Abstract
Purpose
Gastric cancer is a commonly occurring cancer in Asia and one of the leading causes of cancer deaths. However, there is no reliable blood-based screening test for this cancer. Identifying proteins secreted from tumor cells could lead to the discovery of clinically useful biomarkers for early detection of gastric cancer.
Experimental design
A SILAC-based quantitative proteomic approach was employed to identify secreted proteins that were differentially expressed between neoplastic and non-neoplastic gastric epithelial cells. Proteins from the secretome were subjected to SDS-PAGE and SCX-based fractionation, followed by mass spectrometric analysis on an LTQ-Orbitrap Velos mass spectrometer. Immunohistochemical labeling was employed to validate a subset of candidates using tissue microarrays.
Results
We identified 2,205 proteins in the gastric cancer secretome of which 263 proteins were overexpressed >4-fold in gastric cancer-derived cell lines as compared to non-neoplastic gastric epithelial cells. Three candidate proteins, proprotein convertase subtilisin/kexin type 9 (PCSK9), lectin mannose binding 2 (LMAN2) and PDGFA associated protein 1 (PDAP1), were validated by immunohistochemical labeling.
Conclusions and clinical relevance
We report here the largest cancer secretome described to date. The novel biomarkers identified in the current study are excellent candidates for further testing as early detection biomarkers for gastric adenocarcinoma.
Keywords: Secreted proteins, cell supernatants, in vivo labeling, gastric carcinoma, biomarkers, early diagnosis
1. INTRODUCTION
Gastric cancer is the fourth most common malignancy and the second leading cause of cancer deaths in both sexes worldwide [1–3]. Almost a million new cases of gastric cancer occurred worldwide in 2008 [2]. Gastric cancer is often diagnosed at an advanced stage, and currently, there are no suitable strategies to detect it at an early stage [4, 5]. In the context of early detection, it is critical to identify biomarkers that can be detected in blood [6]. However, blood/serum/plasma is difficult to analyse using proteomic methodologies because a few proteins are present at very high abundance with the large majority often at very low concentrations. Secreted proteins from tumor tissues are more likely to enter the systemic circulation and these proteins could potentially be used as biomarkers for early detection [7]. To directly identify proteins with such biomarker potential, secretome of tumor cells can be used for discovery as they may be more challenging to identify from serum/plasma. [8]. Candidates identified in this fashion can then be further tested in serum/plasma or other body fluids.
A number of mass spectrometry (MS)-based studies have been carried out to profile the secretomes of various cancers [3, 9–12]. Stable isotope labeling by amino acids in cell culture (SILAC) has become a method of choice for accurate measurements of protein expression especially in experiments employing cell culture [13]. SILAC coupled with LC-MS/MS analysis has been successfully applied to investigate the secretome of pancreatic [14], esophageal [3] and head and neck cancers [10]. Two recent studies on the analysis of secreted proteins in gastric cancer have identified cathepsin S and granulin as candidate markers for gastric cancer [15, 16]. In this study, we employed a SILAC-based mass spectrometric approach and carried out differential proteomic analysis of secreted proteins from gastric cancer cells. A panel of gastric cancer cell lines and a non-neoplastic gastric epithelial cell line were used in this study. Secretome from the cancer cell lines were pooled in order to minimize the effects of heterogeneity arising from independent analysis of different cell lines. In this study, we identified 2,205 proteins that are secreted by the tumor cells. To our knowledge, this is the largest set of proteins reported from the conditioned media pertaining to gastric cancer. The proteins that were found to be overexpressed in the secretome of gastric cancer cells could prove useful as potential biomarkers after validating them in serum/plasma or other body fluids such as urine.
2. MATERIALS AND METHODS
SILAC labeling and cell culture
The gastric cancer cell lines, AGS, KatoIII, NCI-N87, SNU-1, SNU-5 and SNU-16 were obtained from ATCC. The non-neoplastic gastric epithelial cell line, HFE-145 was derived by immortalization with SV40 large T antigen as previously described [17]. SNU-1, SNU-16 and NCI-N87 were grown in RPMI supplemented with 10% FBS. SNU-5 and KatoIII were grown in IMDM supplemented with 20% FBS, AGS was grown in DMEM-F12 supplemented with 10% FBS. The normal gastric epithelial cell line was grown in RPMI containing 13C6 arginine and 13C6 lysine supplemented with 10% FBS.
2.1. Secretome collection and processing
Equal number of cells from each cell line was grown to ~80% confluence. The cells were washed extensively and then serum-starved in RPMI medium containing appropriate amino acids for 12 hours and processed as previously described [3]. Briefly, following serum starvation, the conditioned media from each cell line was collected, centrifuged at 800 x g for 10 minutes to remove any detached cells floating in the medium. The supernatant was filtered using a 0.22 µm filter. The filtered supernatant was subsequently concentrated using 3 kDa cutoff filters (Millipore, Billerica, MA). Protein concentration was measured using Lowry’s assay. Equal amount of protein from pooled tumor secretome and the normal secretome were mixed.
2.2. Sample fractionation
2.2.1. In-gel digestion
Approximately 250 µg of protein from pooled (neoplastic and non-neoplastic) secretomes were resolved on a 10% SDS-PAGE gel. The gel was stained with colloidal coomassie blue (Invitrogen, Carlsbad, CA). Twenty five gel bands were excised, destained and subjected to in-gel digestion as previously described [18]. Briefly, the excised bands were reduced with 5 mM dithiothreitol in 40 mM ammonium bicarbonate at 60˚C for 30 min followed by alkylation with 10 mM iodoacetamide in 40 mM ammonium bicarbonate for 10 min in the dark. Digestion was carried out using trypsin (modified sequencing grade; Promega, Madison, WI) at 37˚C for 16 hours. The peptides were extracted from the gel slices, dried and stored at −80˚C until LC-MS/MS analysis.
2.2.2. In-solution digestion and SCX fractionation
Approximately 250 µg of protein from tumor and normal mixed secretome were subjected to in solution digestion as described previously [18]. The proteins in solution were reduced with 5 mM dithiothreitol followed by alkylation with 10mM iodoacetamide. Digestion was carried out using trypsin (modified sequencing grade; Promega, Madison, WI) at 37˚C for 16 hours. The digested peptides were fractionated by strong cation exchange chromatography using PolySULFOETHYL A column (PolyLC, Columbia, MD) (100 × 2.1 mm, 5 µm particles with 300 Å pores). Forty SCX fractions (0.5 mL) were collected from a 0–350 mM KCl gradient in the presence of 10 mM potassium phosphate buffer (pH 2.85), containing 25% acetonitrile for 70 min at a flow rate of 0.2 mL/min. Solvent A contained 10 mM potassium phosphate buffer, pH 2.85, 25% acetonitrile and solvent B contained 10 mM potassium phosphate buffer, 350 mM KCl, pH 2.85, 25% acetonitrile.. The fractions were pooled to 12 fractions based on the absorption intensity. The obtained fractions were desalted using C18-stagetips. The cleaned fractions were dried and stored at −80°C until mass spectrometry analysis.
2.3. LC-MS/MS analysis
Nanoflow electrospray ionization tandem mass spectrometric analysis of peptide samples was carried out using LTQ-Orbitrap Velos mass spectrometer (Thermo Scientific, Bremen, Germany) interfaced with Agilent’s 1200 Series nanoflow LC system as previously described [19]. The chromatographic capillary columns used were packed with Magic C18 AQ (Michrom Bioresources, 5 µm particle size, pore size 100Å) reversed phase material in 100% acetonitrile at a pressure of 1000 psi. The peptide sample from each fractionation method was enriched using a trap column (75 µm × 2 cm) at a flow rate of 3 µl/min and separated on an analytical column (75 µm × 10 cm) at a flow rate of 350 nl/min. The peptides were eluted using a linear gradient of 7–30% acetonitrile over 50 min. Mass spectrometry analysis was carried out in a data dependent manner with full scans acquired in the Orbitrap mass analyzer at a mass resolution of 60,000 at 400 m/z. For each cycle, twenty most intense precursor ions from a survey scan were selected for MS/MS and detected at a mass resolution of 15,000 at m/z 400. The fragmentation was carried out using higher-energy collision dissociation as the activation method with 40% normalized collision energy. The ions selected for fragmentation were excluded for 30 s. The automatic gain control for full FT MS was set to 1 million ions and for FT MS/MS was set to 0.1 million ions with a maximum time of accumulation of 750 ms and 100 ms, respectively. For accurate mass measurements, the lock mass option was enabled using the polydimethylcyclosiloxane (m/z, 445.1200025) ion.
2.4 Data analysis
The raw data obtained was processed using Proteome Discoverer (Version 1.3.0.339) software (Thermo Fisher Scientific, Bremen, Germany) workflow and searched using Mascot and Sequest search algorithms against NCBI RefSeq release 49 human protein database containing 32,967 sequences and known contaminants. The search parameters used were oxidation of methionine, SILAC modifications of arginine and lysine (13C6 R and 13C6 K) as variable modifications and carbamidomethylation of cysteine residues as fixed modification. Precursor ion quantitation node was added in the workflow for SILAC quantitation. A maximum of one missed cleavage was allowed for tryptic peptides. False discovery rate was calculated by enabling the peptide sequence analysis using decoy database. Mass error window of 20 ppm and 0.1 Da were allowed for MS and MS/MS, respectively. FDR of 1% was used as a cut-off value for reporting identified peptides. The peptide and protein data were extracted using high peptide confidence and top one peptide rank filters.
2.5. Data submission
The mass spectrometry data reported in this study can be downloaded from proteomecommons.org tranche network using the following hash 2viXRiUSVPO+Acmbw2o8L79QITHkILBelnoJ+D/6V41pA250ygXmUuYCqz8hAWw1oHGErVejZ/+3FIb7sWJCk9Zt7qQAAAAAAAAStQ== The peptide identifications and associated MS/MS spectra are available from Human Proteinpedia [20] (HuPA_00696)
2.6. Immunohistochemical labeling
Tissue arrays prepared by JCR (Universidad de La Frontera, Temuco, Chile) were used for the analysis. Immunohistochemical staining was carried out as previously described [21]. Briefly, formalin fixed paraffin embedded tissue sections were deparaffinized and antigen retrieval was performed for 20 minutes in antigen retrieval buffer. Endogenous peroxidases were quenched using a blocking solution, followed by washes with wash buffer (Phosphate buffered saline with 0.05% Tween). The sections were incubated with primary antibody overnight at 4°C. Anti-PCSK9 was purchased from Epitomics (T2283) and used at 1:50 dilution. Anti-PDAP1 was purchased from PTGlab (Cat # 15081) and used at 1:50 dilution. Anti-LMAN2 was purchased from Sigma Aldrich (Cat # AV46788) and used at 1:250 dilution. Following incubation with respective primary antibodies, the sections were rinsed thrice with wash buffer. The tissue arrays were then incubated with horseradish peroxidase conjugated rabbit secondary antibody. Any excess secondary antibody was washed with wash buffer followed by addition of DAB substrate. The signal was developed using DAB chromogen (DAKO). Tissue sections were then observed under the microscope. The immunohistochemical labeling was assessed by an experienced pathologist and the intensity of staining scored as negative (0), weak (1+) moderate (2+) or strong (3+). Chi square test was carried out to determine the significance of expression of the candidate markers in tumors tissues as compared to normal mucosa.
3. RESULTS AND DISCUSSION
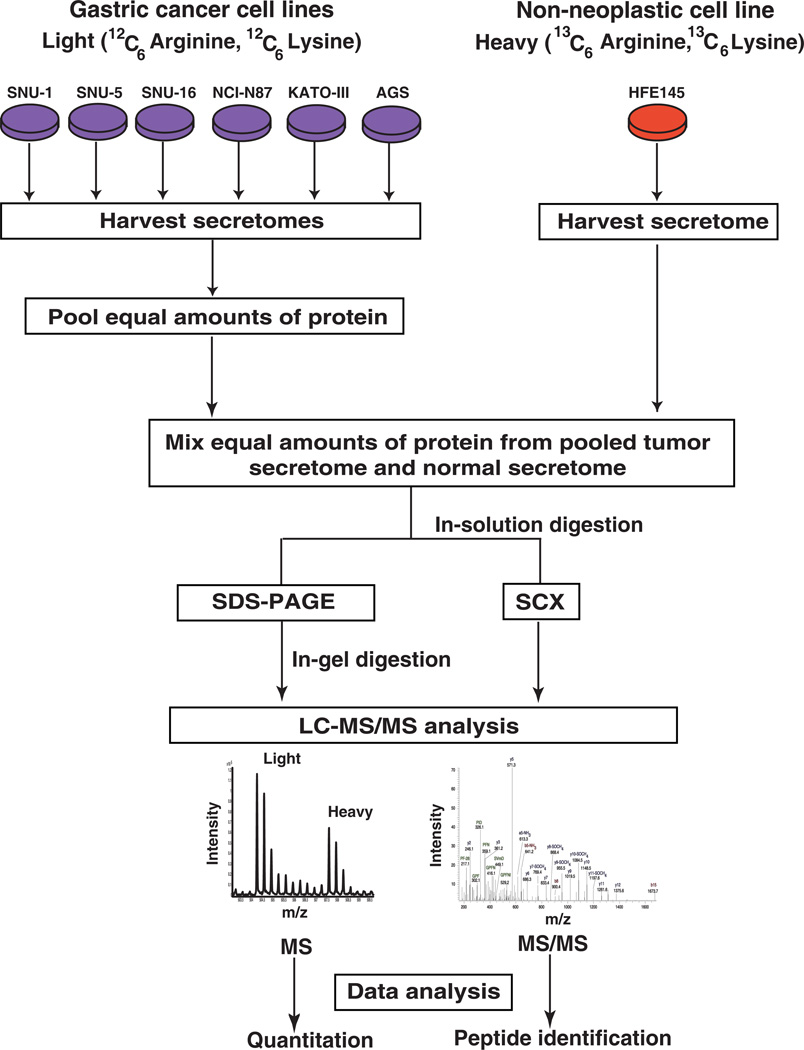
We carried out quantitative proteomic analyses to discover proteins that are secreted from gastric cancer cells. The workflow employed during this analysis is illustrated in Figure 1.
Figure 1. Workflow employed for the analysis of gastric cancer secretome.
SILAC was employed to identify the more abundant proteins in the secretome of gastric cancer cells compared to the normal cells. Gastric cancer cell lines (AGS, SNU-1, SNU-1, SNU-5, SNU-16, NCI-N87, AGS and KATO-III) were grown in medium containing normal arginine and lysine. The normal gastric epithelial cell line was grown in a medium containing heavy arginine and lysine. Equal amounts of protein from pooled tumor secretome and from normal secretome were mixed. Proteins were subjected to fractionation by SDS-PAGE and strong cation exchange chromatography. The fractionated proteins were subjected to LC-MS/MS analysis using an LTQ-Orbitrap Velos mass spectrometer. The data was searched and quantitated using the Proteome Discoverer software suite.
3.1. Quantitative mass spectrometric analysis of the gastric cancer secretome
LC-MS/MS analysis of the pooled secretome from neoplastic and non-neoplastic gastric cells led to the identification of 2,205 proteins from 32 fractions. Of these, 263 proteins were found to be present in higher abundance (>4-fold) and 45 proteins were found to be less abundant (<4-fold) in the secretome of tumor cells when compared to that of normal gastric epithelial cells. A complete list of the identified peptides and the corresponding proteins is provided in Supplementary Tables 1 and 2. Among the identified proteins, 761 were identified based on single peptides, 398 were based on evidence from two unique peptides, and 1,130 proteins were based on evidence from more than two unique peptides. Annotated spectra for all the single peptide identifications are provided in Supplementary Figure 1
3.2. Bioinformatics analysis of the secreted proteins in gastric cancer
Secretory proteins typically contain an N-terminal signal peptide that directs them to the endoplasmic reticulum [18] and are facilitated by vesicular transport to the plasma membrane where the vesicles fuse with the membrane releasing them to the extracellular space. A smaller subset of proteins are also known to be secreted via non-classical secretory mechanisms including endosomal recycling, plasma membrane transporter proteins, membrane blebbing and membrane flip-flop events [22]. Data from the current study was searched against the Human Protein Reference Database (http://www.hprd.org) [23] that contains manually curated information from published literature. Twenty percent of identified proteins contained an N-terminal signal peptide and 14 percent had experimental evidence for their presence in the extracellular compartment. Of the remaining identified proteins, 652 were predicted to be secreted by the non-classical secretory pathways [24]. To ascertain whether identified proteins were found in body fluids, we searched Human Proteinpedia (http://www.humanproteinpedia.org) [20], a community portal for sharing and integration of human protein data and HPRD. We found 70% of identified proteins to have been previously reported in other body fluids (Supplementary Table 1).
3.3. Known proteins differentially expressed in gastric cancer
In the current study, we identified a number of proteins which have previously been associated with gastric cancer. A partial list of these proteins is provided in Table 1. Kallikreins are a subgroup of serine proteases with multiple biological functions [25]. Four members of the kallikreins family were overexpressed in the secretome of gastric cancer cells. KLK10, KLK11 and KLK6 have been previously reported to be overexpressed in gastric cancer [25–27]. In addition to these previously reported kallikreins, we report here a novel kallikrein, KLK14, as being abundantly present in the secretome of gastric cancer. Claudins belong to the family of tight junction proteins involved in maintaining cell polarity in epithelial and endothelial cells [28]. Members of this family have been reported to be dysregulated in gastric cancer [28–30]. Rendon-Huerta et al have reported overexpression of Claudins 6, 7 and 9 in gastric adenocarcinoma. Subsequently, forced overexpression of these proteins in gastric adenocarcinoma cell line, AGS has proven sufficient for gastric tumorigenesis [31]. In agreement with literature, we found 10-fold overexpression of claudin 6 in the secretome of tumor cell lines compared to that of normal cells. This molecule was also found to be 3-fold upregulated at the mRNA level in gastric adenocarcinoma tissues as compared to adjacent non-tumor tissues [32]. Furthermore, we observed 2.8-fold overexpression of tight junction protein 1 (TJP1), which is known to be increased in cells overexpressing claudin 6 [31]. Glutathione S-transferase theta 1 (GSTT1) belongs to the theta family of glutathione S-transferase enzymes that play an essential role in the protection of tissues against harmful toxins and carcinogens. In humans, two theta class isoenzymes, GSTT1 and GSTT2 have been described [33].GSTT1 has been reported to be expressed at significantly higher levels in normal gastric mucosa than in gastric cancer tissues [34]. In concordance with this previous study, we also found GSTT1 to be 2.5-fold downregulated in non-neoplastic gastric epithelial cells as compared to tumor cells. These results indicate that the experimental approach used in the current study is a viable approach for discovering candidate cancer biomarkers.
Table 1.
A partial list of overexpressed proteins previously reported in gastric cancer
| Gene Symbol |
Protein Description | Fold change (Tumor/ Normal) |
Unique Peptides |
Citation |
|---|---|---|---|---|
| MIA | Melanoma-derived growth regulatory protein | Undetectable in normal | 3 | Aung et al, 2003[57] |
| PRSS2 | Protease, serine, 2 | Undetectable in normal | 2 | Rajkumar et al, 2010[58] |
| KLK11 | Kallikrein 11 | 18.1 | 5 | Wen et al, 2011[27] |
| KLK6 | Kallikrein-related peptidase 6 | 41.5 | 10 | Nagahara et al, 2005[26] |
| ADAM10 | Disintegrin and metalloproteinase domain-containing protein 10 | 20.6 | 9 | Wang et al, 2010[59] |
| KLK10 | Kallikrein-related peptidase 10 | 4.1 | 9 | Feng et al, 2006[60] |
| CHI3L1 | Chitinase 3-like 1 | 7.2 | 7 | Bi et al, 2009[61] |
| TGFB2 | Transforming growth factor beta-2 | 13.9 | 5 | Vagenas et al, 2007[62] |
| GDF15 | Growth/differentiation factor 15 | Undetectable in normal | 2 | Lee et al, 2003[63] |
| LGALS3BP | Galectin-3-binding protein | 7.1 | 18 | Park et al, 2007[64] |
| IGFBP6 | Insulin-like growth factor- binding protein 6 | 8.2 | 5 | Yi et al, 2001[65] |
3.4. Novel proteins differentially expressed in gastric cancer
We also found a number of novel candidates that have not been previously reported in the context of gastric cancer. A partial list of these proteins is provided in Table 2. The protein disulphide isomerase (PDI) family of enzymes is a multidomain, multi-functional component of the larger thioredoxin superfamily. PDI proteins are localized to the endoplasmic reticulum and are involved in protein folding [35]. For the first time, we report overexpression of three members of the PDI family in the secretome of gastric cancer. Protein disulphide isomerase family A, member 3 (PDIA3), also known as glucose-regulated protein 58 kDa (GRP58) is known to be involved in the regulation of folding of newly synthesized glycoproteins. It has been reported to be overexpressed in hepatocellular carcinoma and ESCC [3, 36]. In the current study, we observed 3.3-fold overexpression of PDIA3 in gastric cancer. Protein disulphide isomerase family A, member 4 (PDIA4) is a 72k Da protein also known as ERP72. It is a less characterized member of the PDI family and is thought to be a stress-induced protein. Recently, it has been shown to be overexpressed in ESCC [36]. In the current study, we found 2.8-fold overexpression of PDIA4 in secretome from gastric cancer. PDIA6, another member of the PDI family was overexpressed 3.6-fold in the gastric cancer secretome. Future studies will be necessary to elucidate the roles of these PDI family members in gastric tumorigenesis. Other proteins that we report for the first time in the gastric cancer secretome include heparan sulfate proteoglycan 2 (HSPG2), lectin mannose binding 2 (LMAN2), proprotein convertase subtilisin/kexin type 9 (PCSK9), and twisted gastrulation protein (TWSG1). HSPG2 (perlecan), is a glycosaminoglycan known to bind to many extracellular matrix components and cell surface proteins. HSPG2 has been shown to be involved in numerous cellular processes such as cell adhesion, cell-cell communication, cell signaling and ECM-receptor interaction. It is a crucial molecule in the tumor microenvironment and is known to promote tumor cell growth [37]. In our study, we observed 3.4 fold overexpression of HSPG2 in the neoplastic secretome as compared to secretome of non-neoplastic cells. HSPG2 has also been reported to be overexpressed in prostate [38] and pancreatic cancer [14]. TWSG1 was originally discovered as a bone morphogenic protein (BMP) binding protein and involved in BMP signaling in Drosphila [39]. It was considered to be involved in developmental process. Subsequently, it has been shown to be implicated in TGF-beta signaling [40]. It was found to be 4.5-fold overexpressed in gastric cancer secretome. Further studies are needed to decipher the role of this protein in gastric cancer. A novel protein that was downregulated in the gastric cancer secretome was Follistatin- related protein 1 (FSTL1, Figure 2D), which is a secreted myokine that is highly similar to follistatin in sequence and consists of an FS module [41]. FSTL1 regulates endothelial cell function and revascularization in response to ischemic insult by activating nitric oxide synthase-dependent mechanism [42]. In endometrial and ovarian cancers, its expression was downregulated suggesting a tumor suppressor role for FSTL1 [43]. There are no earlier reports of its association with gastric cancer. In our study, it was downregulated 25-fold in gastric cancer.
Table 2.
A partial list of novel proteins overexpressed in gastric cancer secretome
| Gene Symbol |
Protein description | Fold change (Tumor/Normal) |
Unique peptides |
|---|---|---|---|
| F11R | Junctional adhesion molecule A | Undetectable in normal | 3 |
| QPCT | Glutaminyl-peptide cyclotransferase | 20 | 3 |
| PDAP1 | 28 kDa heat- and acid-stable phosphoprotein | 5.2 | 3 |
| LGALS4 | Galectin-4 | 38.7 | 11 |
| TWSG1 | Twisted gastrulation protein homolog 1 | 7.1 | 1 |
| HSPA4L | Heat shock 70 kDa protein 4L | 16.0 | 6 |
| GALNS | N-acetylgalactosamine-6-sulfatase | 7.6 | 1 |
| LMAN2 | Lectin, mannose-binding 2 | 6.0 | 4 |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 | Undetectable in normal | 13 |
| HSPA5 | 78 kDa glucose-regulated protein | 4.8 | 24 |
| PSMC5 | 26S protease regulatory subunit 8 | 2.5 | 4 |
| NCL | Nucleolin | 3.3 | 23 |
| COL1A1 | Alpha 1 type I collagen | 0.2 | 19 |
| COL12A1 | Collagen, type XII, alpha 1 | 0.1 | 27 |
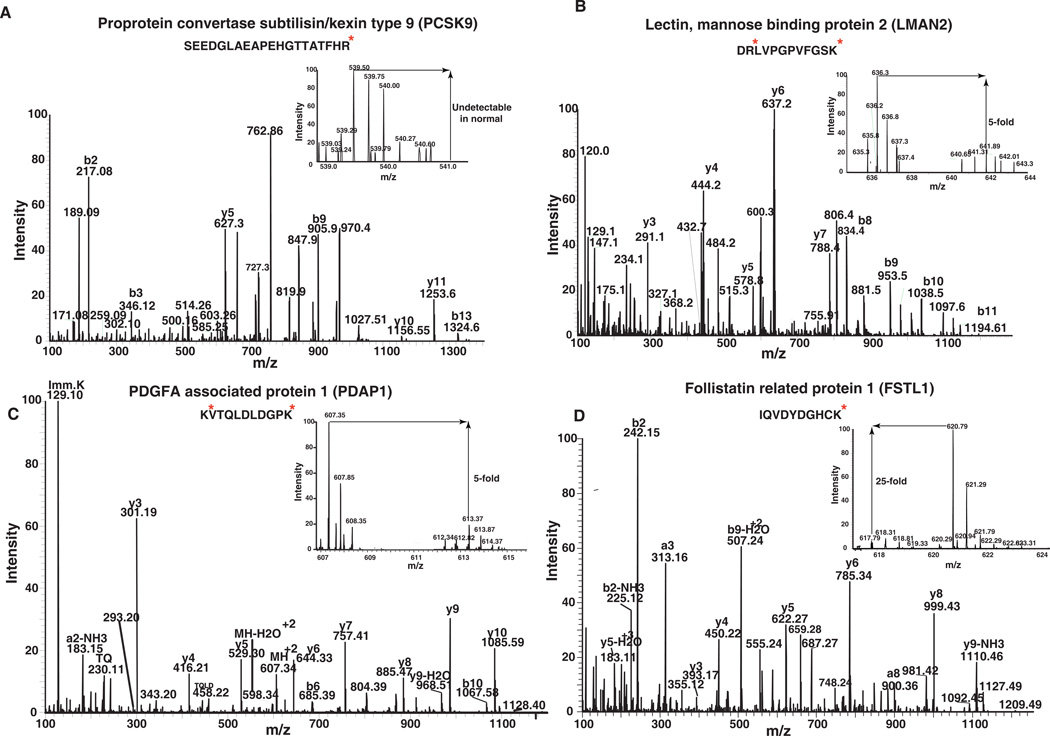
Figure 2. Representative MS and MS/MS spectra of a subset of proteins that were found to be differentially expressed in the gastric cancer secretome.
Representative MS/MS spectra of identified peptides from A) proprotein convertase subtilisin/kexin type 9 (PCSK9) B) Lectin mannose binding 2 (LMAN2) C) PDGFA associated protein 1 (PDAP1) and D) Follistatin related protein 1 (FSTL1) are shown. The MS spectra in the inset provide the relative abundance of the representative peptides in secretome derived from tumor and normal cells.
3.5. Immunohistochemical validation of novel biomarker candidates
Extracellular novel proteins that we found to be highly abundant in the secretome of gastric cancer were validated in a larger set of patient samples using tissue microarrays. A detailed description of the chosen proteins PCSK9, LMAN2, PDAP1 and the pattern of immunohistochemical staining is provided below. Representative MS and MS/MS spectra of some of the peptides identified for these proteins are shown in Figure 2.
3.5.1. Proprotein convertase subtilisin/kexin type 9 (PCSK9)
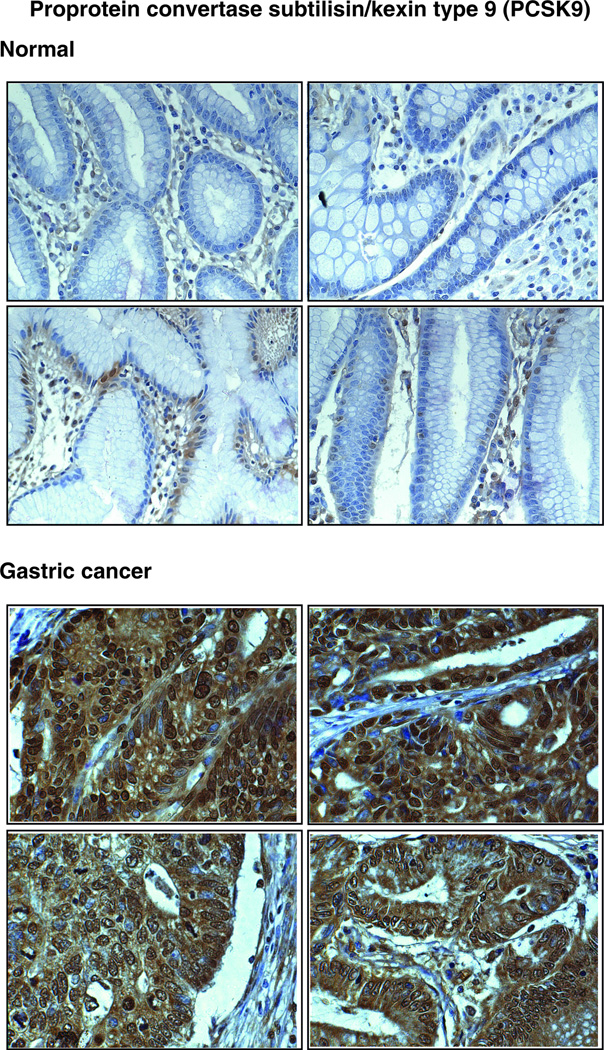
PCSK9 (Figure 2A), is a secreted serine protease and the ninth reported member of the proprotein convertase (PC) family [44]. It was overexpressed 13-fold in the gastric cancer-derived secretome. Members of the PC family are implicated in the activation, inactivation and regulation of cellular localization of proteins that pass through the secretory pathway. A variety of proteins including zymogens, prohormones, adhesion molecules, cytokines and growth factors are processed by PC family members [45]. Other members of the PC family- PC1/PC3, PC2, Furin and PACE4 have been reported to be overexpressed in neuroendocrine or epithelial cancers including pheochromocytomas [46], non-small cell lung carcinoma [47] and small cell lung carcinoma [47]. Furin was shown to be expressed in metastasizing head and neck squamous cell carcinoma [48]. However, there have been no reports pertaining to the involvement of PCSK9 in human cancers. We observed it to be at undetectable levels in the secretome of normal gastric normal mucosa. Immunohistochemical staining clearly showed a significant overexpression of PCSK9 in tumor tissues (Table 3). Figure 3 shows the representative staining pattern of this protein in gastric adenocarcinoma tissues and normal mucosa. It shows predominantly cytoplasmic and extracellular staining. Normal epithelial cells were not stained. However, we observed staining of basal cells in the normal mucosa.
Table 3.
Immunohistochemical validation of potential candidates in gastric cancer
| PCSK9 | PDAP1 | LMAN2 | ||
|---|---|---|---|---|
| Tumor Positive | 60 | 73 | 82 | |
| Strong | 30 | 57 | 43 | |
| Moderate | 12 | 12 | 24 | |
| Weak | 18 | 4 | 15 | |
| Tumor Negative | 40 | 27 | 18 | |
| Normal Positive | 14 | 12 | 10 | |
| p-value | 8.90E-08 | 3.68E-11 | 6.07E-14 | |
Figure 3. Validation of PCSK9 by immunohistochemical labeling.
Representative sections from tissue microarrays for normal gastric tissues and tumor tissues stained with anti-PCSK9 antibody are shown.
3.5.2. Lectin mannose binding protein 2 (LMAN2)
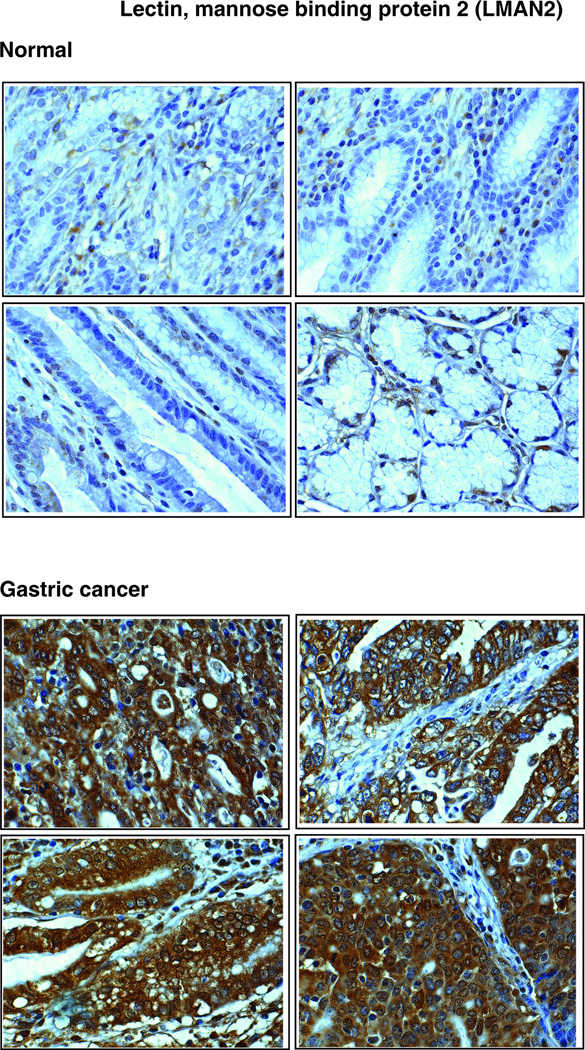
LMAN2 (Figure 2B), a type I integral membrane protein, is an intracellular lectin in the mammalian early secretory pathway [49, 50]. It is also known as vesicular integral membrane protein of 36 kDa (VIP36). LMAN2 is classified as an L-type lectin due to the presence of a luminal carbohydrate recognition domain, which is homologous to the leguminous lectins that have been suggested to play a role in glycoprotein sorting and trafficking [50]. LMAN2 was isolated from epithelial Madin-Darby canine kidney (MDCK) cells [51]. This protein has been shown to be localized to the Golgi apparatus and to structures cycling between the Golgi apparatus and the endoplasmic reticulum (ER) [49]. LMAN2 interacts with high-mannose glycans and has been postulated to bind glycoproteins in the Golgi rather than the ER [52]. Although its sugar binding properties have been characterized in detail, its biological function remains an enigma as no cargo glycoprotein has yet been identified for this lectin. [53]. We report here, for the first time, a 6-fold overexpression of this protein in gastric cancer secretome. LMAN2 was significantly overexpressed in tumor tissues as compared to normal tissues (Table 3). Figure 4 shows a representative staining pattern of this protein in gastric adenocarcinoma tissues and normal mucosa. It predominantly demonstrates cytoplasmic staining. Normal epithelial cells were not stained. However, we were able to observe weak staining of basal cells in the normal mucosa.
Figure 4. Validation of LMAN2 by immunohistochemical labeling.
Representative sections from tissue microarrays for normal gastric tissues and tumor tissues stained with anti-LMAN2 antibody are shown.
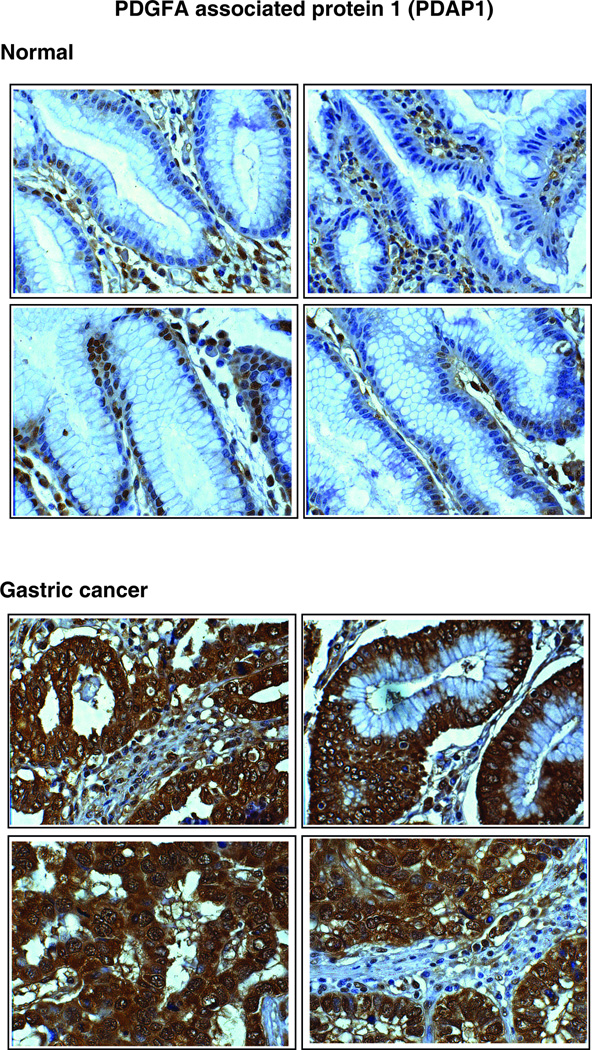
3.5.3. PDGFA associated protein 1 (PDAP1)
PDAP1 (Figure 2C) is a phosphoprotein, isolated from the rat neural retinal cell line, R33. It binds with low affinity to PDGF, a growth factor associated with cellular proliferation and development and modifies its biological activity [54]. PDAP1 was shown to be phosphorylated by casein kinase II, protein kinase C and protein kinase A [55]. PDAP1 was reported to be highly expressed in brain, lung and intestine of new born rats [54]. Recently, PDAP1 was found to be overexpressed in human rectal carcinoma cells [56]. In the current study, we found it to be overexpressed by 5.2-fold in the gastric cancer secretome. PDAP1 was also significantly overexpressed in tumor tissues as compared to normal tissues (Table 3). Figure 5 shows the representative staining pattern of this protein in gastric adenocarcinoma tissues and normal mucosae. It predominantly demonstrates cytoplasmic and stromal staining. Normal epithelial cells were not stained. However, we were able to observe staining of basal cells in the normal mucosa.
Figure 5. Validation of PDAP1 by immunohistochemical labeling.
Representative sections from tissue microarrays for normal gastric tissues and tumor tissues stained with anti-PDAP1 antibody are shown.
4. CONCLUDING REMARKS
Using high resolution mass spectrometry, we identified 2,205 proteins in the secretome, which marks the largest known catalog of secreted proteins in any cancer reported thus far. We identified multiple novel candidate biomarkers for gastric cancer, which can be validated against a larger cohort of patient samples to establish their clinical utility. A large majority of these proteins were known to be localized in the extracellular region or had been reported in body fluids. We validated three novel candidate markers- PCSK9, LMAN2, and PDAP1, and observed 60%, 73% and 82% overexpression, respectively, in the tested gastric adenocarcinoma cases. We believe that candidate biomarkers described in this study have the potential to be developed as valuable biomarkers for early diagnosis and prognosis of gastric cancer.
Supplementary Material
CLINICAL RELEVANCE.
Gastric adenocarcinoma is an aggressive cancer that is associated with poor prognosis and high mortality. Gastric cancer is often diagnosed at an advanced stage resulting in limited therapeutic options. Identification of biomarkers to detect cancer at an early stage has the potential to improve clinical outcome of this disease. Using a SILAC-based quantitative approach coupled with high resolution mass spectrometry, we sought to identify candidate biomarkers for early detection of gastric cancer. Proteins secreted into the bloodstream or other body fluids are attractive candidates as biomarkers for early detection. Because proteomic analysis of serum/plasma is challenging, we analyzed the proteins secreted by tumor cell lines as the secretome contains proteins that could be detected in serum. Our discovery studies provide a list of candidate biomarkers that could now be systematically tested for their utility in serum as biomarkers for early detection of gastric cancer.
ACKNOWLEDGEMENTS
We thank the Department of Biotechnology (DBT) of the Government of India for research support to the Institute of Bioinformatics, Bangalore, India. NAS, HP, NRS and SMP are recipients of Senior Research fellowships from the Council of Scientific and Industrial Research (CSIR), India. YS, SMS and NS are recipients of research fellowships from the University Grants Commission (UGC). T. S. Keshava Prasad is supported by a research grant on “Development of Infrastructure and a Computational Framework for Analysis of Proteomic Data” from DBT. Harsha Gowda is a Wellcome Trust/DBT India Alliance Early Career fellow. We thank Dr. S.K. Shankar and Dr. Anita Mahedavan for providing access to the microscopic facility. This study was supported in part by NIH grants U24CA160036 and G12 MD007597.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest
Supporting information
Supplementary Table 1: A complete list of all the proteins identified in this study.
Supplementary Table 2: List of all the peptides identified from this study.
Supplementary Figures: Annotated MS/MS spectra of all the proteins identified with Single peptide spectral match (PSM).
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Kashyap MK, Harsha HC, Renuse S, Pawar H, et al. SILAC-based quantitative proteomic approach to identify potential biomarkers from the esophageal squamous cell carcinoma secretome. Cancer Biol Ther. 2010;10:796–810. doi: 10.4161/cbt.10.8.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth AD. Curative treatment of gastric cancer: towards a multidisciplinary approach? Crit Rev Oncol Hematol. 2003;46:59–100. doi: 10.1016/s1040-8428(02)00160-9. [DOI] [PubMed] [Google Scholar]
- 5.Ebert MP, Malfertheiner P. Review article: Pathogenesis of sporadic and familial gastric cancer--implications for clinical management and cancer prevention. Aliment Pharmacol Ther. 2002;16:1059–1066. doi: 10.1046/j.1365-2036.2002.01288.x. [DOI] [PubMed] [Google Scholar]
- 6.Brooks JD. Translational genomics: The challenge of developing cancer biomarkers. Genome Res. 2012;22:183–187. doi: 10.1101/gr.124347.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulasingam V, Diamandis EP. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat Clin Pract Oncol. 2008;5:588–599. doi: 10.1038/ncponc1187. [DOI] [PubMed] [Google Scholar]
- 8.Xue H, Lu B, Lai M. The cancer secretome: a reservoir of biomarkers. J Transl Med. 2008;6:52. doi: 10.1186/1479-5876-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polisetty RV, Gupta MK, Nair SC, Ramamoorthy K, et al. Glioblastoma cell secretome: Analysis of three glioblastoma cell lines reveal 148 non-redundant proteins. J Proteomics. 2011;74:1918–1925. doi: 10.1016/j.jprot.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Ralhan R, Masui O, Desouza LV, Matta A, et al. Identification of proteins secreted by head and neck cancer cell lines using LC-MS/MS: Strategy for discovery of candidate serological biomarkers. Proteomics. 2011;11:2363–2376. doi: 10.1002/pmic.201000186. [DOI] [PubMed] [Google Scholar]
- 11.Ji H, Greening DW, Kapp EA, Moritz RL, Simpson RJ. Secretome-based proteomics reveals sulindac-modulated proteins released from colon cancer cells. Proteomics Clin Appl. 2009;3:433–451. doi: 10.1002/prca.200800077. [DOI] [PubMed] [Google Scholar]
- 12.Rangiah K, Tippornwong M, Sangar V, Austin D, et al. Differential secreted proteome approach in murine model for candidate biomarker discovery in colon cancer. J Proteome Res. 2009;8:5153–5164. doi: 10.1021/pr900518v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hathout Y. Approaches to the study of the cell secretome. Expert Rev Proteomics. 2007;4:239–248. doi: 10.1586/14789450.4.2.239. [DOI] [PubMed] [Google Scholar]
- 14.Gronborg M, Kristiansen TZ, Iwahori A, Chang R, et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5:157–171. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Lim SK, Choong LY, Lee H, et al. Cathepsin S mediates gastric cancer cell migration and invasion via a putative network of metastasis-associated proteins. J Proteome Res. 2010;9:4767–4778. doi: 10.1021/pr100492x. [DOI] [PubMed] [Google Scholar]
- 16.Loei H, Tan HT, Lim TK, Lim KH, et al. Mining the Gastric Cancer Secretome: Identification of GRN as a Potential Diagnostic Marker for Early Gastric Cancer. J Proteome Res. 2012 doi: 10.1021/pr201014h. [DOI] [PubMed] [Google Scholar]
- 17.Akhtar M, Cheng Y, Magno RM, Ashktorab H, et al. Promoter methylation regulates Helicobacter pylori-stimulated cyclooxygenase-2 expression in gastric epithelial cells. Cancer Res. 2001;61:2399–2403. [PubMed] [Google Scholar]
- 18.Harsha HC, Molina H, Pandey A. Quantitative proteomics using stable isotope labeling with amino acids in cell culture. Nat Protoc. 2008;3:505–516. doi: 10.1038/nprot.2008.2. [DOI] [PubMed] [Google Scholar]
- 19.Marimuthu A, O'Meally RN, Chaerkady R, Subbannayya Y, et al. A comprehensive map of the human urinary proteome. J Proteome Res. 2011;10:2734–2743. doi: 10.1021/pr2003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathivanan S, Ahmed M, Ahn NG, Alexandre H, et al. Human Proteinpedia enables sharing of human protein data. Nat Biotechnol. 2008;26:164–167. doi: 10.1038/nbt0208-164. [DOI] [PubMed] [Google Scholar]
- 21.Kashyap MK, Marimuthu A, Kishore CJ, Peri S, et al. Genomewide mRNA profiling of esophageal squamous cell carcinoma for identification of cancer biomarkers. Cancer Biol Ther. 2009;8:36–46. doi: 10.4161/cbt.8.1.7090. [DOI] [PubMed] [Google Scholar]
- 22.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 23.Peri S, Navarro JD, Amanchy R, Kristiansen TZ, et al. Development of human protein reference database as an initial platform for approaching systems biology in humans. Genome Res. 2003;13:2363–2371. doi: 10.1101/gr.1680803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendtsen JD, Jensen LJ, Blom N, Von Heijne G, Brunak S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng Des Sel. 2004;17:349–356. doi: 10.1093/protein/gzh037. [DOI] [PubMed] [Google Scholar]
- 25.Yousef GM, Diamandis EP. Tissue kallikreins: new players in normal and abnormal cell growth? Thromb Haemost. 2003;90:7–16. [PubMed] [Google Scholar]
- 26.Nagahara H, Mimori K, Utsunomiya T, Barnard GF, et al. Clinicopathologic and biological significance of kallikrein 6 overexpression in human gastric cancer. Clin Cancer Res. 2005;11:6800–6806. doi: 10.1158/1078-0432.CCR-05-0943. [DOI] [PubMed] [Google Scholar]
- 27.Wen YG, Wang Q, Zhou CZ, Yan DW, et al. Identification and validation of Kallikrein-ralated peptidase 11 as a novel prognostic marker of gastric cancer based on immunohistochemistry. J Surg Oncol. 2011;104:516–524. doi: 10.1002/jso.21981. [DOI] [PubMed] [Google Scholar]
- 28.Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10:235. doi: 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resnick MB, Gavilanez M, Newton E, Konkin T, et al. Claudin expression in gastric adenocarcinomas: a tissue microarray study with prognostic correlation. Hum Pathol. 2005;36:886–892. doi: 10.1016/j.humpath.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal R, Mori Y, Cheng Y, Jin Z, et al. Silencing of claudin-11 is associated with increased invasiveness of gastric cancer cells. PLoS One. 2009;4:e8002. doi: 10.1371/journal.pone.0008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zavala-Zendejas VE, Torres-Martinez AC, Salas-Morales B, Fortoul TI, et al. Claudin-6, 7, or 9 overexpression in the human gastric adenocarcinoma cell line AGS increases its invasiveness, migration, and proliferation rate. Cancer Invest. 2011;29:1–11. doi: 10.3109/07357907.2010.512594. [DOI] [PubMed] [Google Scholar]
- 32.Marimuthu A, Jacob HKC, Jakharia A, Subbannayya Y, Keerthikumar S, Kashyap MK, Goel R, Balakrishnan L, Dwivedi S, Pathare S, Dikshit JB, Maharudraiah J, Singh S, Kumar GSS, Vijayakumar M, Veerendra Kumar KV, Premalatha CS, Tata P, Hariharan R, Roa JC, Prasad TSK, Chaerkady R, Kumar RV, Pandey A. Gene expression profiling of gastric cancer. Journal of Proteomics and Bioinformatics. 2011;4:74–82. [PMC free article] [PubMed] [Google Scholar]
- 33.Juronen E, Tasa G, Uuskula M, Pooga M, Mikelsaar AV. Purification, characterization and tissue distribution of human class theta glutathione S-transferase T1-1. Biochem Mol Biol Int. 1996;39:21–29. doi: 10.1080/15216549600201021. [DOI] [PubMed] [Google Scholar]
- 34.de Bruin WC, Wagenmans MJ, Board PG, Peters WH. Expression of glutathione S-transferase theta class isoenzymes in human colorectal and gastric cancers. Carcinogenesis. 1999;20:1453–1457. doi: 10.1093/carcin/20.8.1453. [DOI] [PubMed] [Google Scholar]
- 35.Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pawar H, Kashyap MK, Sahasrabuddhe NA, Renuse S, et al. Quantitative tissue proteomics of esophageal squamous cell carcinoma for novel biomarker discovery. Cancer Biol Ther. 2011:12. doi: 10.4161/cbt.12.6.16833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010;277:3904–3923. doi: 10.1111/j.1742-4658.2010.07800.x. [DOI] [PubMed] [Google Scholar]
- 38.Datta MW, Hernandez AM, Schlicht MJ, Kahler AJ, et al. Perlecan, a candidate gene for the CAPB locus, regulates prostate cancer cell growth via the Sonic Hedgehog pathway. Mol Cancer. 2006;5:9. doi: 10.1186/1476-4598-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oelgeschlager M, Larrain J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzachanis D, Li L, Lafuente EM, Berezovskaya A, et al. Twisted gastrulation (Tsg) is regulated by Tob and enhances TGF-beta signaling in activated T lymphocytes. Blood. 2007;109:2944–2952. doi: 10.1182/blood-2006-03-006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwijsen A, Blockx H, Van Arnhem W, Willems J, et al. Characterization of a rat C6 glioma-secreted follistatin-related protein (FRP). Cloning and sequence of the human homologue. Eur J Biochem. 1994;225:937–946. doi: 10.1111/j.1432-1033.1994.0937b.x. [DOI] [PubMed] [Google Scholar]
- 42.Ouchi N, Oshima Y, Ohashi K, Higuchi A, et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan QK, Ngan HY, Ip PP, Liu VW, et al. Tumor suppressor effect of follistatin-like 1 in ovarian and endometrial carcinogenesis: a differential expression and functional analysis. Carcinogenesis. 2009;30:114–121. doi: 10.1093/carcin/bgn215. [DOI] [PubMed] [Google Scholar]
- 44.Lopez D. PCSK9: an enigmatic protease. Biochim Biophys Acta. 2008;1781:184–191. doi: 10.1016/j.bbalip.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Taylor NA, Van De Ven WJ, Creemers JW. Curbing activation: proprotein convertases in homeostasis and pathology. FASEB J. 2003;17:1215–1227. doi: 10.1096/fj.02-0831rev. [DOI] [PubMed] [Google Scholar]
- 46.Konoshita T, Gasc JM, Villard E, Takeda R, et al. Expression of PC2 and PC1/PC3 in human pheochromocytomas. Mol Cell Endocrinol. 1994;99:307–314. doi: 10.1016/0303-7207(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 47.Mbikay M, Sirois F, Yao J, Seidah NG, Chretien M. Comparative analysis of expression of the proprotein convertases furin, PACE4, PC1 and PC2 in human lung tumours. Br J Cancer. 1997;75:1509–1514. doi: 10.1038/bjc.1997.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bassi DE, Mahloogi H, Al-Saleem L, Lopez De Cicco R, et al. Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. Mol Carcinog. 2001;31:224–232. doi: 10.1002/mc.1057. [DOI] [PubMed] [Google Scholar]
- 49.Fullekrug J, Scheiffele P, Simons K. VIP36 localisation to the early secretory pathway. J Cell Sci. 1999;112(Pt 17):2813–2821. doi: 10.1242/jcs.112.17.2813. [DOI] [PubMed] [Google Scholar]
- 50.Kamiya Y, Kamiya D, Yamamoto K, Nyfeler B, et al. Molecular basis of sugar recognition by the human L-type lectins ERGIC-53, VIPL, and VIP36. J Biol Chem. 2008;283:1857–1861. doi: 10.1074/jbc.M709384200. [DOI] [PubMed] [Google Scholar]
- 51.Fiedler K, Simons K. A putative novel class of animal lectins in the secretory pathway homologous to leguminous lectins. Cell. 1994;77:625–626. doi: 10.1016/0092-8674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 52.Hauri H, Appenzeller C, Kuhn F, Nufer O. Lectins and traffic in the secretory pathway. FEBS Lett. 2000;476:32–37. doi: 10.1016/s0014-5793(00)01665-3. [DOI] [PubMed] [Google Scholar]
- 53.Reiterer V, Nyfeler B, Hauri HP. Role of the lectin VIP36 in post-ER quality control of human alpha1-antitrypsin. Traffic. 2010;11:1044–1055. doi: 10.1111/j.1600-0854.2010.01078.x. [DOI] [PubMed] [Google Scholar]
- 54.Fischer WH, Schubert D. Characterization of a novel platelet-derived growth factor-associated protein. J Neurochem. 1996;66:2213–2216. doi: 10.1046/j.1471-4159.1996.66052213.x. [DOI] [PubMed] [Google Scholar]
- 55.Shen L, Huang KP, Chen HC, Huang FL. Molecular cloning and characterization of a novel casein kinase II substrate, HASPP28, from rat brain. Arch Biochem Biophys. 1996;327:131–141. doi: 10.1006/abbi.1996.0101. [DOI] [PubMed] [Google Scholar]
- 56.Choi SY, Jang JH, Kim KR. Analysis of differentially expressed genes in human rectal carcinoma using suppression subtractive hybridization. Clin Exp Med. 2011;11:219–226. doi: 10.1007/s10238-010-0130-5. [DOI] [PubMed] [Google Scholar]
- 57.Aung PP, Oue N, Mitani Y, Nakayama H, et al. Systematic search for gastric cancer-specific genes based on SAGE data: melanoma inhibitory activity and matrix metalloproteinase-10 are novel prognostic factors in patients with gastric cancer. Oncogene. 2006;25:2546–2557. doi: 10.1038/sj.onc.1209279. [DOI] [PubMed] [Google Scholar]
- 58.Rajkumar T, Vijayalakshmi N, Gopal G, Sabitha K, et al. Identification and validation of genes involved in gastric tumorigenesis. Cancer Cell Int. 2010;10:45. doi: 10.1186/1475-2867-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang YY, Ye ZY, Li L, Zhao ZS, et al. ADAM 10 is associated with gastric cancer progression and prognosis of patients. J Surg Oncol. 2011;103:116–123. doi: 10.1002/jso.21781. [DOI] [PubMed] [Google Scholar]
- 60.Feng B, Xu WB, Zheng MH, Ma JJ, et al. Clinical significance of human kallikrein 10 gene expression in colorectal cancer and gastric cancer. J Gastroenterol Hepatol. 2006;21:1596–1603. doi: 10.1111/j.1440-1746.2006.04228.x. [DOI] [PubMed] [Google Scholar]
- 61.Bi J, Lau SH, Lv ZL, Xie D, et al. Overexpression of YKL-40 is an independent prognostic marker in gastric cancer. Hum Pathol. 2009;40:1790–1797. doi: 10.1016/j.humpath.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Vagenas K, Spyropoulos C, Gavala V, Tsamandas AC. TGFbeta1, TGFbeta2, and TGFbeta3 protein expression in gastric carcinomas: correlation with prognostics factors and patient survival. J Surg Res. 2007;139:182–188. doi: 10.1016/j.jss.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Lee DH, Yang Y, Lee SJ, Kim KY, et al. Macrophage inhibitory cytokine-1 induces the invasiveness of gastric cancer cells by up-regulating the urokinase-type plasminogen activator system. Cancer Res. 2003;63:4648–4655. [PubMed] [Google Scholar]
- 64.Park YP, Choi SC, Kim JH, Song EY, et al. Up-regulation of Mac-2 binding protein by hTERT in gastric cancer. Int J Cancer. 2007;120:813–820. doi: 10.1002/ijc.22369. [DOI] [PubMed] [Google Scholar]
- 65.Yi HK, Hwang PH, Yang DH, Kang CW, Lee DY. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur J Cancer. 2001;37:2257–2263. doi: 10.1016/s0959-8049(01)00269-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.