized access to care in populations of survivors with rare cancers and short survival is important for future research. Finally, information about access to care in the MEPS was based on survey responses.
Conclusion
Although access to care and use of preventive services in cancer survivors is generally equivalent or greater compared to other individuals, there are important disparities for publicly insured or uninsured cancer survivors aged 18–64 years. Efforts to improve access for these groups are needed, particularly in light of ongoing health insurance expansions in the U.S. Ongoing efforts to improve adherence to preventive service recommendations for the more than 12 million cancer survivors are important.
Supplementary Material
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlander N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review. Bethesda MD: National Cancer Institute; 2011. pp. 1975–2008. [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the U.S.: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng AK, Travis LB. Second primary cancers: an overview. Hematol Oncol Clin North Am. 2008;22(2):271–89. doi: 10.1016/j.hoc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Hudson MM, Landier W, Ganz PA. Impact of survivorship-based research on defining clinical care guidelines. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2085–92. doi: 10.1158/1055-9965.EPI-11-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–30. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 6.Dowling E, Yabroff KR, Mariotto AB, McNeel T, Zeruto C, Buckman D. Burden of illness in adult survivors of childhood cancers: findings from a population-based national sample. Cancer. 2010;116(15):3712–21. doi: 10.1002/cncr.25141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 8.DHHS. Affordable Care Act. Washington, DC: 2011. 11-4-2011. Ref Type: Online Source. [Google Scholar]
- 9.Casillas J, Castellino SM, Hudson MM, et al. Impact of insurance type on survivor-focused and general preventive health care utilization in adult survivors of childhood cancer: the Childhood Cancer Survivor Study (CCSS) Cancer. 2011;117(9):1966–75. doi: 10.1002/cncr.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadley J. Sicker and poorer: the consequences of being uninsured. Med Care Res Rev. 2003;60(2 suppl):3S–75S. doi: 10.1177/1077558703254101. [DOI] [PubMed] [Google Scholar]
- 11.Chien LN, Adams EK, Yang Z. Medicaid enrollment at early stage of disease: the Breast and Cervical Cancer Prevention and Treatment Act in Georgia. Inquiry. 2011;48(3):197–208. doi: 10.5034/inquiryjrnl_48.03.02. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz K, Claxton G, Martin K, Schmidt C. Spending to Survive: Cancer Patients Confront Holes in the Health Insurance System. Kaiser Family Foundation and American Cancer Society. 2009 Feb [Google Scholar]
- 13.Weaver KE, Rowland JH, Bellizzi KM, Aziz NM. Forgoing medical care because of cost: assessing disparities in healthcare access among cancer survivors living in the U.S. Cancer. 2010;116(14):3493–504. doi: 10.1002/cncr.25209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trask PC, Rabin C, Rogers ML, et al. Cancer screening practices among cancer survivors. Am J Prev Med. 2005;28(4):351–6. doi: 10.1016/j.amepre.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Earle CC, Neville BA. Under use of necessary care among cancer survivors. Cancer. 2004;101(8):1712–9. doi: 10.1002/cncr.20560. [DOI] [PubMed] [Google Scholar]
- 16.Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21(8):1447–51. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 17.Snyder CF, Firck KD, Peairs KS, et al. Comparing care for breast cancer survivors to non-cancer controls: a five-year longitudinal study. J Gen Intern Med. 2009;24(4):469–74. doi: 10.1007/s11606-009-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellizzi KM, Rowland JH, Jeffrey DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23(34):8884–93. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 19.Mayer DK, Terrin NC, Menon U, et al. Screening practices in cancer survivors. J Cancer Surviv. 2007;1:17–29. doi: 10.1007/s11764-007-0007-0. [DOI] [PubMed] [Google Scholar]
- 20.Duffy CM, Clark MA, Allsworth JE. Health maintenance and screening in breast cancer survivors in the U.S. Cancer Detect Prev. 2006;30:52–7. doi: 10.1016/j.cdp.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 21.McBean AM, Yu X, Virnig BA. The use of preventive health services among elderly uterine cancer survivors. Am J Obstet Gynecol. 2008;198(1):e1–86.e8. doi: 10.1016/j.ajog.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 22.DeNavas-Walt C, Proctor BD, Smith JC. Income, Poverty, Health Insurance Coverage in the U S: 2008. U.S. Census Bureau, U.S. Department of Commerce; 2009. [Google Scholar]
- 23.Alemayehu B, Warner KE. The lifetime distribution of health care costs. Health Serv Res. 2004;39(3):627–42. doi: 10.1111/j.1475-6773.2004.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen RM, Aday LA. Access to medical care in the U.S.: Realized and potential. Med Care. 1978;16(7):533–46. doi: 10.1097/00005650-197807000-00001. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Preventive Services Task Force. USPSTF A B Recommendations. 2013 www.uspreventiveservicestaskforceorg/uspstf/uspsabrecshtm.
- 26.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–9. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 27.Oeffinger KC, Bhatia S. Second primary cancers in survivors of childhood cancer. Lancet. 2009;31(374):1484–5. doi: 10.1016/S0140-6736(09)61885-7. [DOI] [PubMed] [Google Scholar]
- 28.Hong S, Nekhlyudov L, Didwania A, Olopade O, Ganschow P. Cancer survivorship care: exploring the role of the general internist. J Gen Intern Med. 2009;24(2):S495–S500. doi: 10.1007/s11606-009-1019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DHHS. Healthy People 2020. 2012 www.healthypeople.gov/2020/TopicsObjectives2020/pdfs/HP2020_brochure_with_LHI_508pdf.
- 30.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: colon cancer. 2009 doi: 10.6004/jnccn.2009.0056. www.nccn.org. [DOI] [PubMed]
- 31.Field TS, Doubeni C, Fox MP, et al. Under utilization of surveillance mammography among older breast cancer survivors. J Gen Intern Med. 2008;23(2):158–63. doi: 10.1007/s11606-007-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellison GL, Warren JL, Knopf KB, Brown ML. Racial differences in the receipt of bowel surveillance following potentially curative colorectal cancer surgery. Health Serv Res. 2003;38(6 PT 2):1885–18903. doi: 10.1111/j.1475-6773.2003.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.